Nitric Acid-Treated Carbon Fibers with Enhanced Hydrophilicity for Candida tropicalis Immobilization in Xylitol Fermentation
Abstract
:1. Introduction
2. Results and Discussion
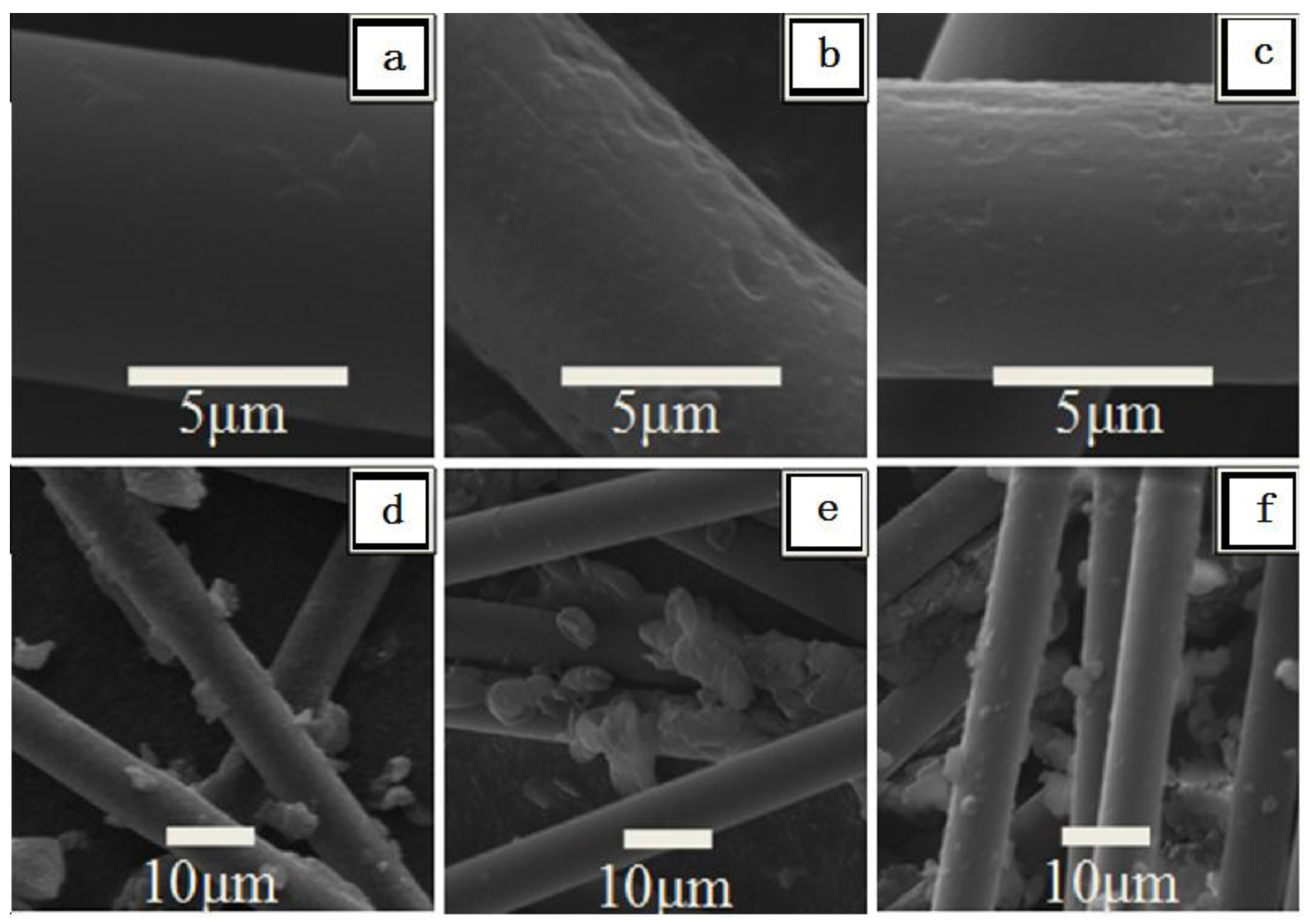
2.1. SEM of Raw and Treated CF
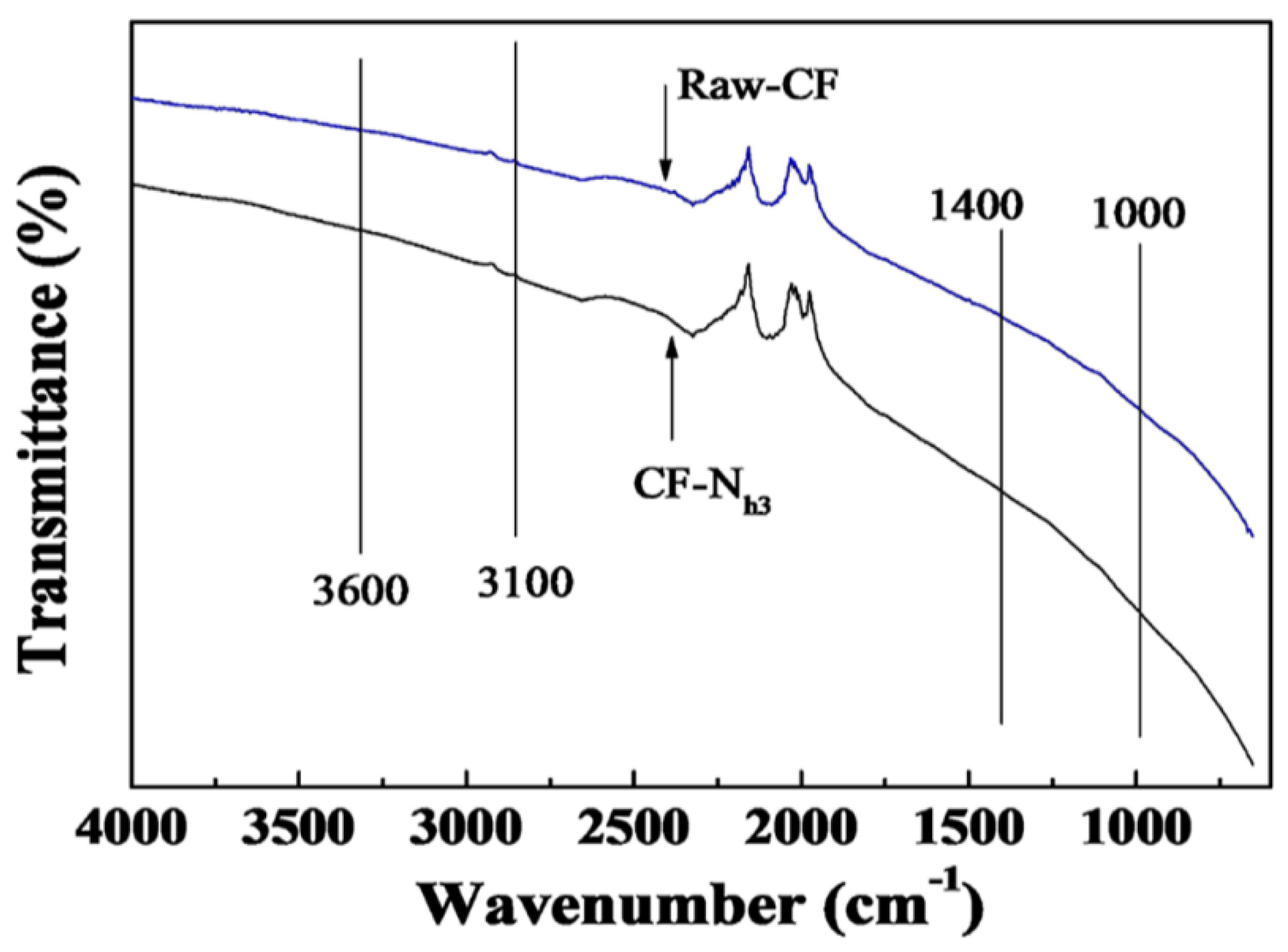
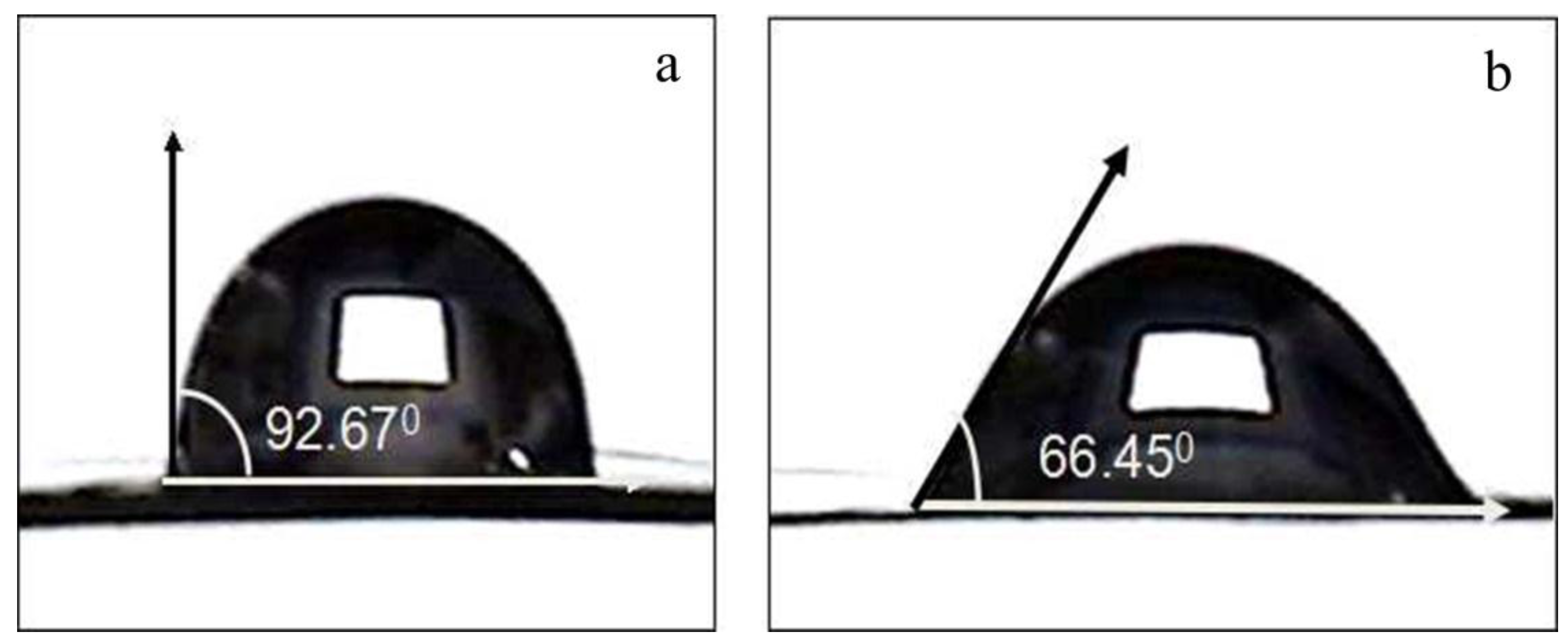
2.2. Effects of Nitric Acid Treatment on Surface Properties of CF and IE
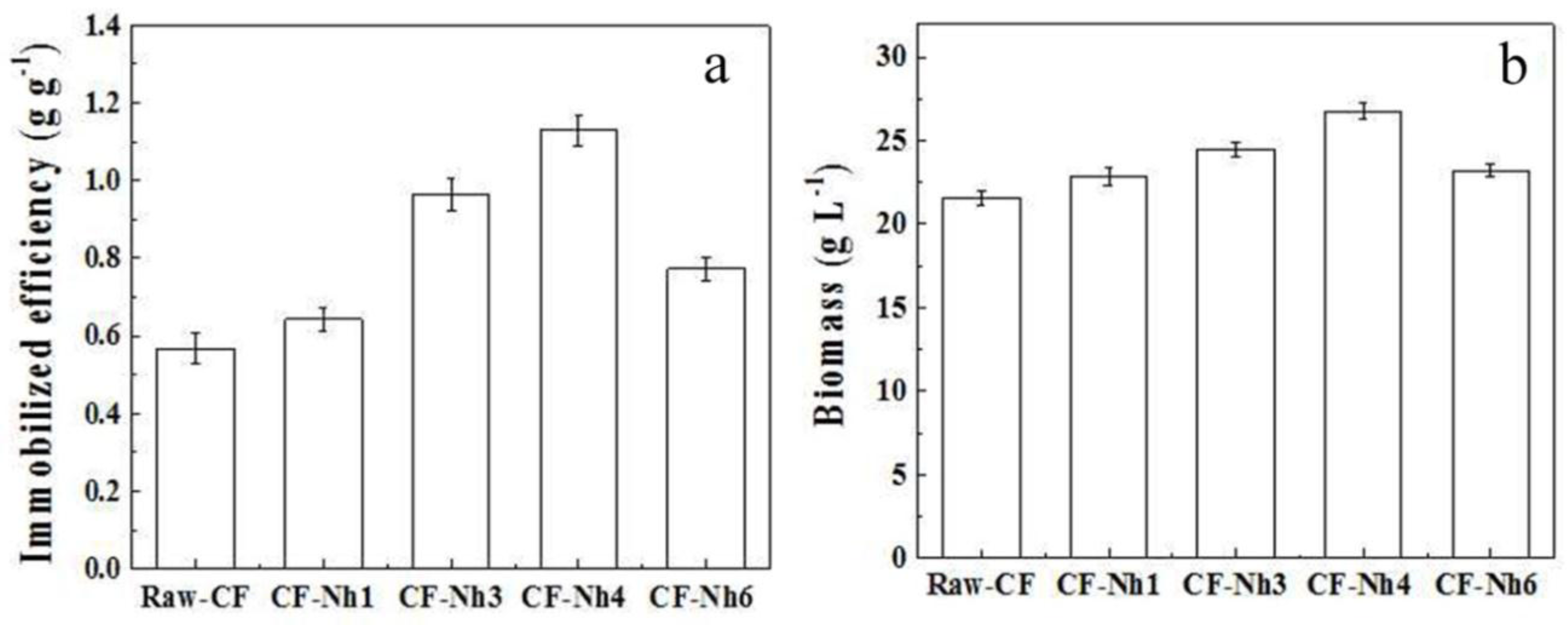
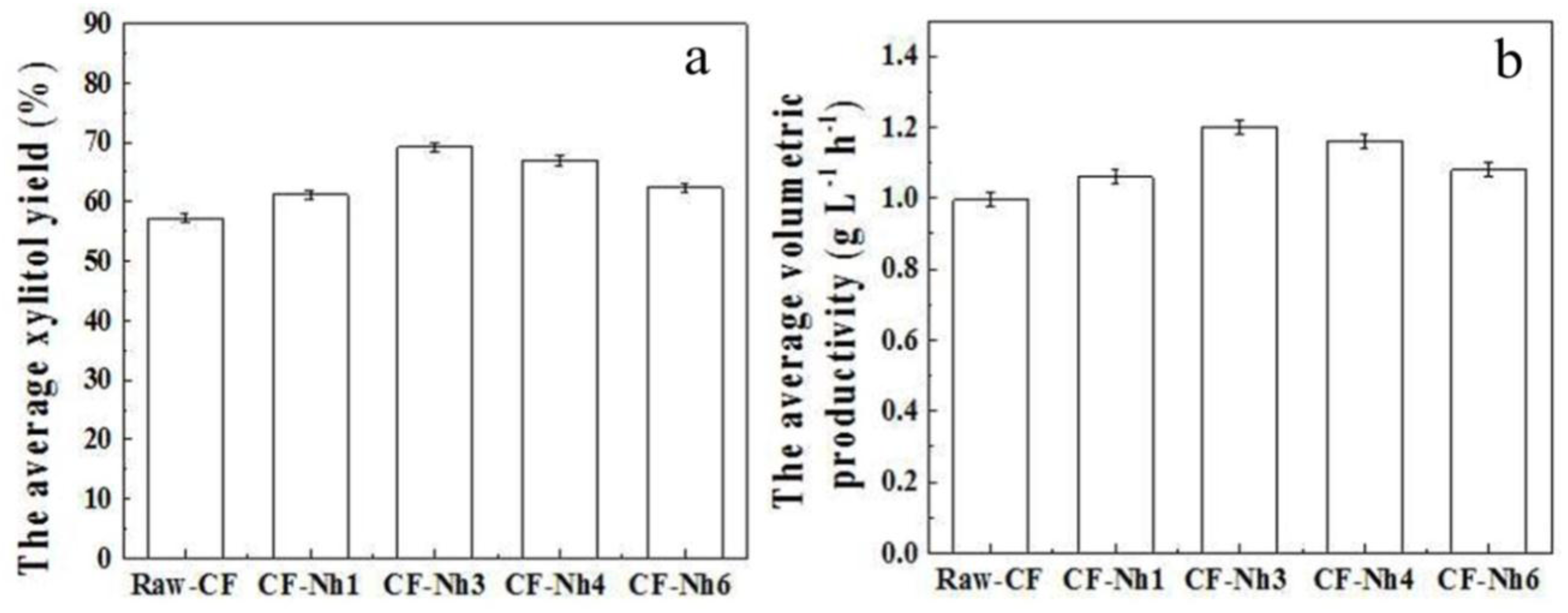
2.3. Multi-Batch Fermentation of Xylitol by C. tropicalis Immobilized on Treated CF
3. Experimental Section
3.1. Immobilization Carriers
3.2. Multi-Batch Xylitol Fermentation by Immobilized Cells
3.3. Measurements
3.4. Statistical Data Treatment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robatjazi, S.M.; Shojaosadati, S.A.; Khalilzadeh, R.; Farahan, E.V.; Balochi, N. Immobilization of magnetic treated Flavobacterium ATCC 27551 using magnetic field and evaluation of the enzyme stability of immobilized bacteria. Bioresour. Technol. 2013, 104, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.S. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Wang, L.; Wu, D.P.; Tang, P.W.; Fan, X.G.; Yuan, Q.P. Xylitol production from corncob hydrolysate using polyurethane foam with immobilized Candida tropicalis. Carbohydr. Polym. 2012, 90, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morita, M.; Sasaki, D.; Hirano, S.; Matsumoto, N.; Watanabe, A.; Ohmura, N.; Igarashi, Y. A bioelectrochemical reactor containing carbon fiber textiles enables efficient methane fermentation from garbage slurry. Bioresour. Technol. 