The Effect of Acceptor and Donor Doping on Oxygen Vacancy Concentrations in Lead Zirconate Titanate (PZT)
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation and Characterisation
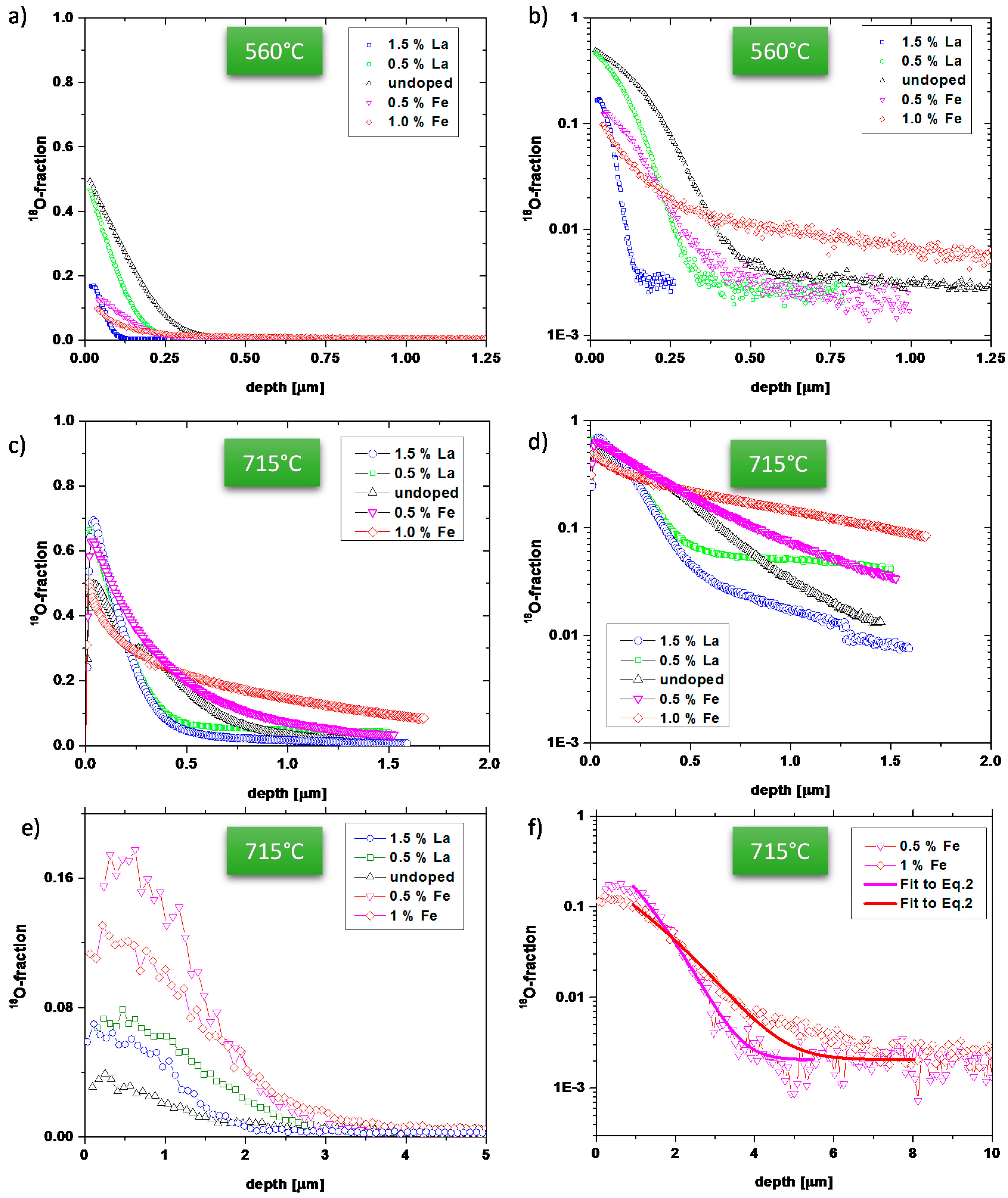
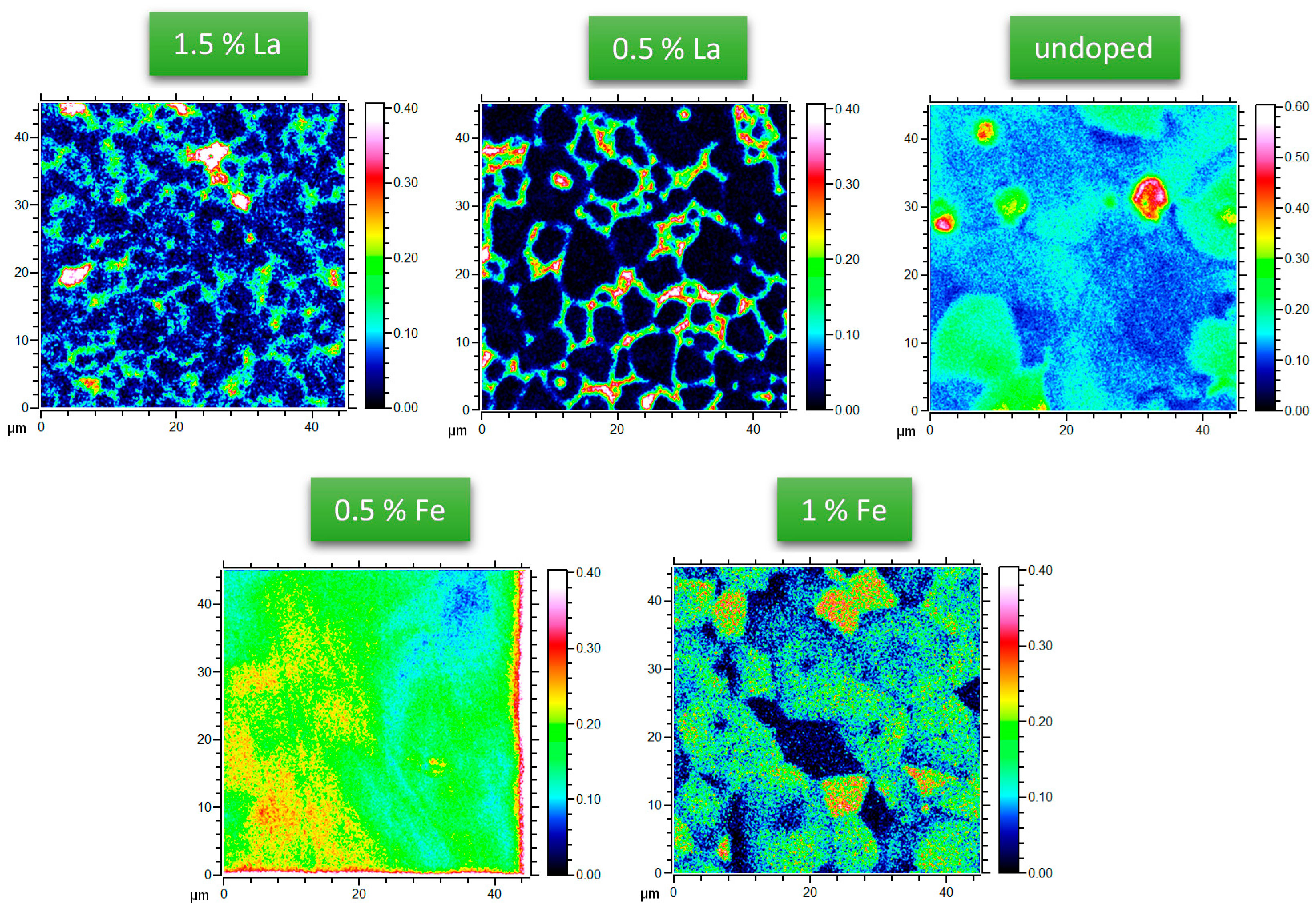
2.2. 18O Diffusion Experiments and ToF-SIMS Analysis
2.3. Impedance Measurements
2.4. P–E Measurements
3. Results and Discussion
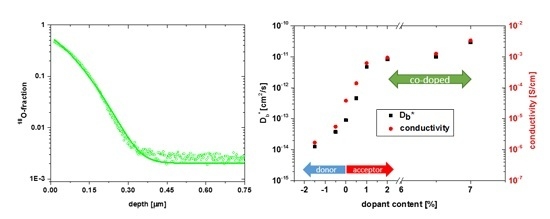
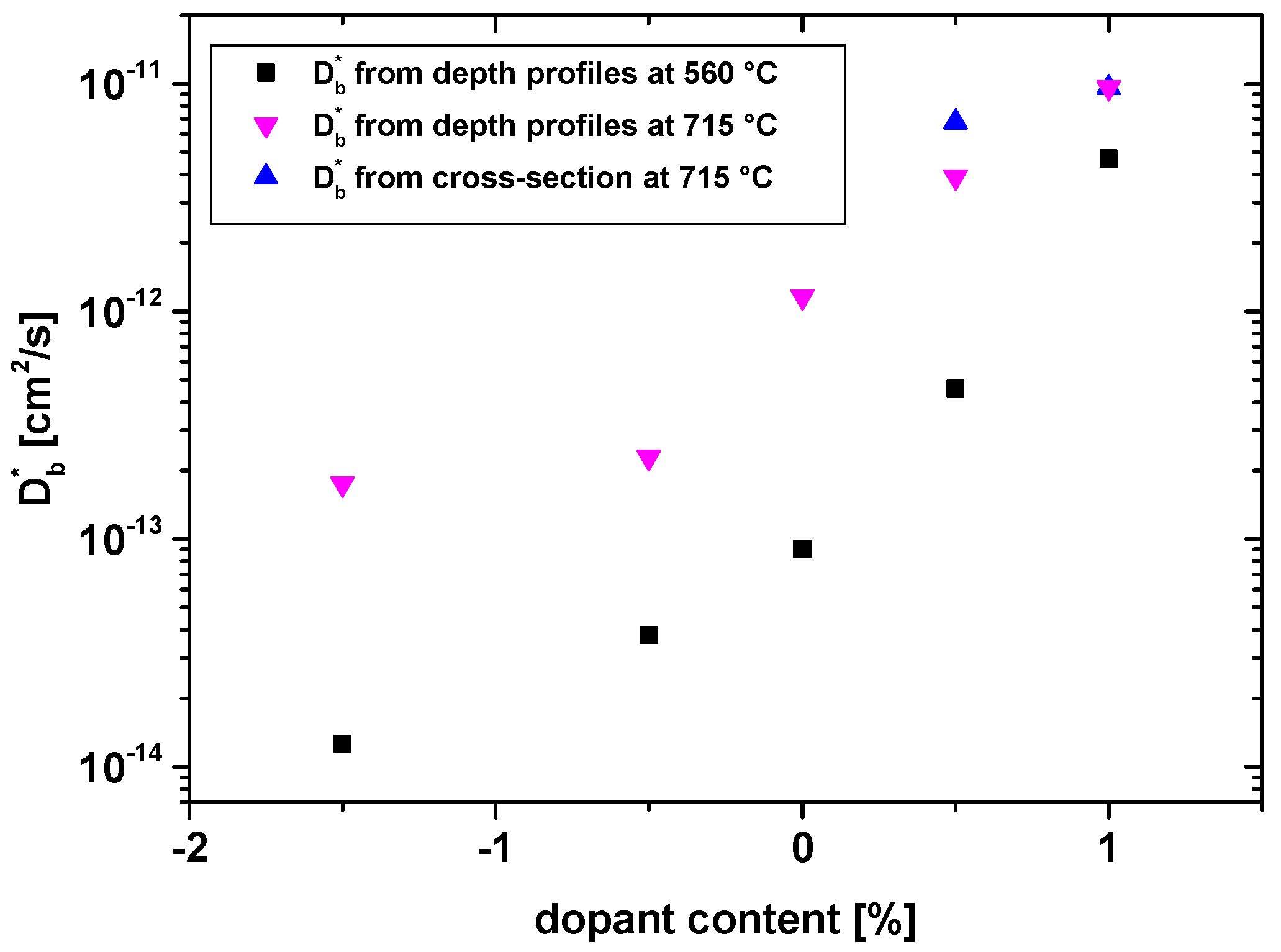
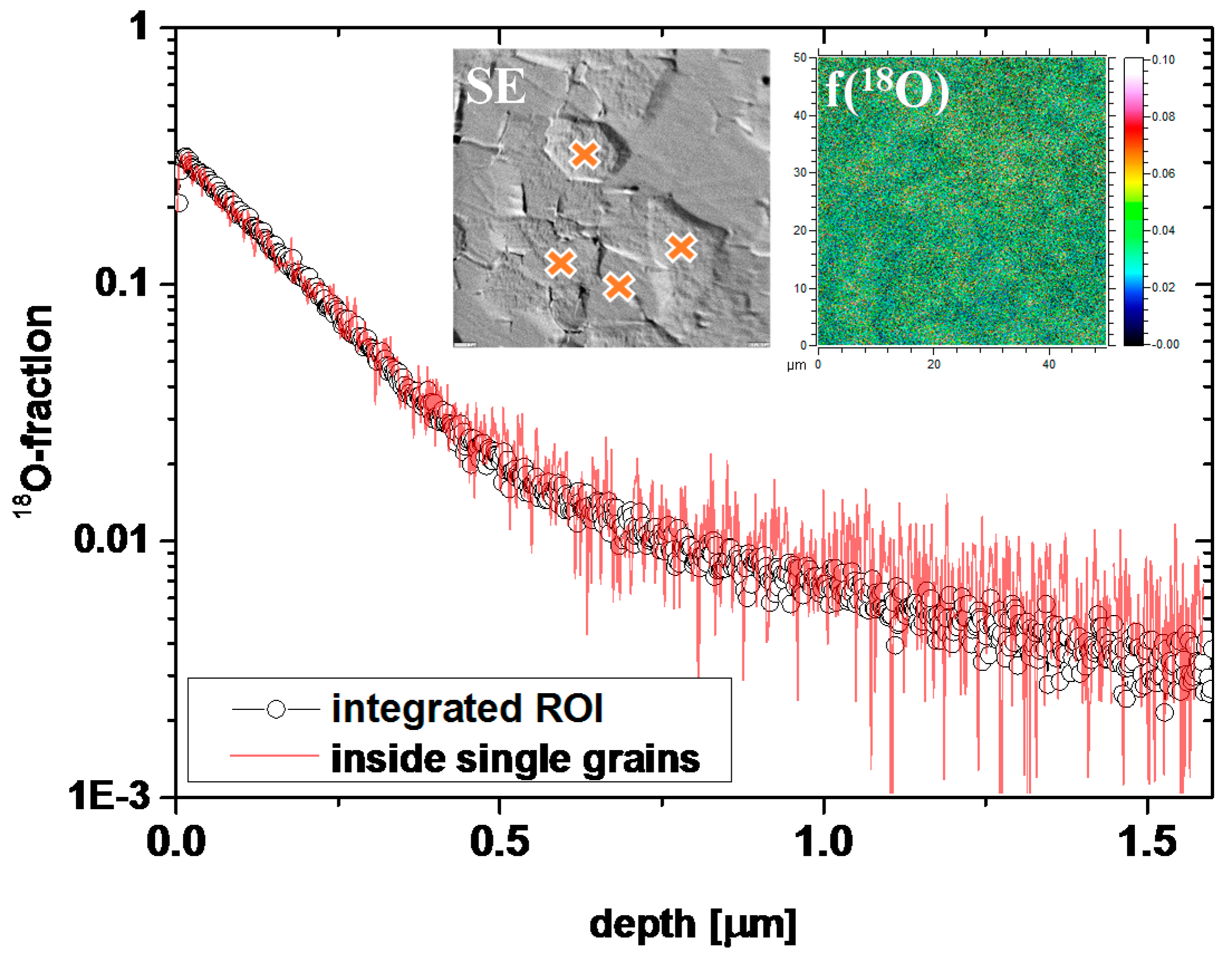
3.1. Oxygen Tracer Diffusion in Differently Doped PZT Samples
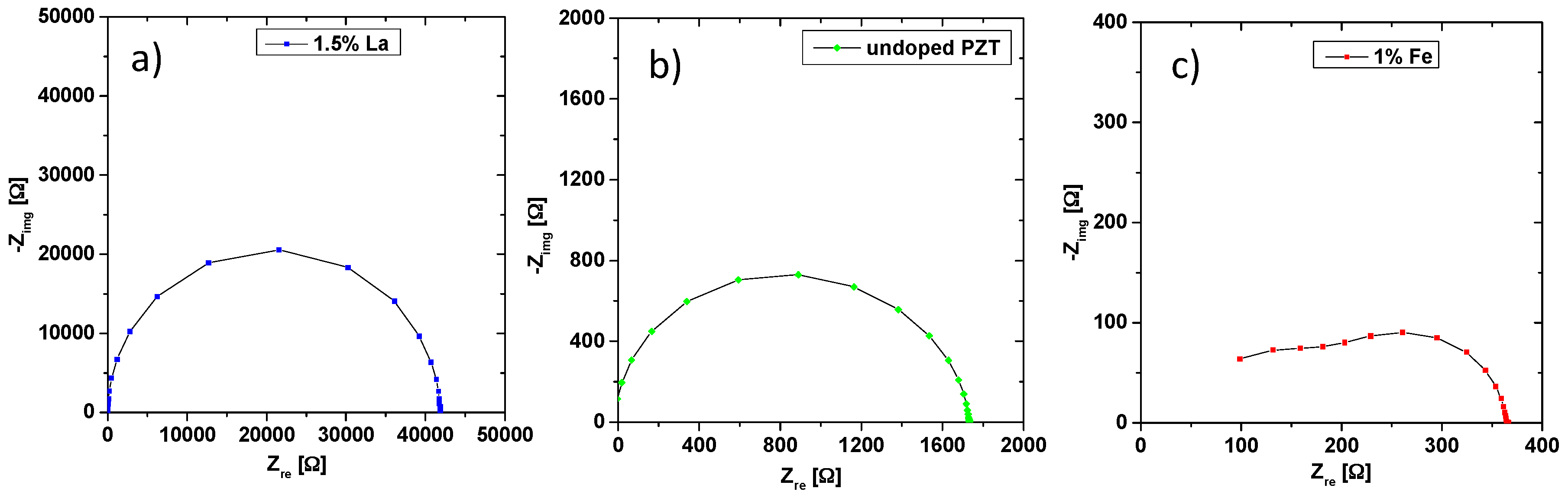
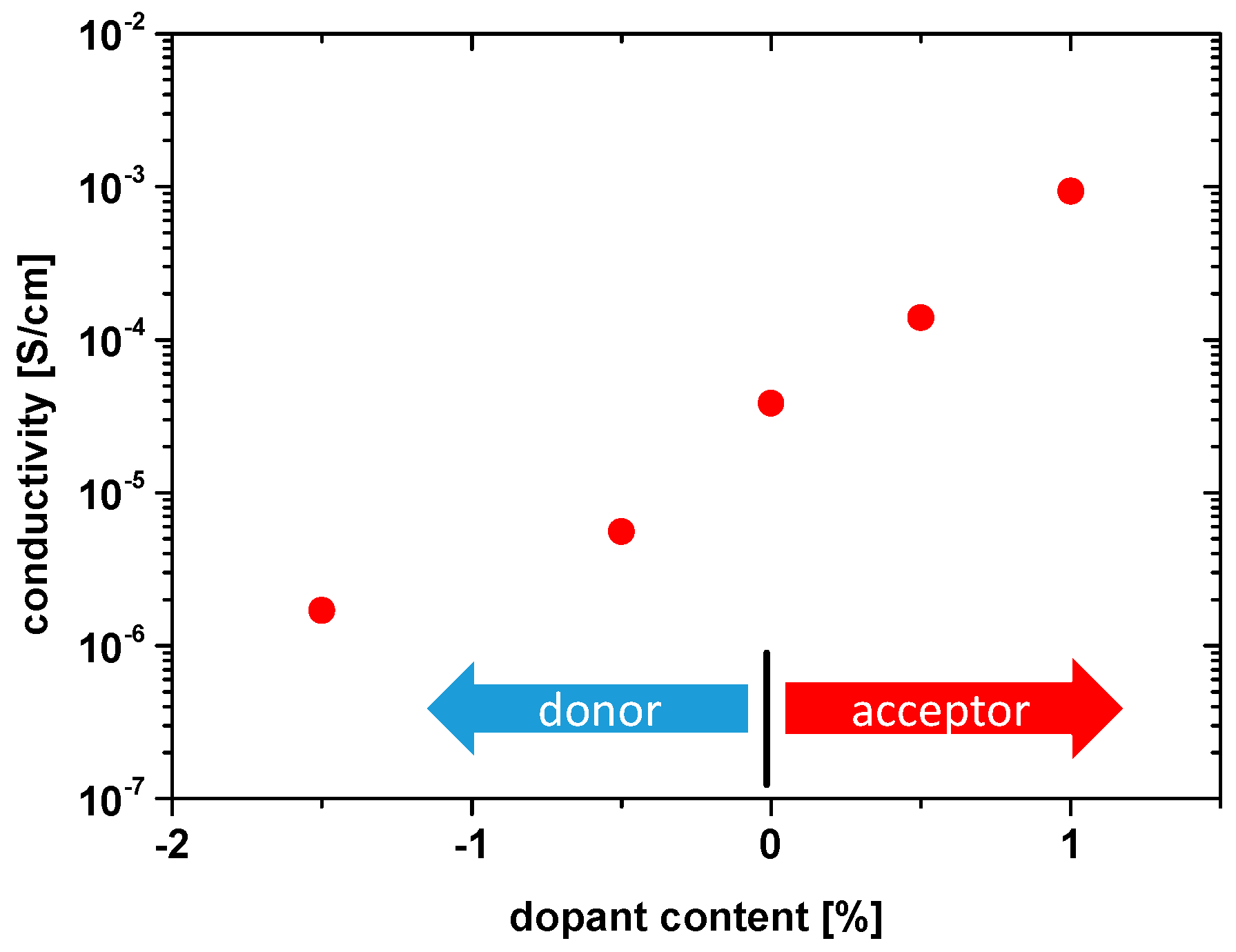
3.2. Electrical Conductivity of Differently Doped PZT Samples
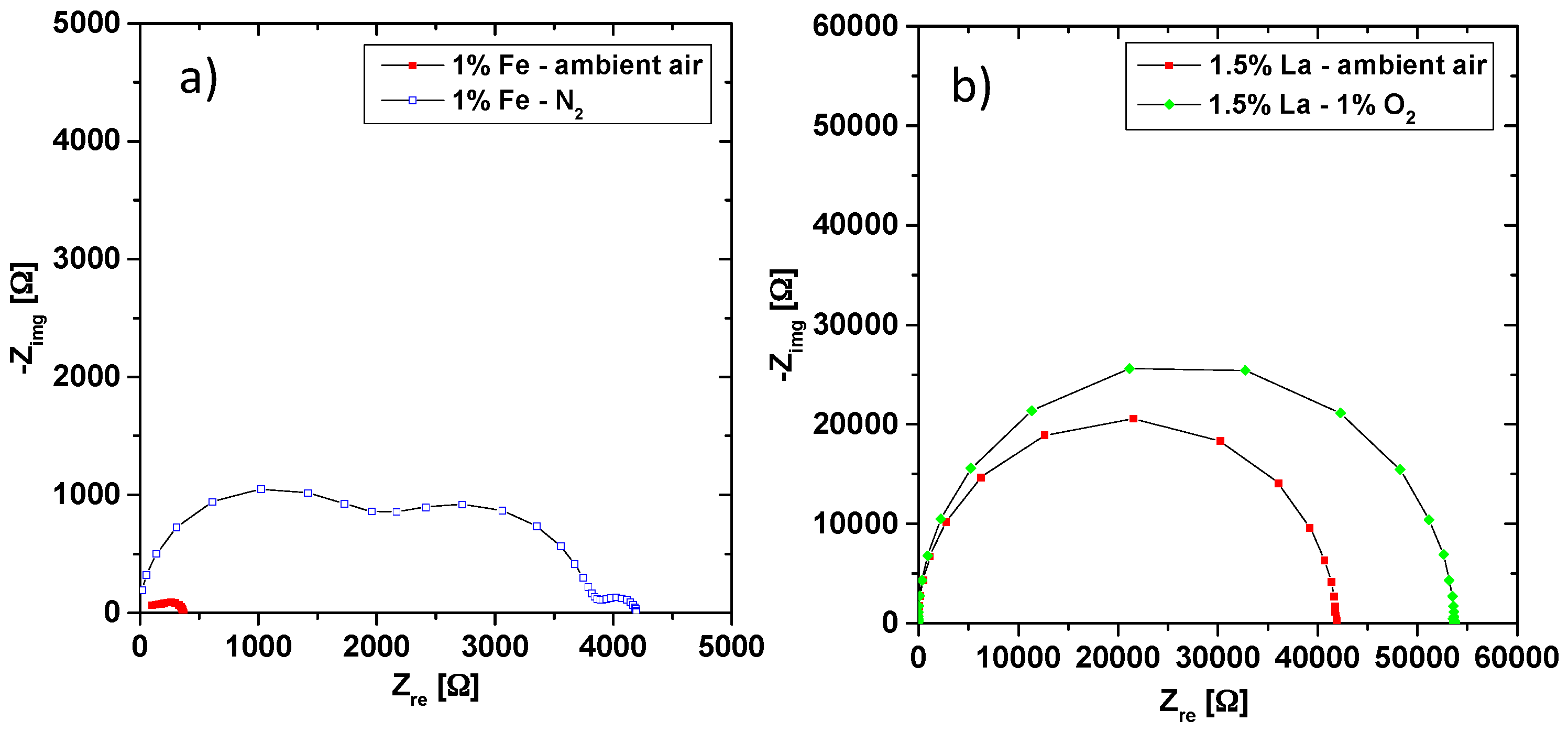
3.3. Co-Doping of Iron and Lanthanum in Net Acceptor Doped PZT
3.4. P–E Curves
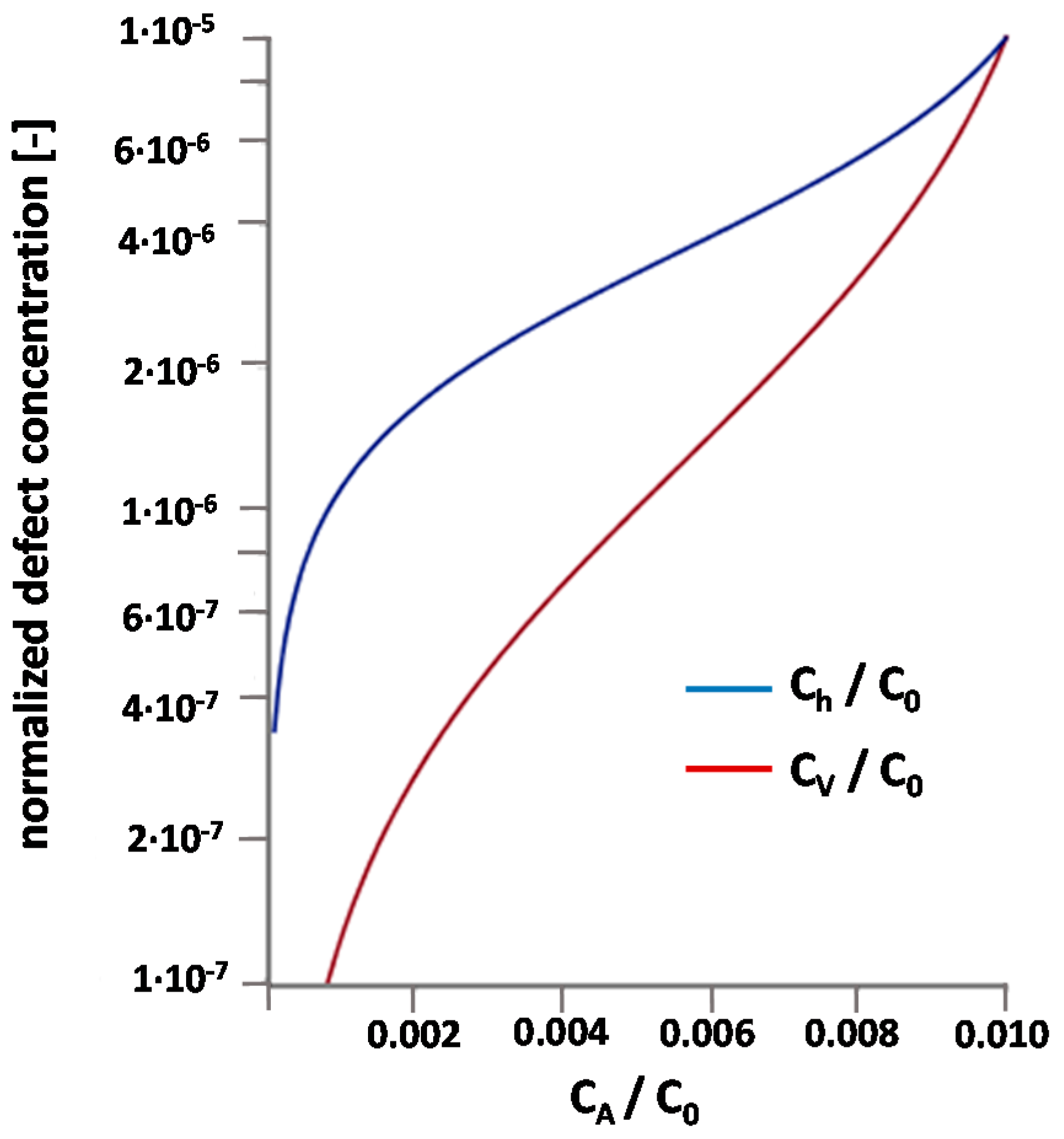
3.5. Problems of Interpretation in Terms of a Simple Defect Chemical Model
3.6. Suggestion of a Modified Defect Chemical Model
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moos, R.; Hardtl, K.H. Defect chemistry of donor-doped and undoped strontium titanate ceramics between 1000 °C and 1400 °C. J. Am. Ceram. Soc. 1997, 80, 2549–2562. [Google Scholar] [CrossRef]
- Heywang, W.; Lubitz, K.; Wersing, W. Piezoelectricity, Evolution and Future of a Technology; Springer Science & Business Media: Berlin, Germany, 2008; Volume 114. [Google Scholar]
