Characterization and Bioactivity Evaluation of (Polyetheretherketone/Polyglycolicacid)-Hydroyapatite Scaffolds for Tissue Regeneration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Scaffold Fabrication
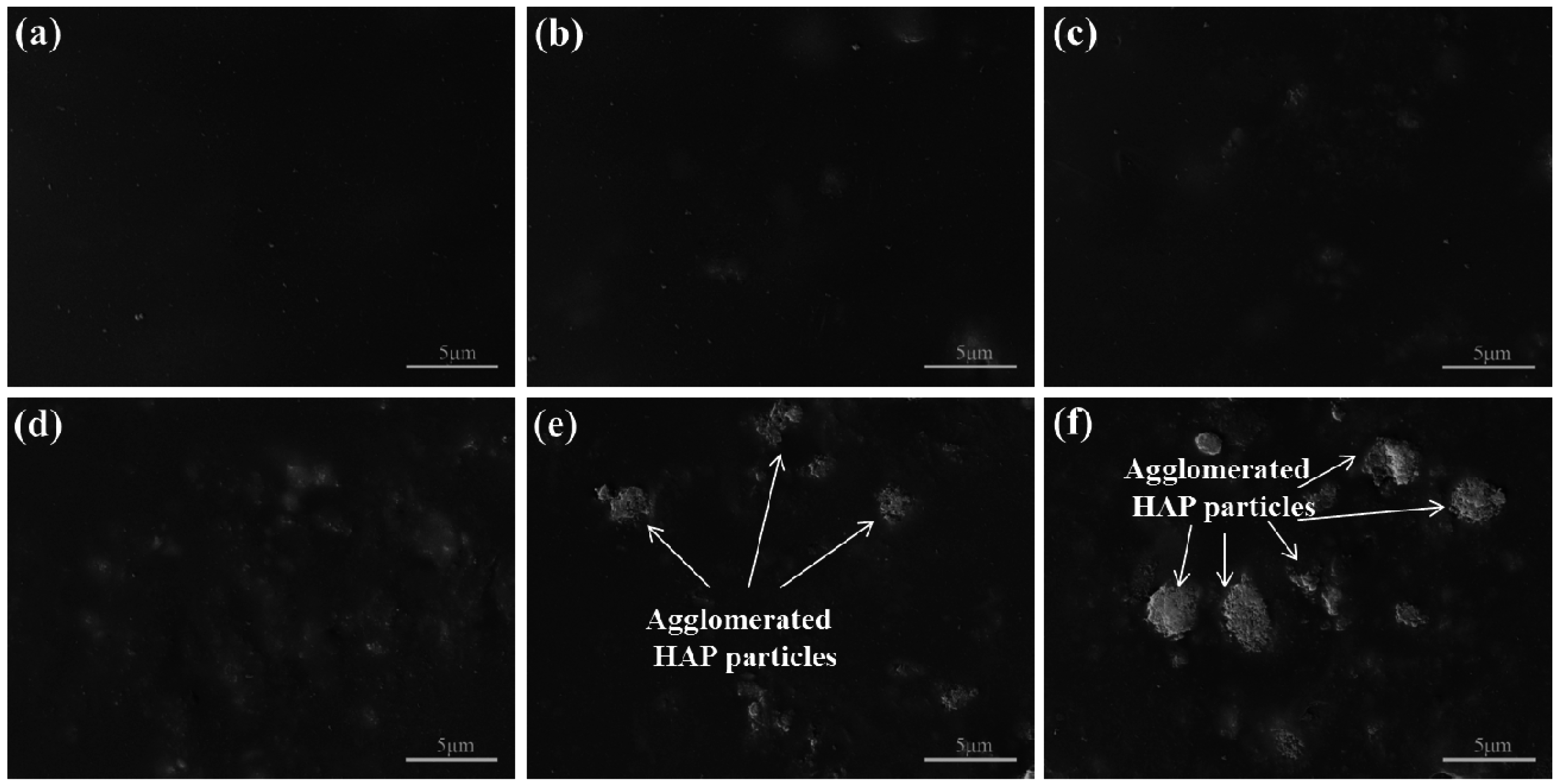
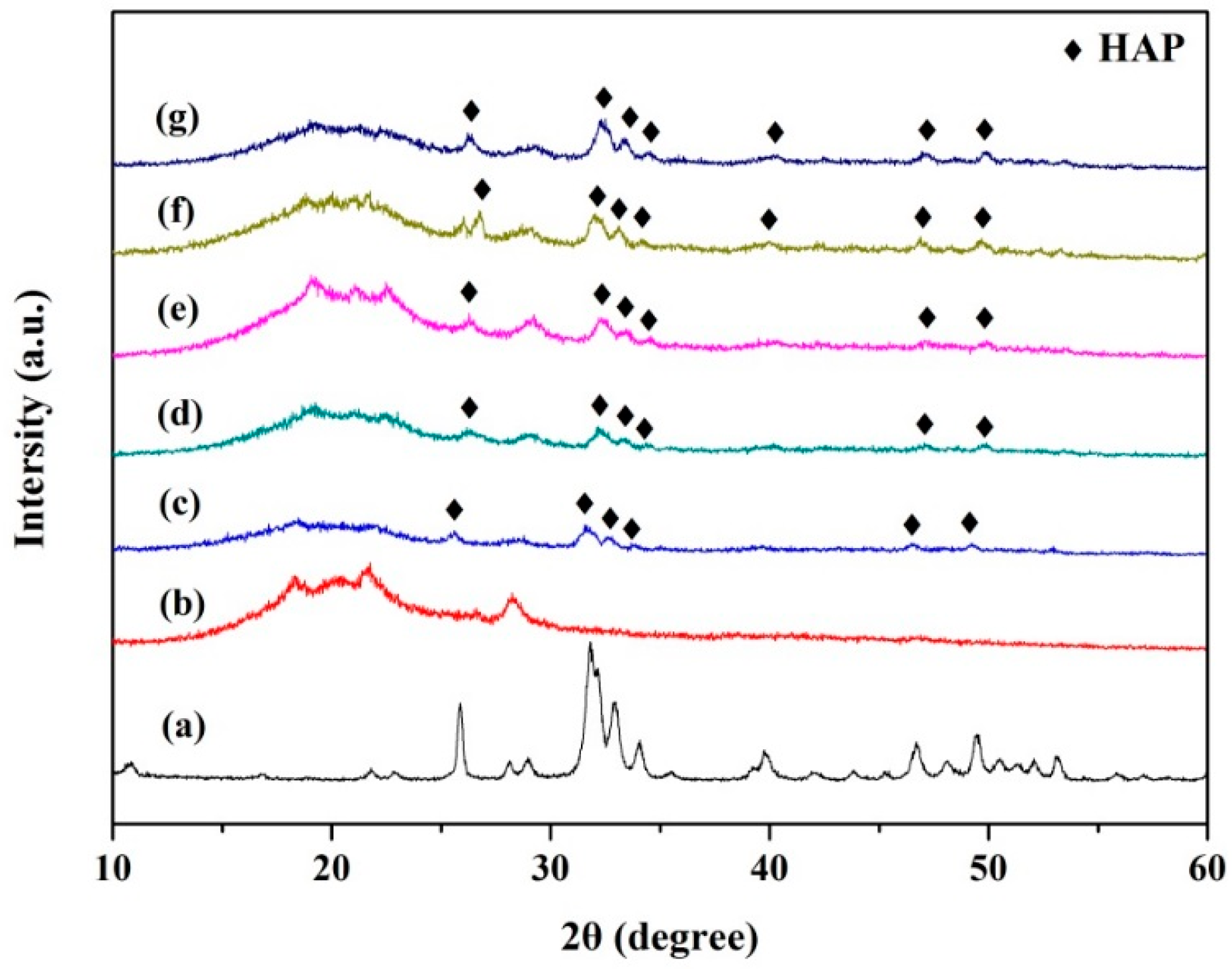
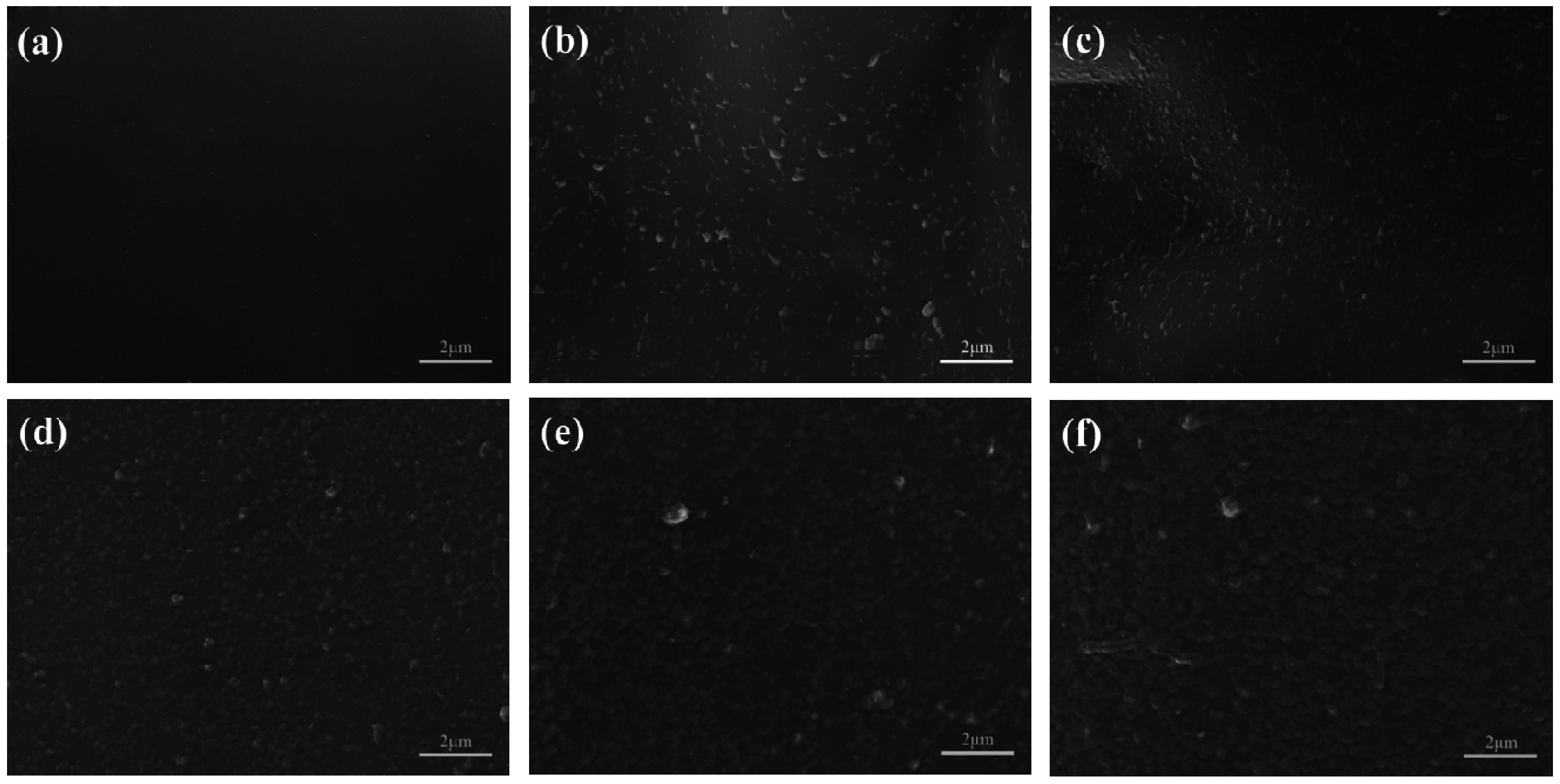
2.2. Microstructure and Composition
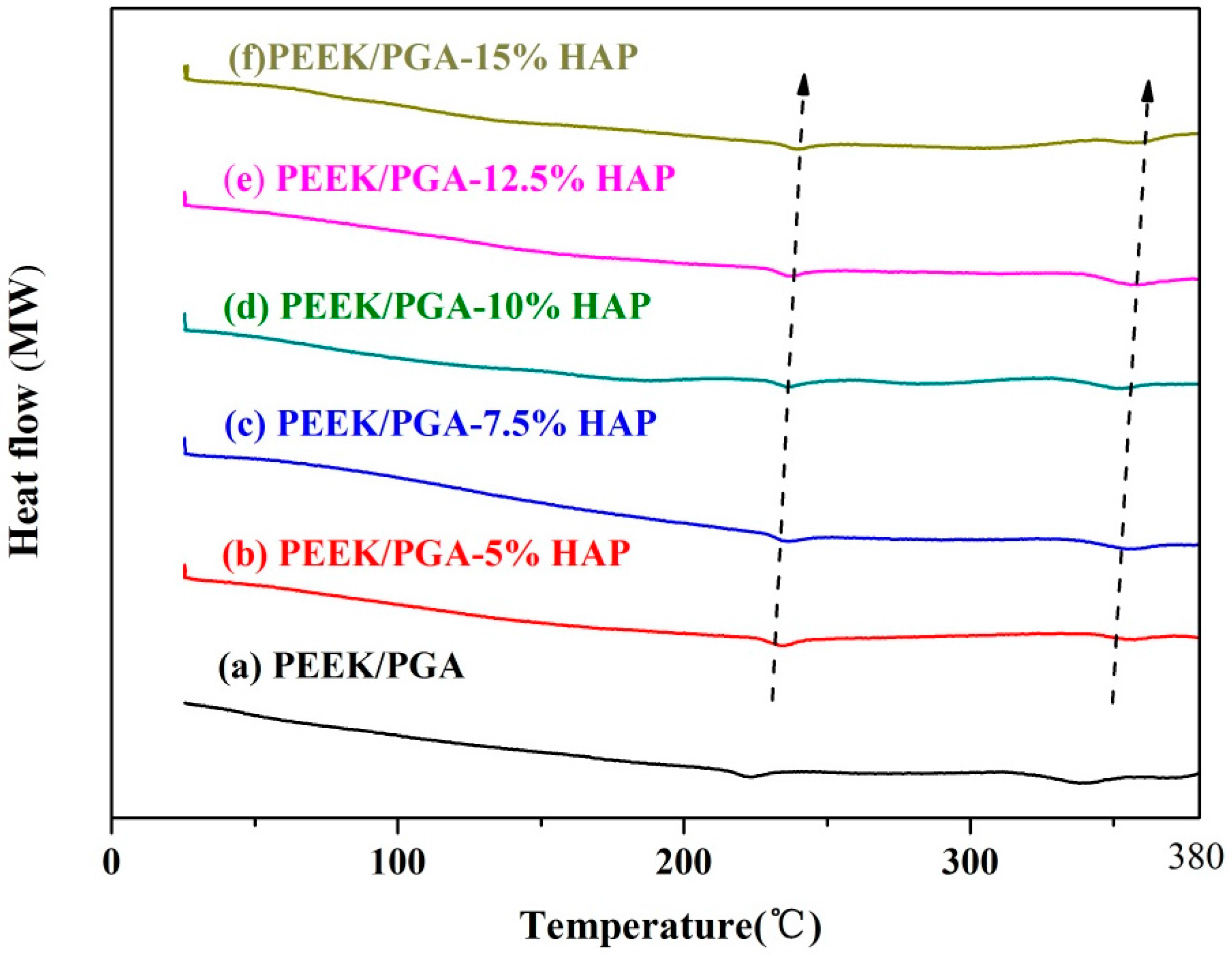
2.3. DSC Studies
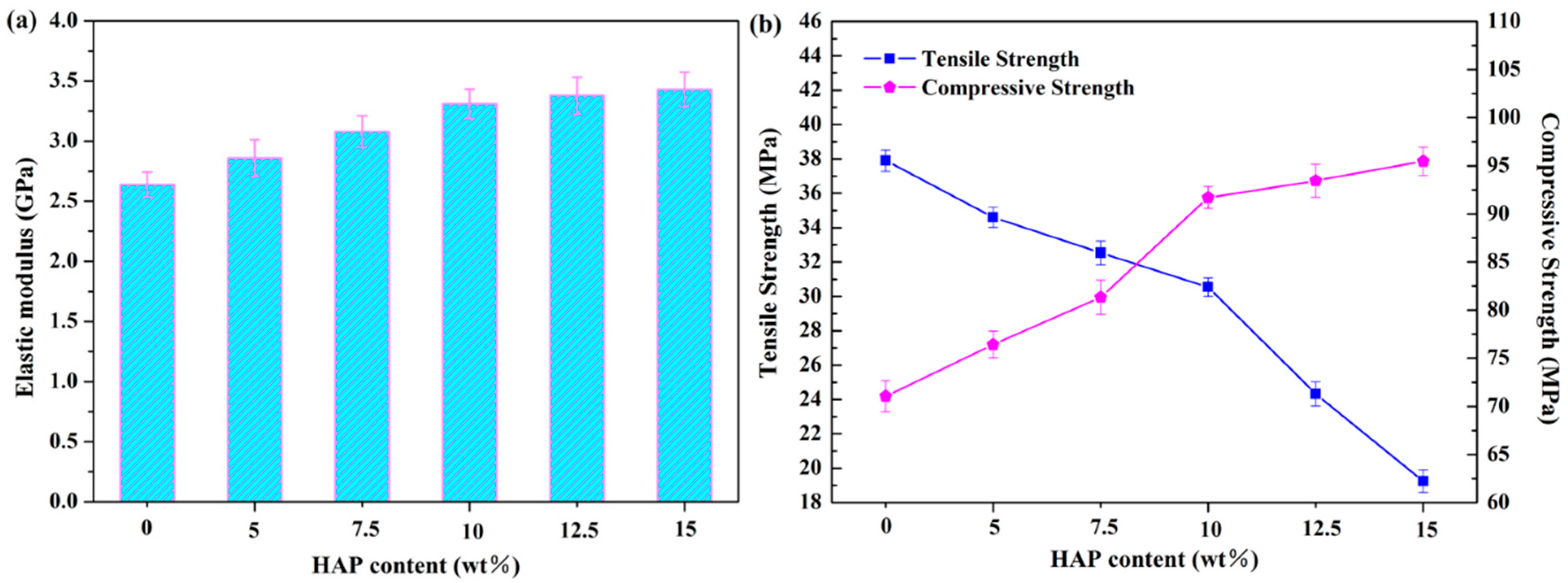
2.4. Mechanical Properties
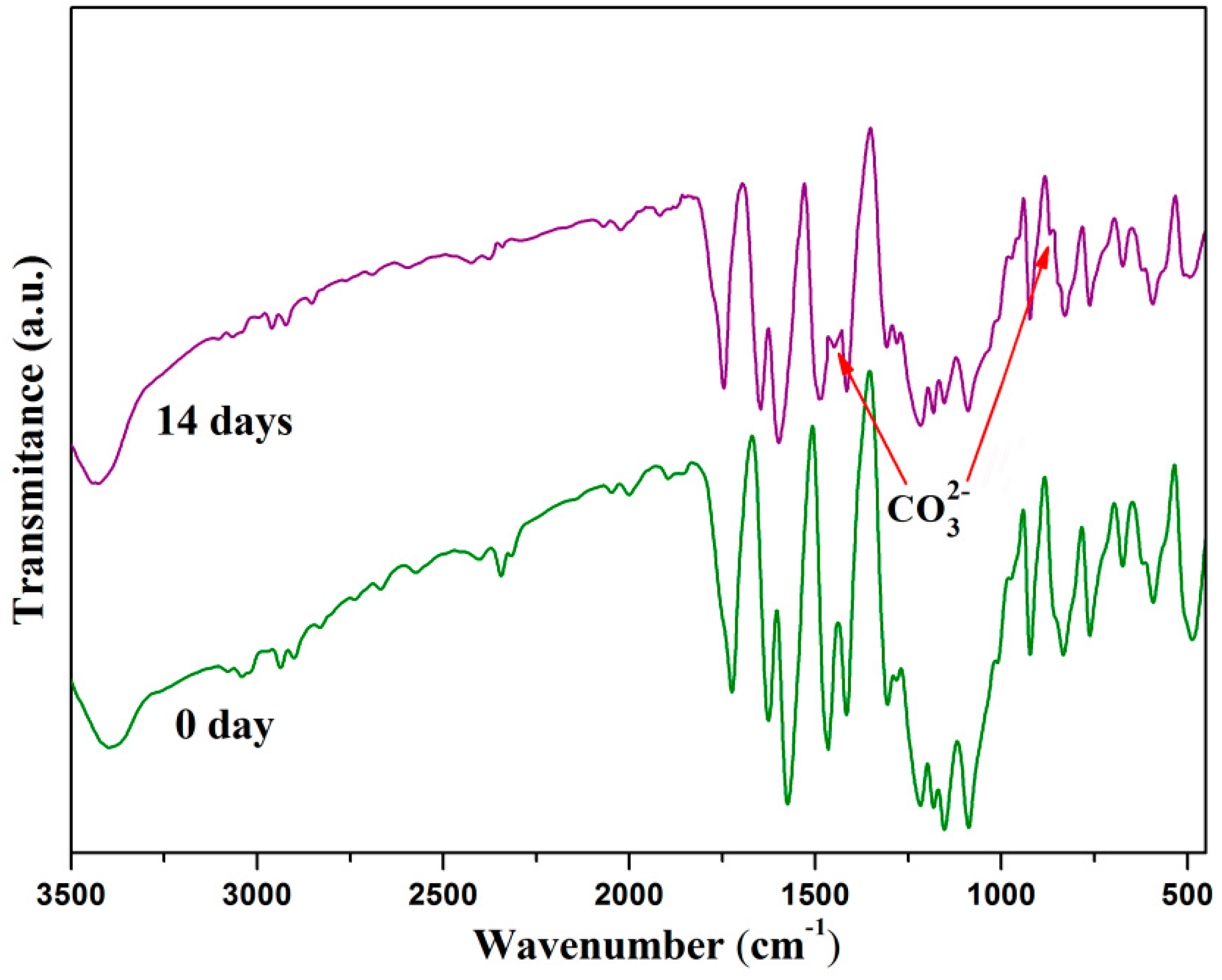
2.5. Bioactivity Evolution
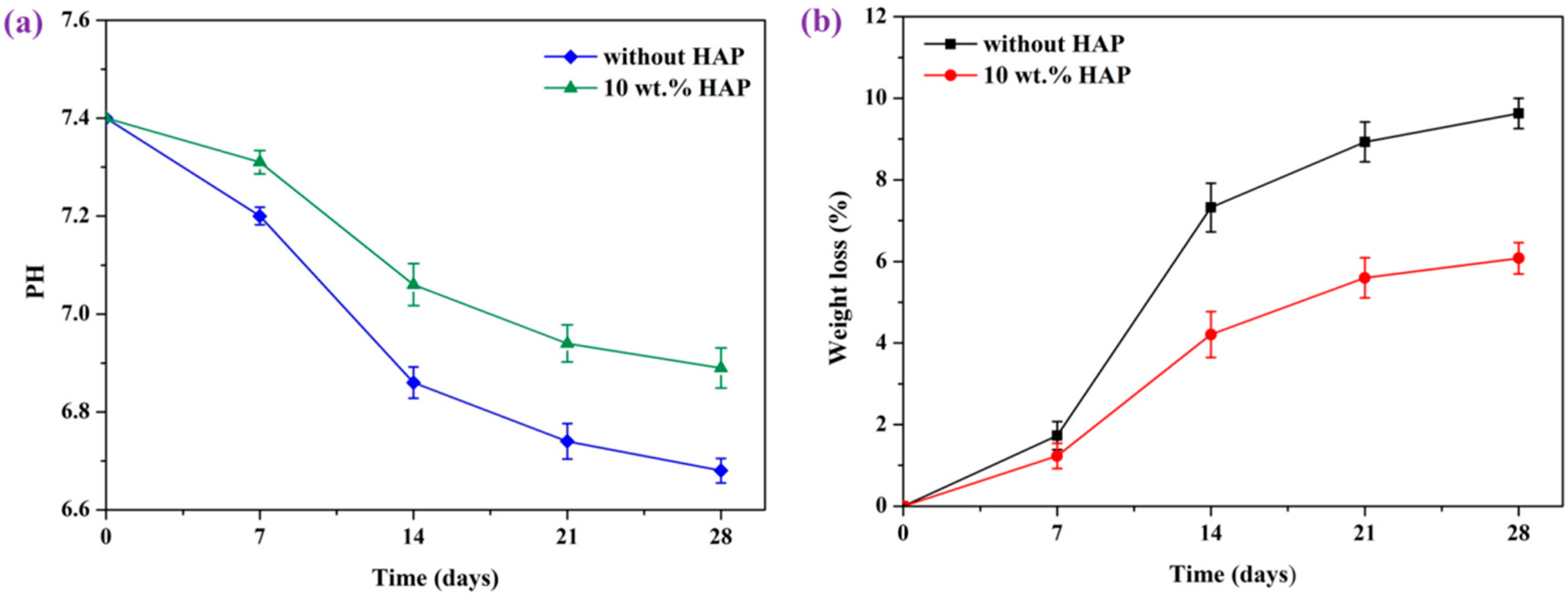
2.6. In Vitro Degradation
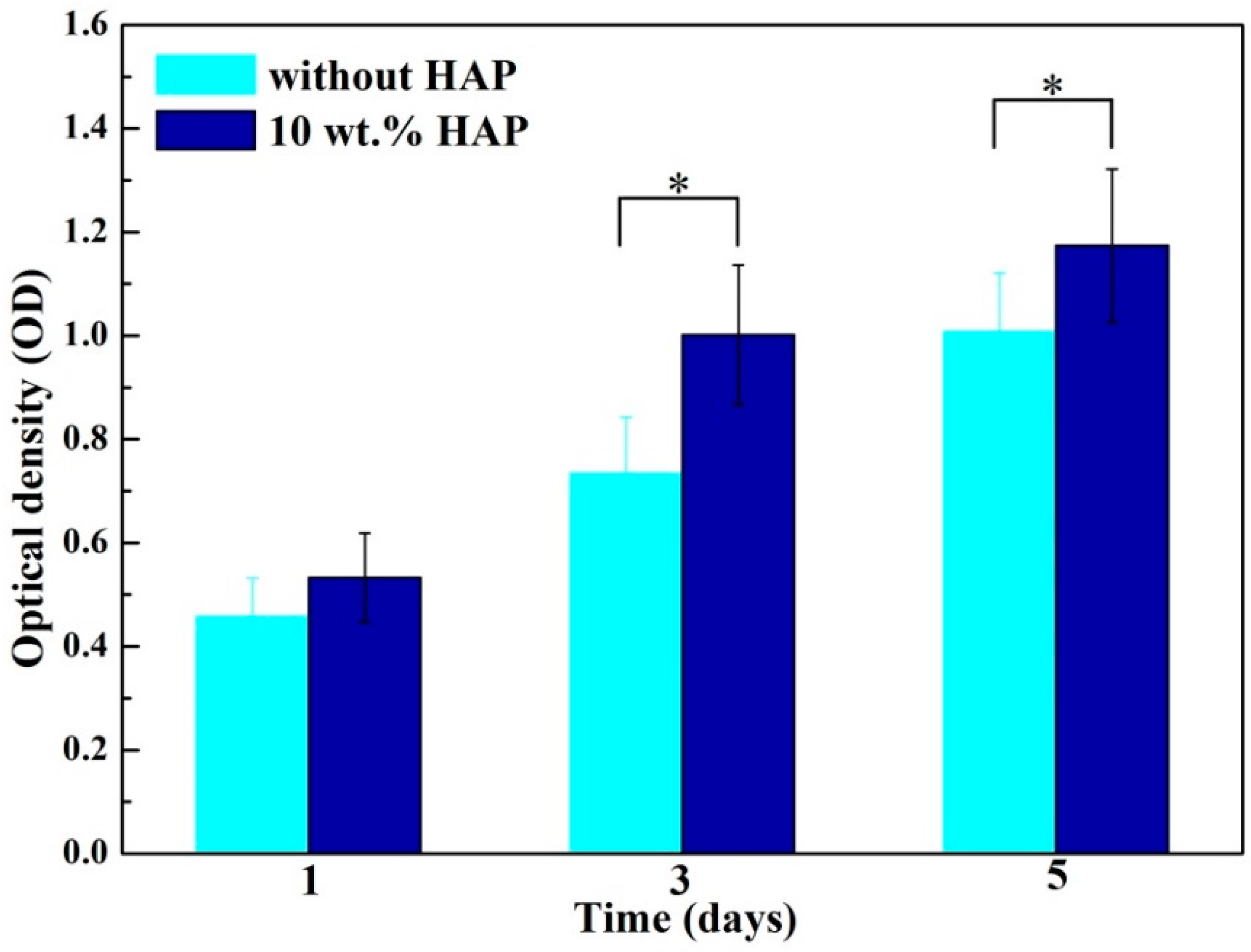
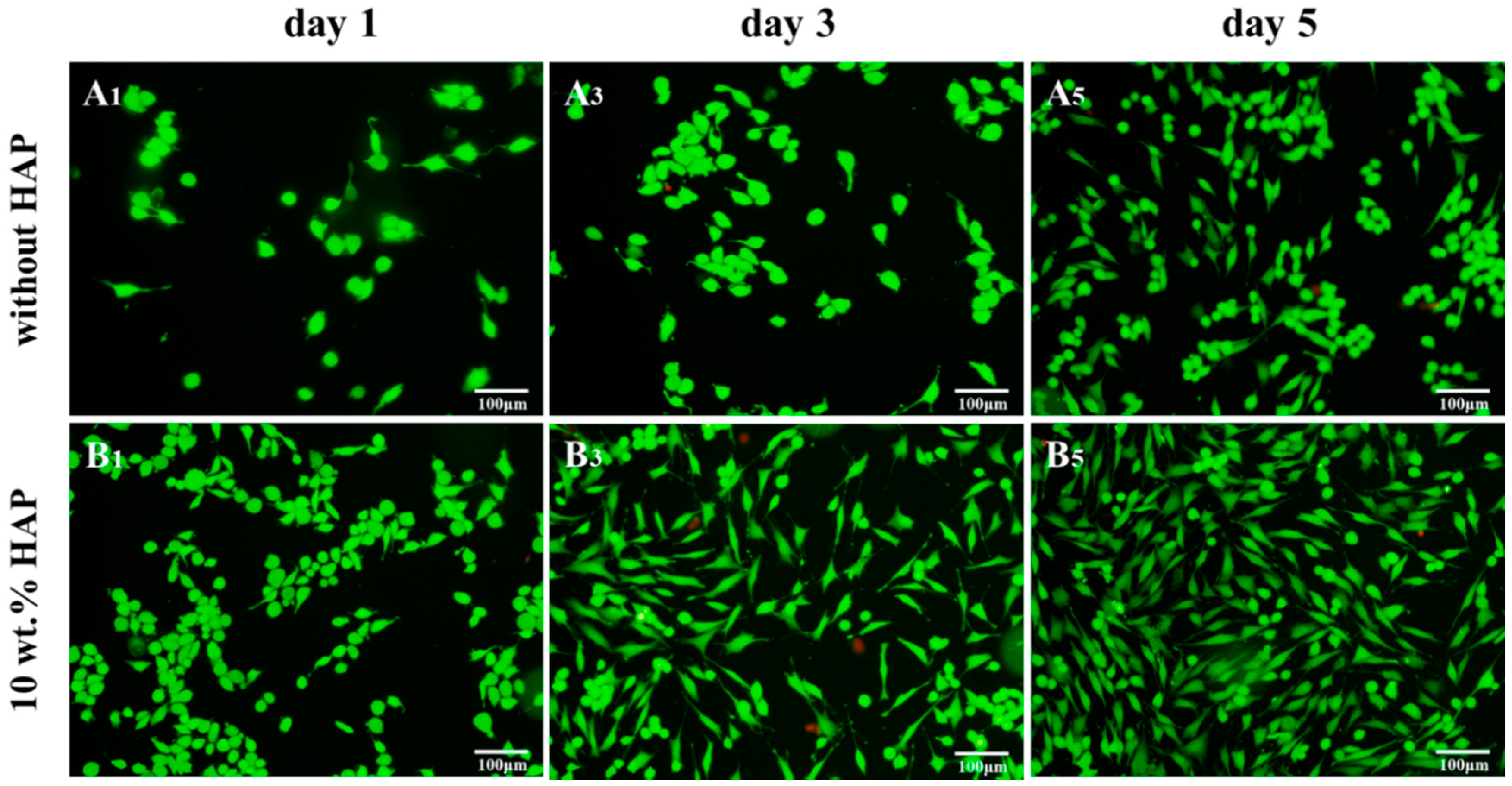
2.7. Biocompatibility Studies
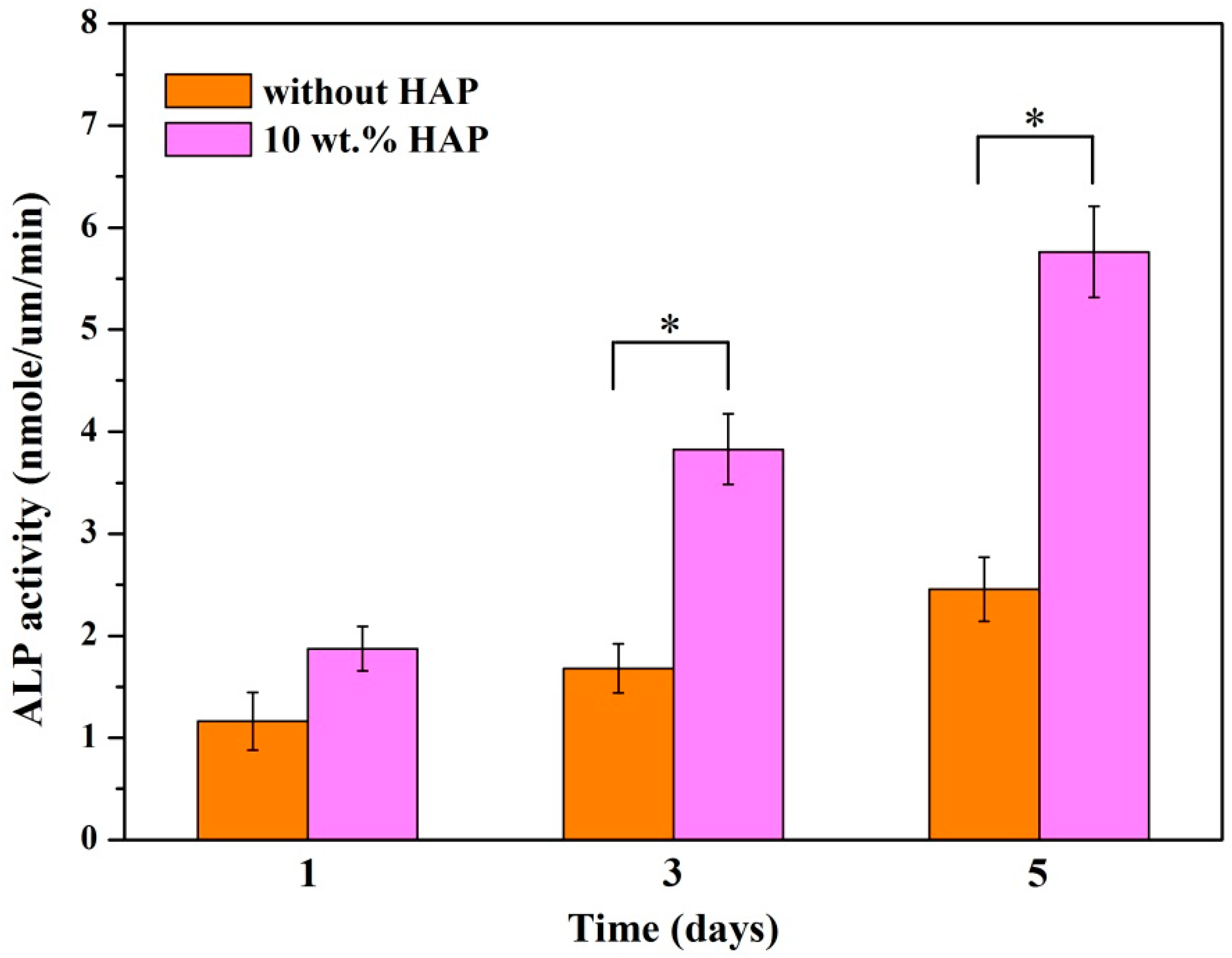
2.8. Alkaline Phosphatase (ALP) Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Porous Scaffolds
3.3. Differential Scanning Calorimetry (DSC)
3.4. Characterization
3.5. In Vitro Biomineralization Properties
3.6. Degradation Behavior
3.7. Cell Culture
3.8. Alkaline Phosphatase (ALP) Assay
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hahn, B.D.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Choi, J.H.; Kim, J.W.; Ahn, C.W.; Kim, H.E.; Yoon, B.H. Osteoconductive hydroxyapatite coated peek for spinal fusion surgery. Appl. Surf. Sci. 2013, 283, 6–11. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Seuss, S.; Boccaccini, A.R. Microstructure and properties of composite polyetheretherketone/bioglass® coatings deposited on ti-6al-7nb alloy for medical applications. Appl. Surf. Sci. 2013, 273, 62–67. [Google Scholar] [CrossRef]
- Vaezi, M.; Yang, S. A novel bioactive peek/ha composite with controlled 3d interconnected ha network. Int. J. Bioprint. 2015, 1, 66–76. [Google Scholar] [CrossRef]
- Revie, R.W. Microbiological Degradation of Polymeric Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; Volume 3, pp. 62–63. [Google Scholar]
- Ozdil, D.; Aydin, H.M. Polymers for medical and tissue engineering applications. J. Chem. Technol. Biotechnol. 2014, 89, 1793–1810. [Google Scholar] [CrossRef]
- Cheung, H.Y.; Lau, K.T.; Lu, T.P.; Hui, D. A critical review on polymer-based bio-engineered materials for scaffold development. Compos. B Eng. 2007, 38, 291–300. [Google Scholar] [CrossRef]
- Lehtonen, T.J.; Tuominen, J.U.; Elina, H. Resorbable composites with bioresorbable glass fibers for load-bearing applications. In vitro degradation and degradation mechanism. Acta Biomater. 2013, 9, 4868–4877. [Google Scholar] [CrossRef] [PubMed]