2011, 102, 6837–6842. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, W.; Santos, J.C.; Canilha, L.; Silva, S.S.; Perego, P.; Converti, A. Xylitol production from sugarcane bagasse hydrolysate: metabolic behaviour of Candida guilliermondii cells entrapped in Ca-alginate. Biochem. Eng. J. 2005, 25, 25–31. [Google Scholar] [CrossRef]
- Branyik, T.; Vicente, A.; Oliveira, R.; Teixeira, J. Physicochemical surface properties of brewing yeast influencing their immobilization onto spent grains in a continuous reactor. Biotechnol. Bioeng. 2004, 88, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.J.; Yang, H.M.; Yu, L.L.; Chen, Y.; Li, Y. Preparation, Surface and Pore Structure of High Surface Area Activated Carbon Fibers from Bamboo by Steam Activation. Materials 2014, 7, 4431–4441. [Google Scholar] [CrossRef]
- Shen, W.; Wang, H.; Guan, R.; Li, Z. Surface modification of activated carbon fiber and its adsorption for vitamin B1 and folic acid. Colloids Surf. A 2008, 331, 263–267. [Google Scholar] [CrossRef]
- Edwards, S.L.; Church, J.S.; Werkmeister, J.A.; Ramshaw, J.A.M. Tubular micro-scale multiwalled carbon nanotube-based scaffolds for tissue engineering. Biomaterials 2009, 30, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Regan, E.M.; Uney, J.B.; Dick, A.D.; Zhang, Y.W.; McGeehan, J.P.; Claseyssens, F.; Kelly, S. Differential patterning of neuronal, glial and neural