Influence of Electrode Design and Contacting Layers on Performance of Electrolyte Supported SOFC/SOEC Single Cells
Abstract
:1. Introduction
2. Results and Discussion
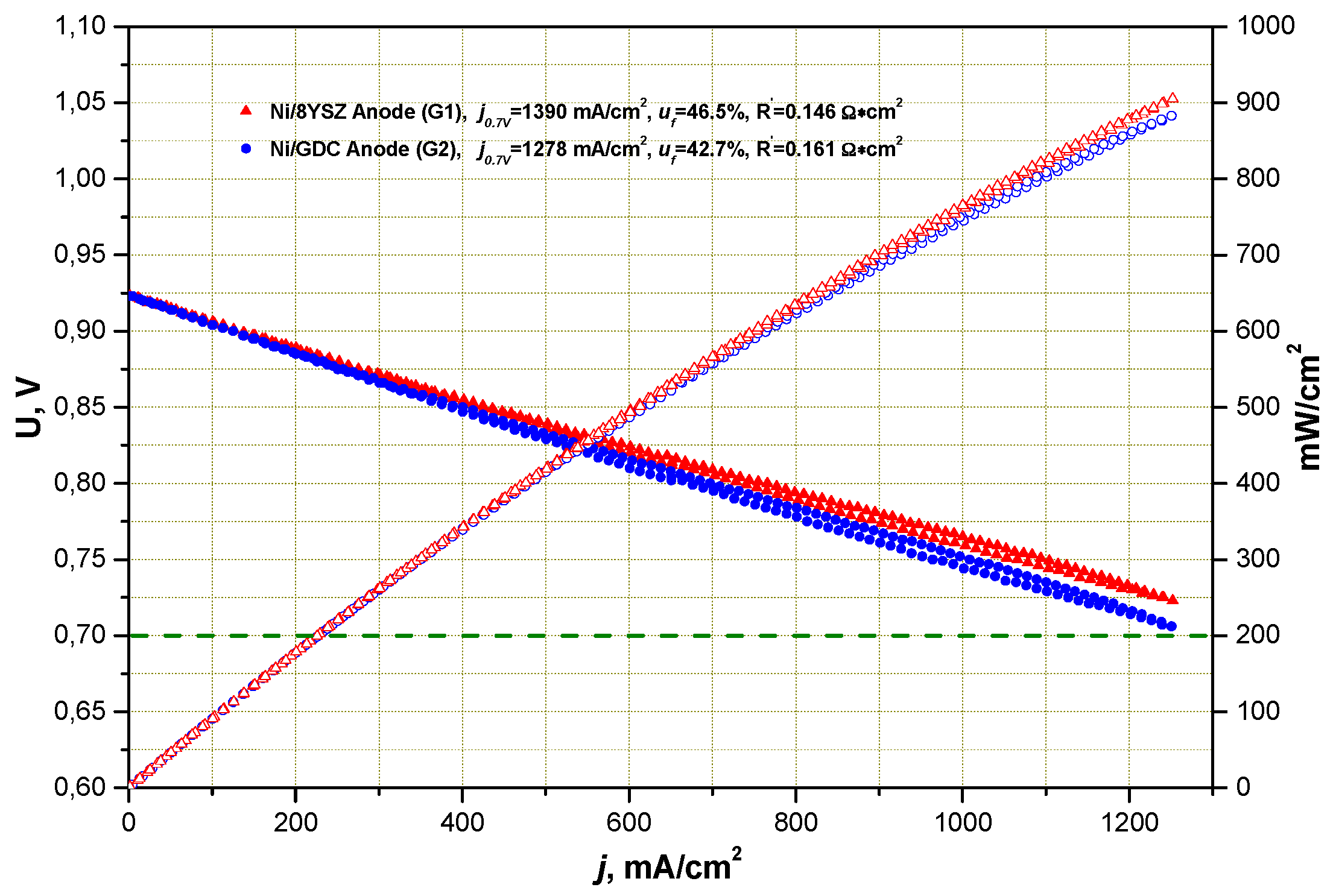
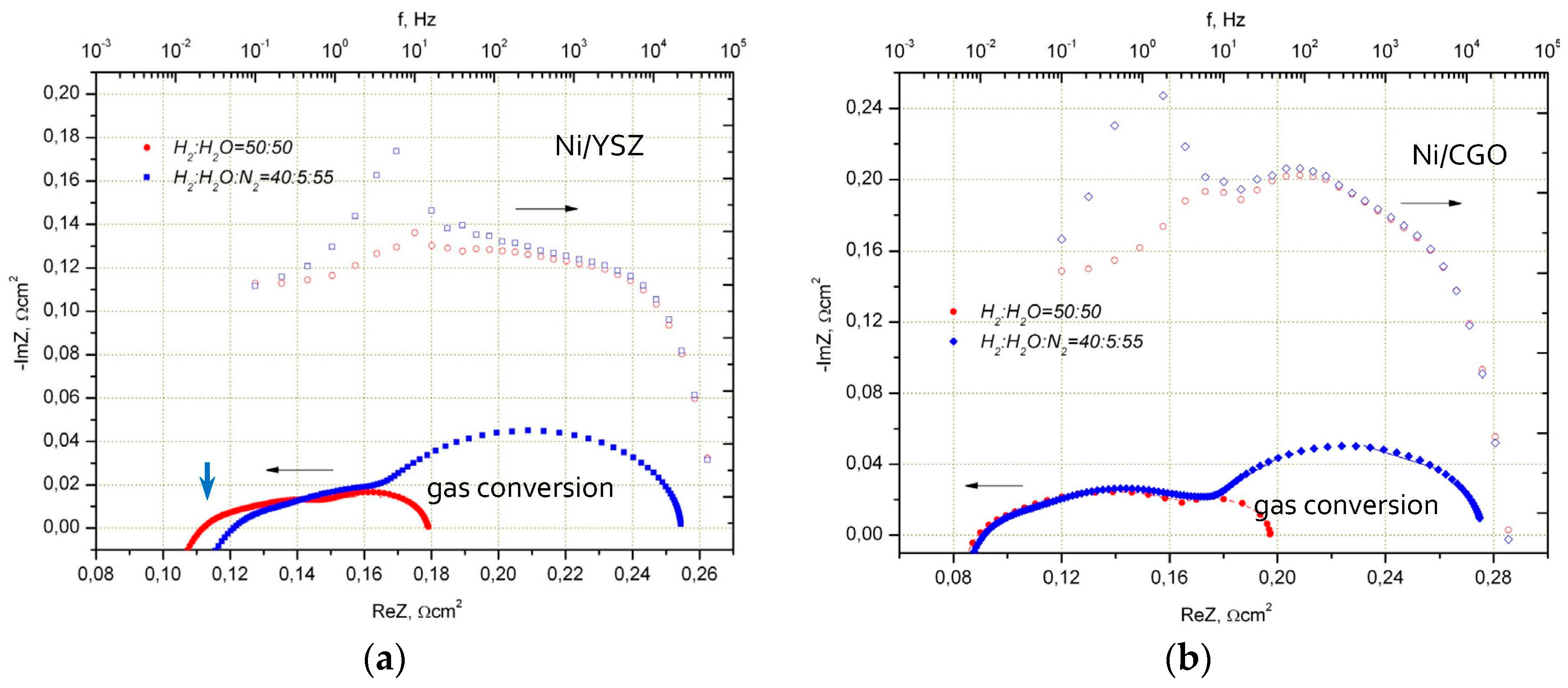
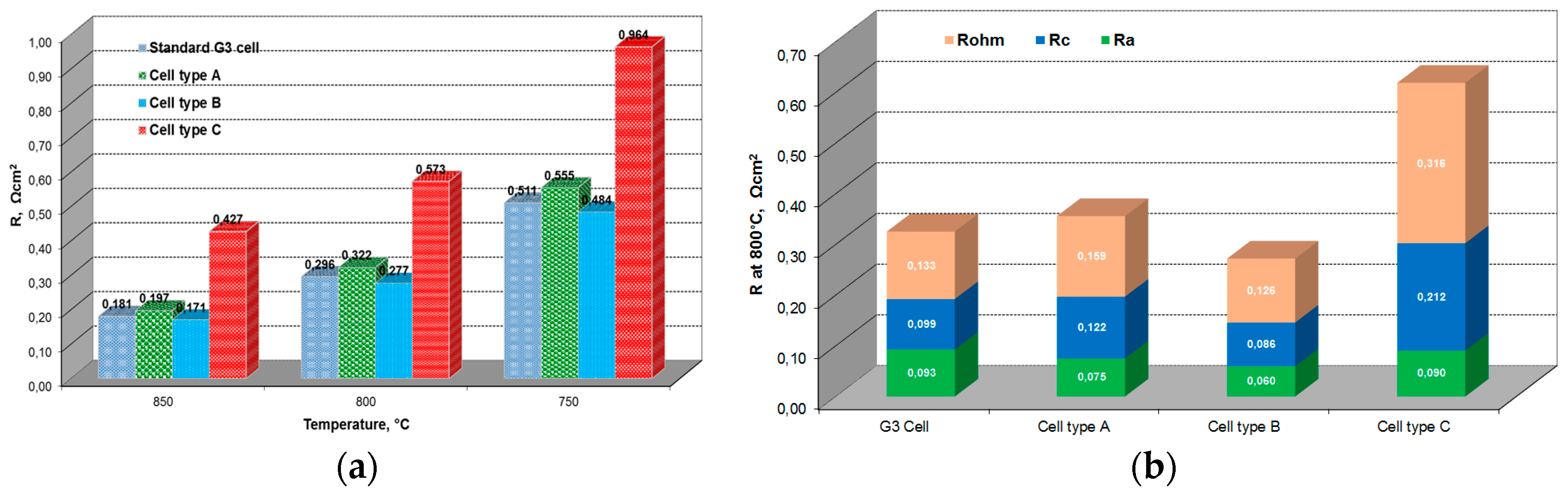
2.1. Influence of Anode Composition on ESC Performance
2.1.1. Performance under Standard SOFC Conditions
