Effect of H2S Plasma Treatment on the Surface Modification of a Polyethylene Terephthalate Surface
Abstract
:1. Introduction
2. Results and Discussion
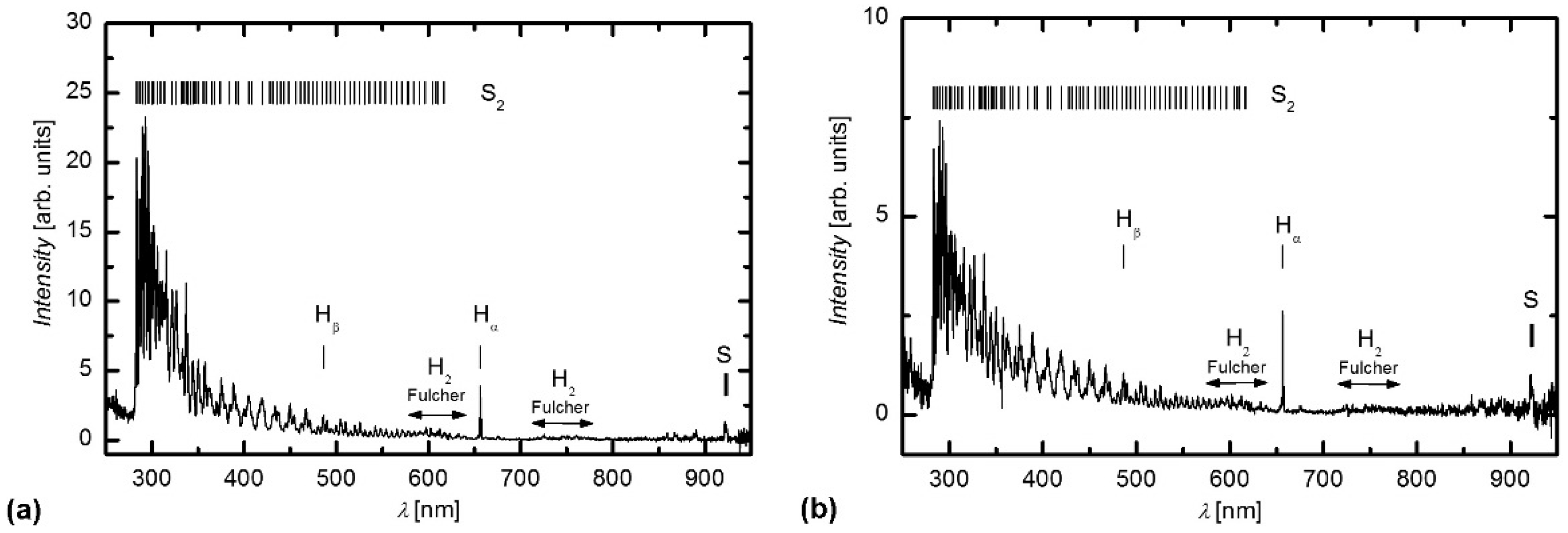
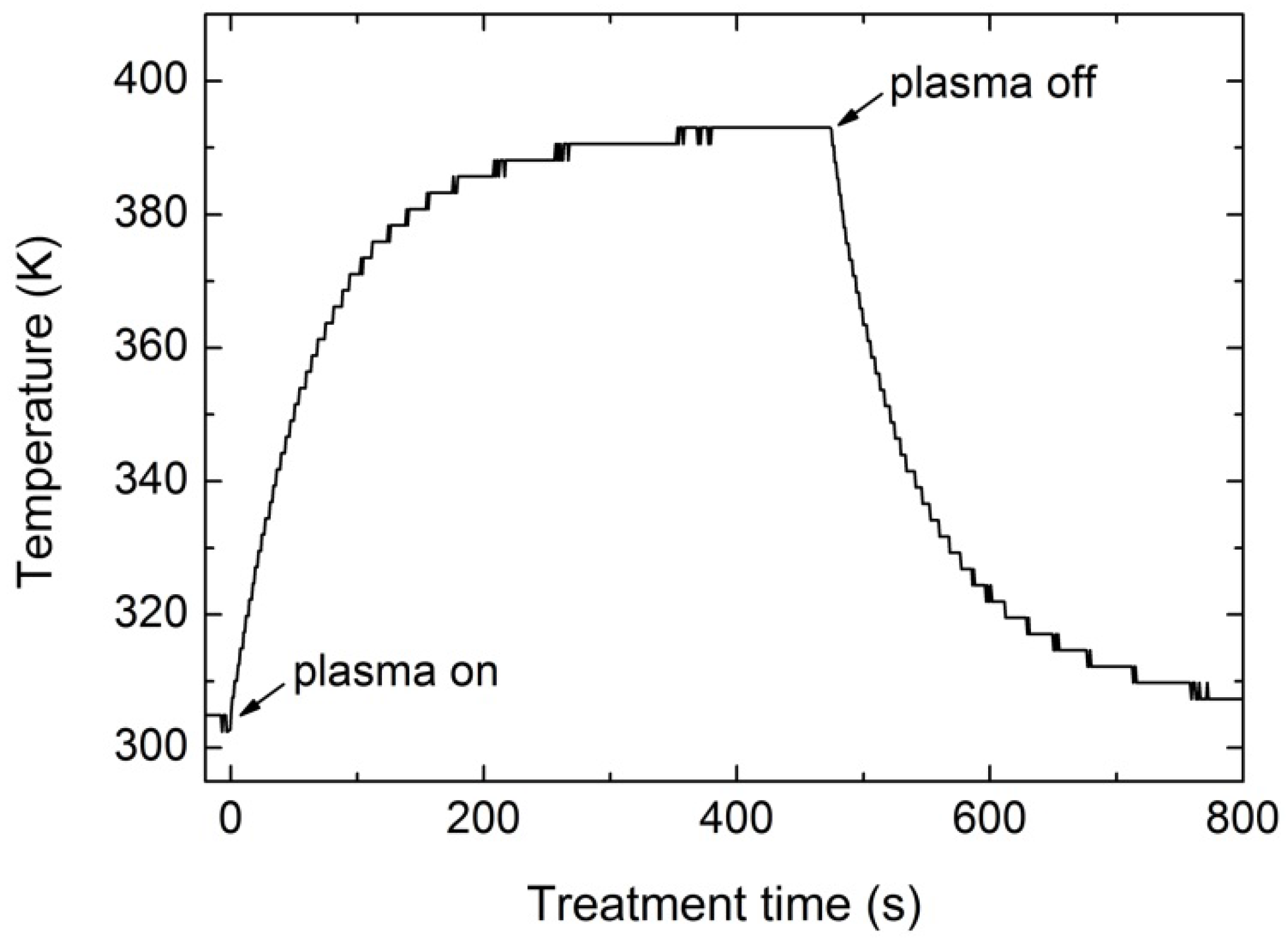
2.1. Plasma Characterization
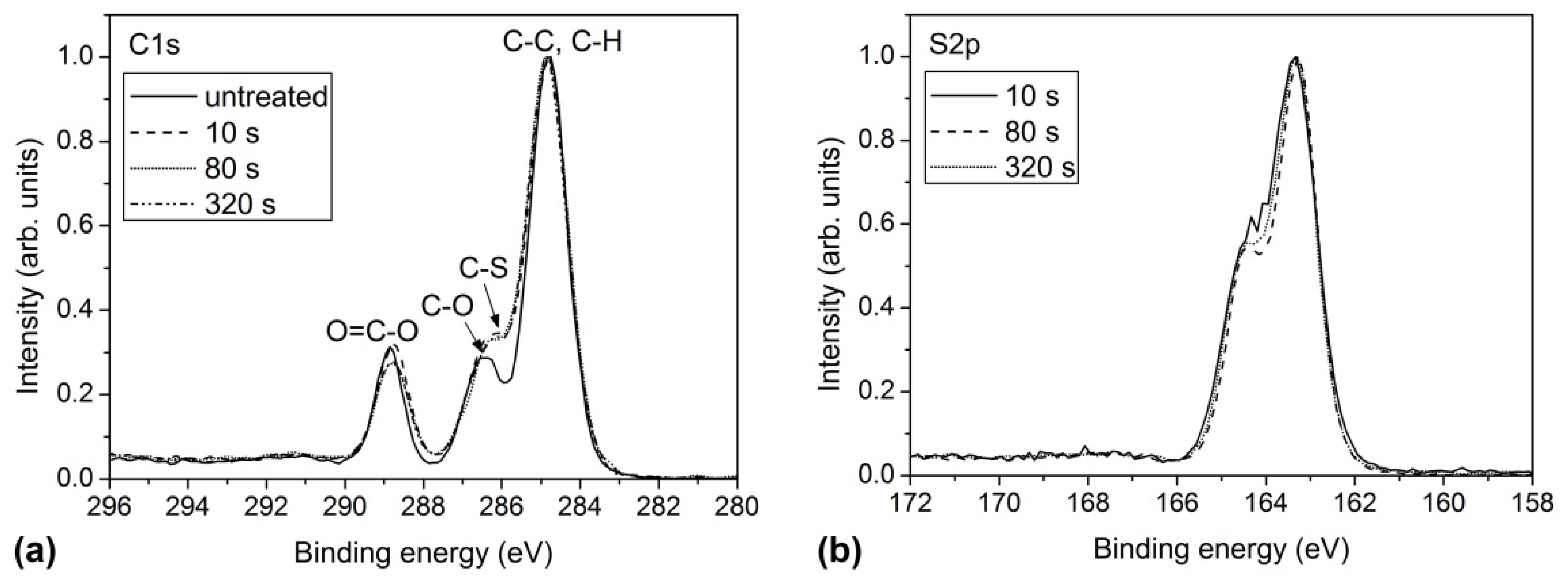
2.2. Surface Chemistry
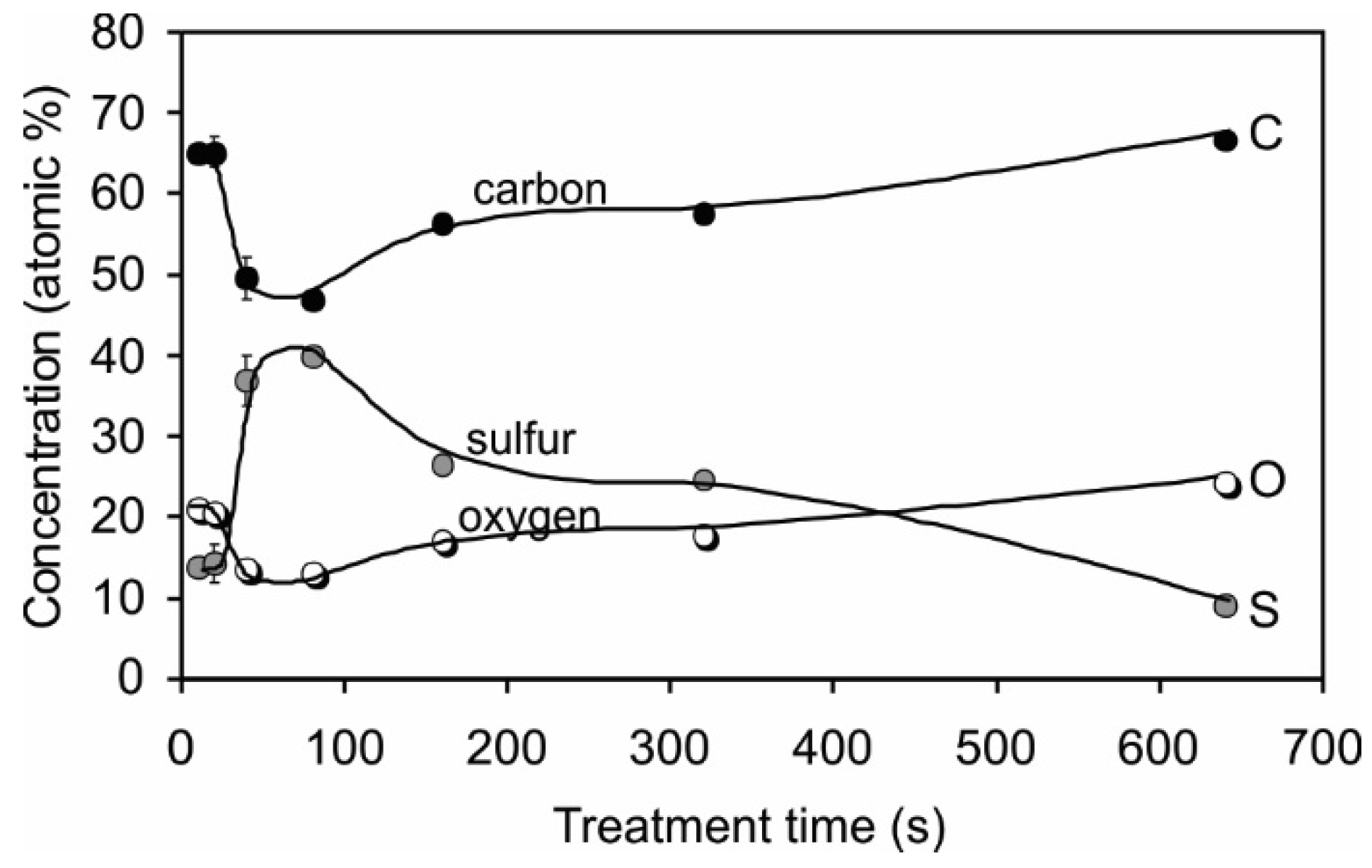
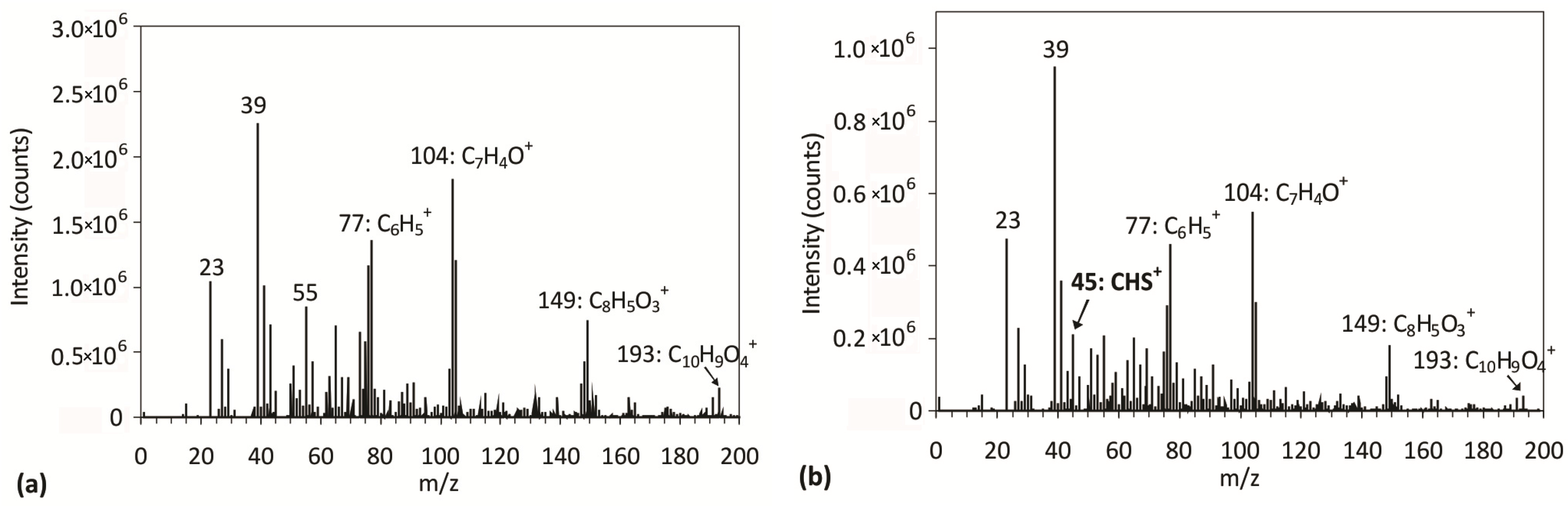
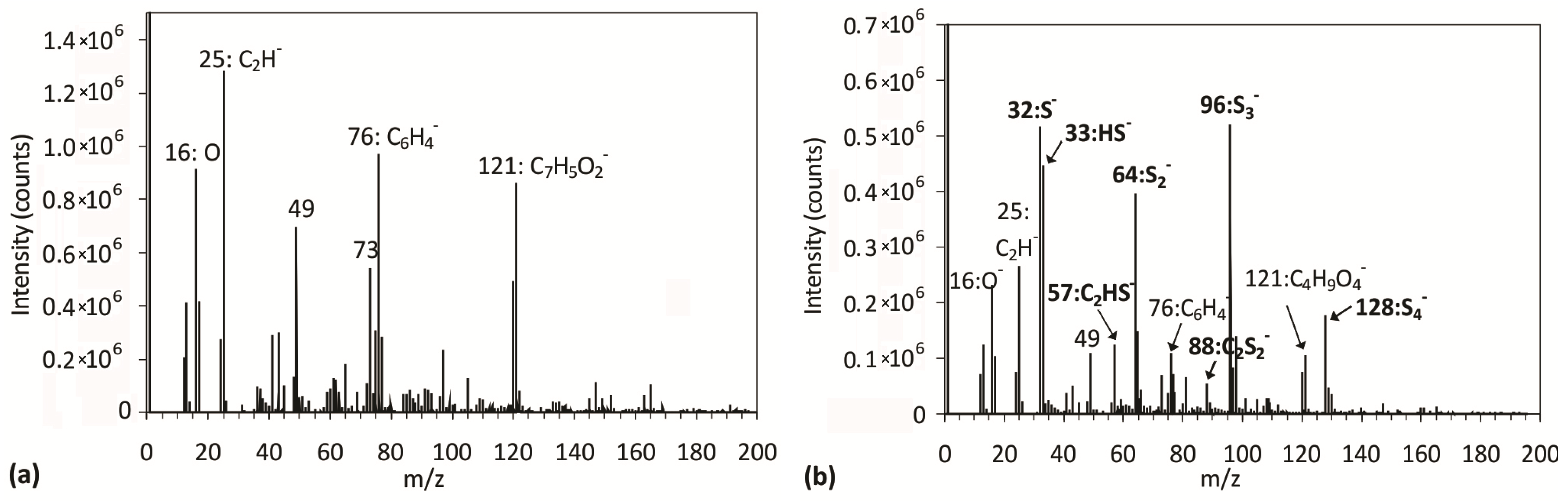

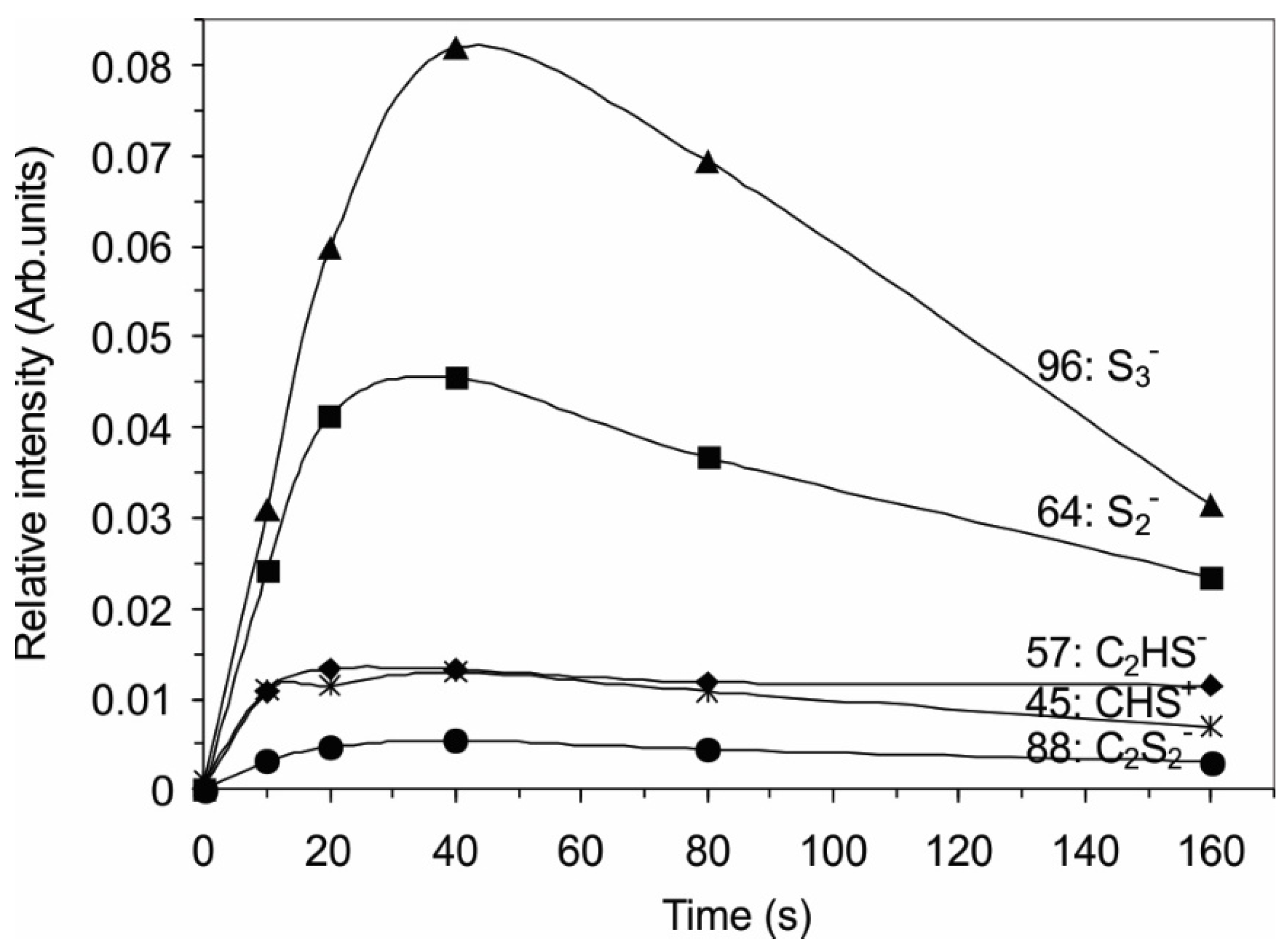
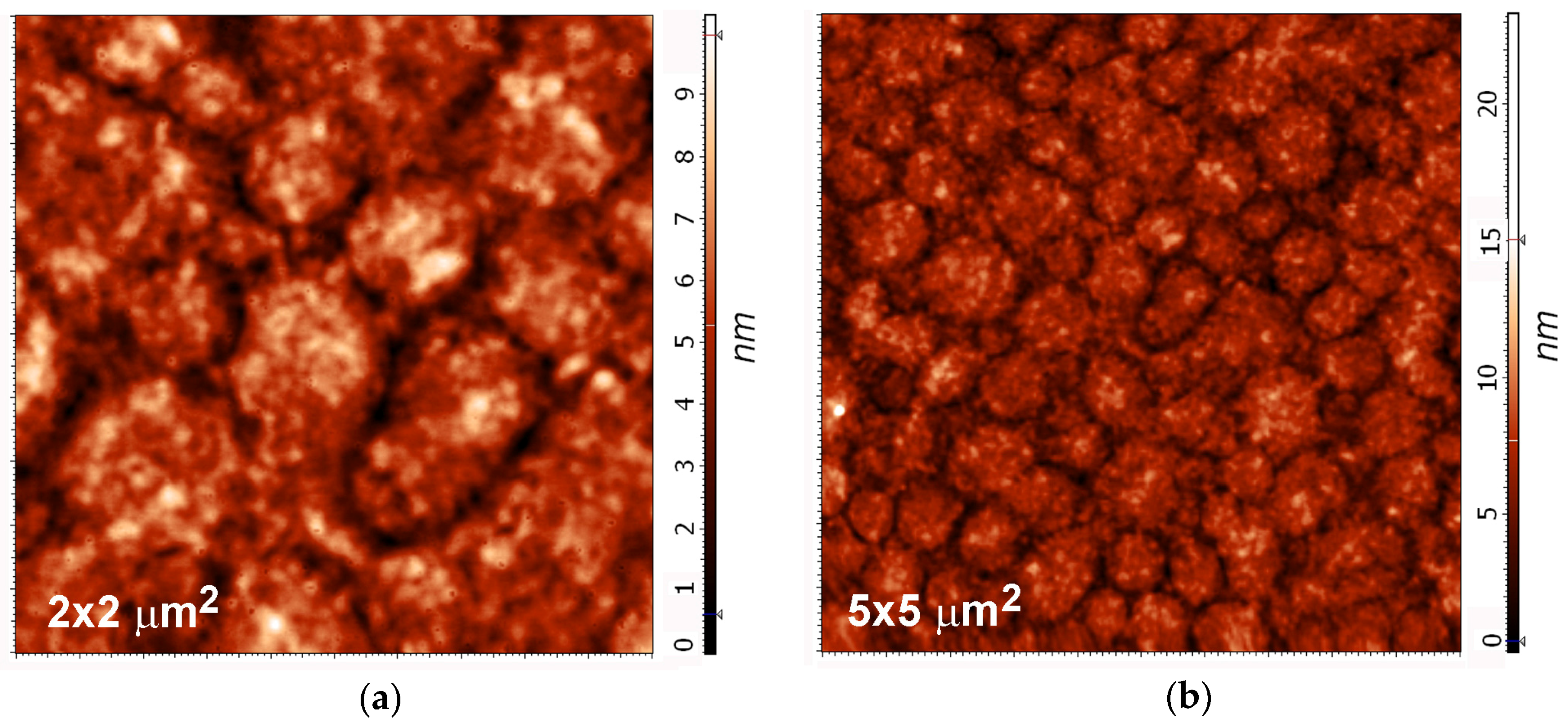
2.3. Surface Morphology, Etching and Aging
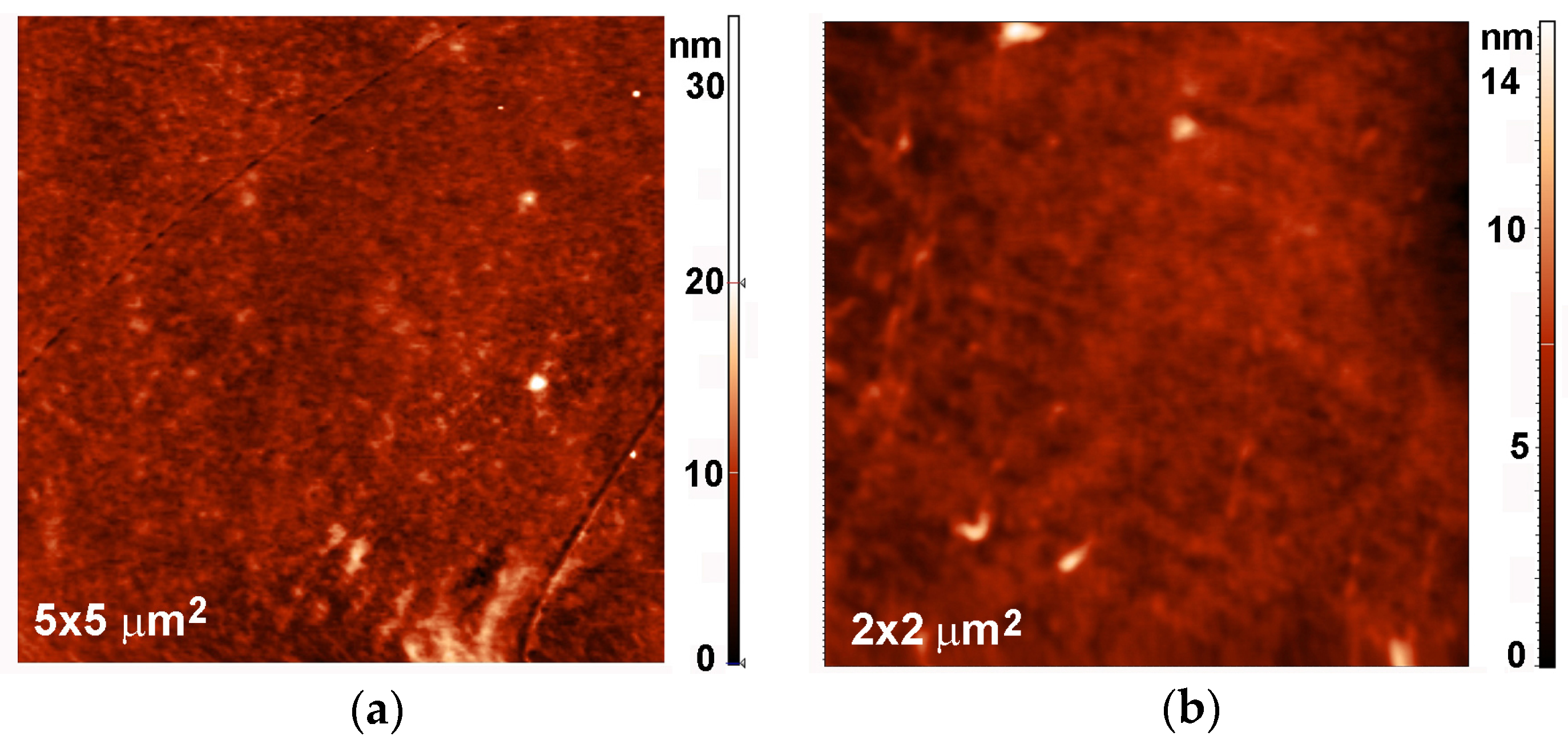
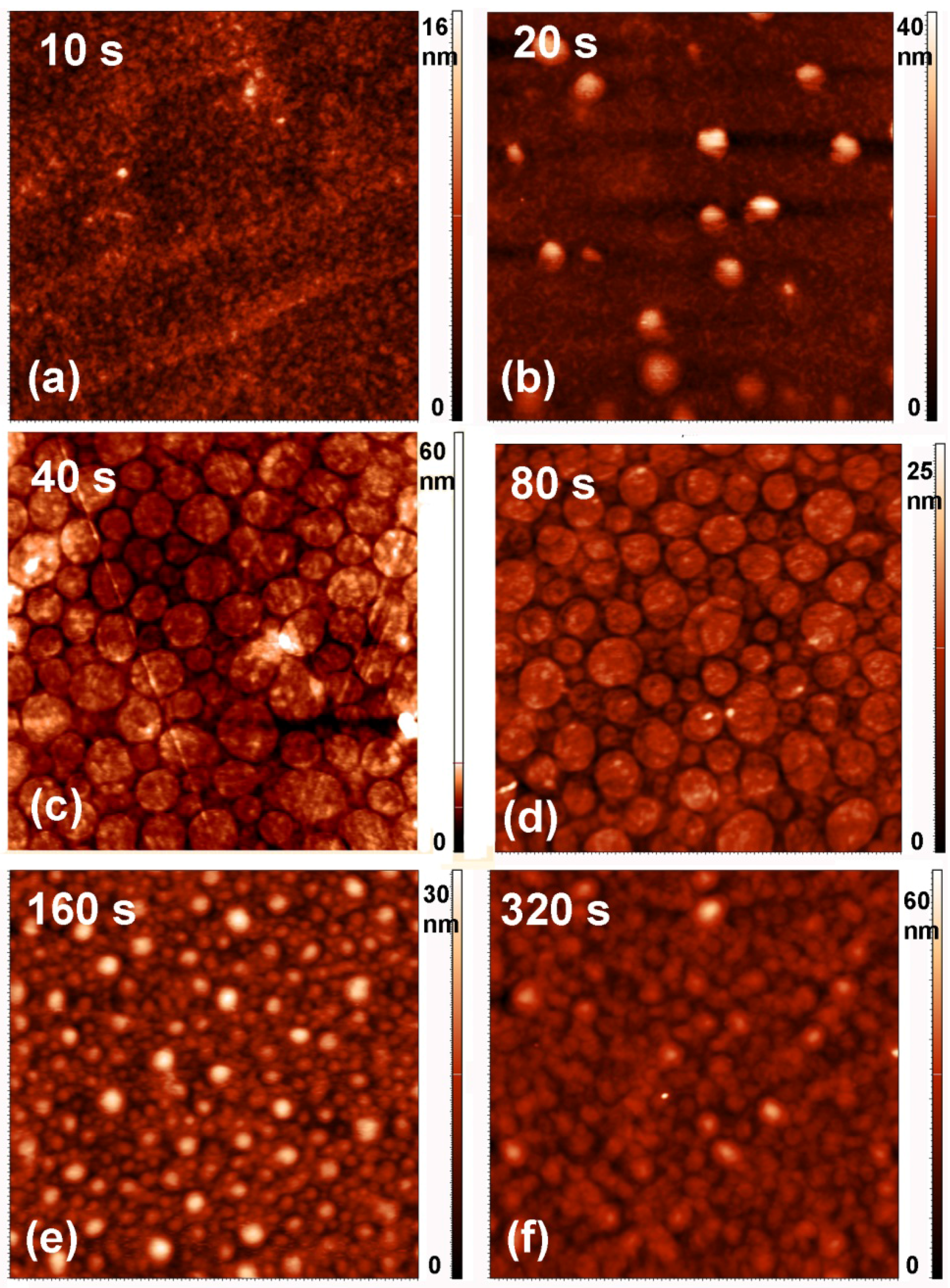
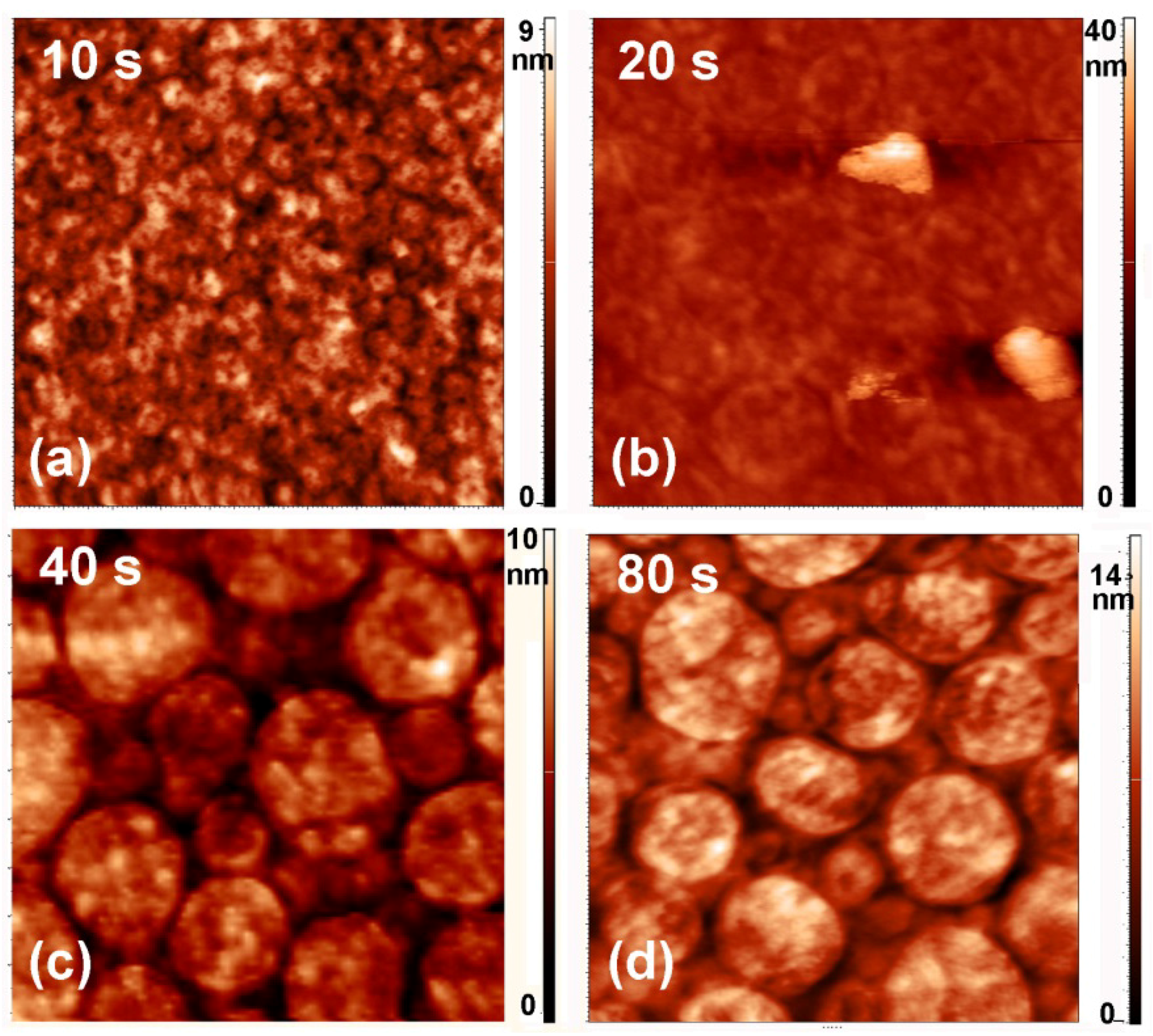
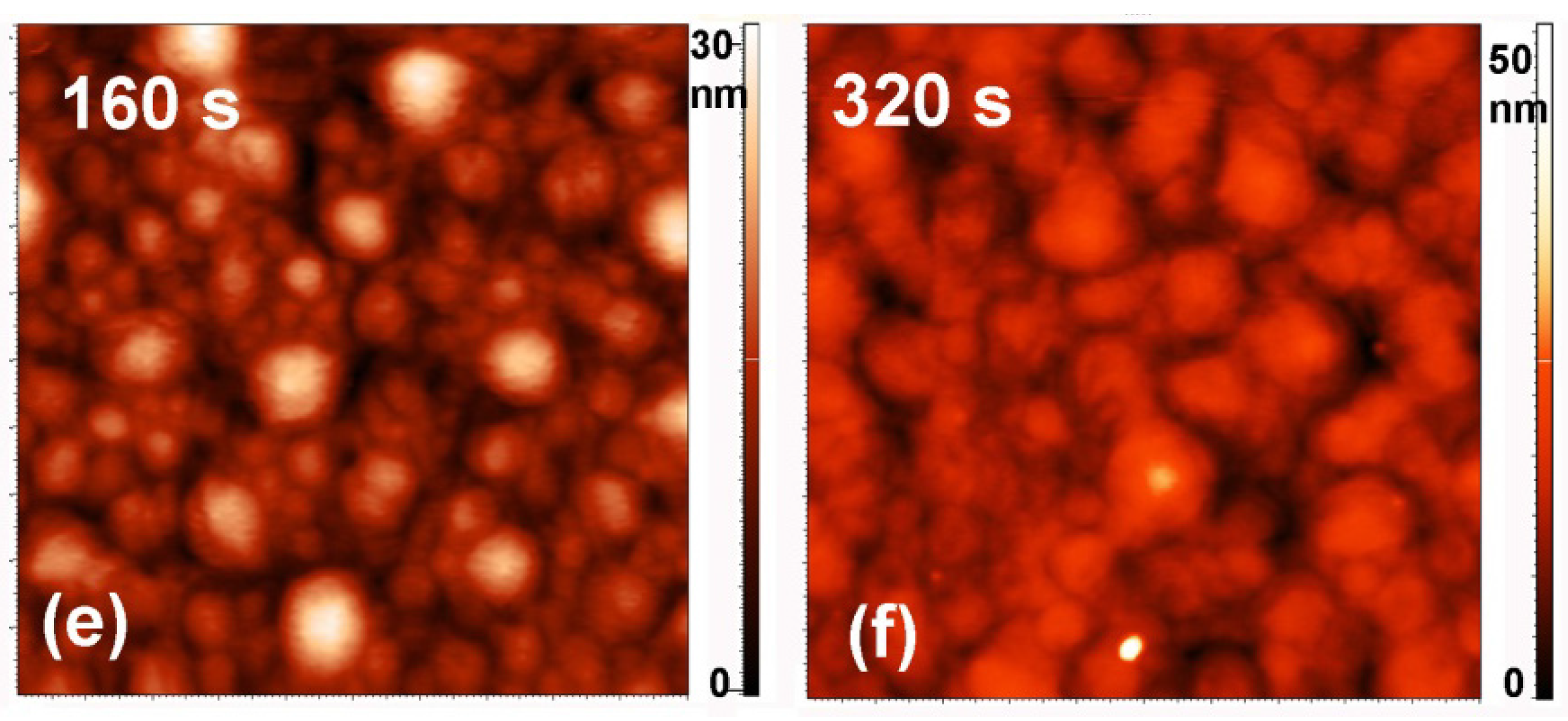
| Treatment Time (s) | Roughness (nm) | |
|---|---|---|
| Measured on 5 × 5 µm2 Area | Measured on 2 × 2 µm2 Area | |
| 0 | 1.2 | 1.2 |
| 10 | 1.2 | 1.2 |
| 20 | 2.4 | 1.6 |
| 40 | 1.7 | 1.4 |
| 80 | 2.3 | 2.1 |
| 160 | 3.3 | 3.1 |
| 320 | 3.8 | 3.2 |
| Aging Period | Element Concentration (atomic %) | ||
|---|---|---|---|
| Carbon | Oxygen | Sulfur | |
| 0 days | 46.9 | 13.1 | 40.0 |
| 1 day | 65.0 | 18.5 | 16.4 |
| 2 days | 67.2 | 19.1 | 13.7 |
| 6 months | 73.8 | 19.8 | 6.4 |
2.4. Surface Wettability
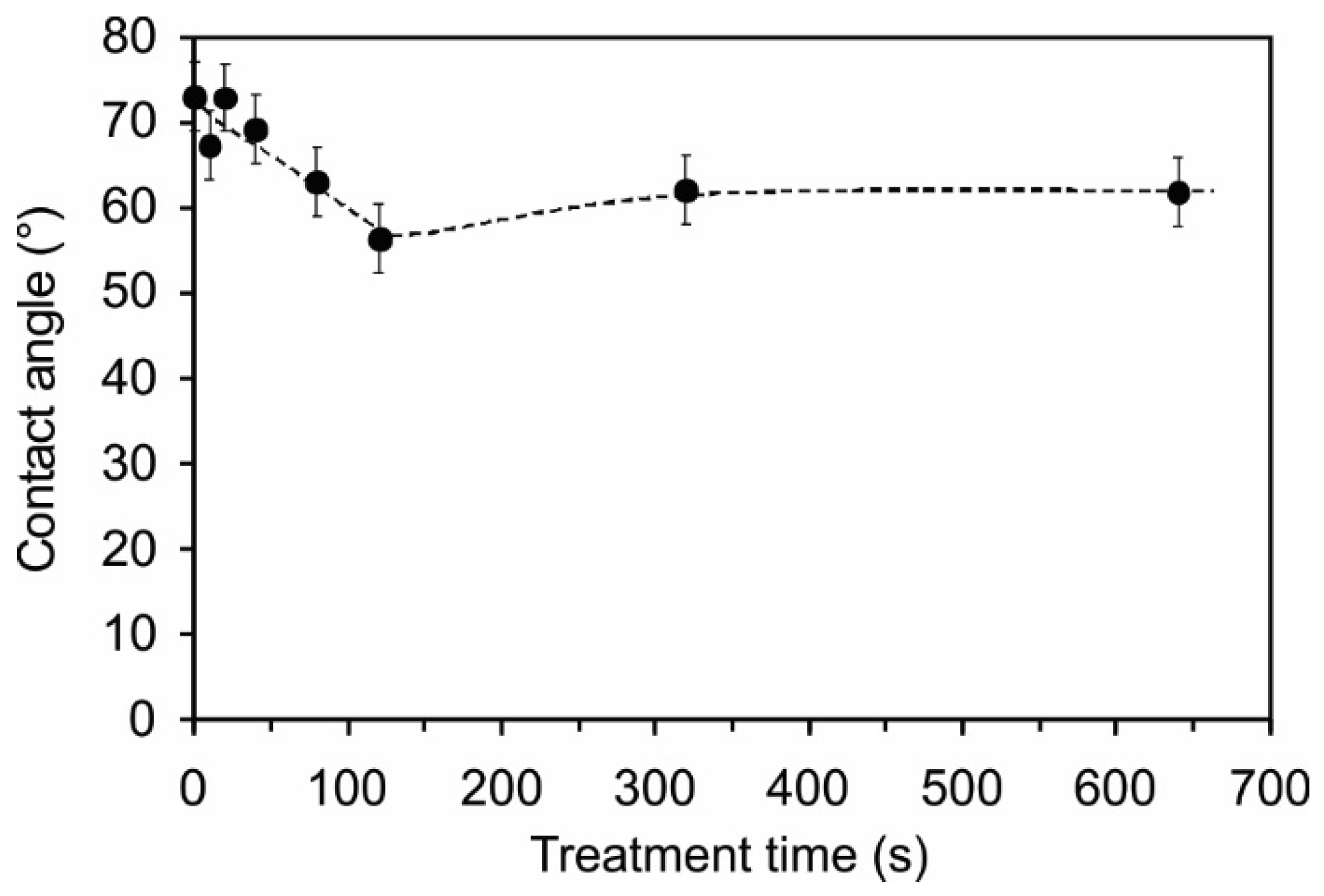
3. Materials and Methods
3.1. Plasma Treatment
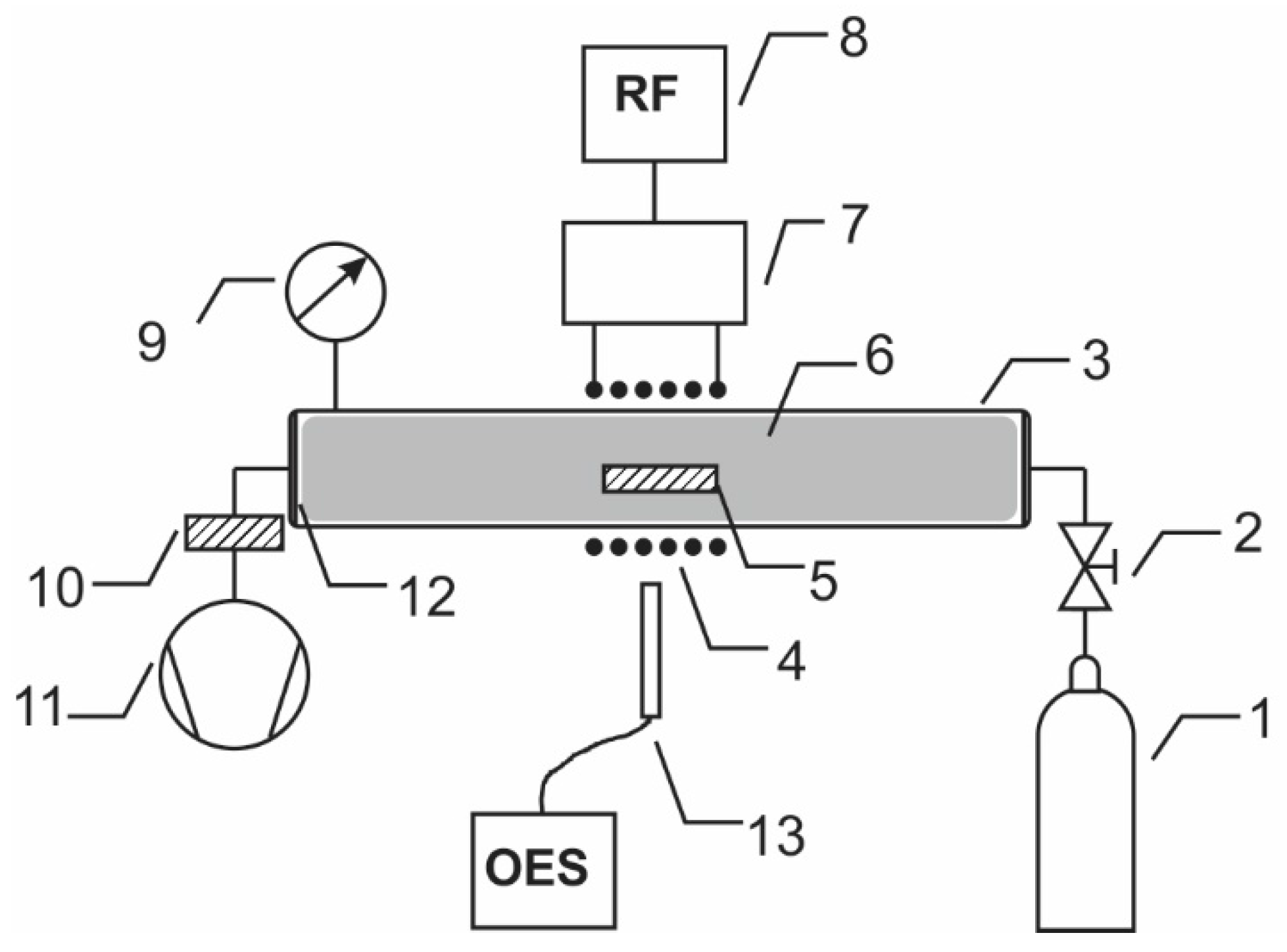