progenitor cells on phosphorus-doped and UV irradiated diamond-like carbon. Biomaterials 2010, 31, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.C.; Yuan, S.Y.; Chang, S.I.; Liao, J.W.; Ko, T.H.; Cheng, C.L. Evaluation of active carbon fibers used in cell biocompatibility and rat cystitis treatment. Carbon 2014, 68, 628–637. [Google Scholar] [CrossRef]
- Rosca, I.; Watari, F.; Uo, M.; Akasaka, T. Oxidation of multiwalled carbon nanotubes by nitric acid. Carbon 2005, 43, 3124–3131. [Google Scholar] [CrossRef]
- Matsumoto, S.; Ohtaki, A.; Hori, K. Carbon fiber as an excellent support material for wastewater treatment biofilms. Environ. Sci. Technol. 2012, 146, 10175–10181. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.L.; Dai, G.Z. Time-gradient nitric acid modification of CF biofilm carrier and surface nature effects on microorganism immobilization behavior in waste water. Biotechnol. Biotechnol. Equip. 2013, 27, 3918–3922. [Google Scholar] [CrossRef]
- Wei, Q.; Lv, D.; Huang, M.H.; Yu, D.S.; You, J.Q. Research on treatment of domestic sewage with aerobic denitrifying bacteria immobilized by carbon fiber. App. Mech. Mater. 2014, 675–677, 627–632. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, J.J.; Liu, F.; Fang, L.C.; Wang, J.B.; Li, D.J.; Wu, J.J. Effects of electrophoretically deposited graphene oxide coatings on interfacial properties of carbon fiber composite. J. Mater. Sci. 2015, 50, 5886–5892. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Mirzadeh, H.; Irani, S. Plasma surface modification of poly (L-lactic acid) and poly (lactic-co-glycolic acid) films for improvement of nerve cells adhesion. Radiat. Phys. Chem. 2008, 77, 280–287. [Google Scholar] [CrossRef]