- Allo, B.A.; Costa, D.O.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactive and biodegradable nanocomposites and hybrid biomaterials for bone regeneration. J. Funct. Biomater. 2012, 3, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Sancak, E.; Erdem, R. Functionalization techniques for electrospun nanofibers for drug delivery applications: A review. Usak Univ. J. Mater. Sci. 2014, 3, 180. [Google Scholar]
- Najeeb, S.; Khurshid, Z.; Matinlinna, J.P.; Siddiqui, F.; Nassani, M.Z.; Baroudi, K. Nanomodified peek dental implants: Bioactive composites and surface modification—A review. Int. J. Dent. 2015, 15, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Puerto, I. In vitro degradation of plcl/nha biodegradable scaffolds. Polym. Plast. Technol. Eng. 2015, 54, 556–564. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhao, Y.; Jin, G.; Tao, L.; Li, J.; Qiao, Y.; Ning, C.; Zhang, X.; Chu, P.K.; Liu, X. Influence of sulfur content on bone formation and antibacterial ability of sulfonated peek. Biomaterials 2016, 83, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Gang, S.; Yang, X.; Fang, M.; Hu, X.; Chen, G.; Deng, X.; Ryu, S. Poly-l-lactic acid/hydroxyapatite hybrid membrane for bone tissue regeneration. J. Biomed. Mater. Res. A 2007, 82, 445–454. [Google Scholar]