3.2. Plasma Characterization
3.3. Temperature Measurements
3.4. Weight-Loss Measurements
3.5. AFM Measurements
3.6. XPS Measurements
3.7. ToF-SIMS Measurements
3.8. Contact Angle Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chiper, A.; Borcia, G. Argon versus helium dielectric barrier discharge for surface modification of polypropylene and poly(methyl methacrylate) films. Plasma Chem. Plasma Process. 2013, 33, 553–568. [Google Scholar] [CrossRef]
- Borcia, C.; Punga, I.L.; Borcia, G. Surface properties and hydrophobic recovery of polymers treated by atmospheric-pressure plasma. Appl. Surf. Sci. 2014, 317, 103–110. [Google Scholar] [CrossRef]
- Jacobs, T.; Carbone, E.; Morent, R.; de Geyter, N.; Reniers, F.; Leys, C. Surface modification of polymer films with a remote atmospheric pressure d.c. glow discharge: Influence of substrate location. Surf. Interface Anal. 2010, 42, 1316–1320. [Google Scholar] [CrossRef]
- Mafra, M.; Belmonte, T.; Poncin-Epaillard, F.; Maliska, A.; Cvelbar, U. Treatment of hexatriacontane by Ar-O2 remote plasma: Formation of the active species. Plasma Process. Polym. 2009, 6, S198–S203. [Google Scholar] [CrossRef]
- Belmonte, T.; Pintassilgo, C.D.; Czerwiec, T.; Henrion, G.; Hody, V.; Thiebaut, J.M.; Loureiro, J. Oxygen plasma surface interaction in treatments of polyolefines. Surf. Coat. Technol. 2005, 200, 26–30. [Google Scholar] [CrossRef]