- Xiong, L.; Qin, X.K.; Liang, H.B.; Huang, S.M.; Lian, Z.Y. Covalent functionalization of carbon fiber with poly(acrylamide) by reversible addition–fragmentation chain transfer polymerization for improving carbon fiber/epoxy interface. Polym. Composite. 2015. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, N.; Schweiss, R.; Lützow, K.; Werner, C.; Groth, T. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials 2004, 25, 2721–2730. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.; Hlady, V.; Tresco, P.A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 1998, 41, 422–430. [Google Scholar] [CrossRef]
- Zhu, N.W.; Chen, X.; Zhang, T.; Wu, P.X.; Li, P.; Wu, J.H. Improved performance of membrane free single-chamber air-cathode microbial fuel cells with nitric acid and ethylenediamine surface treated activated carbon fiber felt anodes. Bioresour. Technol. 2011, 102, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Shi, J.; Langrish, T. Removal of Cr (VI) from aqueous solution using activated carbon treated with nitric acid. Biochem. Eng. J. 2009, 152, 434–439. [Google Scholar]
- Li, Y.; Hu, S. Oxidation of activated carbon fibre and its adsorption of amylase. Mater. Sci-Poland. 2009, 27, 453–461. [Google Scholar]
- Chukov, D.I.; Stepashkin, A.A.; Gorshenkov, M.V.; Tcherdyntsev, V.V.; Kaloshkin, S.D. Surface modification of carbon fiber and its effect on the fiber-matrix interaction of UHMWPE based composites. J. Alloys. Compd. 2014, 586, 459–463. [Google Scholar] [CrossRef]
- Kim, B.K.; Ryu, S.K.; Kim, B.J.; Park, S.J. Adsorption behavior of propylamine on activated carbon fiber surfaces as induced by oxygen functional complexes. J. Colloid. Interf. Sci. 2006, 302, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Pamula, E.; Rouxhet, P.G. Bulk and surface chemical functionalities of type III PAN-based carbon fibres. Carbon 2003, 41, 1905–1915. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Chem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- White, J.S.; Walker, G.M. Influence of cell surface characteristics on adhesion of Saccharomyces cerevisiae to the biomaterial hydroxylapatite. Antonie Van Leeuwenhoek 2011, 99, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Dumitrache, A.; Eberl, H.J.; Allen, D.G. Mathematical modeling to validate on-line CO2 measurements as a metric for cellulolytic biofilm activity in continuous-flow bioreactors. Biochem. Eng. J. 2015, 101, 55–67. [Google Scholar] [CrossRef]
- Wang, L.; Fan, X.G.; Tang, P.; Yuan, Q.P. Xylitol fermentation using hemicellulose hydrolysare prepared by acid pre-impregnated steam explosion of corncob. J. Chem. Technol. Biotechnol. 2013, 88, 2067–2074. [Google Scholar]