- Lee, J.B.; Kim, S.E.; Dong, N.H.; Kwon, I.K.; Choi, B.J. In vitro characterization of nanofibrous plga/gelatin/hydroxyapatite composite for bone tissue engineering. Macromol. Res. 2010, 18, 1195–1202. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.G.; Ding, J.X.; Li, Z.M. Tailor-made poly(l-lactide)/poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds prepared via high-pressure compression molding/salt leaching. RSC Adv. 2016, 6, 47418–47426. [Google Scholar] [CrossRef]
- Raafat, A.I.; Saad Eldin, A.A.; Salama, A.A.; Ali, N.S. Characterization and bioactivity evaluation of (starch/n-vinylpyrrolidone)—Hydroxyapatite nanocomposite hydrogels for bone tissue regeneration. J. Appl. Polym. Sci. 2012, 128, 1697–1705. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yu, H.; Yang, W.; Zhu, Z.; Yue, L. Preparation, degradability, cytotoxicity, and biocompatibility of porous hydroxyapatite whisker-reinforced poly(l-lactide) biocomposite scaffolds. J. Biomater. Sci. Polym. Ed. 2016, 27, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, P.; Pandey, K.N.; Verma, V.; Kumar, V. Poly (ether ether) ketone/poly (ether) imide nanocomposites. Asian J. Res. Chem. 2012, 5, 703–706. [Google Scholar]
- Wang, Y.C.; Lin, M.C.; Wang, D.M.; Hsieh, H.J. Fabrication of a novel porous pga-chitosan hybrid matrix for tissue engineering. Biomaterials 2003, 24, 1047–1057. [Google Scholar] [CrossRef]