- Barni, R.; Zanini, S.; Beretta, D.; Riccardi, C. Experimental study of hydrophobic/hydrophilic transition in SF6 plasma interaction with polymer surfaces. Eur. Phys. J. Appl. Phys. 2007, 38, 263–268. [Google Scholar] [CrossRef]
- Bastos, D.C.; Santos, A.E.F.; da Fonseca, M.D.; Simao, R.A. Inducing surface hydrophobization on cornstarch film by SF6 and HMDSO plasma treatment. Carbohydr. Polym. 2013, 91, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Fatyeyeva, K.; Poncin-Epaillard, F. Sulfur dioxide plasma treatment of the clay (laponite) particles. Plasma Chem. Plasma Process. 2011, 31, 449–464. [Google Scholar] [CrossRef]
- Holländer, A.; Kröpke, S. Polymer surface treatment with SO2-containing plasmas. Plasma Process. Polym. 2010, 7, 390–402. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Sulfonated surfaces by sulfur dioxide plasma surface treatment of plasma polymer films. Plasma Process. Polym. 2009, 6, 583–592. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Jin, L.; Qin, M.; Li, X.; Wang, A.; Song, C.; Hu, Y. Decomposition of hydrogen sulfide in non-thermal plasma aided by supported CdS and ZnS semiconductors. Green Chem. 2013, 15, 1509–1513. [Google Scholar] [CrossRef]