- Haertling, G.H. Ferroelectric ceramics: History and technology. J. Am. Ceram. Soc. 1999, 82, 797–818. [Google Scholar] [CrossRef]
- Raymond, M.V.; Smyth, D.M. Defects and charge transport in perovskite ferroelectrics. J. Phys. Chem. Solids 1996, 57, 1507–1511. [Google Scholar] [CrossRef]
- Rodewald, S.; Fleig, J.; Maier, J. Resistance degradation of iron-doped strontium titanate investigated by spatially resolved conductivity measurements. J. Am. Ceram. Soc. 2000, 83, 1969–1976. [Google Scholar] [CrossRef]
- Yoo, H.-I.; Chang, M.-W.; Oh, T.-S.; Lee, C.-E.; Becker, K. Electrocoloration and oxygen vacancy mobility of Bariumtitanate. J. Appl. Phys. 2007, 102, 093701. [Google Scholar] [CrossRef]
- Smyth, D.M. Defect structure in perovskite titanates. Curr. Opin. Solid State Mater. Sci. 1996, 1, 692–697. [Google Scholar] [CrossRef]
- Maier, J. On the conductivity of polycrystalline materials. Ber. Bunsenges. Phys. Chem. 1986, 90, 26–33. [Google Scholar] [CrossRef]
- Maier, J. Complex oxides: High temperature defect chemistry vs. low temperature defect chemistry. Phys. Chem. Chem. Phys. 2003, 5, 2164–2173. [Google Scholar] [CrossRef]
- Denk, I.; Münch, W.; Maier, J. Partial conductivities in SrTiO3: Bulk polarization experiments, oxygen concentration cell measurements, and defect-chemical modeling. J. Am. Ceram. Soc. 1995, 78, 3265–3272. [Google Scholar] [CrossRef]
