Microstructural Effects of Sulphate Attack in Sustainable Grouts for Micropiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Exposure Media
2.3. Impedance Spectroscopy
2.4. Electrical Resistivity
2.5. Mercury Intrusion Porosimetry
2.6. Compressive Strength
3. Results
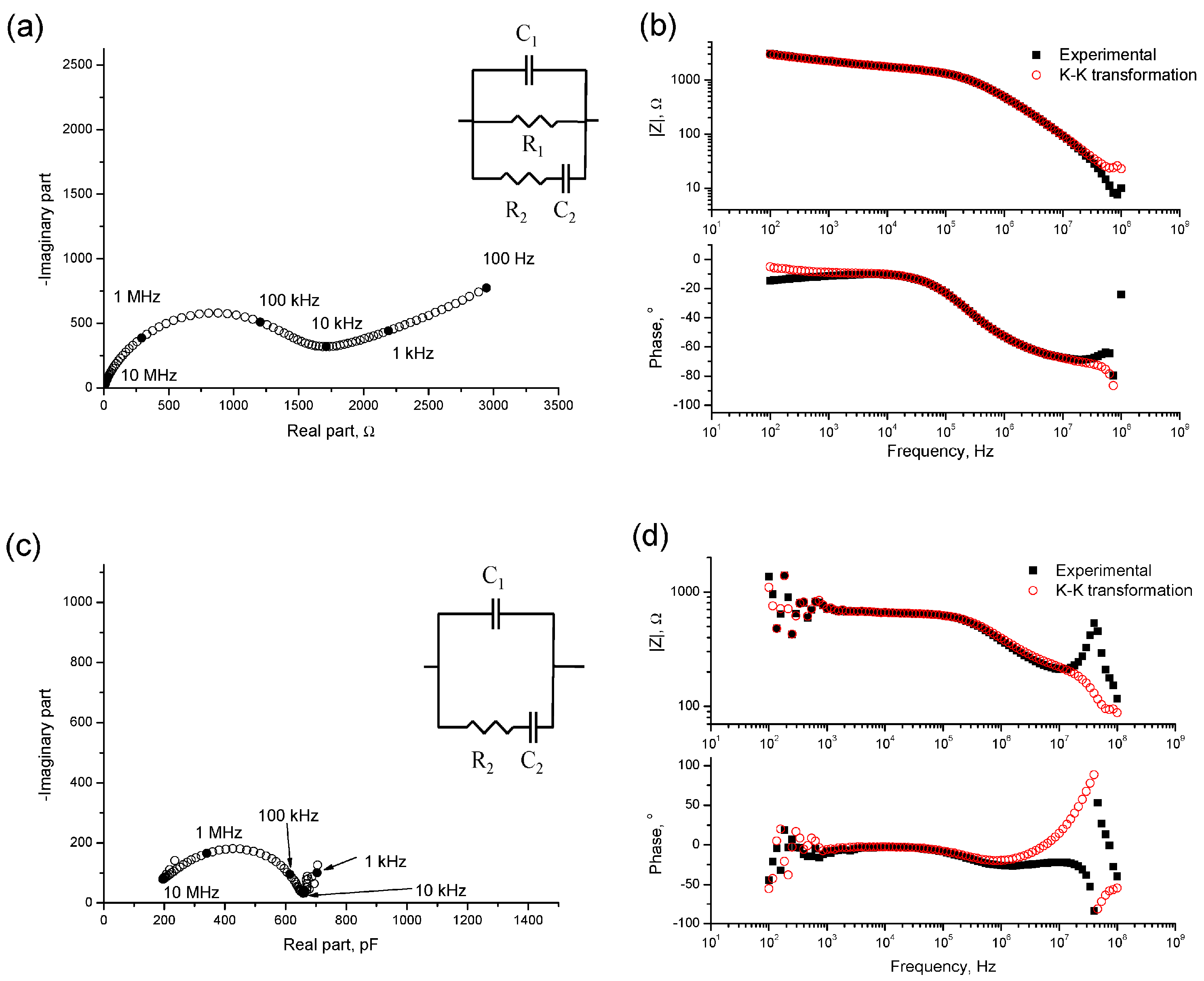
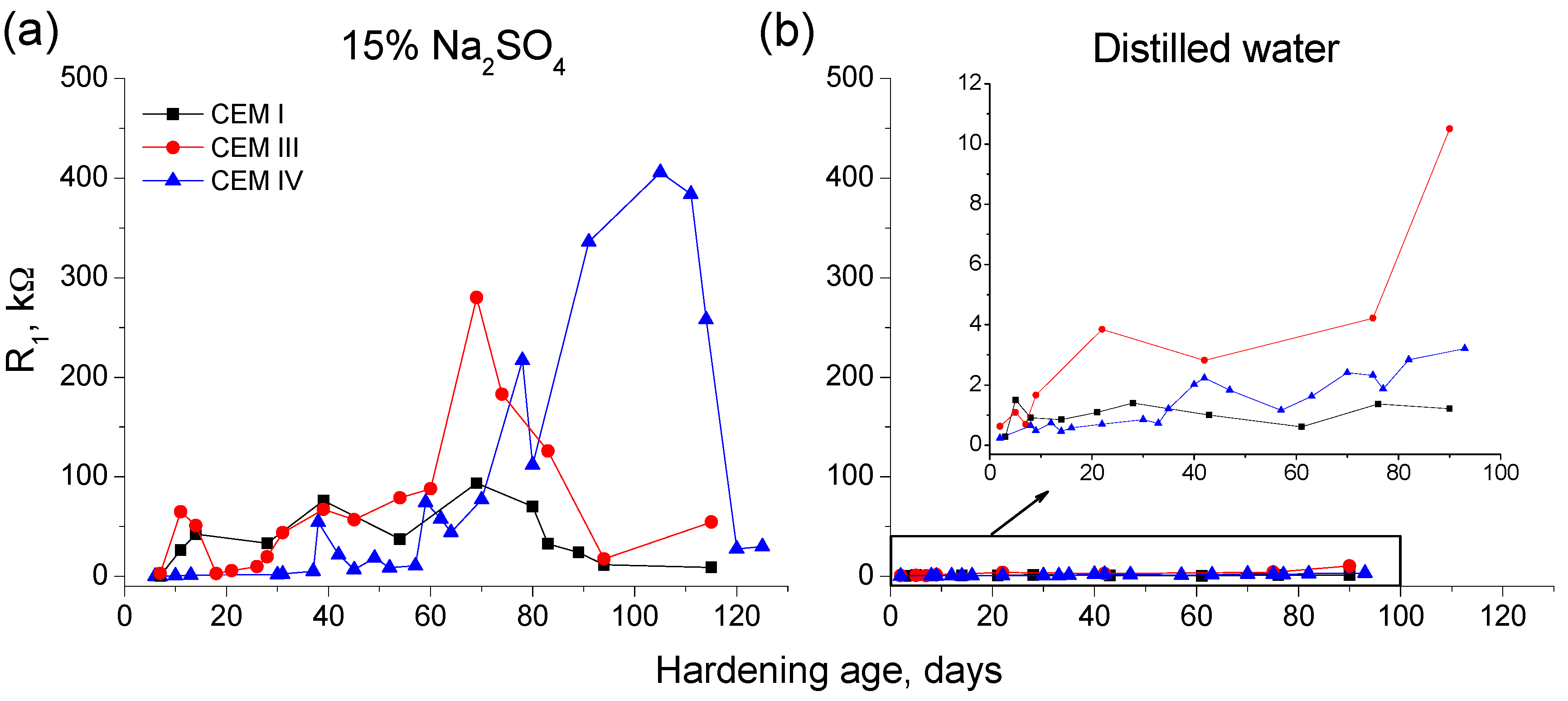
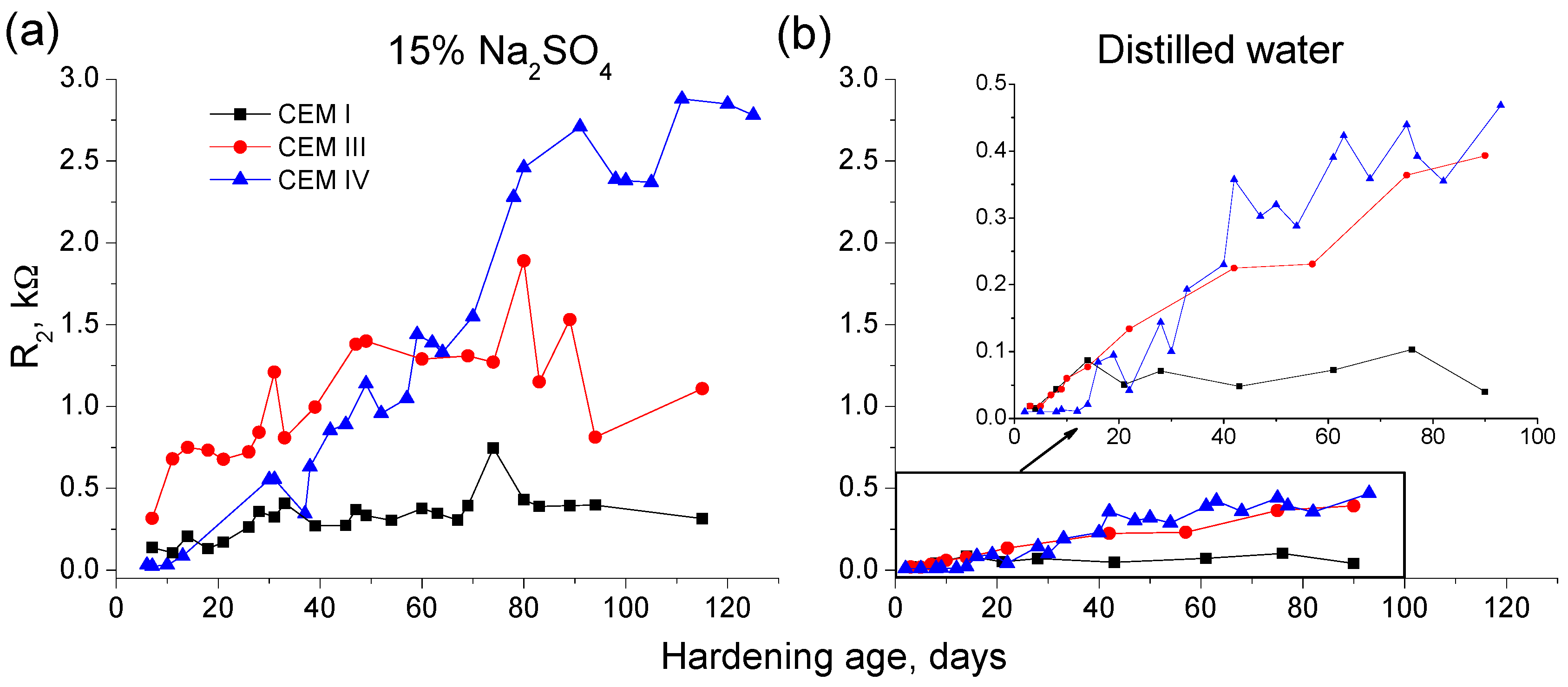
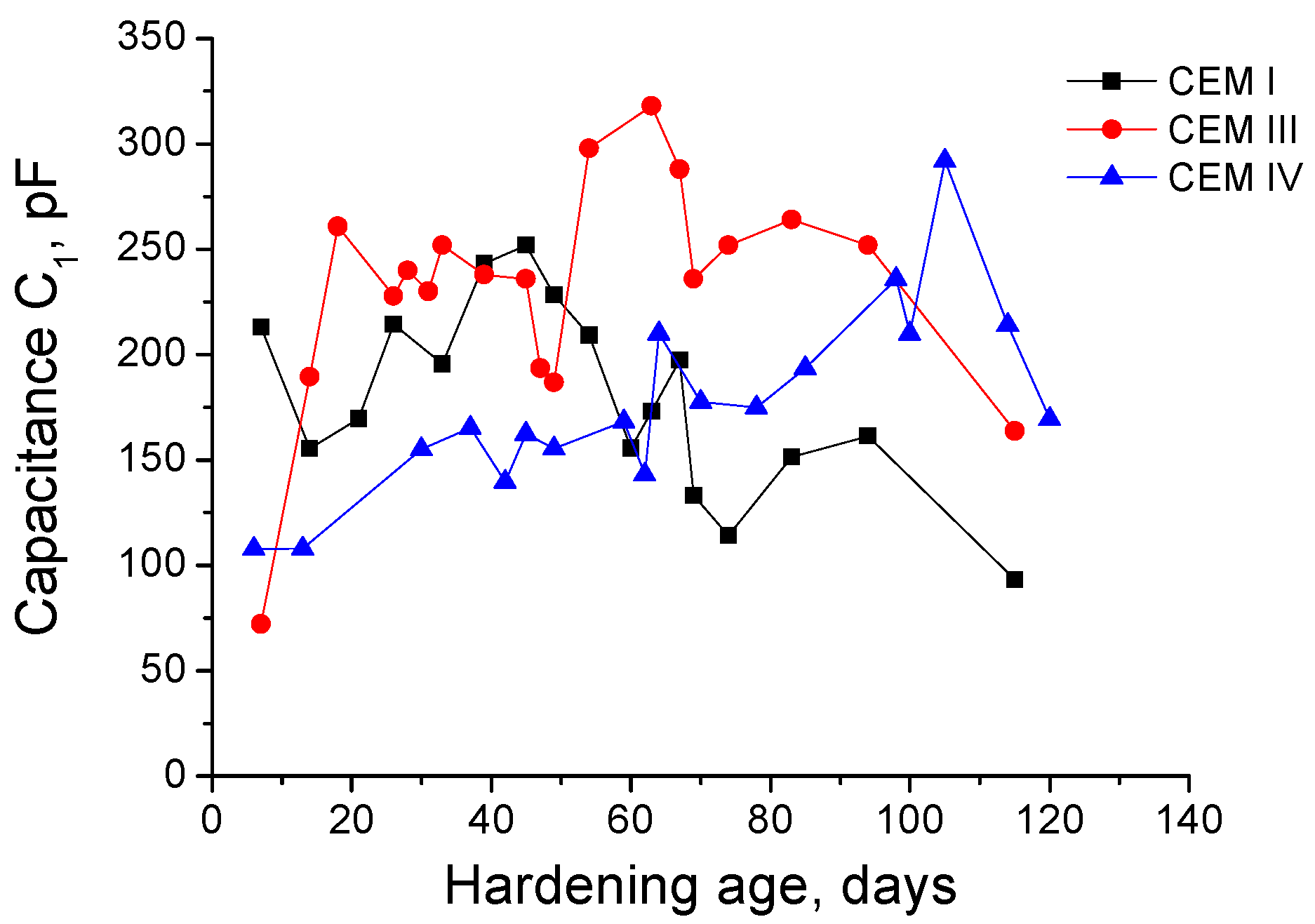
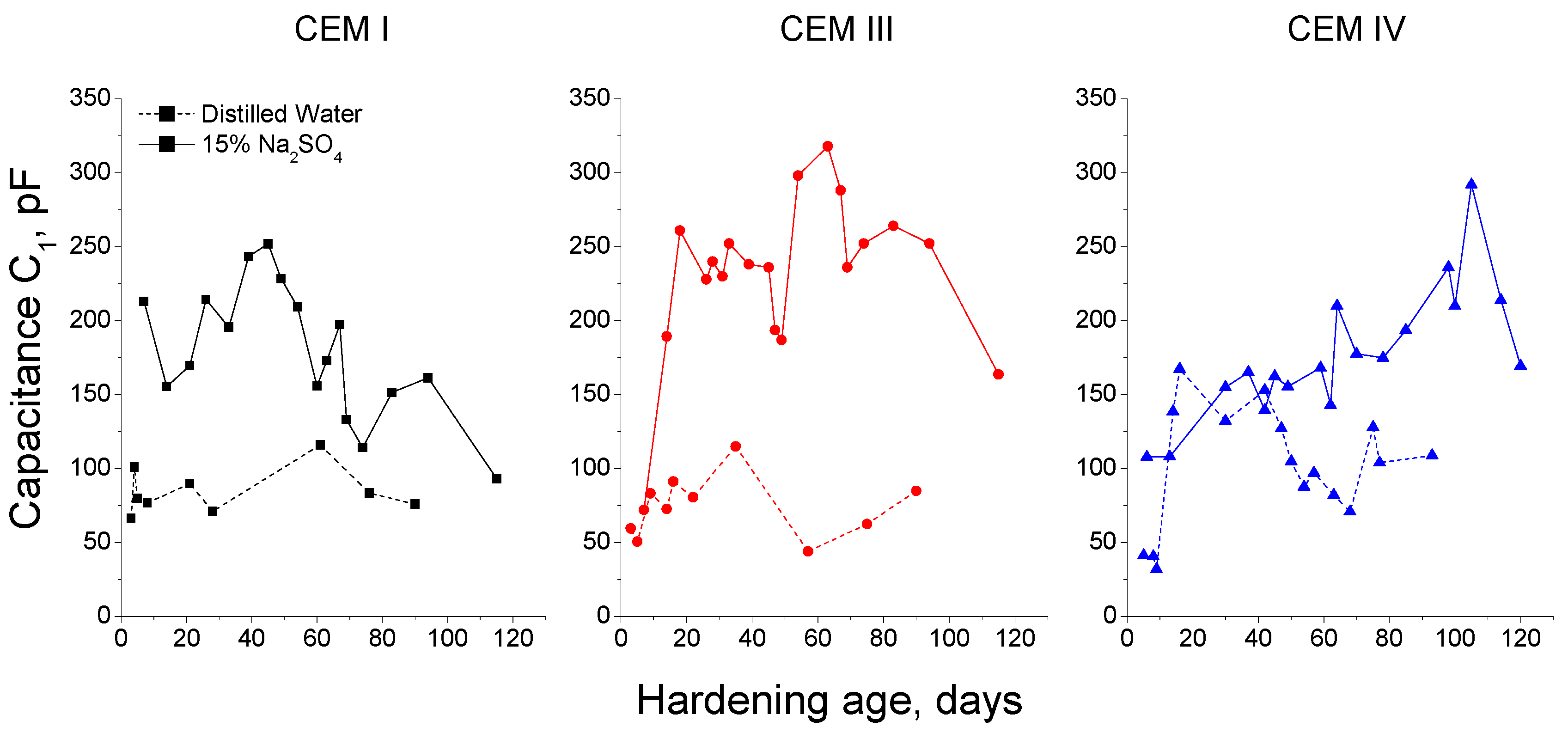
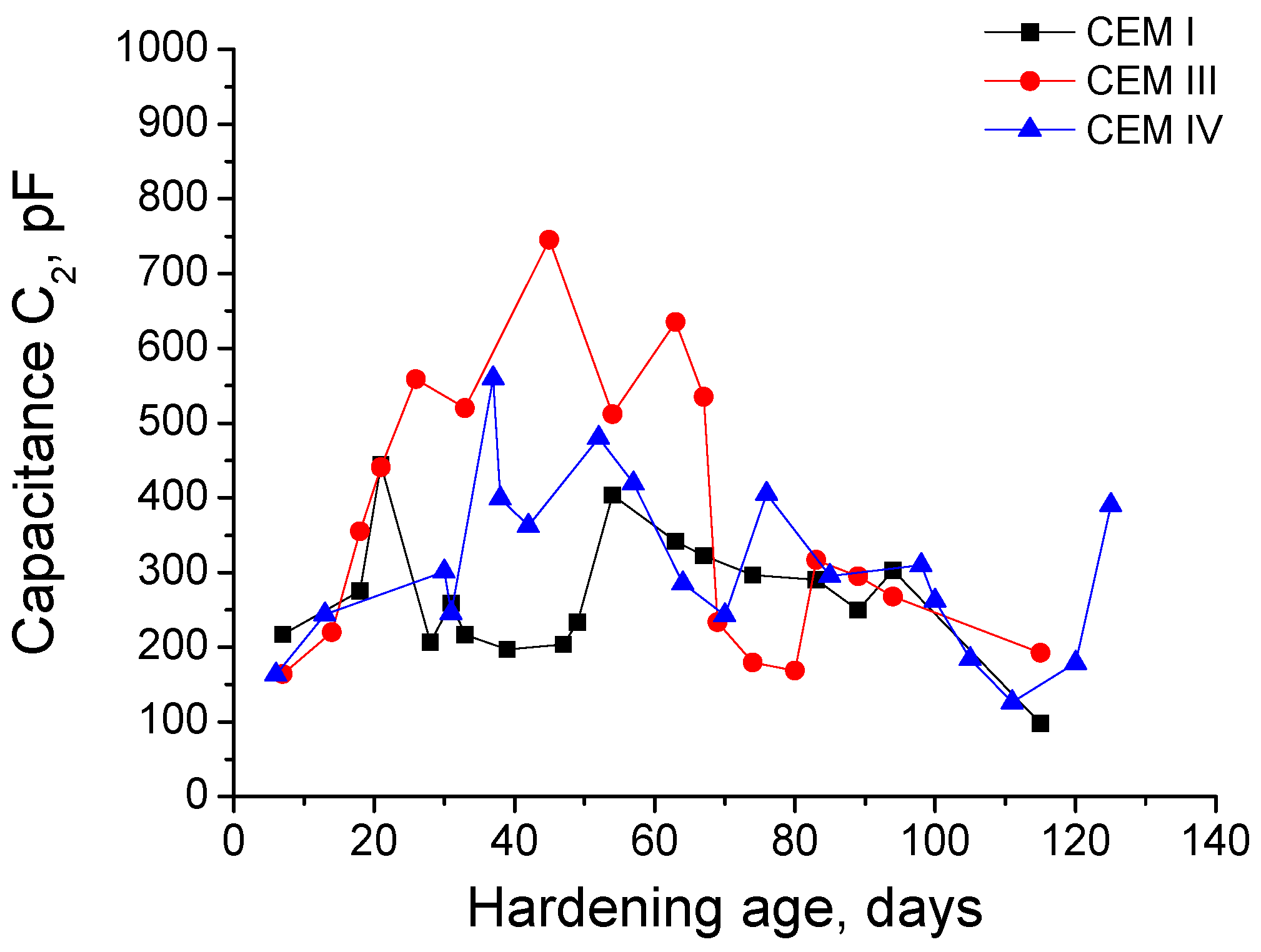
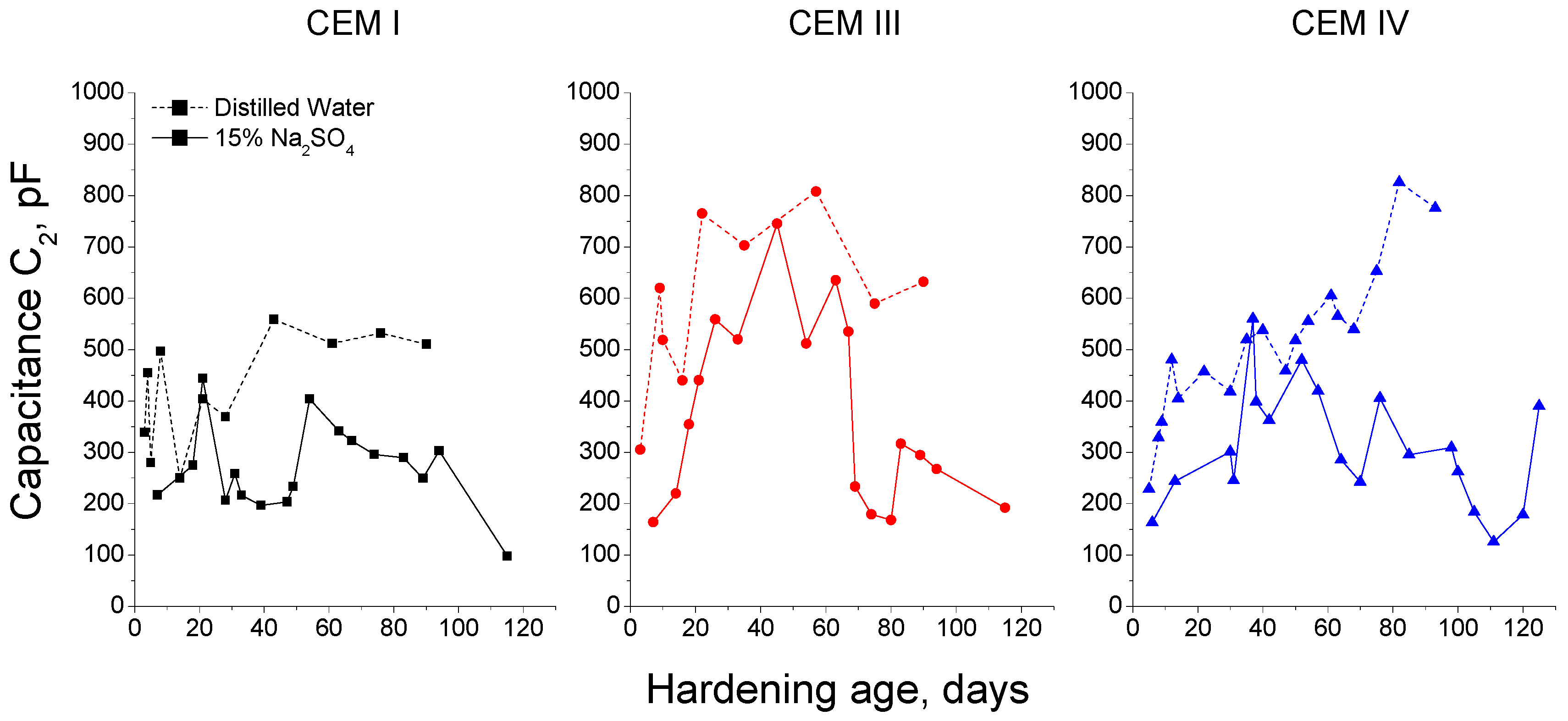
3.1. Impedance Spectroscopy