- Nunnally, T.; Gutsol, K.; Rabinovich, A.; Fridman, A.; Starikovsky, A.; Gutsol, A.; Potter, R.W. Dissociation of H2S in non-equilibrium gliding arc “tornado” discharge. Int. J. Hydrogen Energ. 2009, 34, 7618–7625. [Google Scholar] [CrossRef]
- Reddy, E.L.; Biju, V.M.; Subrahmanyam, C. Production of hydrogen from hydrogen sulfide assisted by dielectric barrier discharge. Int. J. Hydrogen Energ. 2012, 37, 2204–2209. [Google Scholar] [CrossRef]
- Reddy, E.L.; Karuppiah, J.; Subrahmanyam, C. Kinetics of hydrogen sulfide decomposition in a DBD plasma reactor operated at high temperature. J. Energ. Chem. 2013, 22, 382–386. [Google Scholar] [CrossRef]
- John, S.; Hamann, J.C.; Muknahallipatna, S.S.; Legowski, S.; Ackerman, J.F.; Argyle, M.D. Energy efficiency of hydrogen sulfide decomposition in a pulsed corona discharge reactor. Chem. Eng. Sci. 2009, 64, 4826–4834. [Google Scholar] [CrossRef]
- Sassi, M.; Amira, N. Chemical reactor network modeling of a microwave plasma thermal decomposition of H2S into hydrogen and sulfur. Int. J. Hydrogen Energ. 2012, 37, 10010–10019. [Google Scholar] [CrossRef]
- Huang, L.; Xia, L.; Dong, W.; Hou, H. Energy efficiency in hydrogen sulfide removal by non-thermal plasma photolysis technique at atmospheric pressure. Chem. Eng. J. 2013, 228, 1066–1073. [Google Scholar] [CrossRef]
- Reddy, E.L.; Biju, V.M.; Subrahmanyam, C. Production of hydrogen and sulfur from hydrogen sulfide assisted by nonthermal plasma. Appl. Energ. 2012, 95, 87–92. [Google Scholar] [CrossRef]