- Daniels, J.; Härdtl, K.H. Part I: Electrical conductivity at high temperatures of donor-doped barium titanate ceramics. Philips Res. Rep. 1976, 31, 489–504. [Google Scholar]
- Daniels, J.; Härdtl, K.H.; Wernicke, R. Part II. Defect equilibria in acceptor doped barium titanate. Philips Technol. Rev. 1976, 38, 73–82. [Google Scholar]
- De Souza, R.A.; Metlenko, V.; Park, D.; Weirich, T.E. Behavior of oxygen vacancies in single-crystal SrTiO3: Equilibrium distribution and diffusion kinetics. Phys. Rev. B 2012, 85, 174109. [Google Scholar] [CrossRef]
- Waser, R.; Baiatu, T.; Härdtl, K.H. dc Electrical Degradation of Perovskite-Type Titanates Part 1–3. J. Am. Soc. 1990, 73, 1654–1662. [Google Scholar]
- Gainon, D.J. Electron paramagnetic resonance of Fe3+ in the strong axial field of PbTiO3 host. Phys. Rev. 1964, 134, A1300. [Google Scholar] [CrossRef]
- Meštrić, H.; Eichel, R.-A.; Dinse, K.-P.; Ozarowski, A.; van Tol, J.; Brunel, L.C. High-frequency electron paramagnetic resonance investigation of the Fe3+ impurity center in polycrystalline PbTiO3 in its ferroelectric phase. J. Appl. Phys. 2004, 96, 7440–7444. [Google Scholar] [CrossRef]
- Meštrić, H.; Eichel, R.-A.; Kloss, T.; Dinse, K.-P.; Laubach, S.; Schmidt, P.; Schönau, K.; Knapp, M.; Ehrenberg, H. Iron-oxygen vacancy defect centers in PbTiO3: Newman superposition model analysis and density functional calculations. Phys. Rev. B 2005, 71, 134109. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Damjanovic, D.; Setter, N.; Marzari, N. Defect ordering and defect–domain-wall interactions in PbTiO 3: A first-principles study. Phys. Rev. B 2013, 88, 214116. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Wei, X.-K.; Feigl, L.; Damjanovic, D.; Setter, N.; Marzari, N. Asymmetric structure of 90° domain walls and interactions with defects in PbTiO3. Phys. Rev. B 2016, 93, 144102. [Google Scholar] [CrossRef]
- Rojac, T.; Drnovsek, S.; Bencan, A.; Malic, B.; Damjanovic, D. Role of charged defects on the electrical and electromechanical properties of rhombohedral Pb(Zr,Ti)O3 with oxygen octahedra tilts. Phys. Rev. B 2016, 93, 014102. [Google Scholar] [CrossRef]
- Batuk, D.; Batuk, M.; Tsirlin, A.A.; Hadermann, J.; Abakumov, A.M. Trapping of oxygen vacancies at crystallographic shear planes in acceptor-doped Pb-Based ferroelectrics. Angew. Chem. Int. Ed. 2015, 54, 14787–14790. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Kakimoto, K.-I.; Hatano, K.; Doshida, Y. Temperature dependence of mechanical degradation in lead-free alkali niobate ceramics under unipolar loading. Mater. Lett. 2016, 175, 300–304. [Google Scholar] [CrossRef]
- Morozov, M.I.; Einarsrud, M.-A.; Tolchard, J.R.; Geiger, P.T.; Webber, K.G.; Damjanovic, D.; Grande, T. In-situ structural investigations of ferroelasticity in soft and hard rhombohedral and tetragonal PZT. J. Appl. Phys. 2015, 118, 164104. [Google Scholar] [CrossRef]