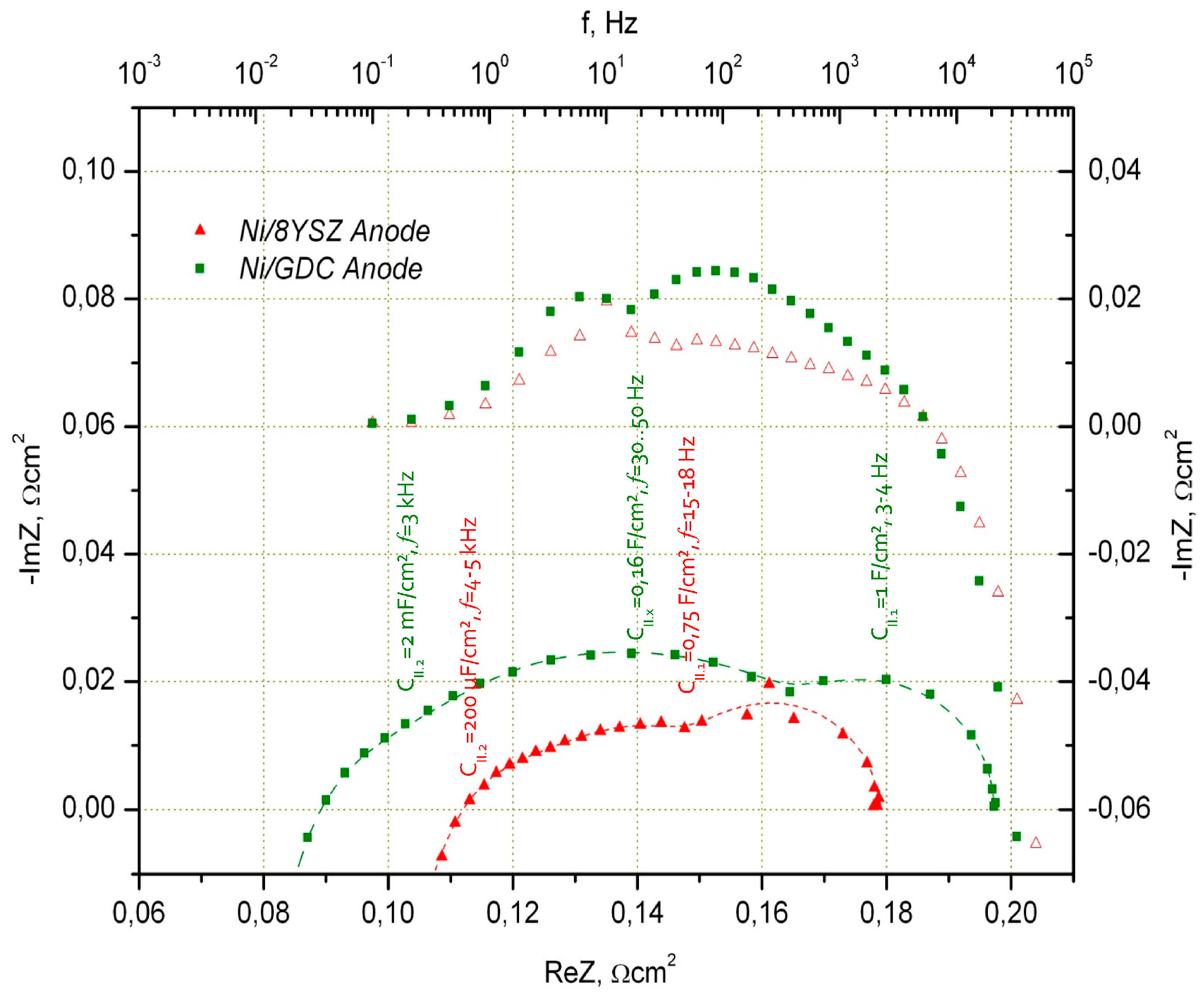
- (i)
- High frequency arc (3 kHz–7 kHz) capacitance had higher values compared to the Ni/8YSZ anode (ca. 2 mF/cm2)
- (ii)
- Low frequency arc (ca. 4 Hz–5 Hz) capacitance is also higher compared to Ni/8YSZ and is in the range of 1 F/cm2 (low frequency adsorption impedance in the arc of air (LSM-based) electrode appears at similar frequencies and the air and Ni/CGO