- Gutsol, K.; Nunnally, T.; Rabinovich, A.; Fridman, A.; Starikovskiy, A.; Gutsol, A.; Kemoun, A. Plasma assisted dissociation of hydrogen sulfide. Int. J. Hydrogen Energ. 2012, 37, 1335–1347. [Google Scholar] [CrossRef]
- Zhao, G.-B.; John, S.; Zhang, J.-J.; Hamann, J.C.; Muknahallipatna, S.S.; Legowski, S.; Ackerman, J.F.; Argyle, M.D. Production of hydrogen and sulfur from hydrogen sulfide in a nonthermal-plasma pulsed corona discharge reactor. Chem. Eng. Sci. 2007, 62, 2216–2227. [Google Scholar] [CrossRef]
- Reddy, E.L.; Karuppiah, J.; Biju, V.M.; Subrahmanyam, C. Catalytic packed bed non-thermal plasma reactor for the extraction of hydrogen from hydrogen sulfide. Int. J. Energ. Res. 2013, 37, 1280–1286. [Google Scholar] [CrossRef]
- Thiry, D.; Francq, R.; Cossement, D.; Guillaume, M.; Cornil, J.; Snyders, R. A detailed description of the chemistry of thiol supporting plasma polymer films. Plasma Process. Polym. 2014, 11, 606–615. [Google Scholar] [CrossRef]
- Thiry, D.; Britun, N.; Konstantinidis, S.; Dauchot, J.-P.; Guillaume, M.; Cornil, J.; Snyders, R. Experimental and theoretical study of the effect of the inductive-to-capacitive transition in propanethiol plasma polymer chemistry. J. Phys. Chem. C 2013, 117, 9843–9851. [Google Scholar] [CrossRef]
