Effects of Ca Content on Formation and Photoluminescence Properties of CaAlSiN3:Eu2+ Phosphor by Combustion Synthesis
Abstract
:1. Introduction
2. Results and Discussion
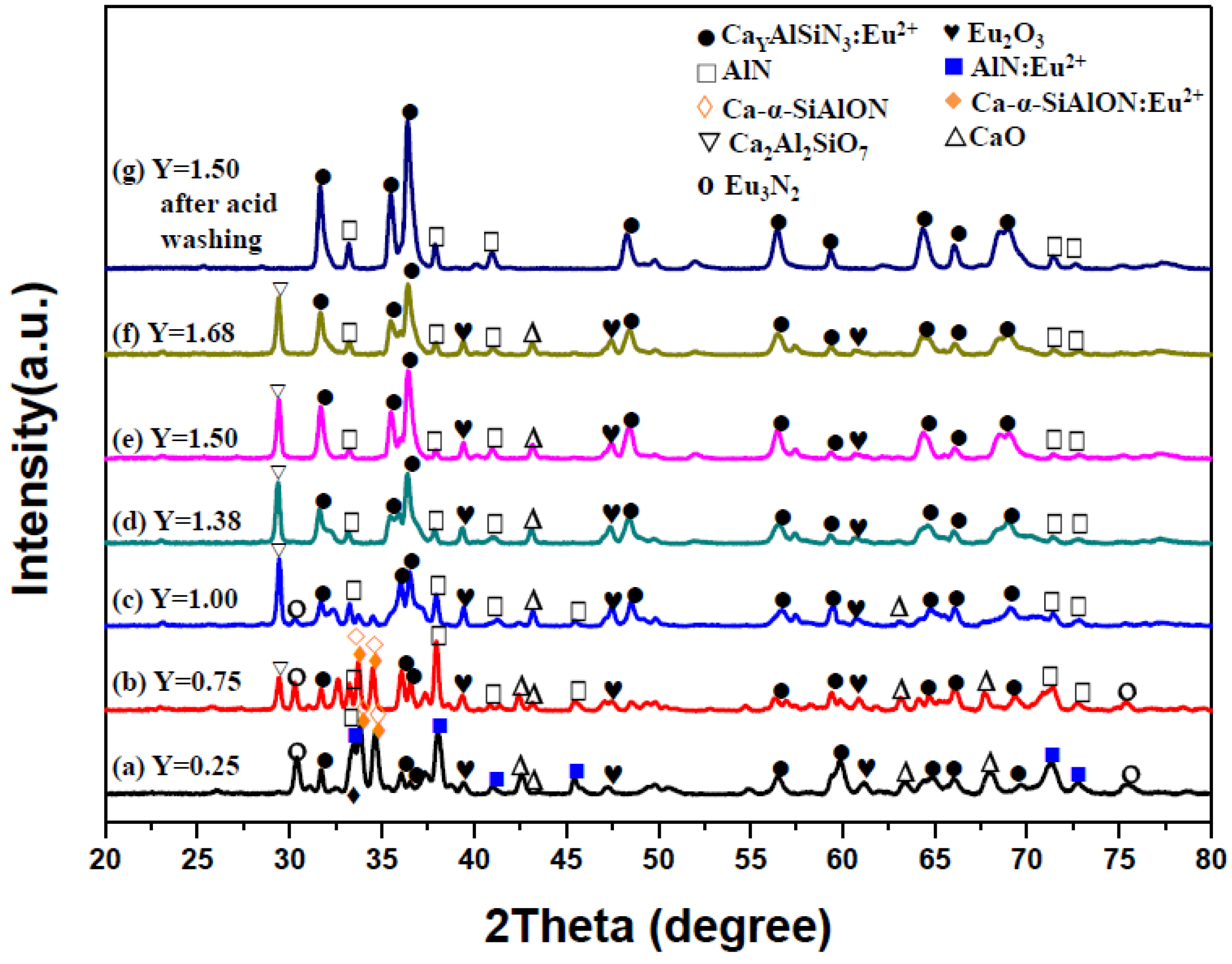
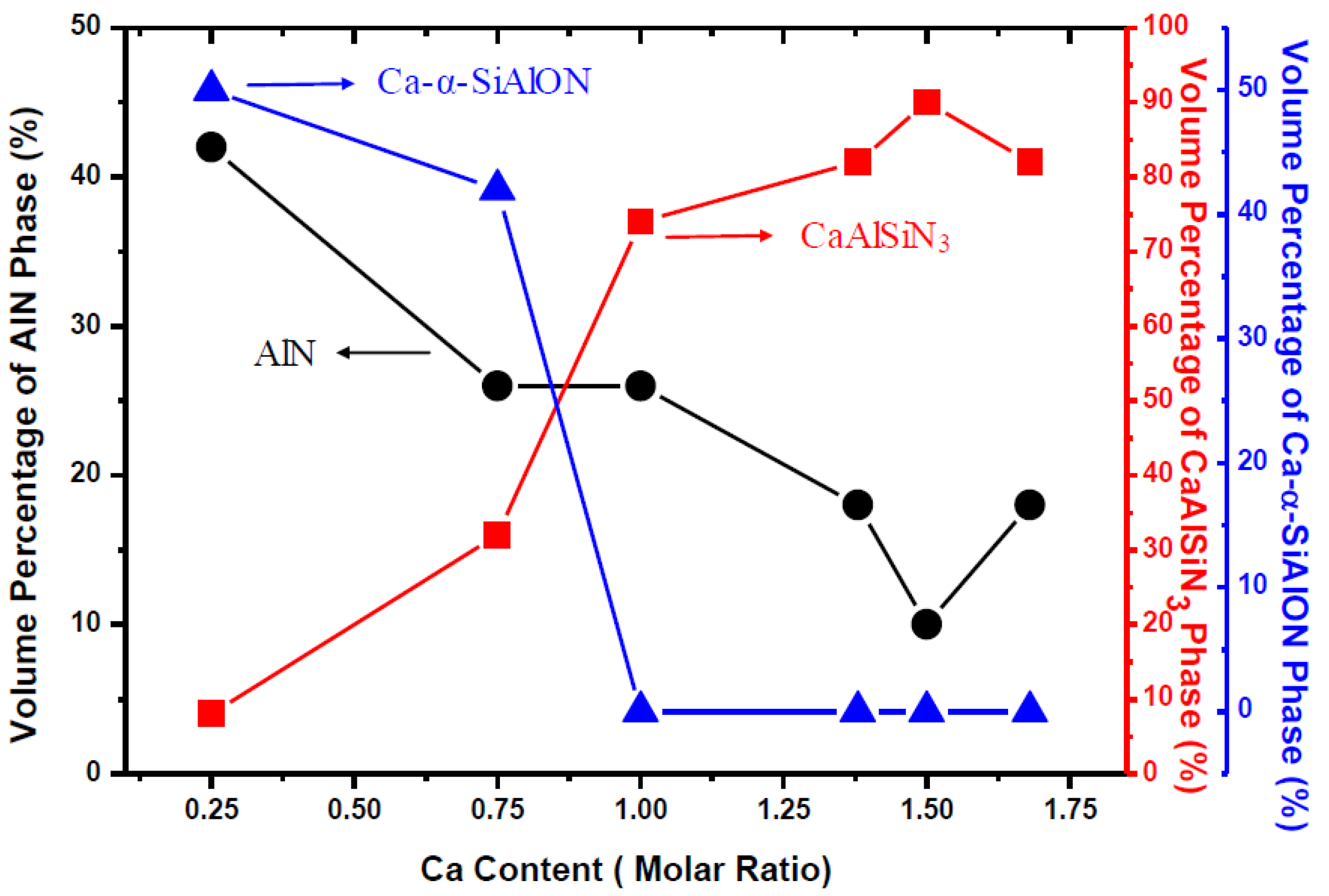
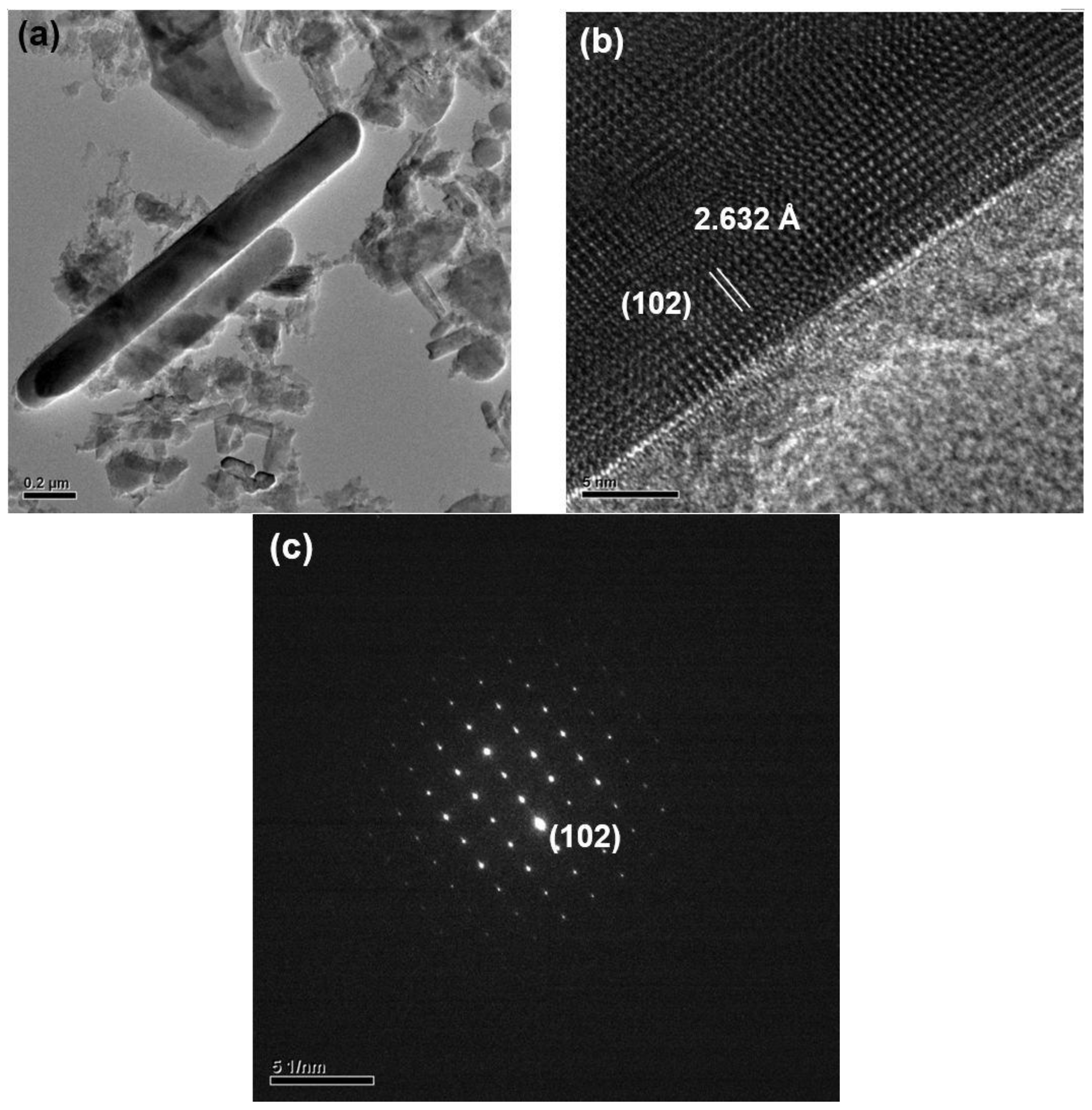
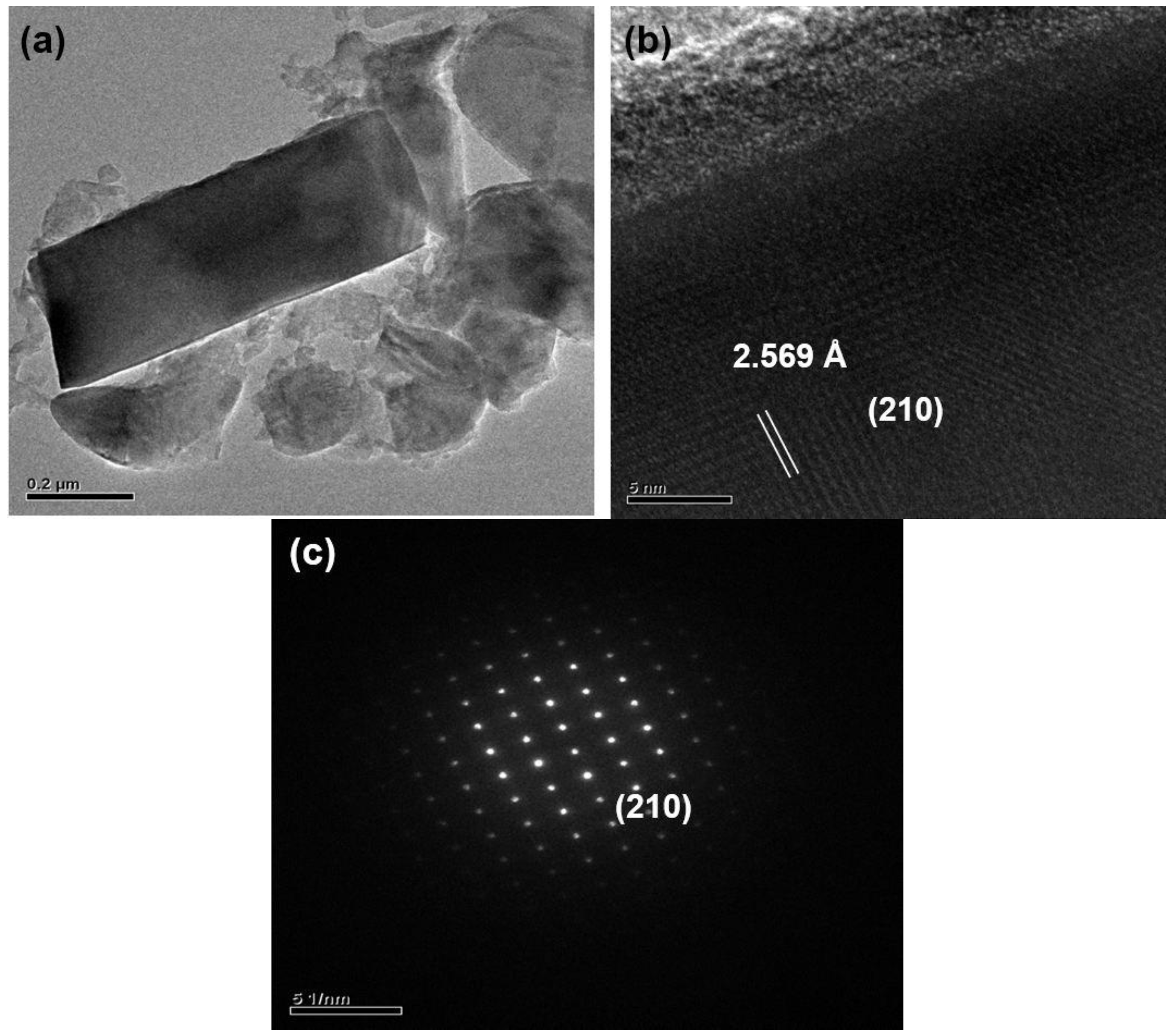
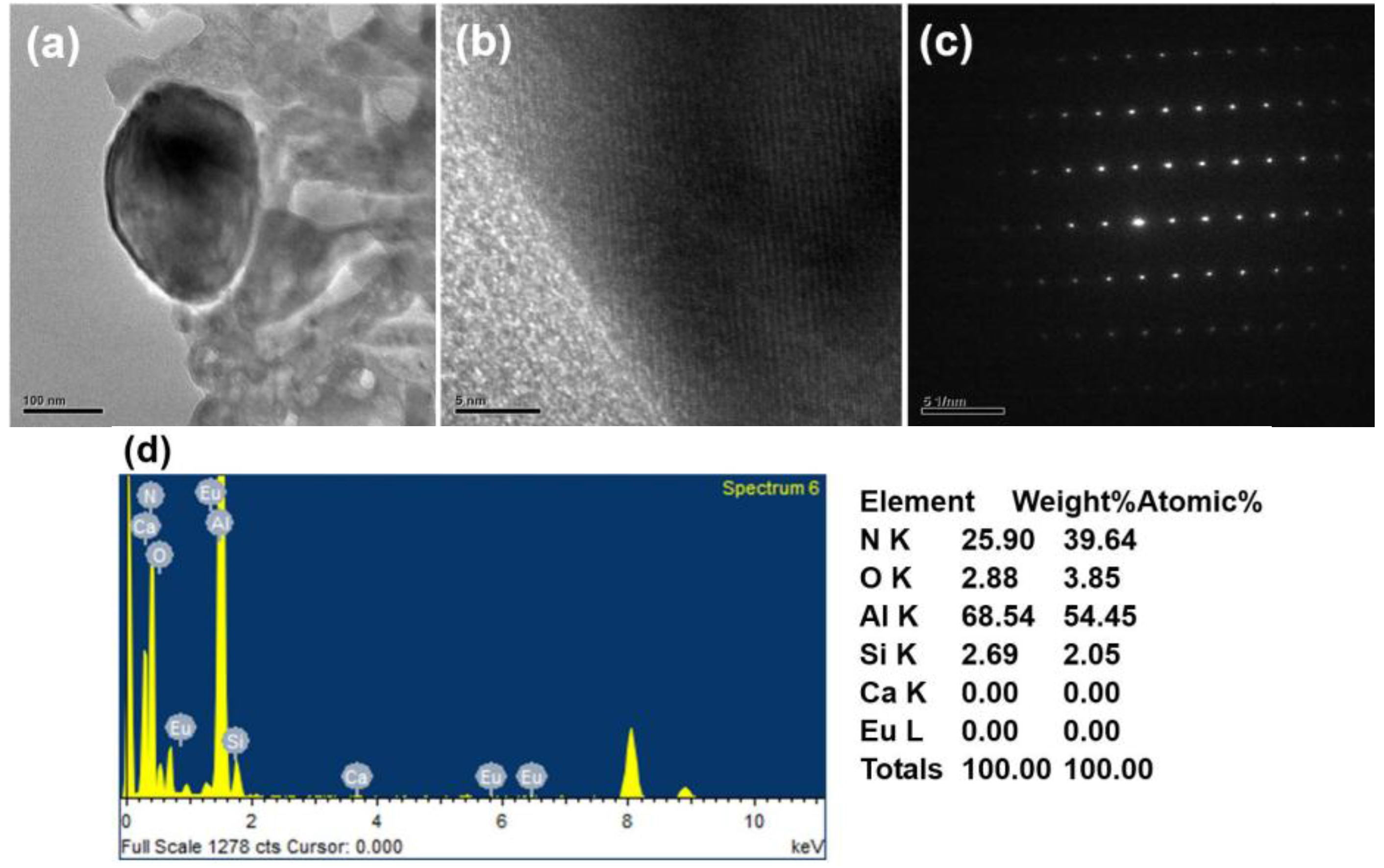
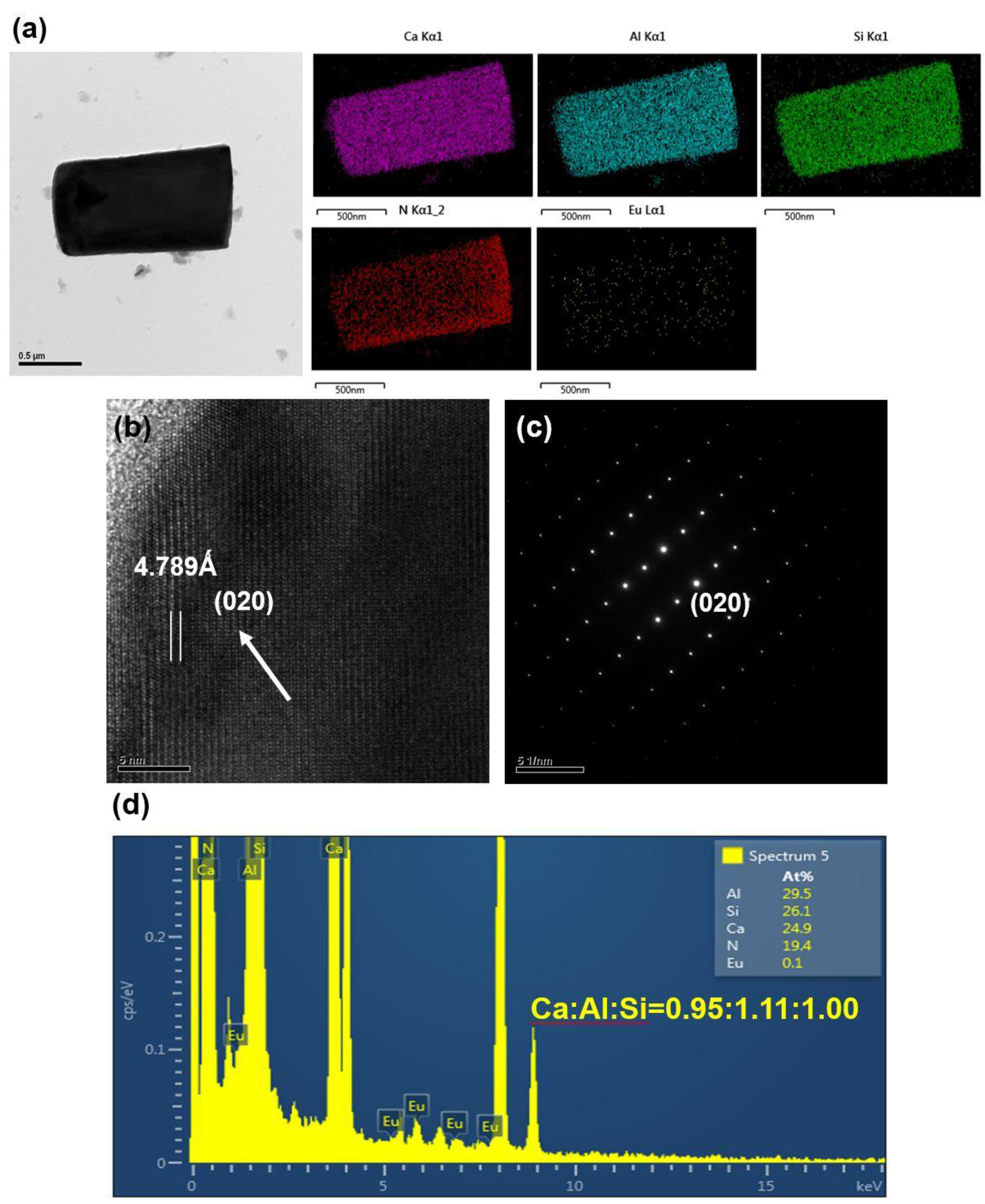
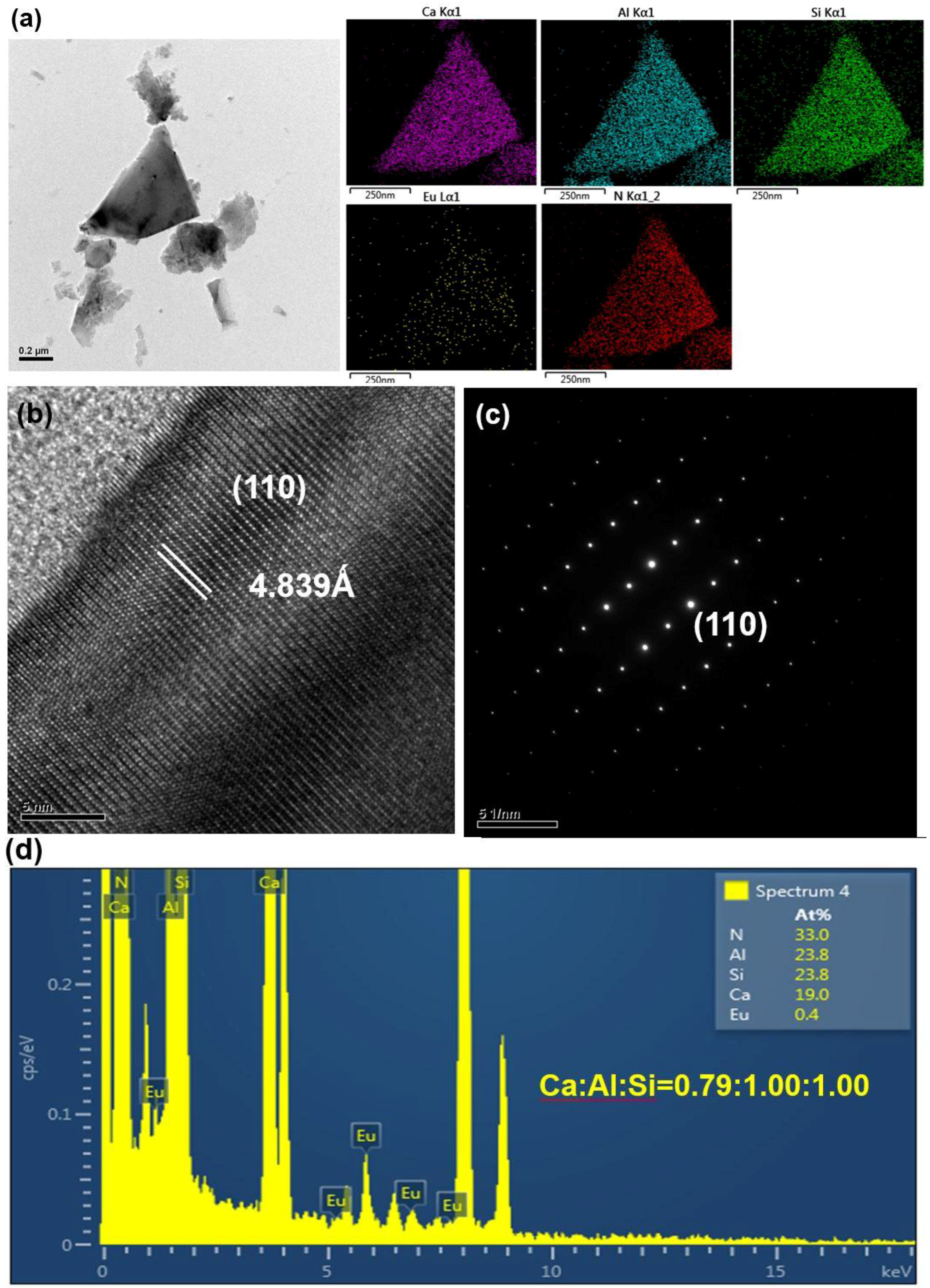
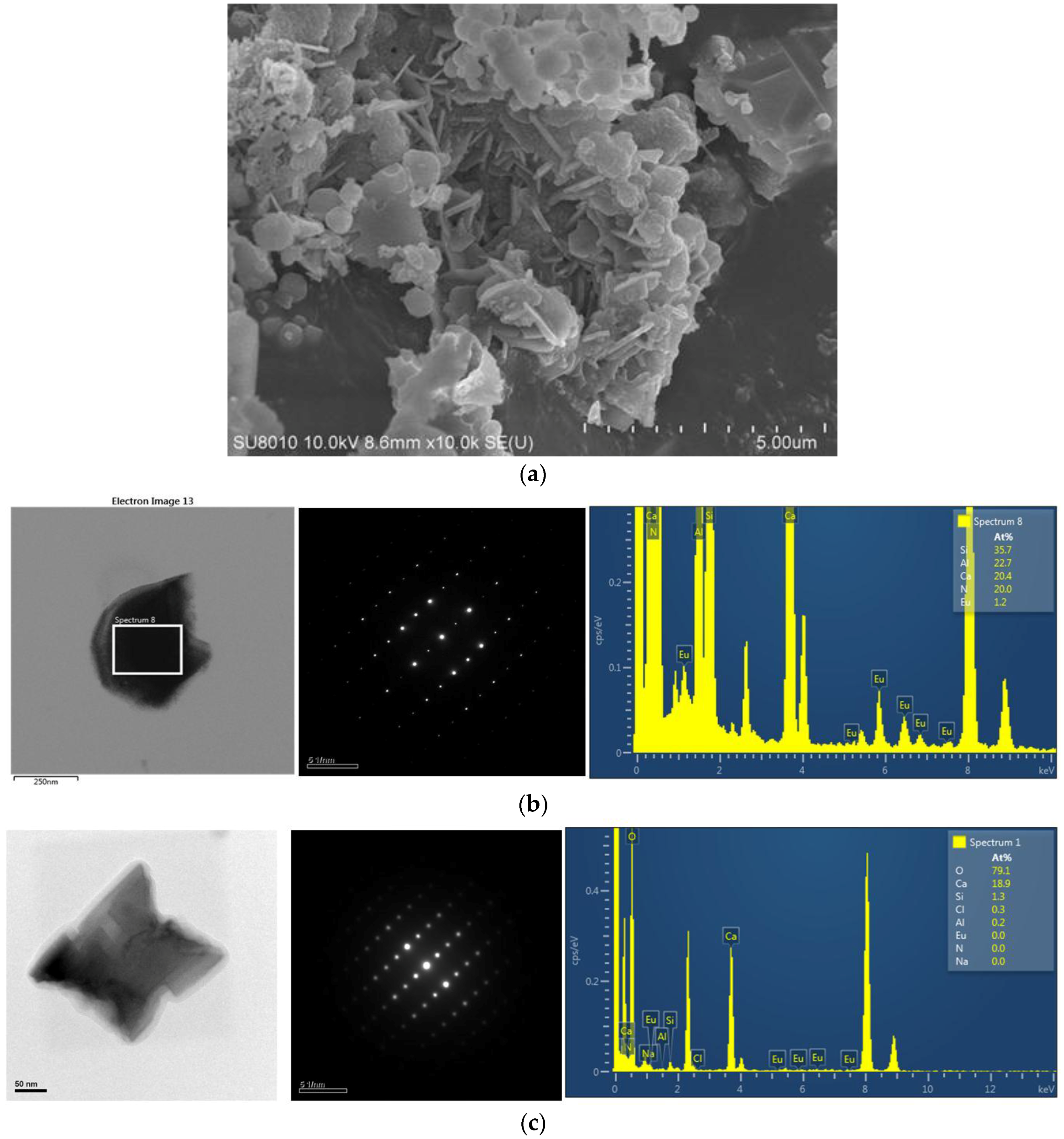
2.1. Effects of Ca Content on Product Formation and Morphology
2.2. Effects of Ca Content on Photoluminescence Properties
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, Y.S.; Zhuang, W.D.; He, H.Q.; Liu, R.H.; Chen, G.T.; Liu, Y.H.; Huang, X.W. High temperature stability of Eu2+-activated nitride red phosphors. J. Rare Earth 2014, 32, 12–16. [Google Scholar] [CrossRef]
- Huang, W.Y.; Yoshimura, F.; Ueda, K.; Pang, W.K.; Su, B.J.; Jang, L.Y.; Chiang, C.Y.; Zhou, W.; Duy, N.H.; Liu, R.S. Domination of second-sphere shrinkage effect to improve photoluminescence of red nitride phosphors. Inorg. Chem. 2014, 53, 12822–12831. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.J.; Hirosaki, N.; Sakuma, K.; Kimura, N. White light-emitting diodes (LEDs) using (oxy)nitride phosphors. J. Phys. D Appl. Phys. 2008, 41, 144013–144018. [Google Scholar] [CrossRef]