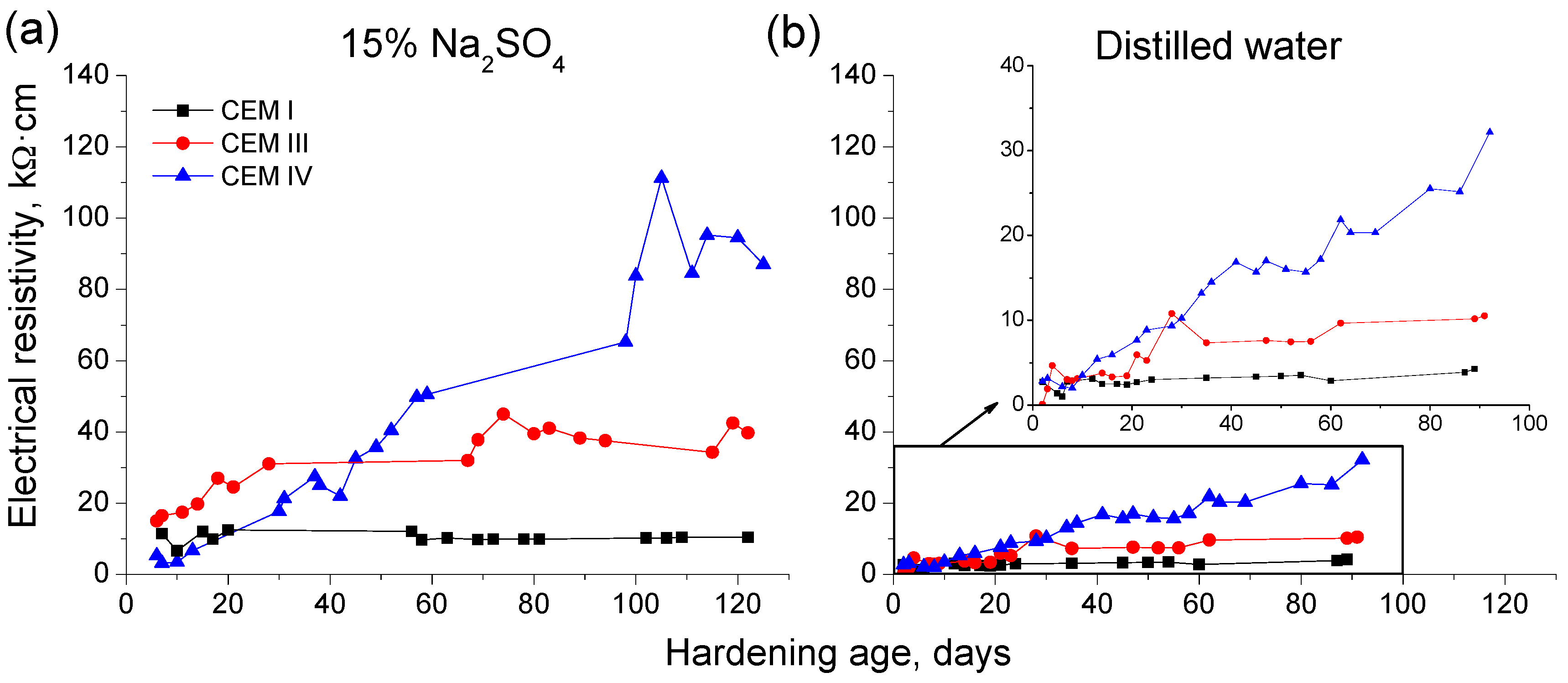
3.2. Electrical Resistivity
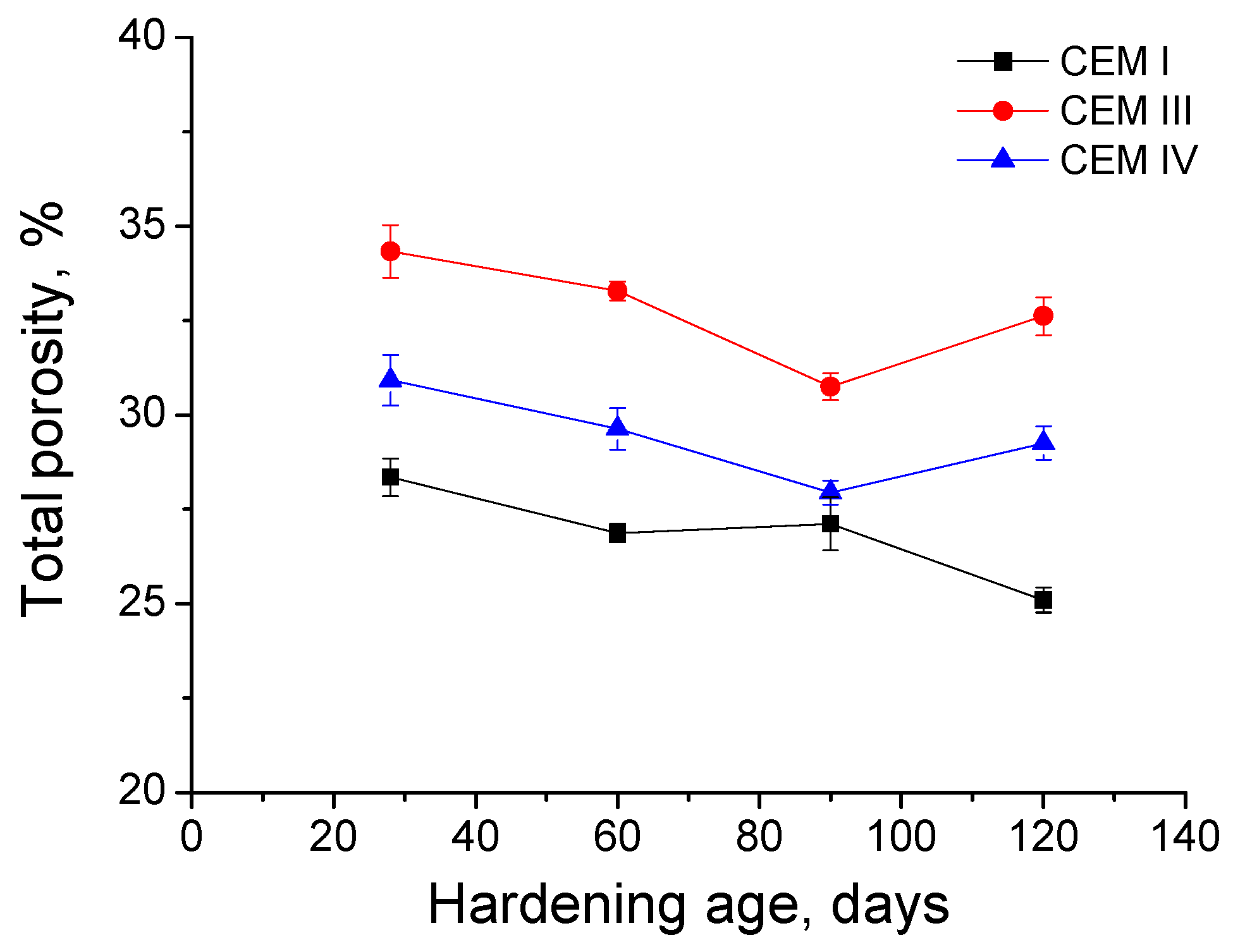
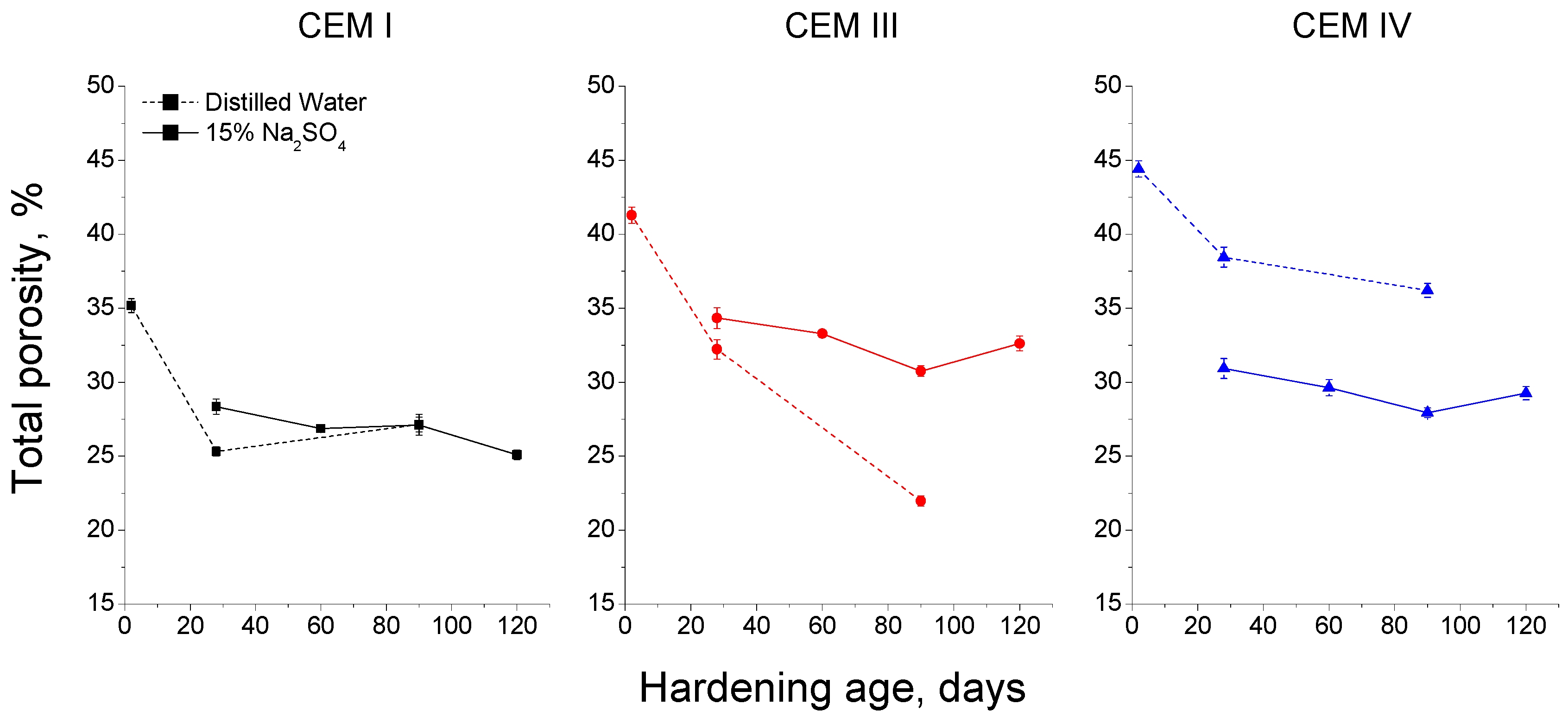
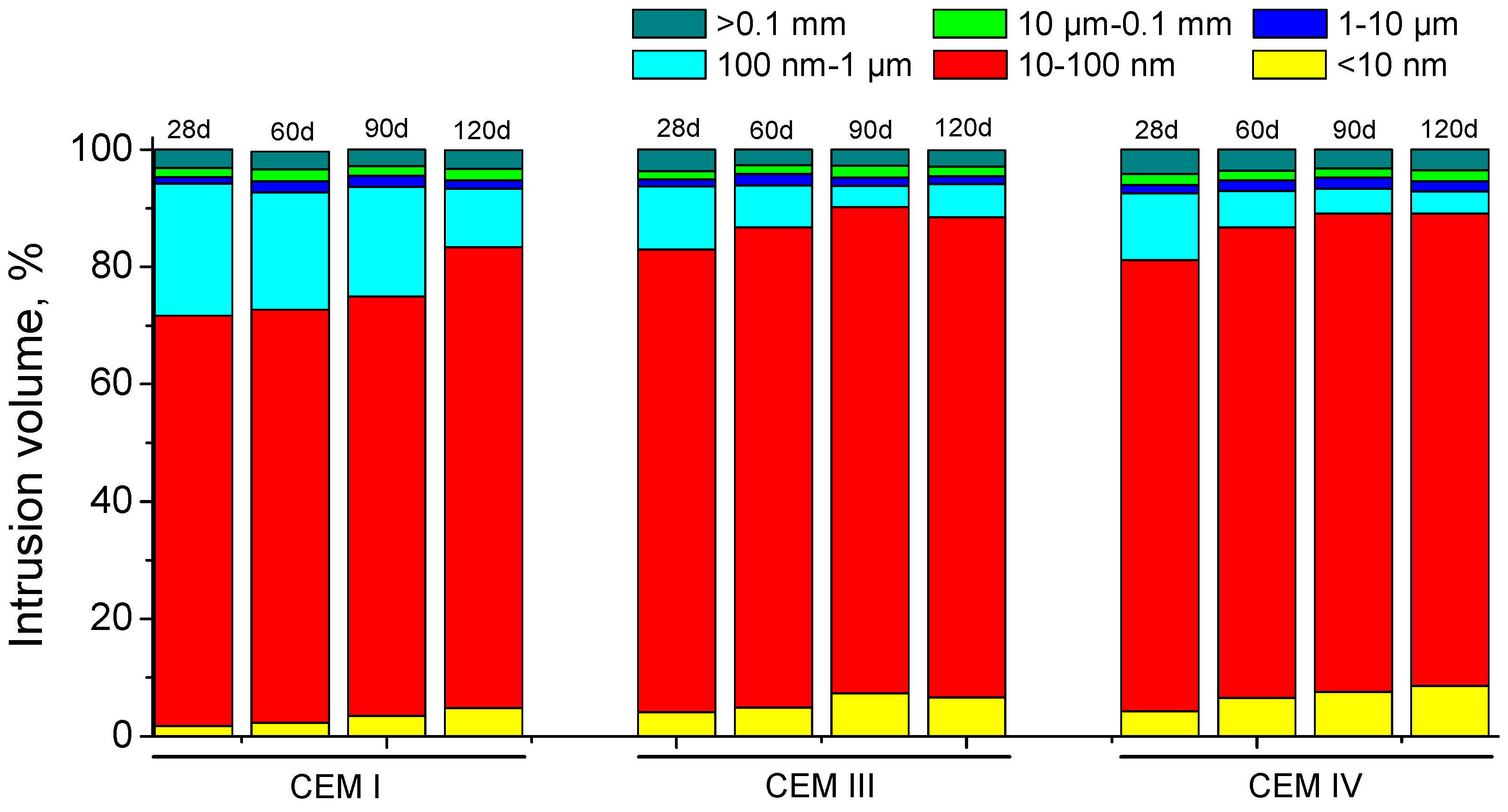
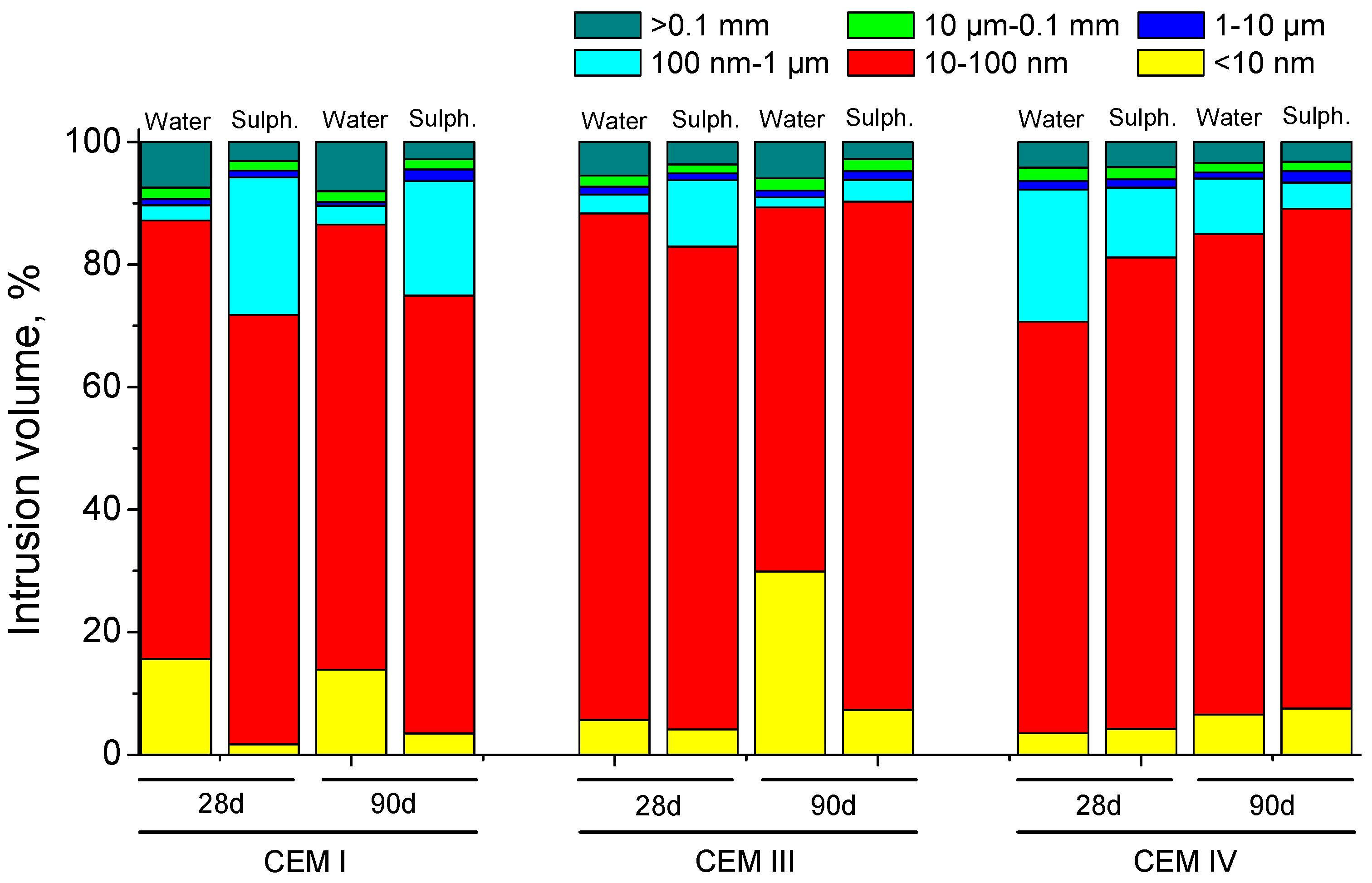
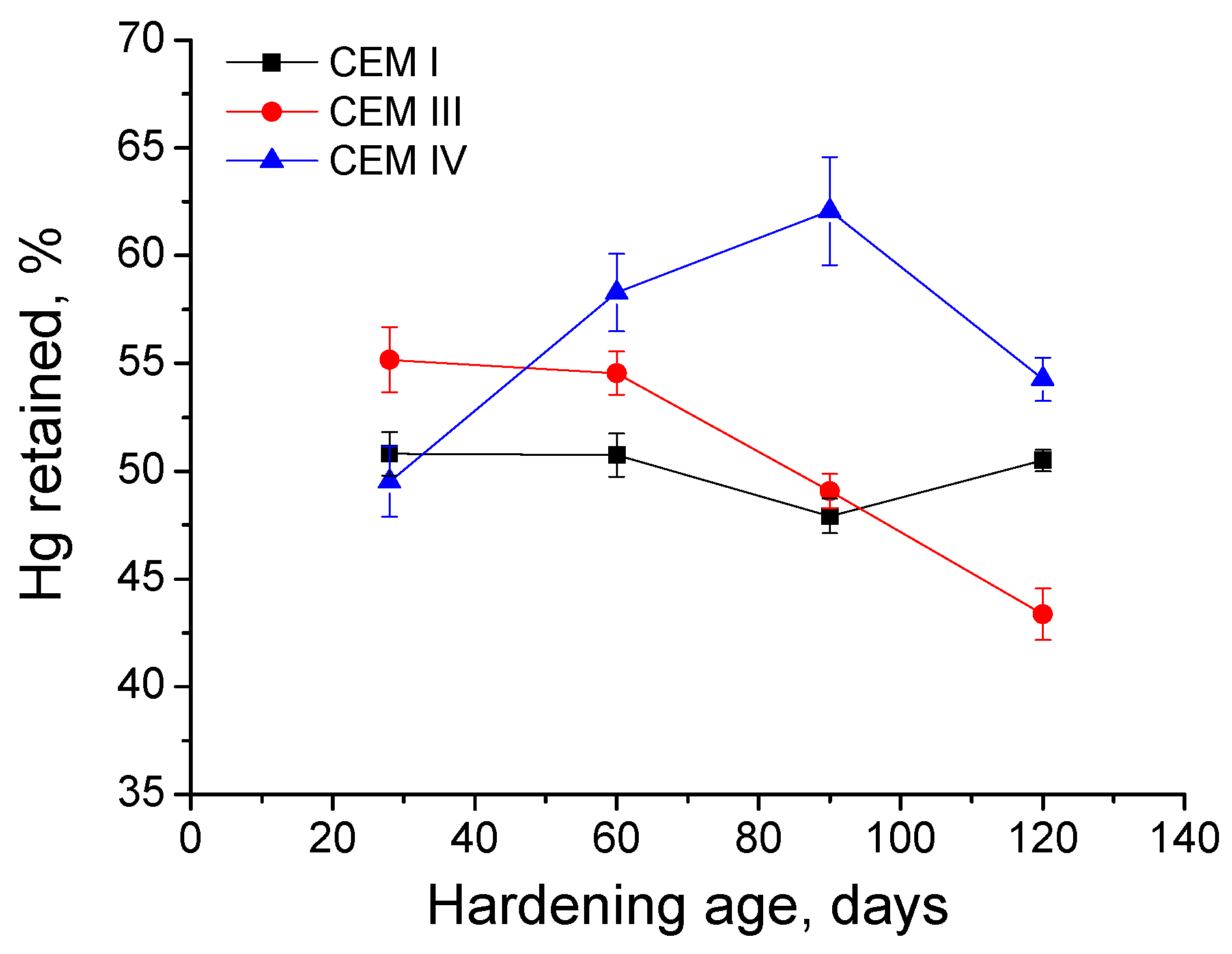
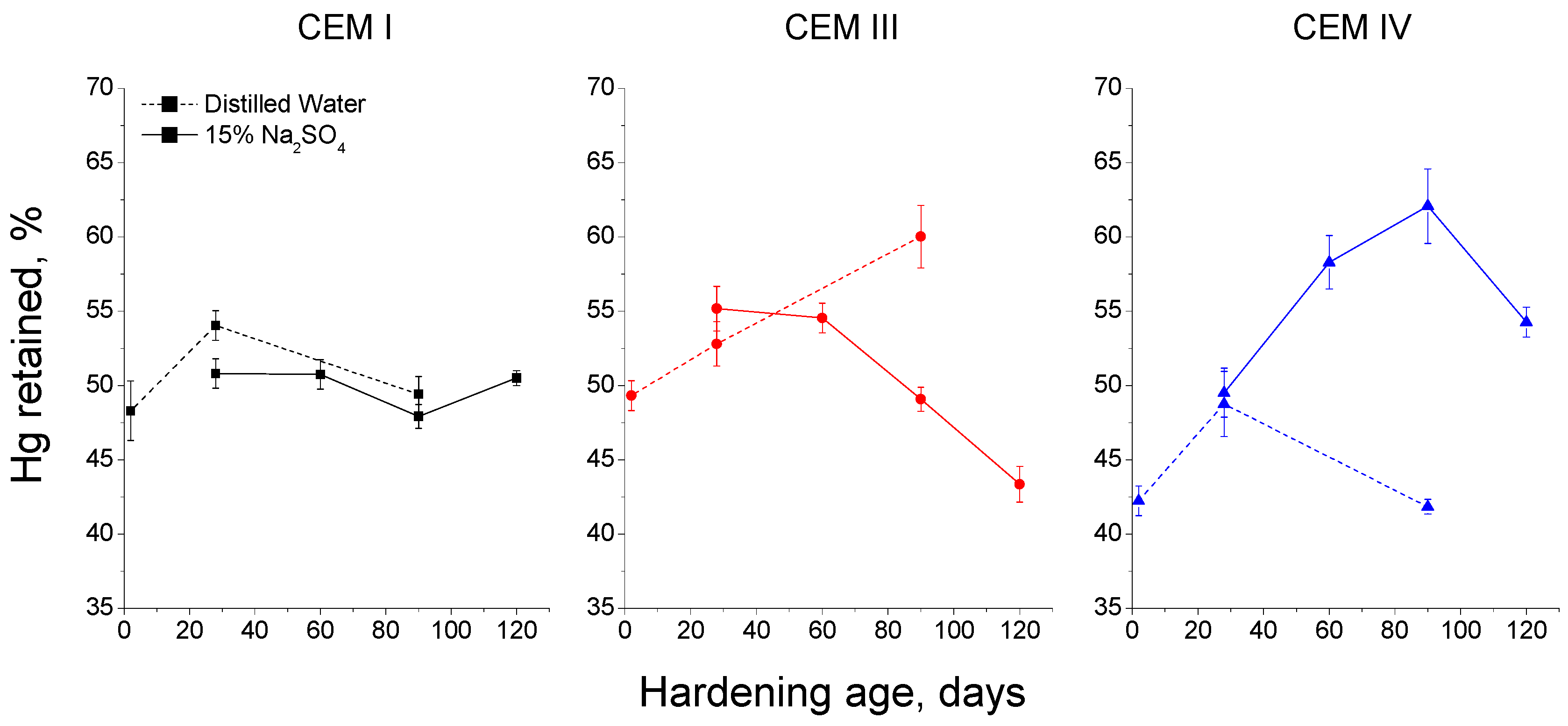
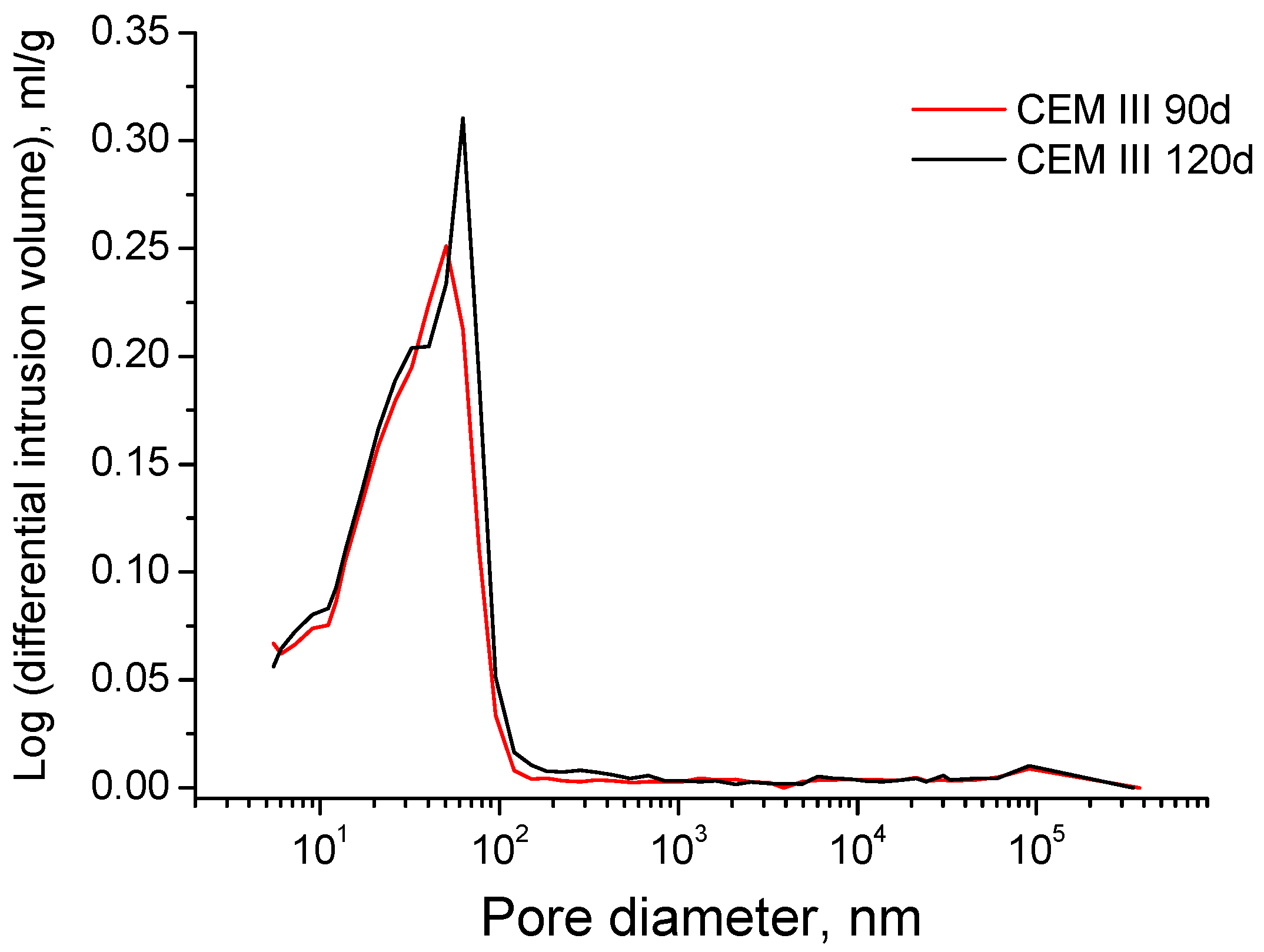
3.3. Mercury Intrusion Porosimetry
3.4. Compressive Strength
4. Discussion
5. Conclusions
- In general, slag and fly ash cement grouts for micropiles showed a more refined microstructure than CEM I ones for both sodium sulphate solution and distilled water hardening media.