- Thiry, D.; Britun, N.; Konstantinidis, S.; Dauchot, J.-P.; Denis, L.; Snyders, R. Altering the sulfur content in the propanethiol plasma polymers using the capacitive-to-inductive mode transition in inductively coupled plasma discharge. Appl. Phys. Lett. 2012, 100. [Google Scholar] [CrossRef]
- Thiry, D.; Aparicio, F.J.; Britun, N.; Snyders, R. Concomitant effects of the substrate temperature and the plasma chemistry on the chemical properties of propanethiol plasma polymer prepared by ICP discharges. Surf. Coat. Technol. 2014, 241, 2–7. [Google Scholar] [CrossRef]
- Akhavan, B.; Jarvis, K.; Majewski, P. Plasma polymerization of sulfur-rich and water-stable coatings on silica particles. Surf. Coat. Technol. 2015, 264, 72–79. [Google Scholar] [CrossRef]
- Akhavan, B.; Jarvis, K.; Majewski, P. Development of oxidized sulfur polymer films through a combination of plasma polymerization and oxidative plasma treatment. Langmuir 2014, 30, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Kiesow, A.; Heilmann, A. Deposition and properties of plasma polymer films made from thiophenes. Thin Solid Films 1999, 343–344, 338–341. [Google Scholar] [CrossRef]
- Bhat, N.V.; Wavhal, D.S. Preparation and characterization of plasma-polymerized thiophene films. J. Appl. Polym. Sci. 1998, 70, 203–209. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Kim, J.-G.; Boo, J.-H. Plasma-polymerized thiophene films for corrosion protection in microelectronic devices. J. Mater. Sci. Lett. 2002, 21, 951–953. [Google Scholar] [CrossRef]