- Wang, L.; Wu, D.P.; Tang, P.W.; Yuan, Q.P. Effect of organic acids found in cottonseed hull hydrolysate on the xylitol fermentation by Candida tropicalis. Bioproc. Biosyst. Eng. 2013, 36, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, C.; Ru, X.L.; Guo, Z.; Wu, D.P.; Zhang, S.B.; Yu, G.H.; Hu, Y.S.; Wang, J.S. Facile synthesis of ZnO hollow microspheres and their high performance in photocatalytic degradation and dye sensitized solar cells. J. Alloy. Compd. 2015, 647, 57–62. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, D.H.; Yang, K.S.; Lee, B.C.; Kim, Y.A.; Endo, M. Electron Beam irradiation-enhanced wettability of carbon fibers. ACS App. Mater. Interf. 2011, 3, 119–123. [Google Scholar] [CrossRef] [PubMed]
| Carriers Properties | Raw-CF | CF-Nh1 | CF-Nh3 | CF-Nh6 |
|---|---|---|---|---|
| Total acidity (mmol∙g−1) | 0.244 ± 0.011 | 0.365 ± 0.014 | 0.949 ± 0.023 | 1.38 ± 0.015 |
| Carriers Properties | Raw-CF | CF-Nh1 | CF-Nh3 | CF-Nh4 | CF-Nh6 |
|---|---|---|---|---|---|
| DM (%) | 1.68 ± 0.21 | 2.77 ± 0.25 | 9.66 ± 0.16 | 12.8 ± 0.20 | 18.7 ± 0.22 |
| CA (°) | 92.67 ± 1.14 | 77.02 ± 1.53 | 66.45 ± 1.86 | 59.31 ± 1.43 | 53.49 ± 1.61 |
| pHpzc | 5.93 ± 0.03 | 5.45 ± 0.01 | 4.02 ± 0.01 | 3.88 ± 0.01 | 2.71 ± 0.04 |
| Carrier Fermentation | Free | Raw-CF | CF-Nh1 | CF-Nh3 | CF-Nh4 | CF-Nh6 |
|---|---|---|---|---|---|---|
| YPS−1 (%) 1 | 57.76± 0.1 | 57.6 ± 0.1 | 60.11 ± 0.1 | 68.66 ± 0.1 | 67.51 ± 0.1 | 60.74 ± 0.1 |
| QP (g∙L−1∙h−1) 2 | 1.00 ± 0.01 | 1.00 ± 0.01 | 1.05 ± 0.02 | 1.19 ± 0.01 | 1.17 ± 0.01 | 1.06 ± 0.01 |
| YPS−1 (%) 3 | 61.92 ± 0.1 | 58.7 ± 0.1 | 61.77 ± 0.1 | 70.13 ± 0.1 | 68.7 ± 0.1 | 64.19 ± 0.2 |