- In the short-term (until approximately 40 hardening days), the microstructure of the grouts exposed to sodium sulphate solution becomes more refined, due to the formation of solid phases as products of clinker and slag hydration and fly ash pozzolanic reactions. Moreover, the ettringite formation as a consequence of sulphate attack would also contribute to this microstructure refinement.
- In the long-term (approximately from 60–90 days, depending on the cement type, up to 120 days of exposure), for grouts in contact with sodium sulphate solution, when enough silting of pores by expansive products formation has been produced, the continuous sulphate attack would damage the rough structures formed in the pore surface, causing a loss of microstructure refinement, independently that the hydration and pozzolanic reactions could be still producing.
- The exposure of grouts to an optimum hardening condition, like the immersion in distilled water, favours the development of hydration and pozzolanic reactions and improves the development of their microstructure, reducing the porosity and increasing the refinement of the pore network.
- The development of microstructure for CEM IV grouts was delayed in comparison with CEM I and III ones. This could be associated with the later beginning of fly ash pozzolanic reactions with respect to clinker and slag hydration.
- The compressive strength of the grouts at 28 days was influenced by the different strength type of cement used. However, the loss of compressive strength for samples submerged in sodium sulphate solution, compared to those hardened in distilled water, was lower for slag and fly ash cement grouts than for CEM I ones.
- Fly ash grouts exposed to sodium sulphate solution showed a better behaviour in relation to microstructure development, compared to those submerged in distilled water. This could be due to the possible effect of 15% Na2SO4 as an activator of pozzolanic reactions of fly ash.
- In general, the microstructure characterization carried out using impedance spectroscopy, mercury intrusion porosimetry and electrical resistivity are in agreement. However, it seems that non-destructive impedance spectroscopy is the most sensitive technique for following the changes in the porous network of slag and fly ash cement grouts for micropiles exposed to an aggressive sodium sulphate medium.