- Meenan, B.J.; Mcclorey, C.; Akay, M. Thermal analysis studies of poly(etheretherketone)/hydroxyapatite biocomposite mixtures. J. Mater. Sci. Mater. Med. 2000, 11, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weng, L.; Song, S.; Zhang, Z.; Tian, S.; Ma, R. Characterization of polyetheretherketone–hydroxyapatite nanocomposite materials. Mater. Sci. Eng. A 2011, 528, 3689–3696. [Google Scholar] [CrossRef]
- Goyal, R.K.; Tiwari, A.N.; Negi, Y.S. Microhardness of peek/ceramic micro- and nanocomposites: Correlation with halpin–tsai model. Mater. Sci. Eng. A 2008, 491, 230–236. [Google Scholar] [CrossRef]
- Li, K.; Che, Y.Y.; Yeung, K.W.K.; Tjong, S.C. Sintered hydroxyapatite/polyetheretherketone nanocomposites: Mechanical behavior and biocompatibility. Adv. Eng. Mater. 2012, 14, B155–B165. [Google Scholar] [CrossRef]
- Sultana, N.; Wang, M. Fabrication of ha/phbv composite scaffolds through the emulsion freezing/freeze-drying process and characterisation of the scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Wei, P.; Li, P.; Gao, C.; Shuai, C.; Peng, S. Calcium silicate ceramic scaffolds toughened with hydroxyapatite whiskers for bone tissue engineering. Mater. Charact. 2014, 97, 47–56. [Google Scholar] [CrossRef]
