Enhanced Visible Light Photocatalytic Degradation of Organic Pollutants over Flower-Like Bi2O2CO3 Dotted with Ag@AgBr
Abstract
:1. Introduction
2. Experimental
2.1. Photocatalyst Synthesis
2.2. Photocatalyst Characterization
2.3. Photocatalytic Activity
2.4. Preparation of the Ag@AgBr/Bi2O2CO3 Film Electrode
3. Results and Discussion
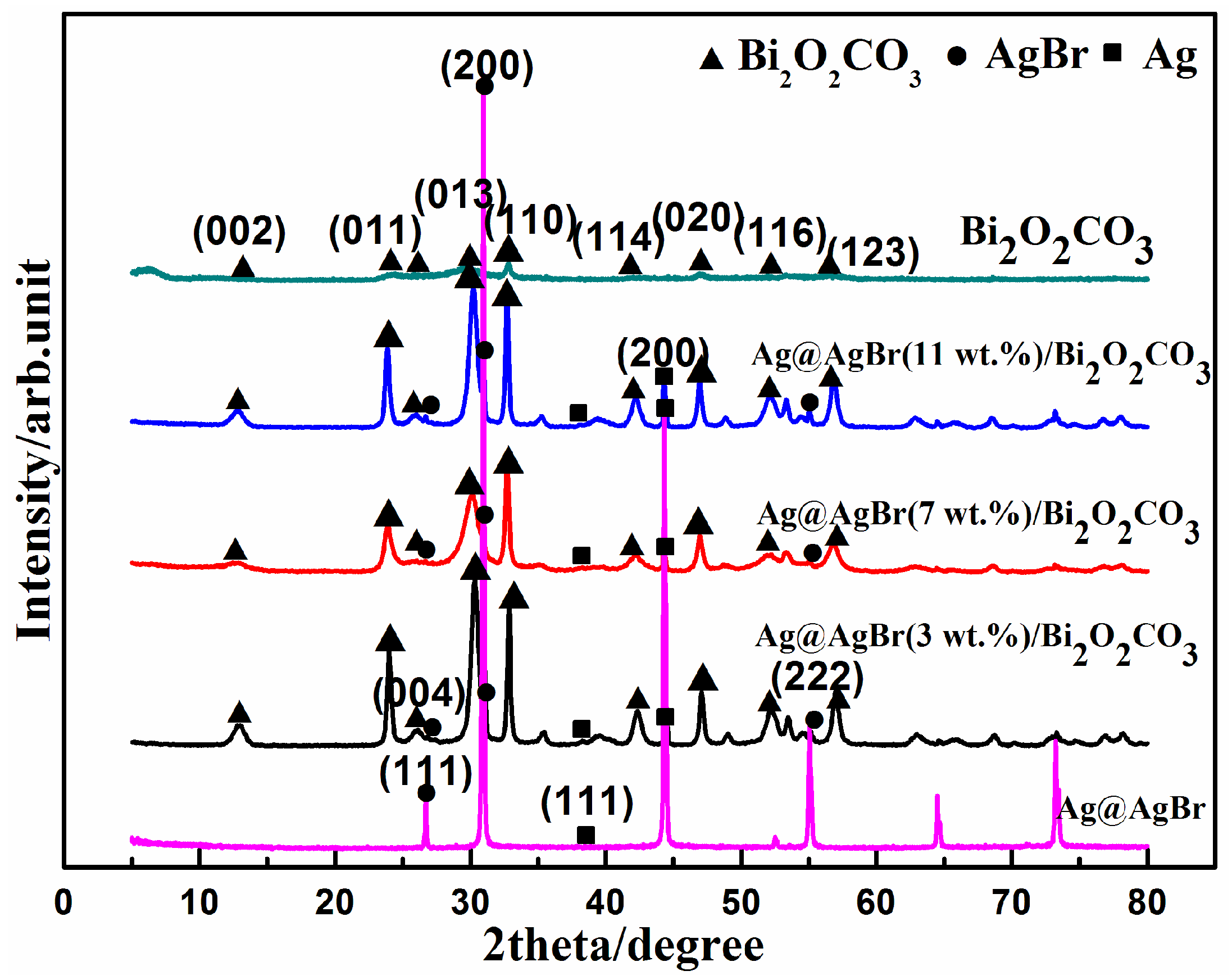
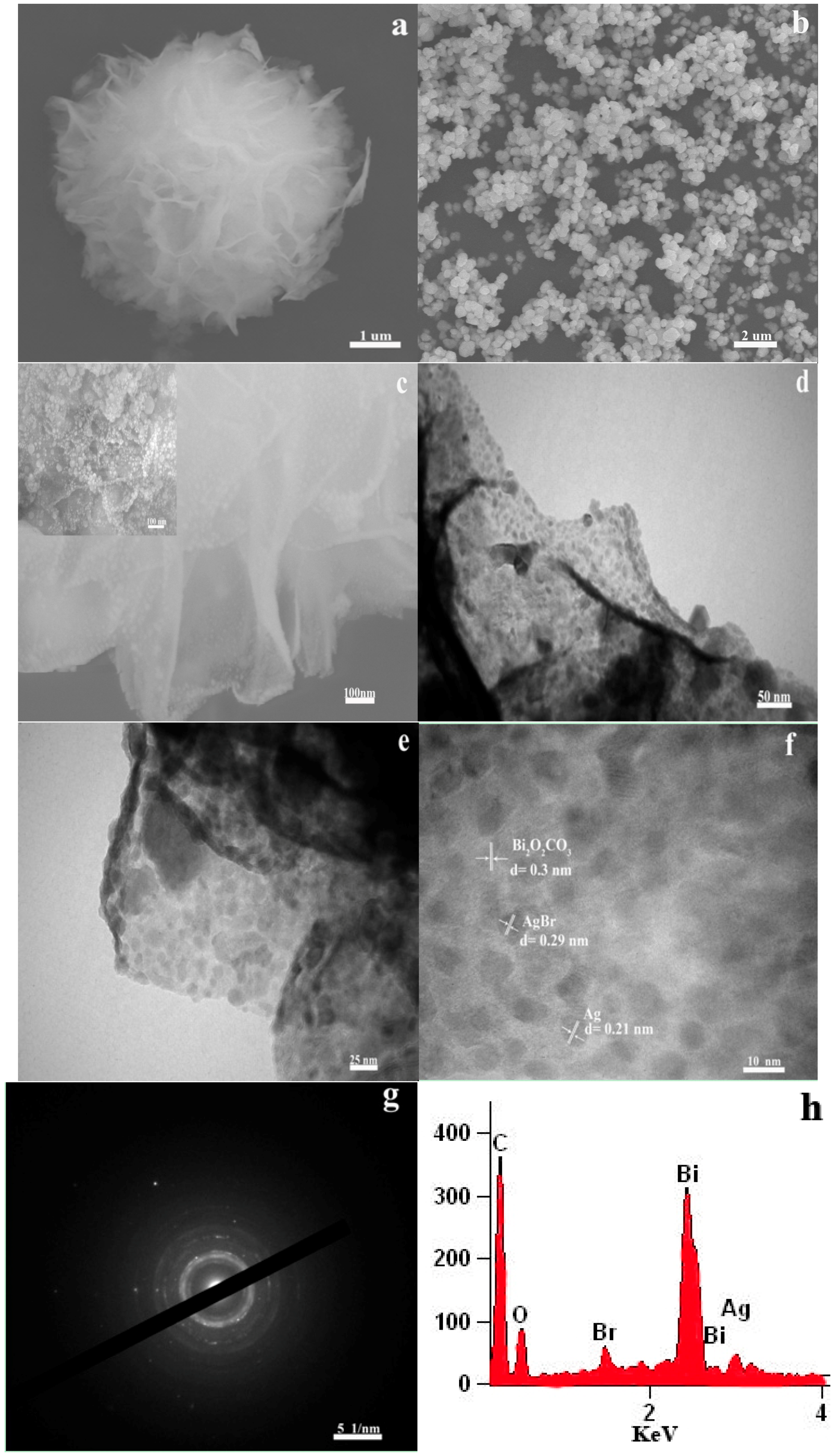
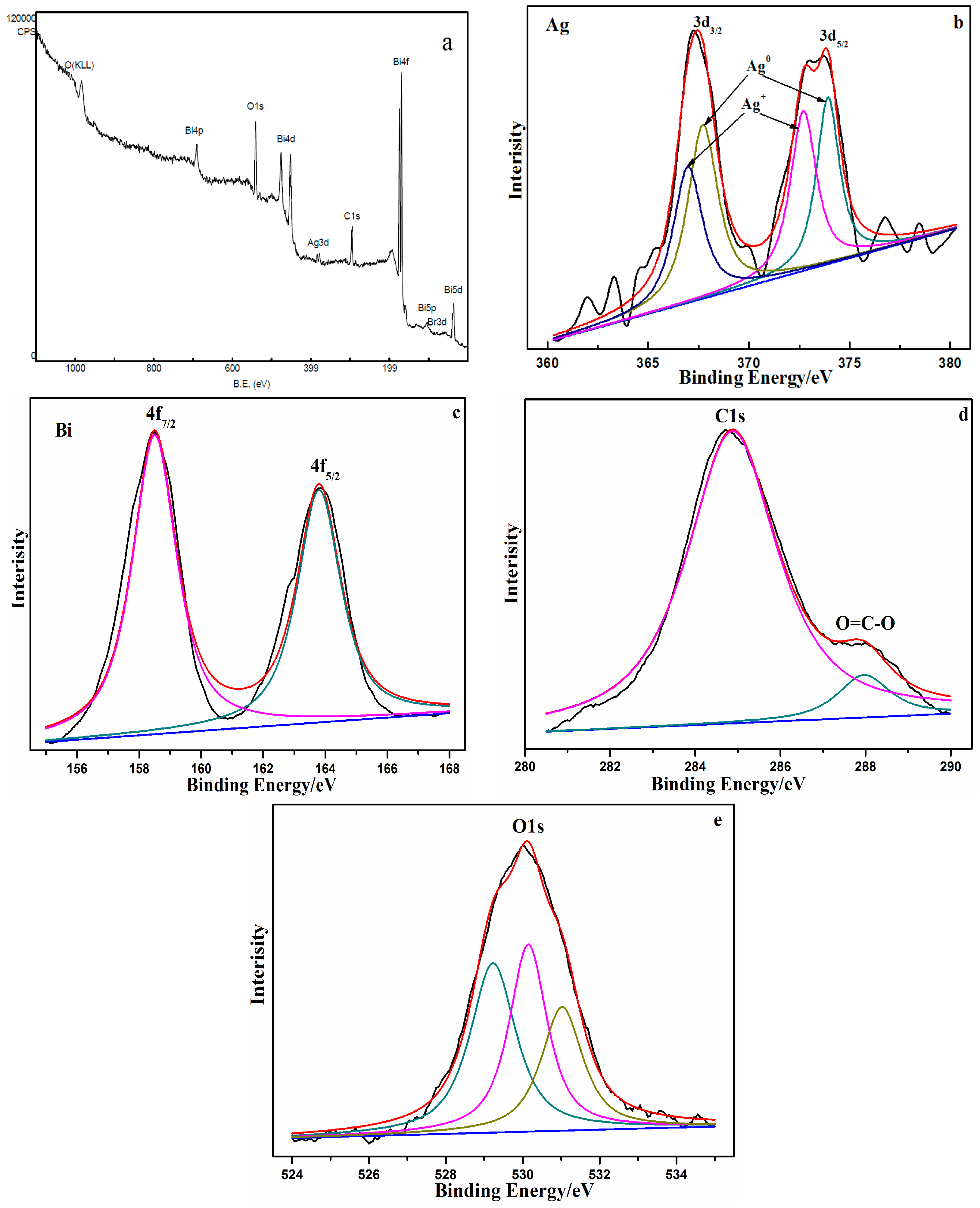