| QP (g∙L−1∙h−1) 4 | 1.08 ± 0.02 | 1.02 ± 0.01 | 1.07 ± 0.01 | 1.22 ± 0.02 | 1.19 ± 0.01 | 1.12 ± 0.01 |
| YPS−1 (%) 5 | 60.87 ± 0.1 | 55.5 ± 0.1 | 61.54 ± 0.1 | 68.73 ± 0.1 | 64.38 ± 0.1 | 62.11 ± 0.1 |
| QP (g∙L−1∙h−1) 6 | 1.06 ± 0.01 | 0.97 ± 0.02 | 1.07 ± 0.01 | 1.20 ± 0.01 | 1.12 ± 0.02 | 1.08 ± 0.01 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, N.; Guo, Z.; Wu, D.; Chen, W.; Chang, Z.; Yuan, Q.; Hui, M.; Wang, J. Nitric Acid-Treated Carbon Fibers with Enhanced Hydrophilicity for Candida tropicalis Immobilization in Xylitol Fermentation. Materials 2016, 9, 206. https://doi.org/10.3390/ma9030206
Wang L, Liu N, Guo Z, Wu D, Chen W, Chang Z, Yuan Q, Hui M, Wang J. Nitric Acid-Treated Carbon Fibers with Enhanced Hydrophilicity for Candida tropicalis Immobilization in Xylitol Fermentation. Materials. 2016; 9(3):206. https://doi.org/10.3390/ma9030206
Chicago/Turabian StyleWang, Le, Na Liu, Zheng Guo, Dapeng Wu, Weiwei Chen, Zheng Chang, Qipeng Yuan, Ming Hui, and Jinshui Wang. 2016. "Nitric Acid-Treated Carbon Fibers with Enhanced Hydrophilicity for Candida tropicalis Immobilization in Xylitol Fermentation" Materials 9, no. 3: 206. https://doi.org/10.3390/ma9030206
APA StyleWang, L., Liu, N., Guo, Z., Wu, D., Chen, W., Chang, Z., Yuan, Q., Hui, M., & Wang, J. (2016). Nitric Acid-Treated Carbon Fibers with Enhanced Hydrophilicity for Candida tropicalis Immobilization in Xylitol Fermentation. Materials, 9(3), 206. https://doi.org/10.3390/ma9030206