- Handel, M.; Hammer, T.R.; Nooeaid, P.; Boccaccini, A.R.; Hoefer, D. 45s5-bioglass®-based 3d-scaffolds seeded with human adipose tissue-derived stem cells induce in vivo vascularization in the cam angiogenesis assay. Tissue Eng. A 2013, 19, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Sun, Y.; Yang, F.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, G.; Tao, L. Fabrication and characterization of nano-composite scaffold of plla/silane modified hydroxyapatite. Med. Eng. Phys. 2010, 32, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Kai, W.C.; Cheng, Z.L.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Preparation of polyetheretherketone composites with nanohydroxyapatite rods and carbon nanofibers having high strength, good biocompatibility and excellent thermal stability. RSC Adv. 2016, 6, 19417–19429. [Google Scholar]
- Fan, X.; Ren, H.; Luo, X.; Wang, P.; Lv, G.; Yuan, H.; Li, H.; Yan, Y. Mechanics, degradability, bioactivity, in vitro, and in vivo biocompatibility evaluation of poly(amino acid)/hydroxyapatite/calcium sulfate composite for potential load-bearing bone repair. J. Biomater. Appl. 2015, 40, A36. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, Z.T.; Caparon, M.G. Citrulline protects streptococcus pyogenes from acid stress using the arginine deiminase pathway and the f1fo-atpase. J. Bacteriol. 