- Jaffe, B.; Cook, W.R.; Jaffe, H. Piezoelectric Ceramics; Academic Press: London, UK; New York, NY, USA, 1971. [Google Scholar]
- Wu, L.; Wei, C.-C.; Wu, T.-S.; Liu, H.-C. Piezoelectric properties of modified PZT ceramics. J. Phys. C Solid State Phys. 1983, 16, 2813. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Pham, M.T.N.; Blank, D.H.A.; Bouwmeester, H.J.M. Ionic and electronic conductivity in lead–zirconate–titanate (PZT). Solid State Ion. 2004, 170, 239–254. [Google Scholar] [CrossRef]
- Slouka, C.; Andrejs, L.; Fleig, J. Defect chemistry and transport properties of Nd-doped Pb(ZrxTi1−x)O3. J. Electroceramics 2014, 33, 221–229. [Google Scholar] [CrossRef]
- Donnelly, N.J.; Randall, C.A. Mixed conduction and chemical diffusion in a PZT buried capacitor structure. Appl. Phys. Lett. 2010, 96, 052906. [Google Scholar] [CrossRef]
- Frömling, T.; Hutter, H.; Fleig, J. Oxide ion transport in donor-doped Pb(ZrxTi1−x)O3: Near-surface diffusion properties. J. Am. Ceram. Soc. 2012, 95, 1692–1700. [Google Scholar] [CrossRef]
- Donnelly, N.J.; Randall, C.A. Impedance Spectroscopy of PZT Ceramics—Measuring Diffusion Coefficients, Mixed Conduction and Pb Loss. In Proceedings of the Applications of Ferroelectrics (ISAF/PFM), 2011 International Symposium on and 2011 International Symposium on Piezoresponse Force Microscopy and Nanoscale Phenomena in Polar Materials, Vancouver, BC, Canada, 24–27 July 2011; pp. 1–5.