- Lin, C.C.; Zheng, Y.S.; Chen, H.Y.; Ruan, C.H.; Xiao, G.W.; Liu, R.S. Improving optical properties of white LED fabricated by a blue LED chip with yellow/red phosphors. J. Electrochem. Soc. 2010, 157, H900–H903. [Google Scholar] [CrossRef]
- Chung, S.L.; Huang, S.C.; Chou, W.C.; Tangguh, W. Phosphors based on nitridosilicates: Synthesis methods and luminescent properties. Curr. Opin. Chem. Eng. 2014, 3, 62–67. [Google Scholar] [CrossRef]
- Li, Y.Q.; van Steen, J.E.J.; van Krevel, J.W.H.; Botty, G.; Delsing, A.C.A.; DiSalvo, F.J.; de With, G.; Hintzen, H.T. Luminescence properties of red-emitting M2Si5N8:Eu2+ (M = Ca, Sr, Ba) LED conversion phosphors. J. Alloy. Compd. 2006, 417, 273–279. [Google Scholar] [CrossRef]
- Piao, X.; Horikawa, T.; Hanzawa, H.; Machida, K.I. Photoluminescence properties of Ca2Si5N8:Eu2+ nitride phosphor prepared by carbothermal reduction and nitridation method. Chem. Lett. 2006, 35, 334–335. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamane, H.; Kijima, N. Crystal structure and luminescence of Sr0.99Eu0.01AlSiN3. J. Solid State Chem. 2008, 181, 1848–1852. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Borovinskaya, I.P. A new class of combustion processes. Combust. Sci. Technol. 1975, 10, 195–201. [Google Scholar] [CrossRef]
- Chung, S.L.; Huang, S.C. Combustion synthesis and photoluminescence properties of red-emitting CaAlSiN3:Eu2+ phosphor for white-LEDs. Materials 2014, 7, 7828–7842. [Google Scholar] [CrossRef]