2015, 197, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Wutticharoenmongkol, P.; Pavasant, P.; Supaphol, P. Osteoblastic phenotype expression of mc3t3-e1 cultured on electrospun polycaprolactone fiber mats filled with hydroxyapatite nanoparticles. Biomacromolecules 2007, 8, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Ngiam, M.; Liao, S.; Patil, A.J.; Cheng, Z.; Chan, C.K.; Ramakrishna, S. The fabrication of nano-hydroxyapatite on plga and plga/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone 2009, 45, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Fukutani, S.; Mori, M. Hydroxyapatite-induced alkaline phosphatase activity of human pulp fibroblasts. J. Mater. Sci. Mater. Med. 1992, 3, 180–183. [Google Scholar] [CrossRef]
- Gupta, D.; Venugopal, J.; Mitra, S.; Dev, V.R.G.; Ramakrishna, S. Nanostructured biocomposite substrates by electrospinning and electrospraying for the mineralization of osteoblasts. Biomaterials 2009, 30, 2085–2094. [Google Scholar] [CrossRef] [PubMed]

| Ion Type | Ion Concentration (mM) | ||||||
|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mg2+ | Ca2+ | Cl− | |||
| Simulated Body fluid | 142.0 | 5.0 | 1.5 | 2.5 | 148.8 | 4.2 | 1.0 |