- Donnelly, N.J.; Randall, C.A. Pb loss in Pb(Zr,Ti)O3 ceramics observed by in situ ionic conductivity measurements. J. Appl. Phys. 2011, 109, 104107. [Google Scholar] [CrossRef]
- Izaki, T.; Haneda, H.; Watanabe, A.; Tanaka, J.; Shirasaki, S.-I.; Tsuji, K. Self diffusion of oxygen in PLZT ceramics: Optical materials and their applications. Nippon Seramikkusu Kyokai Gakujutsu Ronbunshi 1993, 101, 133–138. [Google Scholar] [CrossRef]
- Schloss, L.F.; McIntyre, P.C.; Hendrix, B.C.; Bilodeau, S.M.; Roeder, J.F.; Gilbert, S.R. Oxygen tracer studies of ferroelectric fatigue in Pb (Zr,Ti)O3 thin films. Appl. Phys. Lett. 2002, 81, 3218–3220. [Google Scholar] [CrossRef]
- Cross, J.; Kurihara, K.; Kamehara, N.; Haneda, H.; Sakaguchi, I. Oxygen tracer diffusion in Pb(Zr,Ti)O3 thin film enhanced by catalytic platinum. Appl. Phys. Lett. 2005, 86, 141909. [Google Scholar] [CrossRef]
- Gottschalk, S.; Hahn, H.; Flege, S.; Balogh, A. Oxygen vacancy kinetics in ferroelectric PbZr0.4Ti0.6O3. J. Appl. Phys. 2008, 104, 114106. [Google Scholar] [CrossRef]
- Hummelt, S.; Flege, S.; Ensinger, W. 18Oxygen diffusion in polycrystalline lead lanthanum zirconate titanate. Phys. Status Solidi (RRL)-Rapid Res. Lett. 2010, 4, 203–205. [Google Scholar] [CrossRef]
- Frömling, T.; Schintlmeister, A.; Hutter, H.; Fleig, J. Oxide ion transport in donor-doped Pb(ZrxTi1−x)O3: The role of grain boundaries. J. Am. Ceram. Soc. 2011, 94, 1173–1181. [Google Scholar] [CrossRef]
- Wang, R.-V.; McIntyre, P.C. 18O tracer diffusion in Pb(Zr,Ti)O3 thin films: A probe of local oxygen vacancy concentration. J. Appl. Phys. 2005, 97, 023508. [Google Scholar] [CrossRef]
- De Souza, R.A.; Chater, R.J. Oxygen exchange and diffusion measurements: The importance of extracting the correct initial and boundary conditions. Solid State Ion. 2005, 176, 1915–1920. [Google Scholar] [CrossRef]
- Holzlechner, G.; Kubicek, M.; Hutter, H.; Fleig, J. A novel ToF-SIMS operation mode for improved accuracy and lateral resolution of oxygen isotope measurements on oxides. J. Anal. At. Spectrom. 2013, 28, 1080. [Google Scholar] [CrossRef]
- Kubicek, M.; Holzlechner, G.; Opitz, A.; Larisegger, S.; Hutter, H.; Fleig, J. A novel ToF-SIMS operation mode for sub 100 nm lateral resolution: Application and performance. Appl. Surf. Sci. 2014, 289, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Slouka, C.; Holzlechner, G.; Andrejs, L.; Navickas, E.; Hutter, H.; Fleig, J. Oxygen ion conduction in bulk and grain boundaries of nominally donor-doped lead zirconate titanate (PZT): A combined impedance and tracer diffusion study. J. Am. Ceram. Soc. 2015, 98, 3259–3269. [Google Scholar] [CrossRef]
- Harrison, L. Influence of dislocations on diffusion kinetics in solids with particular reference to the alkali halides. Trans. Faraday Soc. 1961, 57, 1191–1199. [Google Scholar] [CrossRef]
- Whipple, R. CXXXVIII. Concentration contours in grain boundary diffusion. Philos. Mag. 1954, 45, 1225–1236. [Google Scholar] [CrossRef]
- Le Claire, A. The analysis of grain boundary diffusion measurements. Br. J. Appl. Phys. 1963, 14, 351. [Google Scholar] [CrossRef]
- De Souza, R.A.; Zehnpfenning, J.; Martin, M.; Maier, J. Determining oxygen isotope profiles in oxides with Time-of-Flight SIMS. Solid State Ion. 2005, 176, 1465–1471. [Google Scholar] [CrossRef]