fuel electrode arcs are difficult to separate under selected measurement conditions)
- (iii)
- Additional electrochemical process with capacitance of 160 mF/cm2 at 30 Hz–50 Hz is found. This value was not dependent on temperature and H2O content in the fuel (5%–50%).
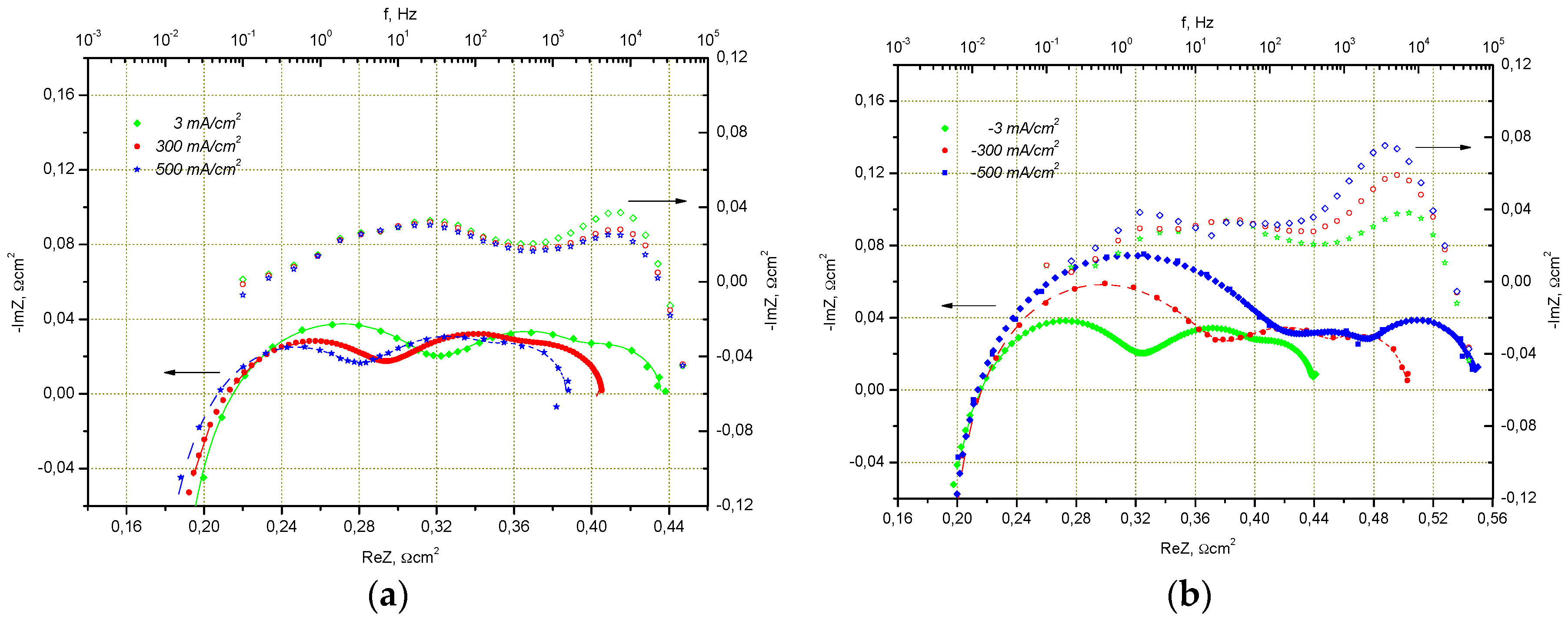
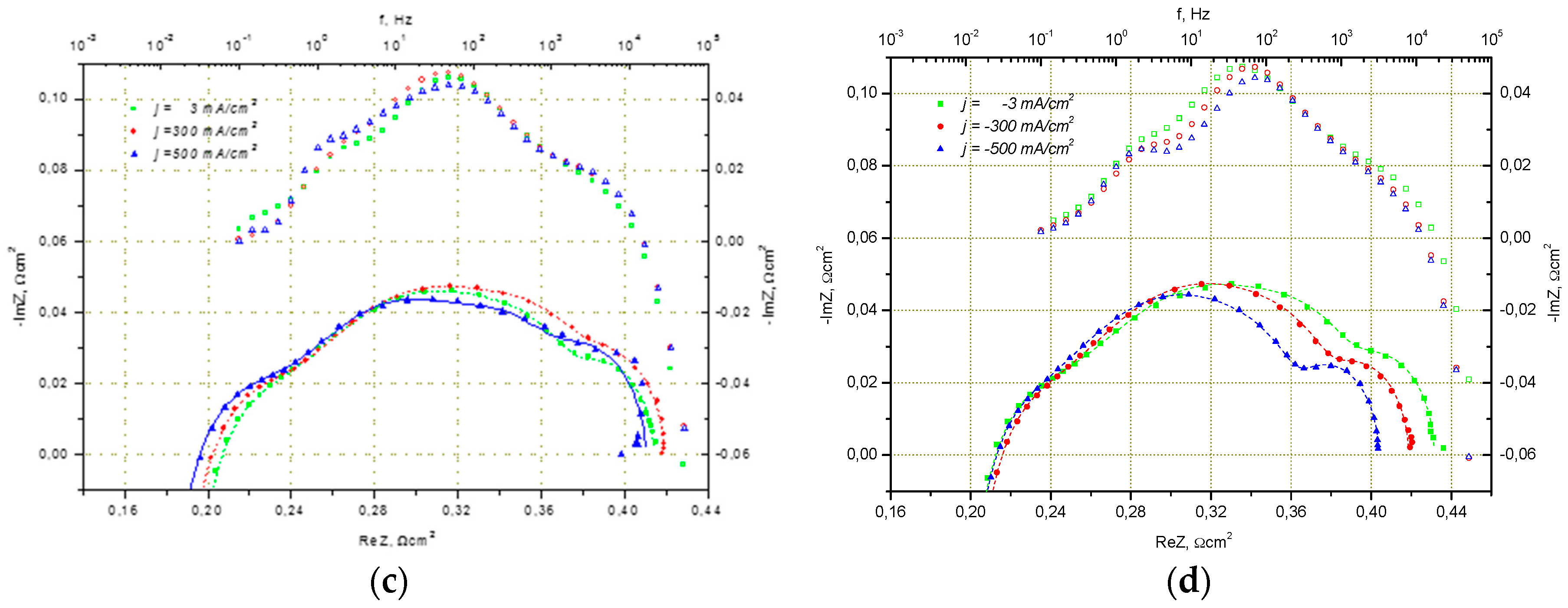
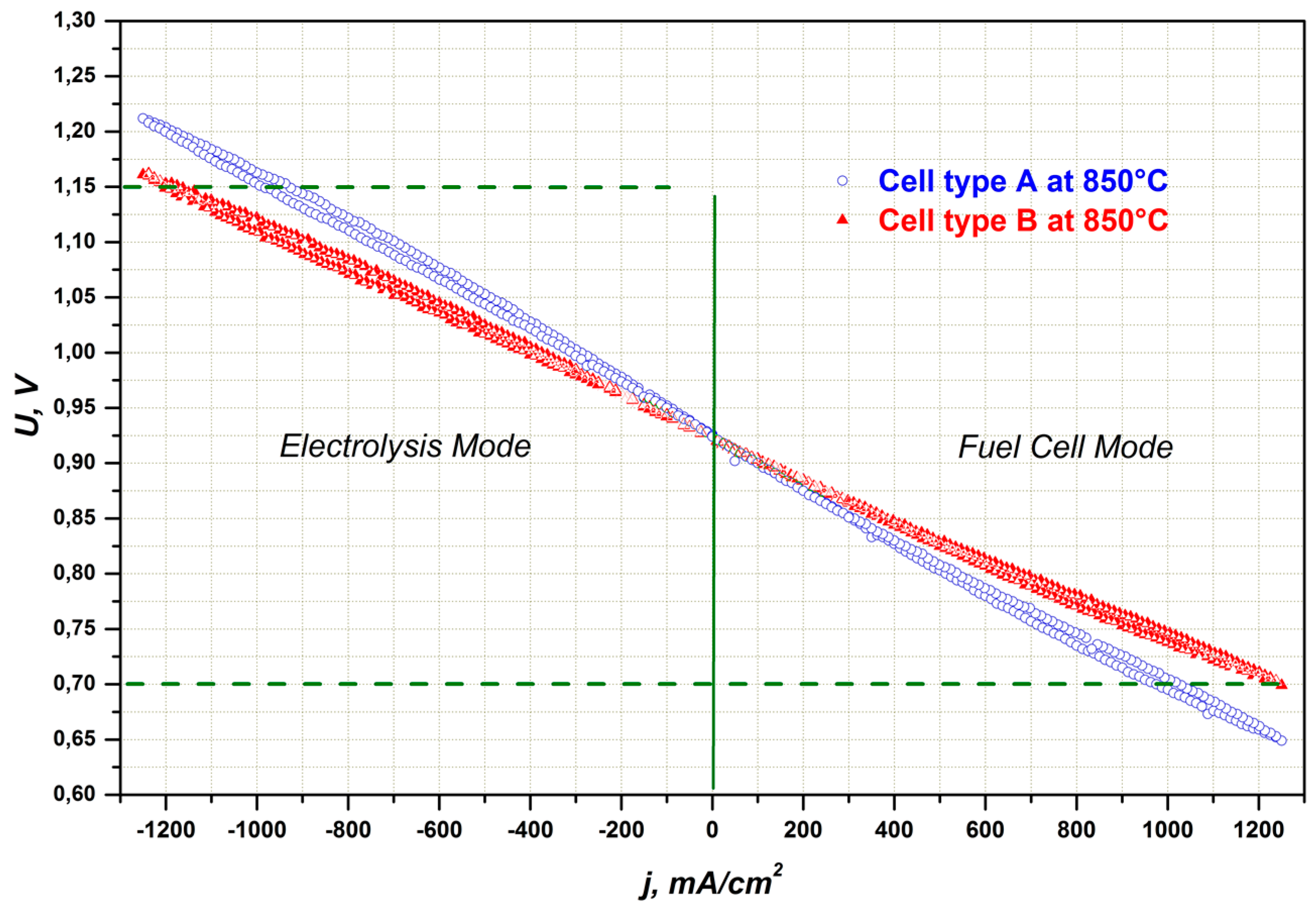