- Kregar, Z.; Biscan, M.; Milosevic, S.; Mozetic, M.; Vesel, A. Interaction of argon, hydrogen and oxygen plasma early afterglow with polyvinyl chloride (PVC) materials. Plasma Process. Polym. 2012, 9, 1020–1027. [Google Scholar] [CrossRef]
- Pearse, R.W.B.; Gaydon, A.G. The Identification of Molecular Spectra; Chapman and Hall: London, UK, 1984. [Google Scholar]
- Thiry, D.; Francq, R.; Cossement, D.; Guerin, D.; Vuillaume, D.; Snyders, R. Establishment of a derivatization method to quantify thiol function in sulfur-containing plasma polymer films. Langmuir 2013, 29, 13183–13189. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.R.; Pitton, Y.; Mathieu, H.J.; Landolt, D.; Moser, E.M. Surface analysis of polyethyleneterephthalate by ESCA and TOF-SIMS. Fresen. J. Anal. Chem. 1997, 358, 251–254. [Google Scholar] [CrossRef]
- Lee, T.C.P. Properties and Applications of Elastomeric Polysulfides; Rapra Technology: Shrewsbury, UK, 1999. [Google Scholar]
- Kozhevnikov, V.F.; Payne, W.B.; Olson, J.K.; McDonald, C.L.; Inglefield, C.E. Physical properties of sulfur near the polymerization transition. J. Chem. Phys. 2004, 121, 7379–7386. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Setsuhara, Y.; Takenaka, K.; Shiratani, M.; Sekine, M.; Hori, M. Effects of photoirradiation in UV and VUV regions during plasma exposure to polymers. Thin Solid Films 2011, 519, 6810–6814. [Google Scholar] [CrossRef]