- Suehiro, T.; Xie, R.J.; Hirosaki, N. Gas-reduction-nitridation synthesis of CaAlSiN3:Eu2+ fine powder phosphors for solid-state lighting. Ind. Eng. Chem. Res. 2014, 53, 2713–2717. [Google Scholar] [CrossRef]
- Li, J.W.; Watanabe, T.; Sakamoto, N.; Wada, H.; Setoyama, T.; Yoshimura, M. Synthesis of a multinary nitride, Eu-doped CaAlSiN3, from alloy at low temperatures. Chem. Mater. 2008, 20, 2095–2105. [Google Scholar] [CrossRef]
- Yin, L.J.; Zhu, Q.Q.; Yu, W.; Hao, L.Y.; Xu, X.; Hu, F.C.; Lee, M.H. Europium location in the AlN:Eu green phosphor prepared by a gas-reduction-nitridation route. J. Appl. Phys. 2012, 111. [Google Scholar] [CrossRef]
- Yin, L.J.; Xu, X.; Yu, W.; Yang, J.G.; Yang, L.X.; Yang, X.F.; Hao, L.Y.; Liu, X.J. Synthesis of Eu2+-doped AlN phosphors by carbothermal reduction. J. Am. Ceram. Soc. 2010, 93, 1702–1707. [Google Scholar] [CrossRef]
- Inoue, K.; Hirosaki, N.; Xie, R.J.; Takeda, T. Highly efficient and thermally stable blue-emitting AlN:Eu2+ phosphor for ultraviolet white light-emitting diodes. J. Phys. Chem. C 2009, 113, 9392–9397. [Google Scholar] [CrossRef]
- Li, H.L.; Hirosaki, N.; Xie, R.J.; Suehiro, T.; Mitomo, M. Fine yellow α-SiAlON:Eu phosphors for white LEDs prepared by the gas-reduction-nitridation method. Sci. Technol. Adv. Mater. 2007, 8, 601–606. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Xie, R.J.; Huang, Q. Facile synthesis of Ca-α-SiAlON:Eu2+ phosphor by the microwave sintering method and its photoluminescence properties. Chin. Sci. Bull. 2012, 58, 708–712. [Google Scholar] [CrossRef]
- Ge, Y.Y.; Chen, Y.; Wang, Q.; Cui, W.; Zou, Y.F.; Xie, Z.P.; Yuan, X.Y.; Chen, K.X. Effect of NH4Cl additive on combustion synthesis of Eu-doped Ca-α-SiAlON phosphors. J. Alloys Compd. 2016, 654, 404–409. [Google Scholar] [CrossRef]
- Li, G.H.; Chen, J.J.; Mao, Z.Y.; Song, W.W.; Sun, T.; Wang, D.J. Carbothermal synthesis of CaAlSiN3:Eu2+ red-emitting phosphors and the photoluminescent properties. J Mater Sci Mater Electron. 2015, 26, 10201–10206. [Google Scholar] [CrossRef]
- Xie, R.J.; Mitomo, M.; Xu, F.F.; Uheda, K.; Bando, Y. Preparation of Ca-α-sialon ceramics with compositions along the Si3N4–1/2 Ca3N2:3AlN line. Z. Metallkd. 2001, 92, 931–936. [Google Scholar]
- Cai, J.J.; Pan, H.H.; Wang, Y. Luminescence properties of red-emitting Ca2Al2SiO7:Eu3+ nanoparticles prepared by sol-gel method. Rare Met. 2011, 30, 374–380. [Google Scholar] [CrossRef]
- Li, Y.Q.; Hirosaki, N.; Xie, R.J.; Takeda, T.; Mitomo, M. Yellow-orange-emitting CaAlSiN3:Ce3+ phosphor: Structure, photoluminescence, and application in white LEDs. Chem. Mater. 2008, 20, 6704–6714. [Google Scholar] [CrossRef]