- Donnelly, N.J.; Shrout, T.R.; Randall, C.A.; Reaney, I.M. Thermochemical reactions between PZT and Ag/Pd powders: Relevance to cofiring of multilayer actuators. J. Am. Ceram. Soc. 2008, 91, 1013–1018. [Google Scholar] [CrossRef]
- De Souza, R.A.; Martin, M. Using 18O/16O exchange to probe an equilibrium space-charge layer at the surface of a crystalline oxide: Method and application. Phys. Chem. Chem. Phys. 2008, 10, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Kessel, M.; De Souza, R.A.; Martin, M. Oxygen diffusion in single crystal barium titanate. Phys. Chem. Chem. Phys. 2015, 17, 12587–12597. [Google Scholar] [CrossRef] [PubMed]
- Fleig, J. The grain boundary impedance of random microstructures: Numerical simulations and implications for the analysis of experimental data. Solid State Ion. 2002, 150, 181–193. [Google Scholar] [CrossRef]
- Fleig, J.; Maier, J. Microcontact impedance measurements of individual highly conductive grain boundaries: General aspects and application to AgCl. Phys. Chem. Chem. Phys. 1999, 1, 3315–3320. [Google Scholar] [CrossRef]
- Cui, Z.H.; Gregori, G.; Ding, A.L.; Guo, X.X.; Maier, J. Electrical transport properties of transparent PLZT ceramics: Bulk and grain boundaries. Solid State Ion. 2012, 208, 4–7. [Google Scholar] [CrossRef]
- Fleig, J.; Maier, J. The impedance of ceramics with highly resistive grain boundaries: Validity and limits of the brick layer model. J. Eur. Ceram. Soc. 1999, 19, 693–696. [Google Scholar] [CrossRef]
- De Souza, R. Oxygen diffusion in SrTiO3 and related perovskite oxides. Adv. Funct. Mater. 2015, 25, 6326–6342. [Google Scholar] [CrossRef]
- Mastrikov, Y.A.; Merkle, R.; Kotomin, E.A.; Kuklja, M.M.; Maier, J. Formation and migration of oxygen vacancies in La1−xSrxCo1−yFeyO3−δ perovskites: Insight from ab initio calculations and comparison with Ba1−x SrxCo1−y FeyO3−δ. Phys. Chem. Chem. Phys. 2013, 15, 911–918. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.A.; Fleig, J.; Merkle, R.; Maier, J. SrTiO3: A model electroceramic: Dedicated to Professor Dr. Dr. hc Manfred Rühle on the Occasion of his 65th Birthday. Z. Metall. 2003, 94, 218–225. [Google Scholar] [CrossRef]
- Claus, J.; Leonhardt, M.; Maier, J. Tracer diffusion and chemical diffusion of oxygen in acceptor doped SrTiO 3. J. Phys. Chem. Solids 2000, 61, 1199–1207. [Google Scholar] [CrossRef]
- Andrejs, L.; Fleig, J. Resistance degradation in donor-doped PZT ceramic stacks with Ag/Pd electrodes: I. Phenomenology of processes. J. Eur. Ceram. Soc. 2013, 33, 779–794. [Google Scholar] [CrossRef]
- Raymond, M.V.; Smyth, D.M. Defects and transport in Pb(Zr½Ti½)O3. Ferroelectrics 1993, 144, 129–135. [Google Scholar] [CrossRef]
- Dih, J.; Fulrath, R. Electrical conductivity in lead zirconate-titanate ceramics. J. Am. Ceram. Soc. 1978, 61, 448–451. [Google Scholar] [CrossRef]
- Eichel, R.-A. Structural and dynamic properties of oxygen vacancies in perovskite oxides—analysis of defect chemistry by modern multi-frequency and pulsed EPR techniques. Phys. Chem. Chem. Phys. 2011, 13, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Eyraud, L.; Gonnard, P.; Claudel, B. Causes of instability and aging of piezoelectric power ceramics. J. Am. Ceram. Soc. 1990, 73, 1854–1856. [Google Scholar] [CrossRef]