- In view of the results obtained in this research, the micropiles made using fly ash and slag cement grouts could have a good performance in contact with aggressive sodium sulphate media, even better than ordinary Portland cement ones.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Armour, T.; Groneck, P.; Keeley, J.; Sharma, S. Micropile Design and Construction Guidelines—Implementation Manual Report FHWA-SA-97-070; Federal Highway Administration–US Department of Transportation: Washington, DC, USA, 2000; p. 376. [Google Scholar]
- De Carreteras, D.G.; de Fomento, M.; Madrid, S. Guía para el Proyecto y la Ejecución de Micropilotes en Obras de Carretera; Ministerio de Fomento: Paseo de la Castellana, Spain, 2005. [Google Scholar]
- AENOR. UNE-EN 14199: Ejecución de Trabajos Geotécnicos Especiales: Micropilotes; AENOR: Madrid, Spain, 2006; p. 54. [Google Scholar]
- AENOR. UNE-EN 197-1:2011. Composición, Especificaciones y Criterios de Conformidad de los Cementos Comunes; AENOR: Madrid, Spain, 2011; p. 30. [Google Scholar]
- Bijen, J. Benefits of slag and fly ash. Constr. Build. Mater. 1996, 10, 309–314. [Google Scholar] [CrossRef]
- Demirboğa, R. Thermal conductivity and compressive strength of concrete incorporation with mineral admixtures. Build. Environ. 2007, 42, 2467–2471. [Google Scholar] [CrossRef]
- Ganjian, E.; Pouya, H.S. The effect of Persian Gulf tidal zone exposure on durability of mixes containing silica fume and blast furnace slag. Constr. Build. Mater. 2009, 23, 644–652. [Google Scholar] [CrossRef]
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Influencia de diferentes condiciones de curado en la estructura porosa y en las propiedades a edades tempranas de morteros que contienen ceniza volante y escoria de alto horno. Mater. Constr. 2012, 63, 219–234. [Google Scholar]
- Ponikiewski, T.; Gołaszewski, J. The effect of high-calcium fly ash on selected properties of self-compacting concrete. Arch. Civ. Mech. Eng. 2014, 14, 455–465. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of fly ash on Portland cement systems. Cem. Concr. Res. 1999, 29, 1727–1736. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, C.; Sun, W. Fly ash effects. Cem. Concr. Res. 2004, 34, 2057–2060. [Google Scholar] [CrossRef]
- Glinicki, M.; Jóźwiak-Niedźwiedzka, D.; Gibas, K.; Dąbrowski, M. Influence of blended cements with calcareous fly ash on chloride ion migration and carbonation resistance of concrete for durable structures. Materials 2016, 9, 18. [Google Scholar] [CrossRef]
- Wedding, P.; Manmohan, D.; Mehta, P. Influence of pozzolanic, slag, and chemical admixtures on pore size distribution and permeability of hardened cement pastes. Cem. Concr. Aggreg. 1981, 3, 63–67. [Google Scholar] [CrossRef]
- Bouikni, A.; Swamy, R.N.; Bali, A. Durability properties of concrete containing 50% and 65% slag. Constr. Build. Mater. 2009, 23, 2836–2845. [Google Scholar] [CrossRef]
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Durability related transport properties of OPC and slag cement mortars hardened under different environmental conditions. Constr. Build. Mater. 2012, 27, 176–183. [Google Scholar] [CrossRef]
- Thomas, M.D.; Matthews, J. Performance of pfa concrete in a marine environment—10-year results. Cem. Concr. Compos. 2004, 26, 5–20. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Scott, A.; Bremner, T.; Bilodeau, A.; Day, D. Performance of slag concrete in marine environment. ACI Mater. J. 2008, 105, 628–634. [Google Scholar]
- Chalee, W.; Ausapanit, P.; Jaturapitakkul, C. Utilization of fly ash concrete in marine environment for long term design life analysis. Mater. Des. 2010, 31, 1242–1249. [Google Scholar] [CrossRef]
- Bonakdar, A.; Mobasher, B. Multi-parameter study of external sulfate attack in blended cement materials. Constr. Build. Mater. 2010, 24, 61–70. [Google Scholar] [CrossRef]
- Tixier, R.; Mobasher, B. Modeling of damage in cement-based materials subjected to external sulfate attack. I: Formulation. J. Mater. Civ. Eng. 2003, 15, 305–313. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look. Part 1: Summary of experimental results. Cem. Concr. Res. 2002, 32, 915–921. [Google Scholar] [CrossRef]
- Neville, A. The confused world of sulfate attack on concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Kalousek, G.L.; Porter, L.C.; Benton, E.J. Concrete for long-time service in sulfate environment. Cem. Concr. Res. 1972, 2, 79–89. [Google Scholar] [CrossRef]
- Monteiro, P.J.M.; Kurtis, K.E. Time to failure for concrete exposed to severe sulfate attack. Cem. Concr. Res. 2003, 33, 987–993. [Google Scholar] [CrossRef]
- Ramyar, K.; İnan, G. Sodium sulfate attack on plain and blended cements. Build. Environ. 2007, 42, 1368–1372. [Google Scholar] [CrossRef]
- Bentz, D.P.; Garboczi, E.J. Simulation studies of the effects of mineral admixtures on the cement paste-aggregate interfacial zone. ACI Mater. J. 1991, 88, 518–529. [Google Scholar]
- Baghabra Al-Amoudi, O.S. Attack on plain and blended cements exposed to aggressive sulfate environments. Cem. Concr. Compos. 2002, 24, 305–316. [Google Scholar] [CrossRef]
- Cao, H.T.; Bucea, L.; Ray, A.; Yozghatlian, S. The effect of cement composition and pH of environment on sulfate resistance of Portland cements and blended cements. Cem. Concr. Compos. 1997, 19, 161–171. [Google Scholar] [CrossRef]
- Tian, B.; Cohen, M.D. Does gypsum formation during sulfate attack on concrete lead to expansion? Cem. Concr. Res. 2000, 30, 117–123. [Google Scholar] [CrossRef]
- Živica, V. Sulfate resistance of the cement materials based on the modified silica fume. Constr. Build. Mater. 2000, 14, 17–23. [Google Scholar] [CrossRef]
- Irassar, E.F.; Di Maio, A.; Batic, O.R. Sulfate attack on concrete with mineral admixtures. Cem. Concr. Res. 1996, 26, 113–123. [Google Scholar] [CrossRef]
- Mehta, P.K. Effect of fly ash composition on sulfate resistance of cement. J. Am. Concr. Inst. 1986, 83, 994–1000. [Google Scholar]
- Geiseler, J.; Kollo, H.; Lang, E. Influence of blast furnace cements on durability of concrete structures. ACI Mater. J. 1995, 92, 252–257. [Google Scholar]
- Popovics, J.S.; Subramaniam, K.V.L. Review of ultrasonic wave reflection applied to early-age concrete and cementitious materials. J. Nondestruct. Eval. 2014. [Google Scholar] [CrossRef]
- Kogbara, R.B.; Iyengar, S.R.; Grasley, Z.C.; Masad, E.A.; Zollinger, D.G. Non-destructive evaluation of concrete mixtures for direct LNG containment. Mater. Des. 2015, 82, 260–272. [Google Scholar] [CrossRef]
- Henry, M.; Darma, I.S.; Sugiyama, T. Analysis of the effect of heating and re-curing on the microstructure of high-strength concrete using X-ray CT. Constr. Build. Mater. 2014, 67, 37–46. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Shen, P.; Shui, Z. Microstructural development of hydrating portland cement paste at early ages investigated with non-destructive methods and numerical simulation. J. Nondestruct. Eval. 2013, 32, 228–237. [Google Scholar] [CrossRef]
- Chaix, J.-F.; Garnier, V.; Corneloup, G. Concrete damage evolution analysis by backscattered ultrasonic waves. NDT E Int. 2003, 36, 461–469. [Google Scholar] [CrossRef]
- Demčenko, A.; Visser, H.A.; Akkerman, R. Ultrasonic measurements of undamaged concrete layer thickness in a deteriorated concrete structure. NDT E Int. 2016, 77, 63–72. [Google Scholar] [CrossRef]
- McCarter, W.J.; Brousseau, R. The, A.C. response of hardened cement paste. Cem. Concr. Res. 1990, 20, 891–900. [Google Scholar] [CrossRef]
- Díaz, B.; Nóvoa, X.R.; Pérez, M.C. Study of the chloride diffusion in mortar: A new method of determining diffusion coefficients based on impedance measurements. Cem. Concr. Compos. 2006, 28, 237–245. [Google Scholar] [CrossRef]
- Sánchez, I.; López, M.P.; Ortega, J.M.; Climent, M.Á. Impedance spectroscopy: An efficient tool to determine the non-steady-state chloride diffusion coefficient in building materials. Mater. Corros. 2011, 62, 139–145. [Google Scholar] [CrossRef]
- Sánchez, I.; Antón, C.; de Vera, G.; Ortega, J.M.; Climent, M.A. Moisture Distribution in partially saturated concrete studied by impedance spectroscopy. J. Nondestruct. Eval. 2013, 32, 362–371. [Google Scholar] [CrossRef]
- Sánchez, I.; Nóvoa, X.R.; de Vera, G.; Climent, M.A. Microstructural modifications in Portland cement concrete due to forced ionic migration tests. Study by impedance spectroscopy. Cem. Concr. Res. 2008, 38, 1015–1025. [Google Scholar] [CrossRef]
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Impedance spectroscopy study of the effect of environmental conditions in the microstructure development of OPC and slag cement mortars. Arch. Civ. Mech. Eng. 2015, 15, 569–583. [Google Scholar] [CrossRef]
- AENOR. UNE-EN 445:2009. Lechadas Para Tendones de Pretensado. Métodos de Ensayo; AENOR: Madrid, Spain, 2009; p. 18. [Google Scholar]
- AENOR. UNE-EN 447:2009. Lechadas Para Tendones de Pretensado. Requisitos Básicos; AENOR: Madrid, Spain, 2009; p. 14. [Google Scholar]