2.1.2. Electrode Optimization for Performance in SOEC Mode
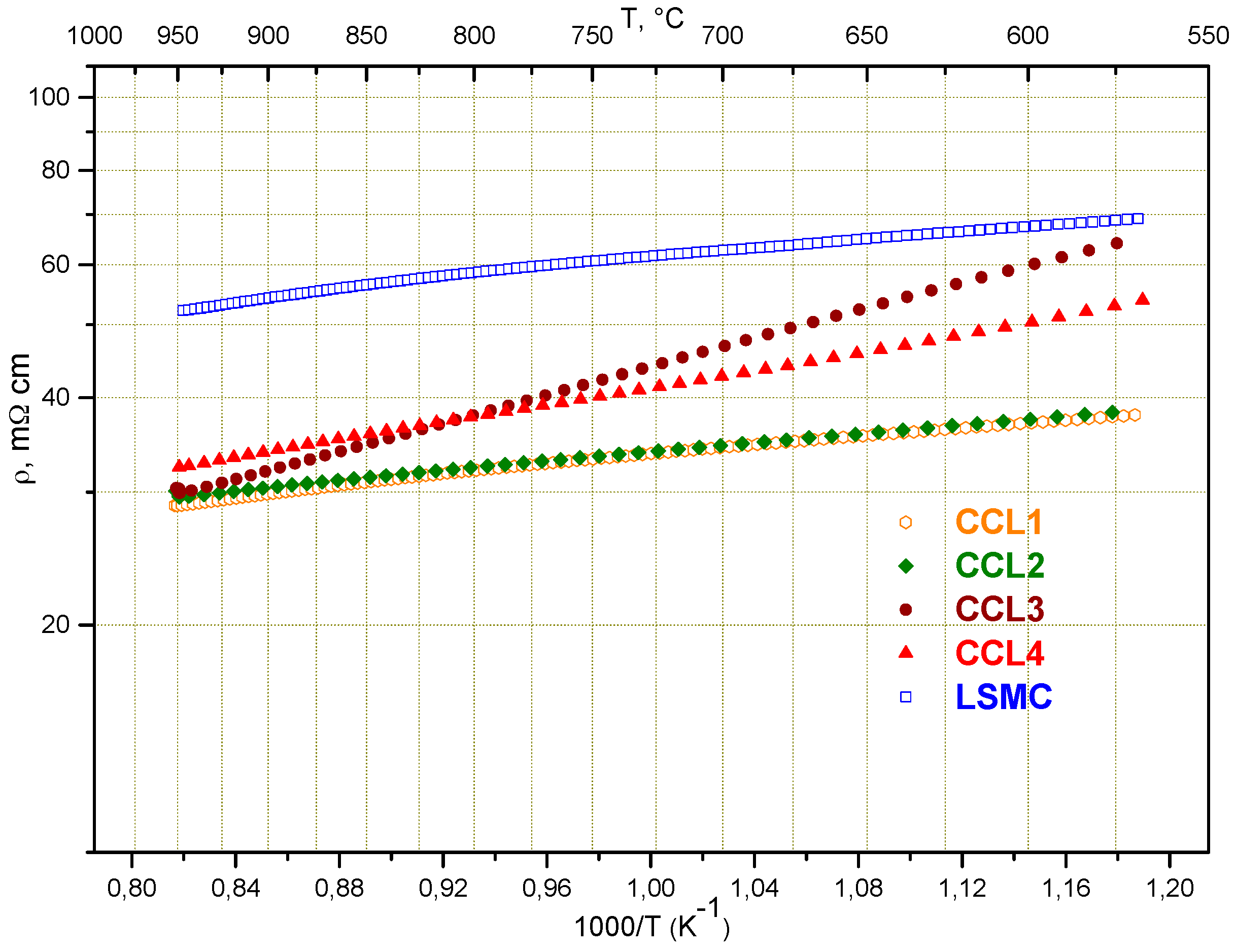
2.2. Influence of Contacting
3. Materials and Methods
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Otterstedt, R.; Rietveld, G.; Huiberts, R.; Indec, B.V. On the road toward commercialization. In On Solid Oxide Fuel Cells (SOFC-IX), Proceedings of the International Symposium, San Diego, CA, USA, 17–18 October 2005; Singhal, S.C., Mizusaki, J., Eds.; The Electrochemical Society: Pennington, NJ, USA, 2005; Volume 1, pp. 476–481. [Google Scholar]
- Sfeir, J.; Mai, A.; Iwanschitz, B.; Weissen, U.; Denzler, R.; Haberstock, D.; Hocker, T.; Roos, M. Status of SOFC Stack and Material Development at Hexis. In Proceedings of the 8th European Solid Oxide Fuel Cell Forum, Lucern, Switzerland, 30 June–4 July 2008.