| Human blood plasma | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 17.0 | 1.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, C.; Shuai, C.; Wu, P.; Yuan, F.; Feng, P.; Yang, Y.; Guo, W.; Fan, X.; Su, T.; Peng, S.; et al. Characterization and Bioactivity Evaluation of (Polyetheretherketone/Polyglycolicacid)-Hydroyapatite Scaffolds for Tissue Regeneration. Materials 2016, 9, 934. https://doi.org/10.3390/ma9110934
Shuai C, Shuai C, Wu P, Yuan F, Feng P, Yang Y, Guo W, Fan X, Su T, Peng S, et al. Characterization and Bioactivity Evaluation of (Polyetheretherketone/Polyglycolicacid)-Hydroyapatite Scaffolds for Tissue Regeneration. Materials. 2016; 9(11):934. https://doi.org/10.3390/ma9110934
Chicago/Turabian StyleShuai, Cijun, Chenying Shuai, Ping Wu, Fulai Yuan, Pei Feng, Youwen Yang, Wang Guo, Xiaohan Fan, Ting Su, Shuping Peng, and et al. 2016. "Characterization and Bioactivity Evaluation of (Polyetheretherketone/Polyglycolicacid)-Hydroyapatite Scaffolds for Tissue Regeneration" Materials 9, no. 11: 934. https://doi.org/10.3390/ma9110934
APA StyleShuai, C., Shuai, C., Wu, P., Yuan, F., Feng, P., Yang, Y., Guo, W., Fan, X., Su, T., Peng, S., & Gao, C. (2016). Characterization and Bioactivity Evaluation of (Polyetheretherketone/Polyglycolicacid)-Hydroyapatite Scaffolds for Tissue Regeneration. Materials, 9(11), 934. https://doi.org/10.3390/ma9110934