- Dierre, B.; Takeda, T.; Sekiguchi, T.; Suehiro, T.; Takahashi, K.; Yamamoto, Y.; Xie, R.J.; Hirosaki, N. Local analysis of Eu2+ emission in CaAlSiN3. Sci. Technol. Adv. Mater. 2013, 14. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, F.Y.; Piao, X.Q.; Sun, Z.; Horikawa, T.; Machida, K.I. Synthesis and photoluminescence properties of divalent europium doped-CaAlSi1+2xN3+2xOx (x = 0–1) solid solution phosphors. ECS J. Solid State Sci. Technol. 2013, 2, R131–R134. [Google Scholar] [CrossRef]
- Li, S.X.; Liu, X.J.; Liu, J.Q.; Li, H.L.; Mao, R.H.; Huang, Z.R.; Xie, R.J. Synthesis, composition optimization, and tunable red emission of CaAlSiN3:Eu2+ phosphors for white light-emitting diodes. J. Mater. Res. 2015, 30, 2919–2927. [Google Scholar] [CrossRef]
- Dexter, D.L. A theory of sensitized luminescence in solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Yang, J.J.; Wang, T.; Chen, D.C.; Chen, G.D.; Liu, Q.L. An investigation of Eu2+-doped CaAlSiN3 fabricated by an alloy-nitridation method. Mater. Sci. Eng. B 2012, 177, 1596–1604. [Google Scholar] [CrossRef]
- Chung, S.L.; Chou, W.C. Combustion synthesis of Ca2Si5N8:Eu2+ phosphors and their luminescent properties. J. Am. Ceram. Soc. 2013, 96, 2086–2092. [Google Scholar] [CrossRef]
- Li, J.W.; Watanabe, T.; Wada, H.; Setoyama, T.; Yoshimura, M. Low-temperature crystallization of Eu-doped red-emitting CaAlSiN3 from alloy-derived ammonometallates. Chem. Mater. 2007, 19, 3592–3594. [Google Scholar] [CrossRef]