- Glauche, A.; Betz, T.; Mosch, S.; Trofimenko, N.; Kusnezoff, M. Long-term, redox and thermal cycling stability of electrolyte supported cells. ESC Trans. 2009, 25, 411–419. [Google Scholar]
- Glauche, A.; Betz, T.; Ise, M. Product Development for SOFC and SOE Applications. ECS Trans. 2001, 35, 157–165. [Google Scholar]
- Cooley, N. NexTech materials demonstrates world’s largest SOFC platform. Int. J. Hydrogen Energy 2009, 34, 8454. [Google Scholar] [CrossRef]
- Trofimenko, N.; Kusnezoff, M.; Klemm, D.; Schimanke, D. Development of Electrolyte Supported Cells based on a Thin 3YSZ Substrate: Through Optimized Contact Layer to High Power Density. ECS Trans. 2015, 68, 1933–1942. [Google Scholar] [CrossRef]
- Mai, A.; Schuler, J.A.; Fleischhauer, F.; Nerlich, V.; Schuler, A. Hexis and the SOFC System Galileo 1000 N: Experiences from Lab and Field Testing. ECS Trans. 2015, 68, 109–116. [Google Scholar] [CrossRef]
- Mermelstein, J.; Posdziech, O. Development and Demonstration of a Novel Reversible SOFC System for Utility and Micro Grid Energy Storage. In Proceedings of the 12th European Solid Oxide Fuel cell Forum, Lucern, Switzerland, 5–8 July 2016.
- Kusnezoff, M.; Beckert, W.; Trofimenko, N.; Jacobs, B.; Dosch, C.; Megel, S.; Rachau, M.; Wieprecht, C.; Gipp, D. Electrochemical MEA Characterization: Area Specific Resistance Corrected to Fuel Utilization as Universal Characteristic for Cell Performance. ECS Trans. 2015, 68, 2555–2563. [Google Scholar] [CrossRef]
- Sammes, N.M.; Tompsett, G.A.; Cai, Z. The chemical reaction between ceria and fully stabilised zirconia. Solid State Ion. 1999, 121, 121–125. [Google Scholar] [CrossRef]
- Tsoga, A.; Naoumidis, A.; Stöver, D. Total electrical conductivity and defect structure of ZrO2–CeO2–Y2O3–Gd2O3 solid solutions. Solid State Ion. 2000, 135, 403–409. [Google Scholar] [CrossRef]
- Schubert, S.K.; Kusnezoff, M.; Michaelis, A.; Bredikhin, S. Comparison of the performances of single cell solid oxide fuel cell stacks with Ni/8YSZ and Ni/10CGO anodes with H2S containing fuel. J. Power Sources 2012, 217, 364–372. [Google Scholar] [CrossRef]
- Xiong, Y.; Yamaji, K.; Horita, T.; Sakai, N.; Yokokawa, H. Hole and Electron Conductivities of 20 mol %-REO1.5 Doped CeO2 (RE = Yb, Y, Gd, Sm, Nd, La). J. Electrochem. Soc. 2004, 151, A407–A412. [Google Scholar] [CrossRef]
- Wang, S.; Kobayashi, T.; Dokiya, M.; Hashimoto, T. Electrical and Ionic Conductivity of Gd-Doped Ceria. J. Electrochem. Soc. 2000, 147, 3606–3609. [Google Scholar] [CrossRef]
- Hauch, A.; Ebbesen, S.D.; Jensen, S.H.; Mogensen, M. Solid Oxide Electrolysis Cells: Microstructure and Degradation of the Ni/Yttria-Stabilized Zirconia Electrode. J. Electrochem. Soc. 2008, 155, B1184–B1193. [Google Scholar] [CrossRef]
- Knibbe, R.; Traulsen, M.L.; Hauch, A.; Ebbesen, S.D.; Mogensen, M. Solid Oxide Electrolysis Cells: Degradation at High Current Densities. J. Electrochem. Soc. 2010, 157, B1209–B1217. [Google Scholar] [CrossRef]
- Mawdsley, J.R.; David Carter, J.; Jeremy Kropf, A.; Yildiz, B.; Maroni, V.A. Post-test evaluation of oxygen electrodes from solid state electrolysis stacks. Int. J. Hydrogen Energy 2009, 34, 4198–4207. [Google Scholar] [CrossRef]
- Virkar, A. Failure of ion-conducting materials by internal precipitation under electrolytic conditions. In Engineered Ceramics: Current Status and Future Prospects; Ohji, T., Singh, M., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; pp. 59–76. [Google Scholar]
- Virkar, A. Mechanism of oxygen electrode delamination in solid oxide electrolyzer cells. Int. J. Hydrogen Energy 2010, 35, 9527–9543. [Google Scholar] [CrossRef]
- Venkateswaran, V.; Curry, T.; Iakomini, C.; Olenick, J. Highly efficient solid oxide electrolyzer and Sabatier system. In Advances in Solid Oxide Fuel Cells and Electronic Ceramics; Kusnezoff, M., Kirihara, S., Shimamura, K., Wang, J., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; pp. 105–114. [Google Scholar]
- Kellner, M.; Betz, T.; Kusnezoff, M.; Trofimenko, N.; Mosch, S. Development and Manufacturing of Electrolyte-Supported Cells with High Power Density and Durability. In Proceedings of the 8th European Solid Oxide Fuel Cell Forum, Lucerne, Switzerland, 30 June–4 July 2008.
- Mosch, S.; Trofimenko, N.; Kusnezoff, M.; Betz, T.; Kellner, M. Fabrication and electrochemical characterisation of cathodes for Solid Oxide Fuel Cells. In Proceedings of the 7th European Solid Oxide Fuel Cell Forum, Lucerne, Switzerland, 3–7 July 2006.