- Inaguma, Y.; Tanaka, K.; Tsuchiya, T.; Mori, D.; Katsumata, T.; Ohba, T.; Hiraki, K.-I.; Takahashi, T.; Saitoh, H. Synthesis, structural transformation, thermal stability, valence state, and magnetic and electronic properties of PbNiO3 with perovskite-and LiNbO3-type structures. J. Am. Chem. Soc. 2011, 133, 16920–16929. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Saito, H.; Yoshida, M.; Katsumata, T.; Ohba, T.; Inaguma, Y.; Tsurui, T.; Shikano, M. High-Pressure Synthesis of a Novel PbFeO3; MRS Proceedings; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Goodenough, J.B.; Zhou, J. Varied roles of Pb in transition-metal PbMO3 perovskites (M = Ti, V, Cr, Mn, Fe, Ni, Ru). Sci. Technol. Adv. Mater. 2015, 16. Available online: http://china.tandfonline.com/doi/full/10.1088/1468-6996/16/3/036003 (accessed on 17 November 2016). [Google Scholar]
- Yamamoto, T.; Ohno, T. A hybrid density functional study on the electron and hole trap states in anatase titanium dioxide. Phys. Chem. Chem. Phys. 2012, 14, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Warren, W.; Robertson, J.; Dimos, D.; Tuttle, B.; Smyth, D. Transient hole traps in PZT. Ferroelectrics 1994, 153, 303–308. [Google Scholar] [CrossRef]
- Frantti, J.; Lantto, V.; Kakihana, M. Raman scattering studies of Pb(ZrxTi1−x)O3 and Pb1–3y/2Ndy(ZrxTi1−x)O3 ceramics with uv and visible laser lights. Jpn. J. Appl. Phys. 1998, 37, 5406. [Google Scholar]
- Robertson, J.; Warren, W.; Tuttle, B. Band states and shallow hole traps in Pb(Zr,Ti)O3 ferroelectrics. J. Appl. Phys. 1995, 77, 3975–3980. [Google Scholar] [CrossRef]

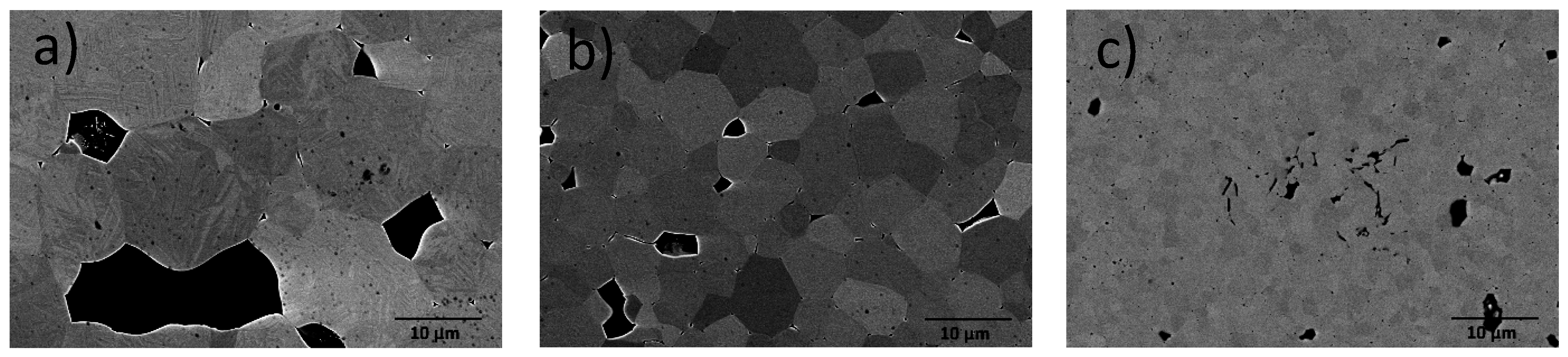
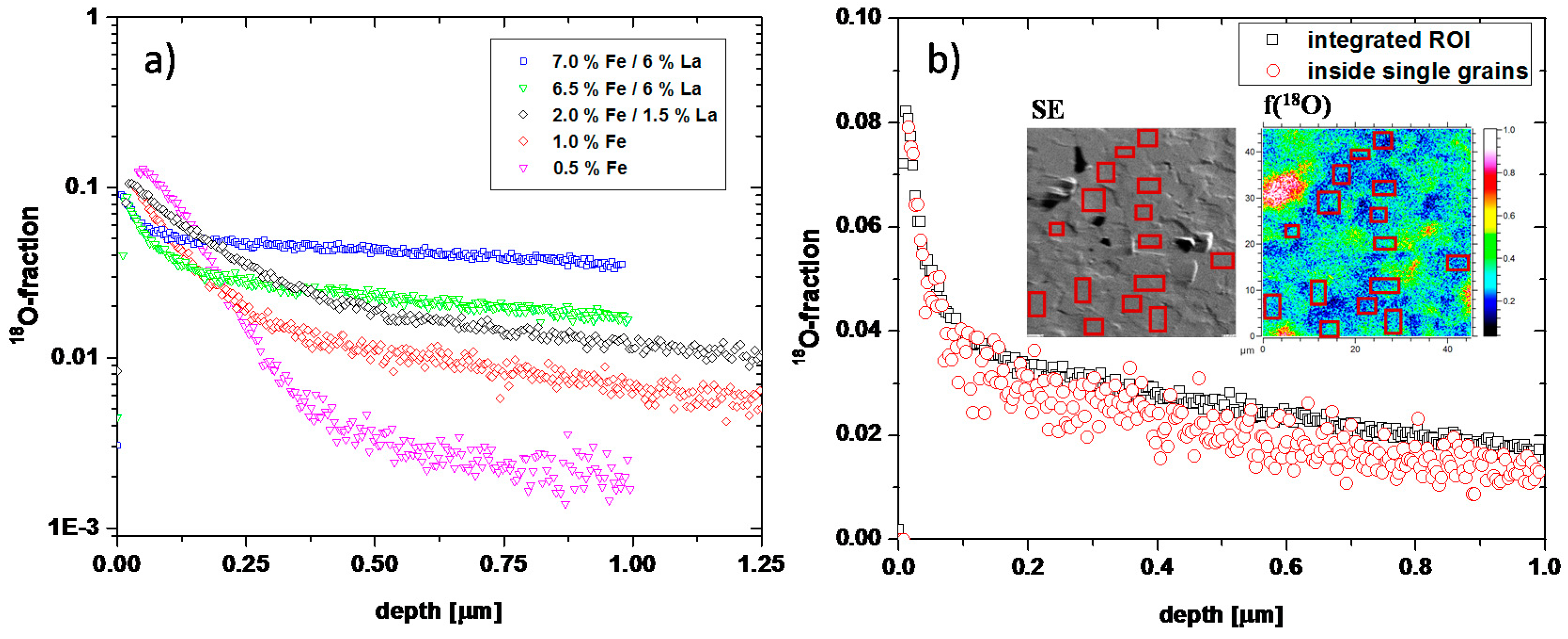
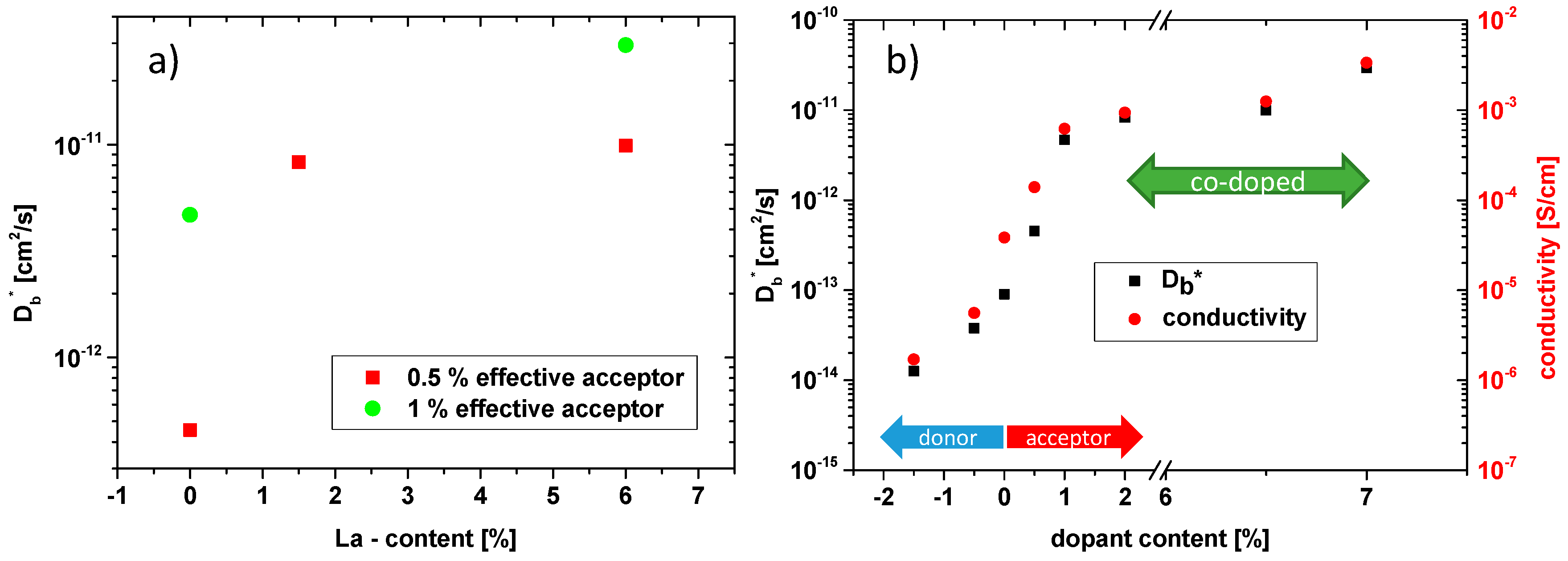
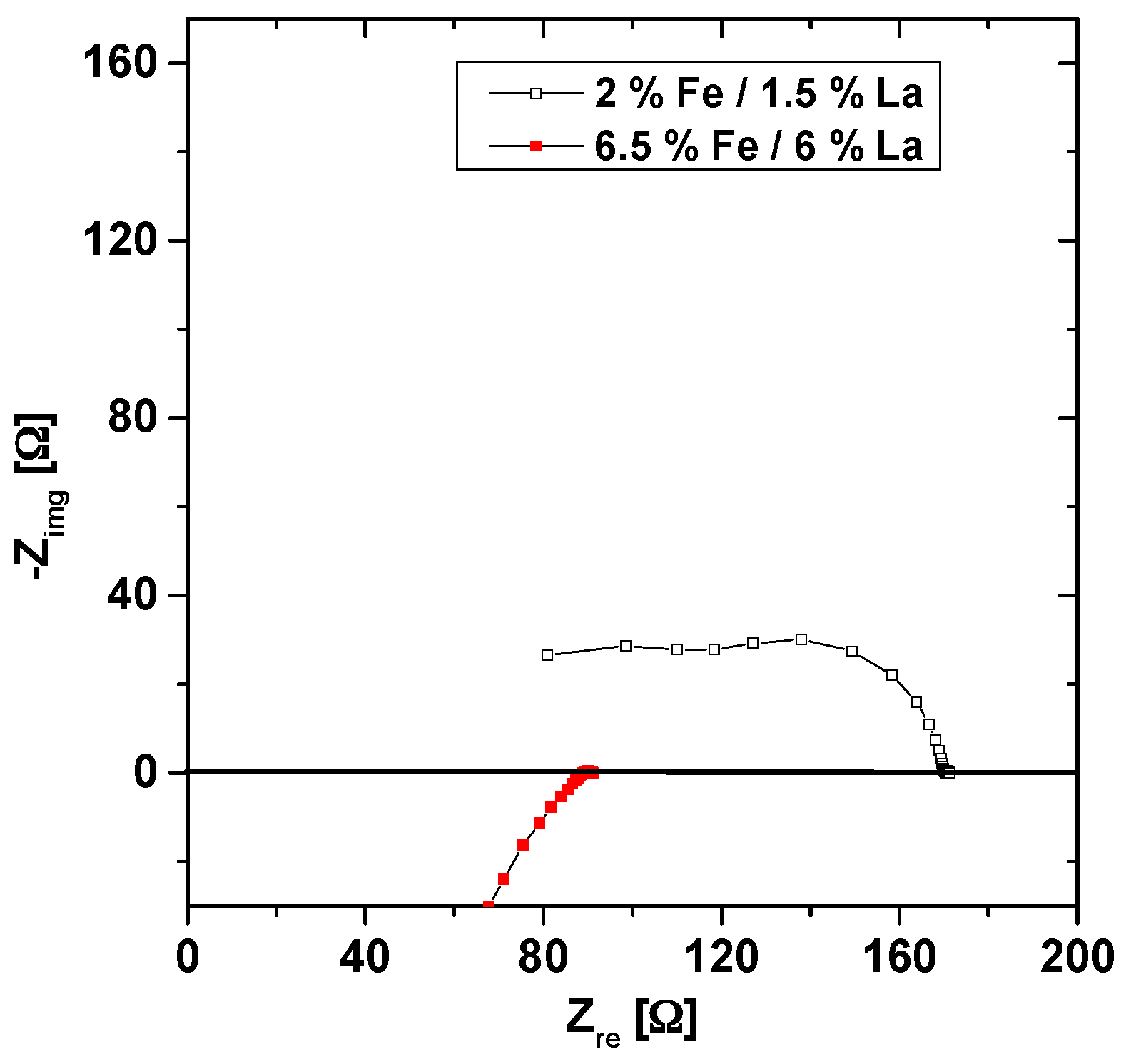

| Dopants | Net Acceptor Doping (Singly Charged) | Composition |
|---|---|---|
| Undoped | 0% | PbZr0.60Ti0.40O3 |
| 0.5% La | - | Pb0.9925La0.005Zr0.6Ti0.4O3 |
| 1.5% La | - | Pb0.9775La0.015Zr0.6Ti0.4O3 |
| 0.5% Fe | 0.5% | PbZr0.60Ti0.395Fe0.005O3 |
| 1% Fe | 1% | PbZr0.60Ti0.39Fe0.01O3 |
| 2% Fe, 1.5% La | 0.5% | Pb0.985La0.015Zr0.60Ti0.38Fe0.02O3 |
| 6.5% Fe, 6% La | 0.5% | Pb0.94La0.06Zr0.60Ti0.335Fe0.065O3 |
| 7% Fe, 6% La | 1% | Pb0.94La0.06Zr0.60Ti0.33Fe0.07O3 |
| Samples | |||
|---|---|---|---|
| 0.5% La/1.5% La | 3.3 | 3.0 | 3.63 |
| undoped/0.5% La | 6.3 | 2.4 | 16.5 |
| 0.5% Fe/undoped | 4.0 | 5.1 | 3.2 |
| 1% Fe/0.5% Fe | 6.7 | 10.2 | 4.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slouka, C.; Kainz, T.; Navickas, E.; Walch, G.; Hutter, H.; Reichmann, K.; Fleig, J. The Effect of Acceptor and Donor Doping on Oxygen Vacancy Concentrations in Lead Zirconate Titanate (PZT). Materials 2016, 9, 945. https://doi.org/10.3390/ma9110945
Slouka C, Kainz T, Navickas E, Walch G, Hutter H, Reichmann K, Fleig J. The Effect of Acceptor and Donor Doping on Oxygen Vacancy Concentrations in Lead Zirconate Titanate (PZT). Materials. 2016; 9(11):945. https://doi.org/10.3390/ma9110945
Chicago/Turabian StyleSlouka, Christoph, Theresa Kainz, Edvinas Navickas, Gregor Walch, Herbert Hutter, Klaus Reichmann, and Jürgen Fleig. 2016. "The Effect of Acceptor and Donor Doping on Oxygen Vacancy Concentrations in Lead Zirconate Titanate (PZT)" Materials 9, no. 11: 945. https://doi.org/10.3390/ma9110945
APA StyleSlouka, C., Kainz, T., Navickas, E., Walch, G., Hutter, H., Reichmann, K., & Fleig, J. (2016). The Effect of Acceptor and Donor Doping on Oxygen Vacancy Concentrations in Lead Zirconate Titanate (PZT). Materials, 9(11), 945. https://doi.org/10.3390/ma9110945