- Xie, R.J.; Hirosaki, N.; Suehiro, T.; Xu, F.F.; Mamoru, M. A simple, efficient synthetic route to Sr2Si5N8:Eu2+-based red phosphors for white light-emitting diodes. Chem. Mater. 2006, 18, 5578–5583. [Google Scholar] [CrossRef]
- Uheda, K.; Hirosaki, N.; Yamamoto, Y.; Naito, A.; Nakajima, T.; Yamamotoa, H. Luminescence properties of a red phosphor, CaAlSiN3:Eu2+, for white light-emitting diodes. Electrochem. Solid State Lett. 2006, 9, H22–H25. [Google Scholar] [CrossRef]
- Xie, R.J.; Mitomo, M.; Uheda, K.; Xu, F.F.; Akimune, Y. Preparation and luminescence spectra of calcium- and rare-earth (R = Eu,Tb, and Pr)-codoped α-SiAlON ceramics. J. Am. Ceram. Soc. 2002, 85, 1229–1234. [Google Scholar] [CrossRef]
- Xie, R.J.; Hirosaki, N. Silicon-based oxynitride and nitride phosphors for white LEDs—A review. Sci. Technol. Adv. Mater. 2007, 8, 588–600. [Google Scholar] [CrossRef]
- Zeuner, M.; Pagano, S.; Schnick, W. Nitridosilicates and oxonitridosilicates: From ceramic materials to structural and functional diversity. Angew. Chem. Int. Ed. Engl. 2011, 50, 7754–7775. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.N.; Chung, S.L. Combustion synthesis method for synthesis of aluminum nitride powder using aluminum containers. J. Mater. Res. 2001, 16, 3518–3525. [Google Scholar] [CrossRef]