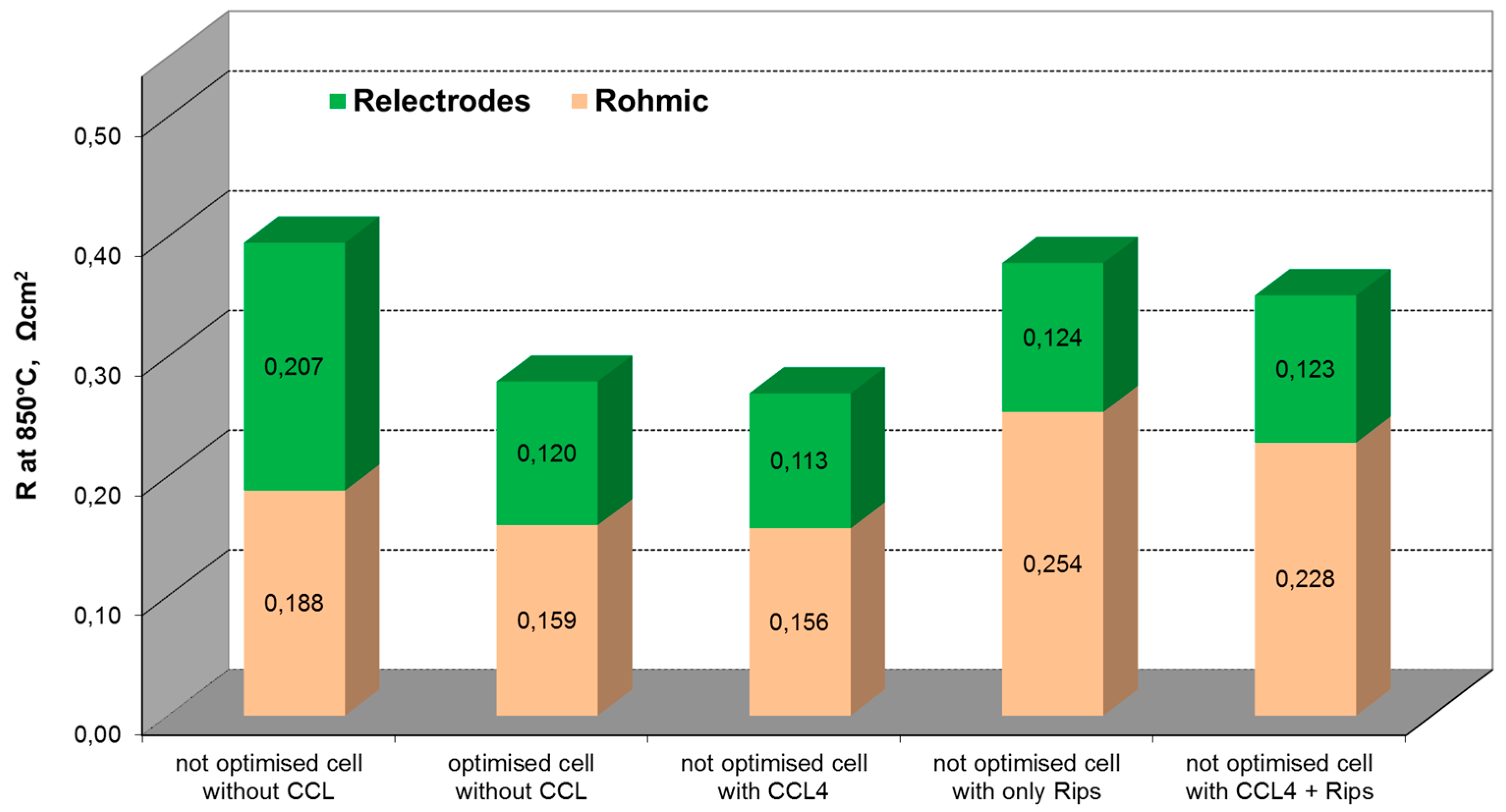

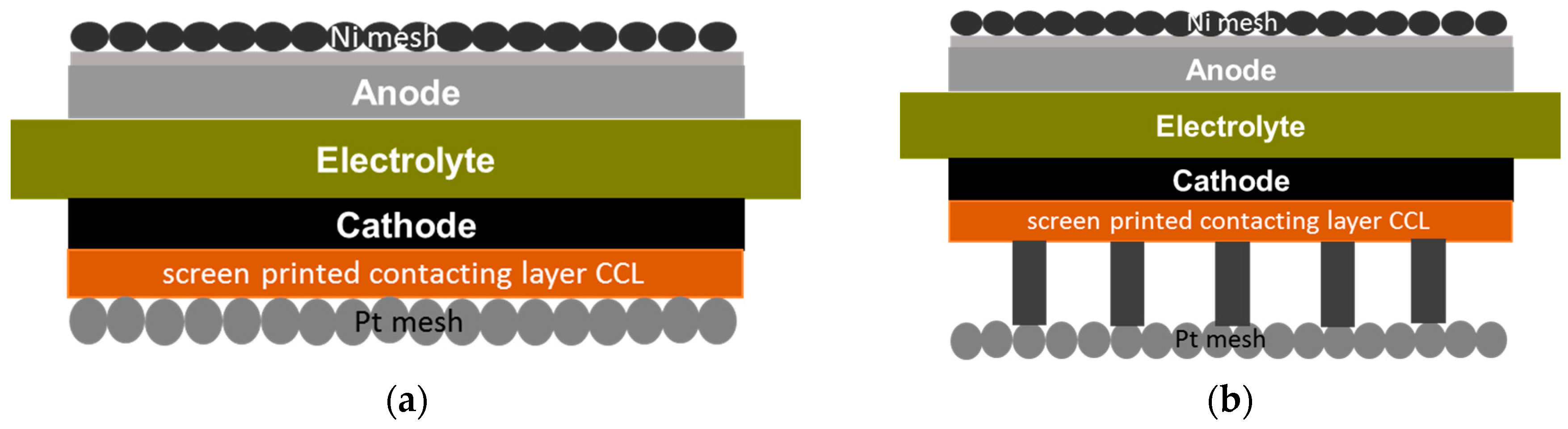
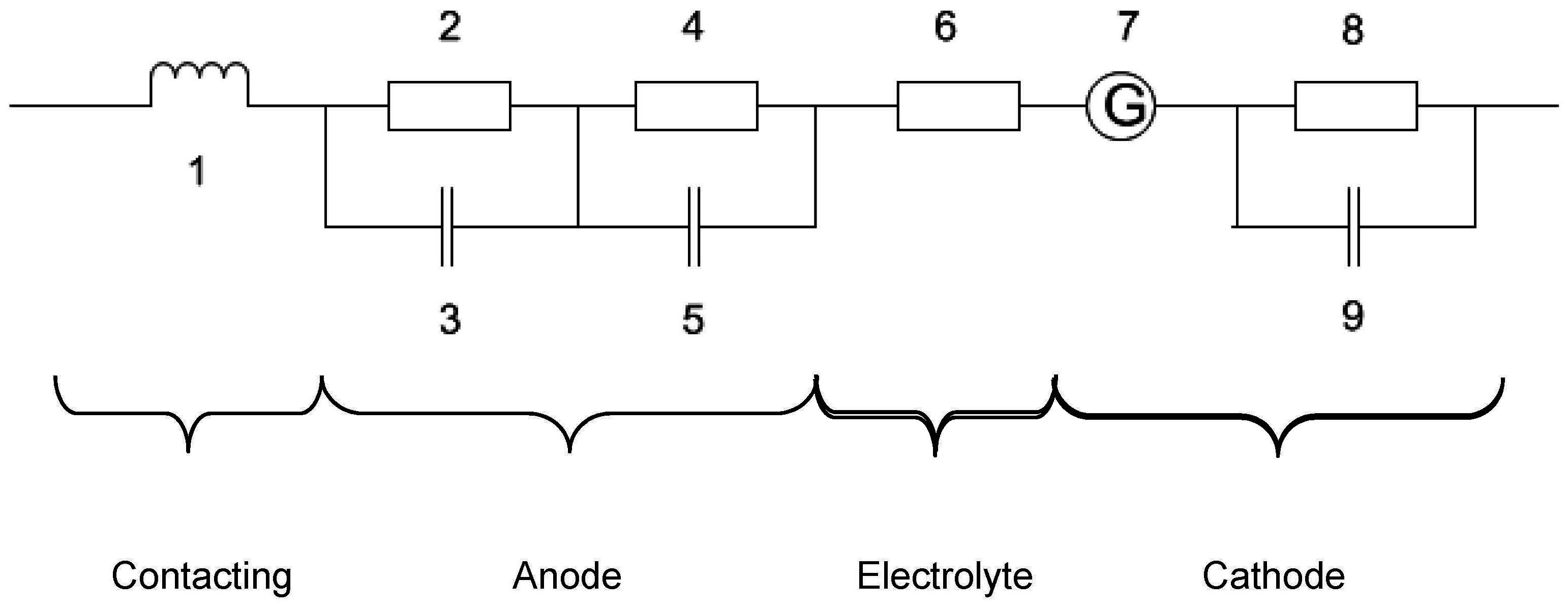
| Temperature (°C) | ASR (Ω·cm2) | ∆ASR (Ω·cm2) | Voltage (mV) at 300 mA/cm2 in H2/H2O/N2 = 40/5/55 |
|---|---|---|---|
| 850 | 0.178 | ±0.010 | 935 |
| 800 | 0.286 | ±0.013 | 915 |
| 750 | 0.508 | ±0.019 | 865 |
| 700 | 0.918 | ±0.036 | 745 |
| Cell Type | Current Density (mA/cm2) | Temperature (°C) | Testing Period (h) | Measured Voltage (mV) | ASR (Ω·cm2) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | After 200 h | Final | Initial | After 200 h | Final | ||||
| A | −300 | 850 | 1370 | 952 | 964 | 963 | 0.336 | 0.381 | 0.378 |
| B | −300 | 850 | 1270 | 917 | 918 | 929 | 0.224 | 0.229 | 0.264 |
| B | −500 | 850 | 1180 | 962 | 963 | 974 | 0.238 | 0.240 | 0.264 |
| B | −500 | 800 | 5200 | 1035 | 1043 | 1068 | 0.340 | 0.360 | 0.413 |
| CCL | Temperature (°C) | ASR (Ω·cm2) | Working Voltage (mV) (at 300 mA/cm2 in H2:H2O:N2 = 40:5:55) |
|---|---|---|---|
| no | 850 | 0.359 | 878 |
| CCL1 | 850 | 0.292 | 898 |
| CCL2 | 850 | 0.287 | 898 |