- Zaplotnik, R.; Vesel, A.; Mozetic, M. Transition from E to H mode in inductively coupled oxygen plasma: Hysteresis and the behaviour of oxygen atom density. Eur. Phys. Lett. 2011, 95. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesel, A.; Kovac, J.; Primc, G.; Junkar, I.; Mozetic, M. Effect of H2S Plasma Treatment on the Surface Modification of a Polyethylene Terephthalate Surface. Materials 2016, 9, 95. https://doi.org/10.3390/ma9020095
Vesel A, Kovac J, Primc G, Junkar I, Mozetic M. Effect of H2S Plasma Treatment on the Surface Modification of a Polyethylene Terephthalate Surface. Materials. 2016; 9(2):95. https://doi.org/10.3390/ma9020095
Chicago/Turabian StyleVesel, Alenka, Janez Kovac, Gregor Primc, Ita Junkar, and Miran Mozetic. 2016. "Effect of H2S Plasma Treatment on the Surface Modification of a Polyethylene Terephthalate Surface" Materials 9, no. 2: 95. https://doi.org/10.3390/ma9020095
APA StyleVesel, A., Kovac, J., Primc, G., Junkar, I., & Mozetic, M. (2016). Effect of H2S Plasma Treatment on the Surface Modification of a Polyethylene Terephthalate Surface. Materials, 9(2), 95. https://doi.org/10.3390/ma9020095