- Lin, C.N.; Chung, S.L. Combustion synthesis method for synthesis of aluminum nitride powder using aluminum containers (II). J. Mater. Res. 2004, 19, 3037–3045. [Google Scholar] [CrossRef]

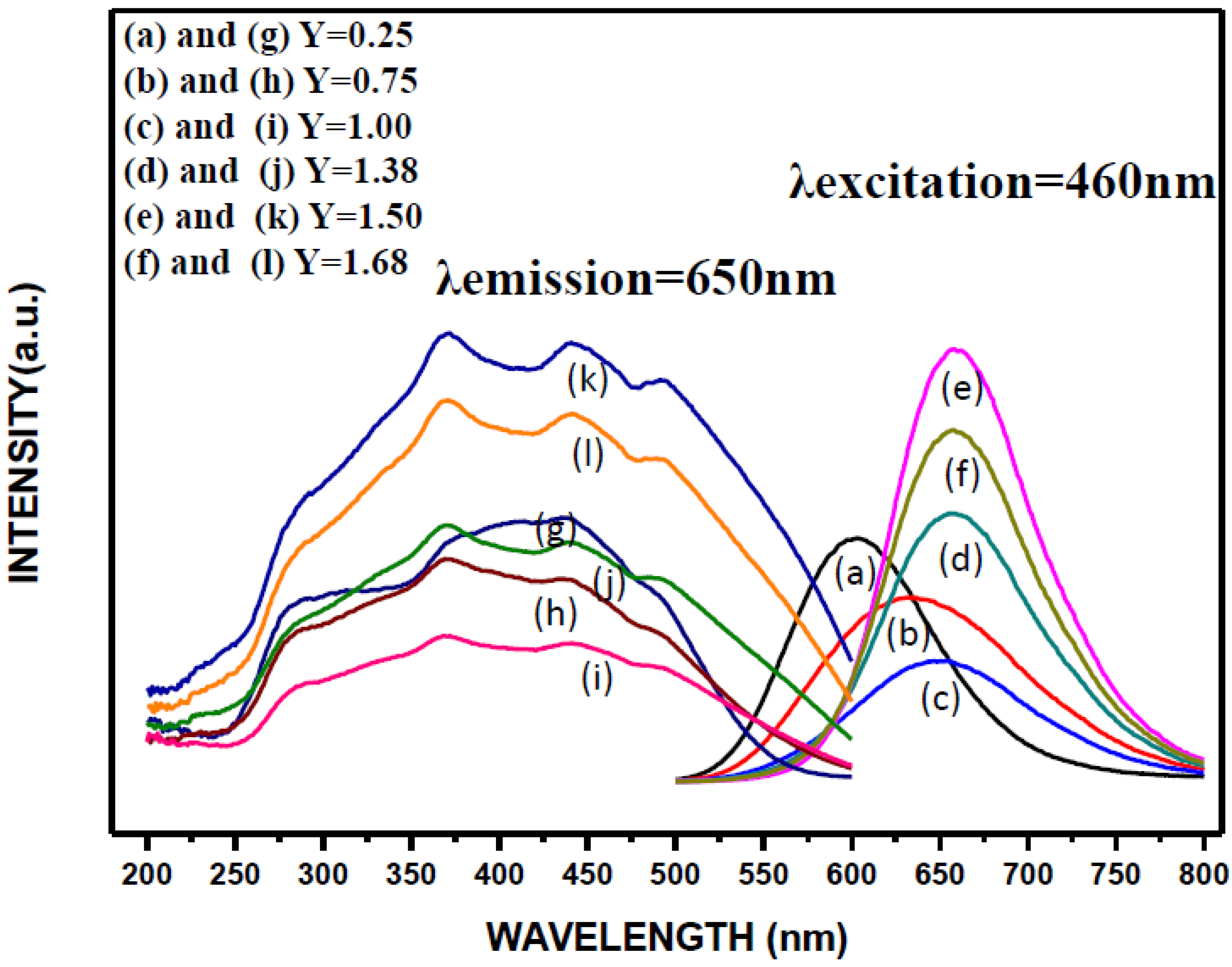
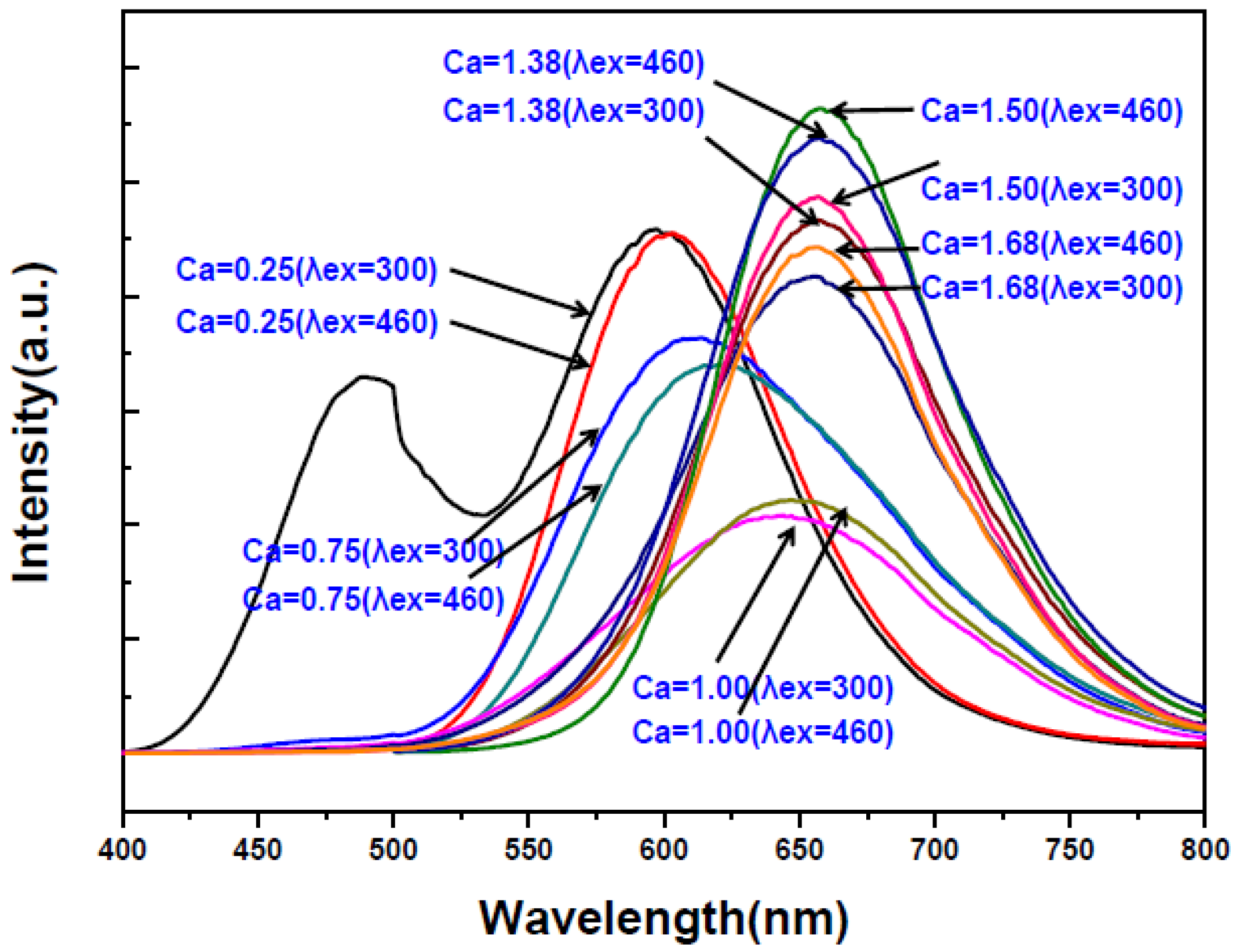
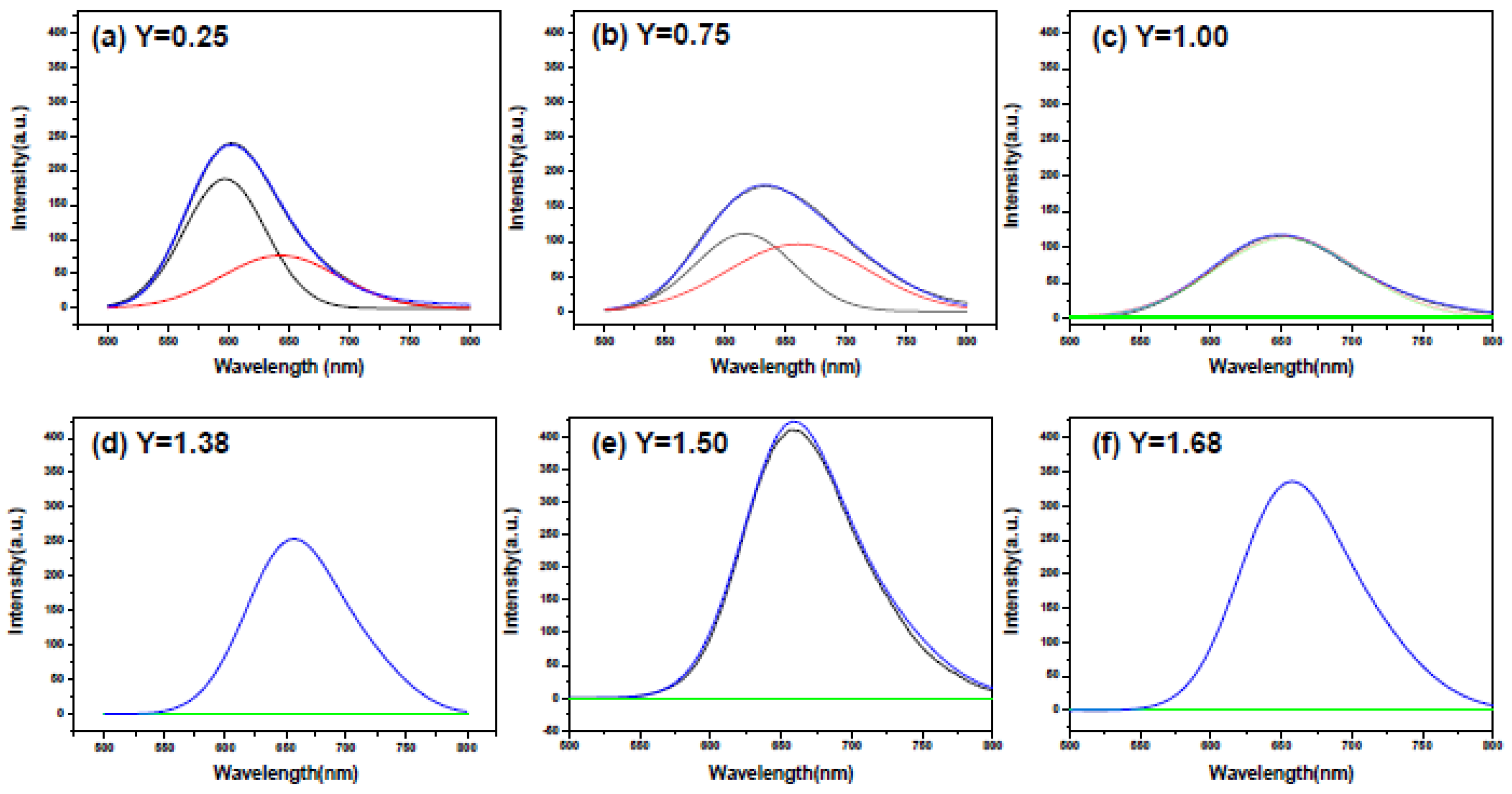
| Reactant Composition | Cationic Molar Ratios of the Products | ||||||
|---|---|---|---|---|---|---|---|
| Ca (Y) | Al | Si | Eu | Ca | Al | Si | Eu |
| 0.25 | 1.00 | 1.00 | 0.02 | 0.12 | 1.06 | 1.00 | 0.010 |
| 0.75 | 1.00 | 1.00 | 0.02 | 0.35 | 1.19 | 1.00 | 0.011 |
| 1.00 | 1.00 | 1.00 | 0.02 | 0.57 | 1.14 | 1.00 | 0.013 |
| 1.38 | 1.00 | 1.00 | 0.02 | 0.64 | 1.15 | 1.00 | 0.014 |
| 1.50 | 1.00 | 1.00 | 0.02 | 0.77 | 1.18 | 1.00 | 0.015 |
| 1.68 | 1.00 | 1.00 | 0.02 | 0.88 | 1.11 | 1.00 | 0.018 |
| Reactant Composition | Cationic Molar Ratios of the Products | ||||||
|---|---|---|---|---|---|---|---|
| Ca (Y) | Al | Si | Eu | Ca | Al | Si | Eu |
| 0.25 | 1.00 | 1.00 | 0.02 | 0.26 | 0.68 | 1.00 | 0.004 |
| 0.75 | 1.00 | 1.00 | 0.02 | 0.39 | 0.73 | 1.00 | 0.006 |
| 1.00 | 1.00 | 1.00 | 0.02 | 0.60 | 0.71 | 1.00 | 0.007 |
| 1.38 | 1.00 | 1.00 | 0.02 | 0.83 | 0.81 | 1.00 | 0.013 |
| 1.50 | 1.00 | 1.00 | 0.02 | 0.86 | 0.87 | 1.00 | 0.013 |
| 1.68 | 1.00 | 1.00 | 0.02 | 0.88 | 0.90 | 1.00 | 0.011 |
| Reagent | Particle Size (um) | Purity (%) | Source |
|---|---|---|---|
| Si | 1–5 | 99 | Alfa Aesar (Ward Hill, MA, USA) |