| CCL3 | 850 | 0.293 | 897 |
| CCL4 | 851 | 0.264 | 902 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusnezoff, M.; Trofimenko, N.; Müller, M.; Michaelis, A. Influence of Electrode Design and Contacting Layers on Performance of Electrolyte Supported SOFC/SOEC Single Cells. Materials 2016, 9, 906. https://doi.org/10.3390/ma9110906
Kusnezoff M, Trofimenko N, Müller M, Michaelis A. Influence of Electrode Design and Contacting Layers on Performance of Electrolyte Supported SOFC/SOEC Single Cells. Materials. 2016; 9(11):906. https://doi.org/10.3390/ma9110906
Chicago/Turabian StyleKusnezoff, Mihails, Nikolai Trofimenko, Martin Müller, and Alexander Michaelis. 2016. "Influence of Electrode Design and Contacting Layers on Performance of Electrolyte Supported SOFC/SOEC Single Cells" Materials 9, no. 11: 906. https://doi.org/10.3390/ma9110906
APA StyleKusnezoff, M., Trofimenko, N., Müller, M., & Michaelis, A. (2016). Influence of Electrode Design and Contacting Layers on Performance of Electrolyte Supported SOFC/SOEC Single Cells. Materials, 9(11), 906. https://doi.org/10.3390/ma9110906