- Lee, S.T.; Moon, H.Y.; Swamy, R.N. Sulfate attack and role of silica fume in resisting strength loss. Cem. Concr. Compos. 2005, 27, 65–76. [Google Scholar] [CrossRef]
- Türker, F.; Aköz, F.; Koral, S.; Yüzer, N. Effects of magnesium sulfate concentration on the sulfate resistance of mortars with and without silica fume. Cem. Concr. Res. 1997, 27, 205–214. [Google Scholar] [CrossRef]
- Behfarnia, K.; Farshadfar, O. The effects of pozzolanic binders and polypropylene fibers on durability of SCC to magnesium sulfate attack. Constr. Build. Mater. 2013, 38, 64–71. [Google Scholar] [CrossRef]
- Ortega, J.M.; Albaladejo, A.; Pastor, J.L.; Sánchez, I.; Climent, M.A. Influence of using slag cement on the microstructure and durability related properties of cement grouts for micropiles. Constr. Build. Mater. 2013, 38, 84–93. [Google Scholar] [CrossRef]
- Pastor, J.L.; Ortega, J.M.; Flor, M.; López, M.P.; Sánchez, I.; Climent, M.A. Microstructure and durability of fly ash cement grouts for micropiles. Constr. Build. Mater. 2016, 117, 47–57. [Google Scholar] [CrossRef]
- Ortega, J.M.; Pastor, J.L.; Albaladejo, A.; Sánchez, I.; Climent, M.A. Durability and compressive strength of blast furnace slag-based cement grout for special geotechnical applications. 2014, 64. [Google Scholar] [CrossRef]
- AENOR. UNE-EN 196-1:2005. Métodos de Ensayo de Cementos. Parte 1: Determinación de Resistencias Mecánicas; AENOR: Madrid, Spain, 2005. [Google Scholar]
- Abadir, M.F.; El Saidy, M.I.; El Sersy, H.H.; Ibrahim, O.A. Estimation of the activation energies in thermal decomposition of some hydrated cement clinker. J. Eng. Appl. Sci. 2006, 53, 815–827. [Google Scholar]
- Bernal Camacho, J.; Mahmoud Abdelkader, S.; Reyes Pozo, E.; Moragues Terrades, M. Estudio de la influencia de los medios con presencia de sulfatos en hormigones con cementos sulforresistentes y adiciones minerales. Parte 1: Hormigones expuestos a sulfato sódico (Na2SO4). Rev. Constr. 2013, 12, 14–29. [Google Scholar] [CrossRef]
- Gollop, R.S.; Taylor, H.F.W. Microstructural and microanalytical studies of sulfate attack. I. Ordinary portland cement paste. Cem. Concr. Res. 1992, 22, 1027–1038. [Google Scholar] [CrossRef]
- Gollop, R.S.; Taylor, H.F.W. Microstructural and microanalytical studies of sulfate attack. IV. Reactions of a slag cement paste with sodium and magnesium sulfate solutions. Cem. Concr. Res. 1996, 26, 1013–1028. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution ASTM C 1012-04; ASTM International: Wesk Conshohocken, PA, USA, 2004; p. 6. [Google Scholar]
- Cabeza, M.; Merino, P.; Miranda, A.; Nóvoa, X.R.; Sanchez, I. Impedance spectroscopy study of hardened Portland cement paste. Cem. Concr. Res. 2002, 32, 881–891. [Google Scholar] [CrossRef]
- Climent, M.A.; Ortega, J.M.; Sánchez, I. Cement mortars with fly ash and slag—Study of their microstructure and resistance to salt ingress in different environmental conditions. In Concrete Repair, Rehabilitation and Retrofitting III, Proceedings of the 3rd International Conference on Concrete Repair, Rehabilitation and Retrofitting, ICCRRR2012, Cape Town, South Africa, 3–5 September 2012; CRC Press: Boca Raton, FL, USA; pp. 345–350.
- Keddam, M.; Takenouti, H.; Nóvoa, X.R.; Andrade, C.; Alonso, C. Impedance measurements on cement paste. Cem. Concr. Res. 1997, 27, 1191–1201. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy; Barsoukov, E., Macdonald, J.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Vladikova, D.; Zoltowski, P.; Makowska, E.; Stoynov, Z. Selectivity study of the differential impedance analysis—Comparison with the complex non-linear least-squares method. Electrochim. Acta 2002, 47, 2943–2951. [Google Scholar] [CrossRef]
- Cabeza, M.; Keddam, M.; Nóvoa, X.R.; Sánchez, I.; Takenouti, H. Impedance spectroscopy to characterize the pore structure during the hardening process of Portland cement paste. Electrochim. Acta 2006, 51, 1831–1841. [Google Scholar] [CrossRef]
- AENOR. UNE 83987:2014. Durabilidad del Hormigón. Métodos de Ensayo. Determinación de los Coeficientes de Difusión de los Iones Cloruro en el Hormigón Endurecido. Método Multirrégimen; AENOR: Madrid, Spain, 2014; p. 9. [Google Scholar]
- Polder, R.; Andrade, C.; Elsener, B.; Vennesland, Ø.; Gulikers, J.; Weidert, R.; Raupach, M. Test methods for on site measurement of resistivity of concrete. Mater. Struct. 2000, 33, 603–611. [Google Scholar] [CrossRef]
- Lübeck, A.; Gastaldini, A.L.G.; Barin, D.S.; Siqueira, H.C. Compressive strength and electrical properties of concrete with white Portland cement and blast-furnace slag. Cem. Concr. Compos. 2012, 34, 392–399. [Google Scholar] [CrossRef]
- Diamond, S. Aspects of concrete porosity revisited. Cem. Concr. Res. 1999, 29, 1181–1188. [Google Scholar] [CrossRef]
- Diamond, S. Mercury porosimetry. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Palankar, N.; Ravi Shankar, A.U.; Mithun, B.M. Durability studies on eco-friendly concrete mixes incorporating steel slag as coarse aggregates. J. Clean. Prod. 2016, 129, 437–448. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look. Part 2: Proposed mechanisms. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- El Sokkary, T.; Assal, H.; Kandeel, A. Effect of silica fume or granulated slag on sulphate attack of ordinary portland and alumina cement blend. Ceram. Int. 2004, 30, 133–138. [Google Scholar] [CrossRef]
- Çakır, Ö.; Aköz, F. Effect of curing conditions on the mortars with and without GGBFS. Constr. Build. Mater. 2008, 22, 308–314. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Acceleration of the reactivity of fly ash by chemical activation. Cem. Concr. Res. 1995, 25, 15–21. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lee, H.K.; Lee, K.M. Strength and microstructural characteristics of chemically activated fly ash–cement systems. Cem. Concr. Res. 2003, 33, 425–431. [Google Scholar] [CrossRef]
- Leng, F.; Feng, N.; Lu, X. An experimental study on the properties of resistance to diffusion of chloride ions of fly ash and blast furnace slag concrete. Cem. Concr. Res. 2000, 30, 989–992. [Google Scholar] [CrossRef]
- Nochaiya, T.; Wongkeo, W.; Chaipanich, A. Utilization of fly ash with silica fume and properties of Portland cement–fly ash–silica fume concrete. Fuel 2010, 89, 768–774. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, M.; Zhu, J. Damage evolution in cement mortar due to erosion of sulphate. Corros. Sci. 2008, 50, 2478–2483. [Google Scholar] [CrossRef]

| Element | Kg/m3 |
|---|---|
| Cement | 1270 |
| Distilled water | 635 |
| Technique | Exposure Medium | Type of Sample | Exposure Time | Samples Tested | Standard |
|---|---|---|---|---|---|
| Impedance spectroscopy | 15 wt % Na2SO4 | Disks 10 cm diameter and 2 cm thickness | Up to 120 days | 5 | n/a |
| Distilled water | Disks 10 cm diameter and 1 cm thickness | Up to 90 days | 5 | n/a | |
| Electrical resistivity | 15 wt % Na2SO4 | Cylinders 30 cm height and 7.5 cm diameter | Up to 120 days | 4 | UNE 83988-2 |
| Distilled water | Cylinders 30 cm height and 15 cm diameter | Up to 90 days | 4 | UNE 83988-2 | |
| Mercury intrusion porosimetry | 15 wt % Na2SO4 | Pieces of disks 10 cm diameter and 2 cm thickness | 28, 60, 90 and 120 days | 2 | n/a |
| Distilled water | Pieces of disks 10 cm diameter and 1 cm thickness | 2, 28 and 90 days | 2 | n/a | |
| Compressive strength | 15 wt % Na2SO4 | Prisms 4 cm × 4 cm × 16 cm | 28 days | 3 | UNE-EN 196-1 |
| Distilled water | Prisms 4 cm × 4 cm × 16 cm | 28 days | 3 | UNE-EN 196-1 |
| Cement Type | Exposure Medium | |
|---|---|---|
| Distilled Water | 15% Na2SO4 | |
| CEM I | 49.7 MPa (1.56) | 43 MPa (2.65) |
| CEM III | 42.7 MPa (2.23) | 38.7 MPa (2.08) |
| CEM IV | 31.1 MPa (1.88) | 30.1 MPa (1.39) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega Álvarez, J.M.; Esteban Pérez, M.D.; Rodríguez Escribano, R.R.; Pastor Navarro, J.L.; Sánchez Martín, I. Microstructural Effects of Sulphate Attack in Sustainable Grouts for Micropiles. Materials 2016, 9, 905. https://doi.org/10.3390/ma9110905
Ortega Álvarez JM, Esteban Pérez MD, Rodríguez Escribano RR, Pastor Navarro JL, Sánchez Martín I. Microstructural Effects of Sulphate Attack in Sustainable Grouts for Micropiles. Materials. 2016; 9(11):905. https://doi.org/10.3390/ma9110905
Chicago/Turabian StyleOrtega Álvarez, José Marcos, María Dolores Esteban Pérez, Raúl Rubén Rodríguez Escribano, José Luís Pastor Navarro, and Isidro Sánchez Martín. 2016. "Microstructural Effects of Sulphate Attack in Sustainable Grouts for Micropiles" Materials 9, no. 11: 905. https://doi.org/10.3390/ma9110905
APA StyleOrtega Álvarez, J. M., Esteban Pérez, M. D., Rodríguez Escribano, R. R., Pastor Navarro, J. L., & Sánchez Martín, I. (2016). Microstructural Effects of Sulphate Attack in Sustainable Grouts for Micropiles. Materials, 9(11), 905. https://doi.org/10.3390/ma9110905