| Ca | <963 | 99.5 | Alfa Aesar (Heysham, England) |
| Eu2O3 | 3.0 | 99.999 | Baogang Group (Baotou, China) |
| NaN3 | d50 ≈ 150 | 99 | Johnson Matthey (Tokyo, Japan) |
| NH4Cl | - | 99 | Panreac (Barcelona, Spain) |
| Al | d50 ≈ 3 | 99 | First Chemical Work (Taipei, Taiwan) |
| α-Si3N4 | d50 ≈ 10 | 99.999 | Seminc Company (Ward Hill, MA, USA) |
| Mg | d50 ≈ 20 | 99 | Nihon Shiyaku (Osaka, Japan) |
| Fe3O4 | 102 | 99 | Nihon Shiyaku (Osaka, Japan) |
| N2 | Gas | 99 | Yun Shan (Tainan, Taiwan) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, S.-L.; Huang, S.-C. Effects of Ca Content on Formation and Photoluminescence Properties of CaAlSiN3:Eu2+ Phosphor by Combustion Synthesis. Materials 2016, 9, 178. https://doi.org/10.3390/ma9030178
Chung S-L, Huang S-C. Effects of Ca Content on Formation and Photoluminescence Properties of CaAlSiN3:Eu2+ Phosphor by Combustion Synthesis. Materials. 2016; 9(3):178. https://doi.org/10.3390/ma9030178
Chicago/Turabian StyleChung, Shyan-Lung, and Shu-Chi Huang. 2016. "Effects of Ca Content on Formation and Photoluminescence Properties of CaAlSiN3:Eu2+ Phosphor by Combustion Synthesis" Materials 9, no. 3: 178. https://doi.org/10.3390/ma9030178
APA StyleChung, S.-L., & Huang, S.-C. (2016). Effects of Ca Content on Formation and Photoluminescence Properties of CaAlSiN3:Eu2+ Phosphor by Combustion Synthesis. Materials, 9(3), 178. https://doi.org/10.3390/ma9030178