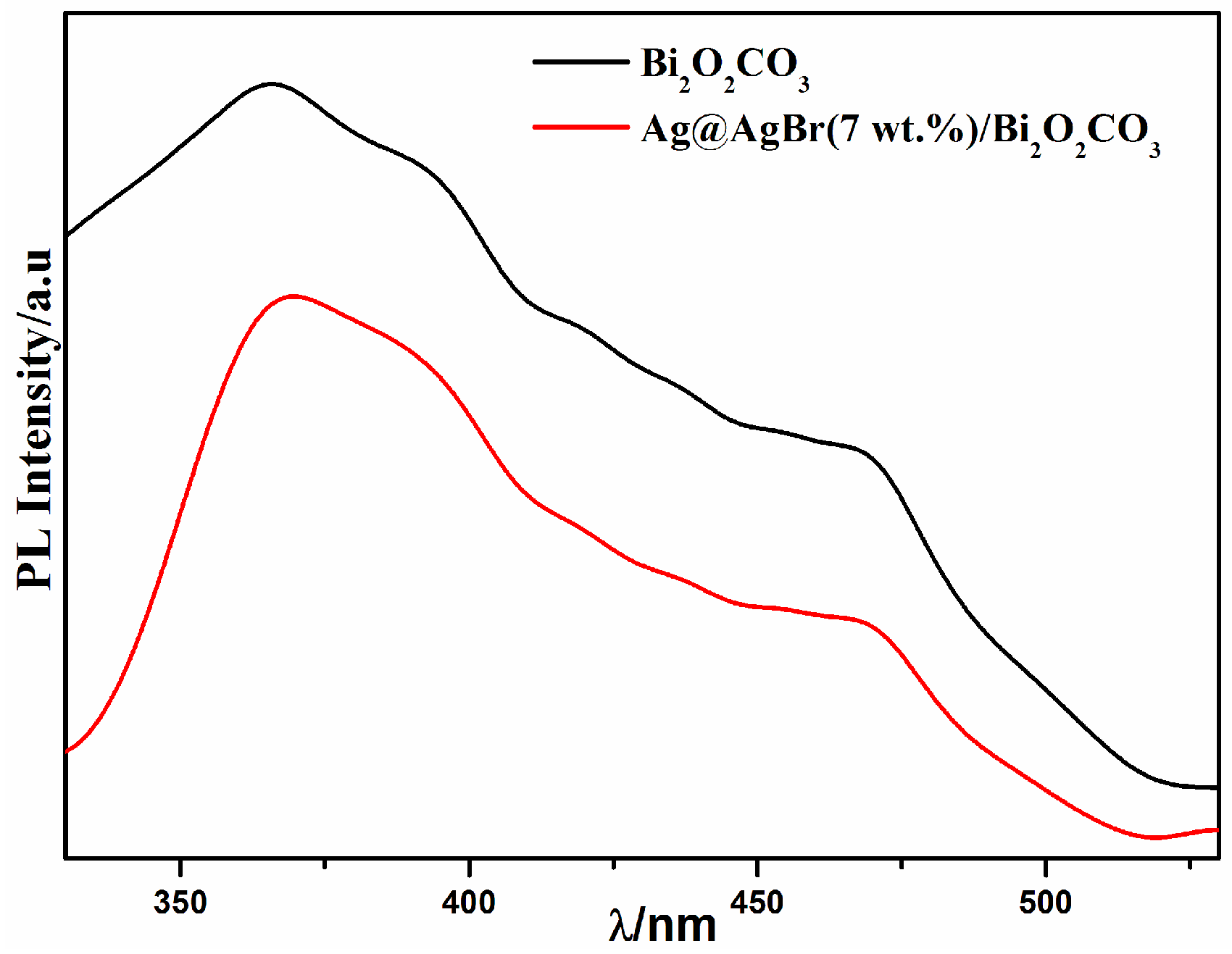
3.1. Catalyst Characterization
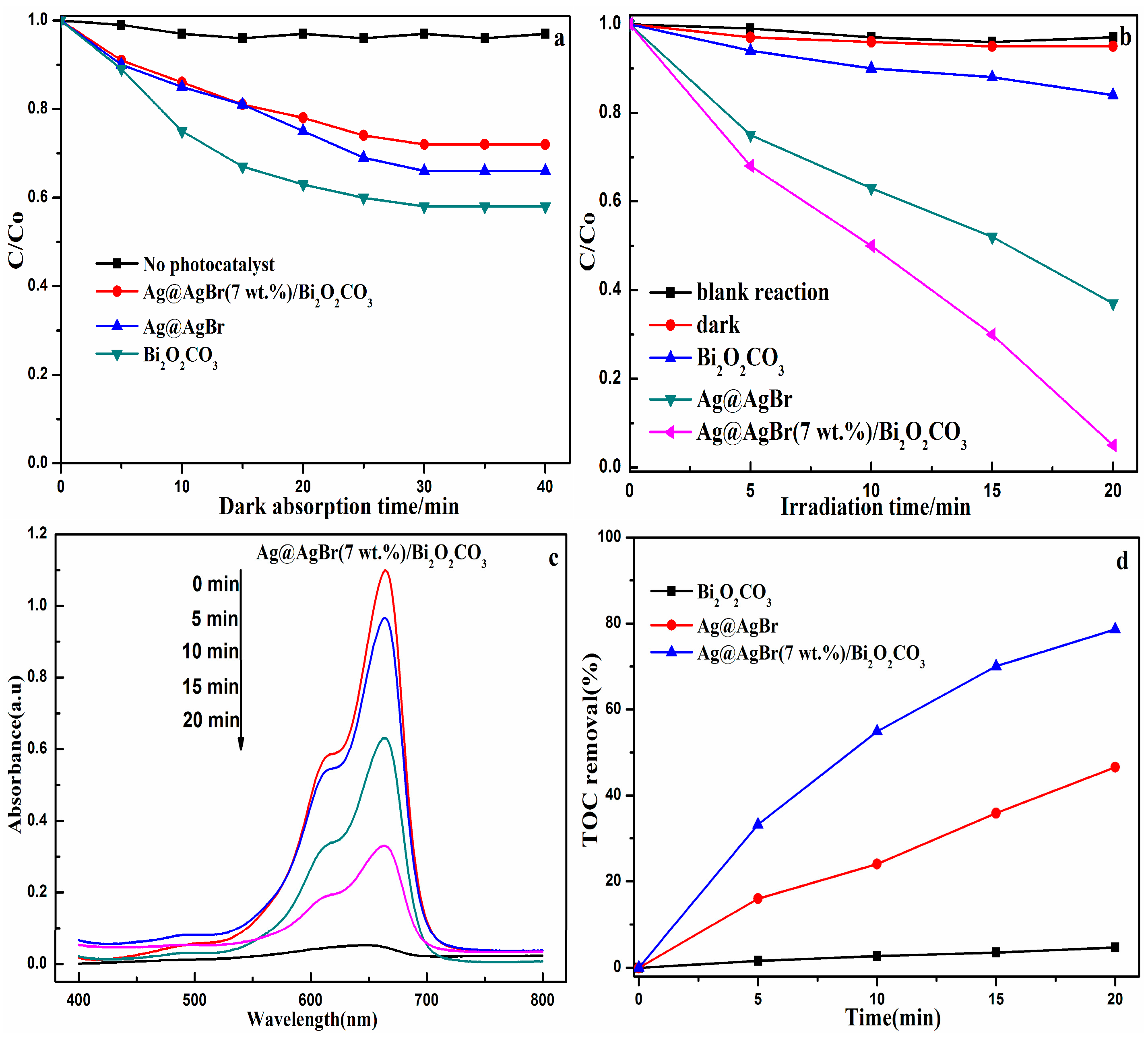
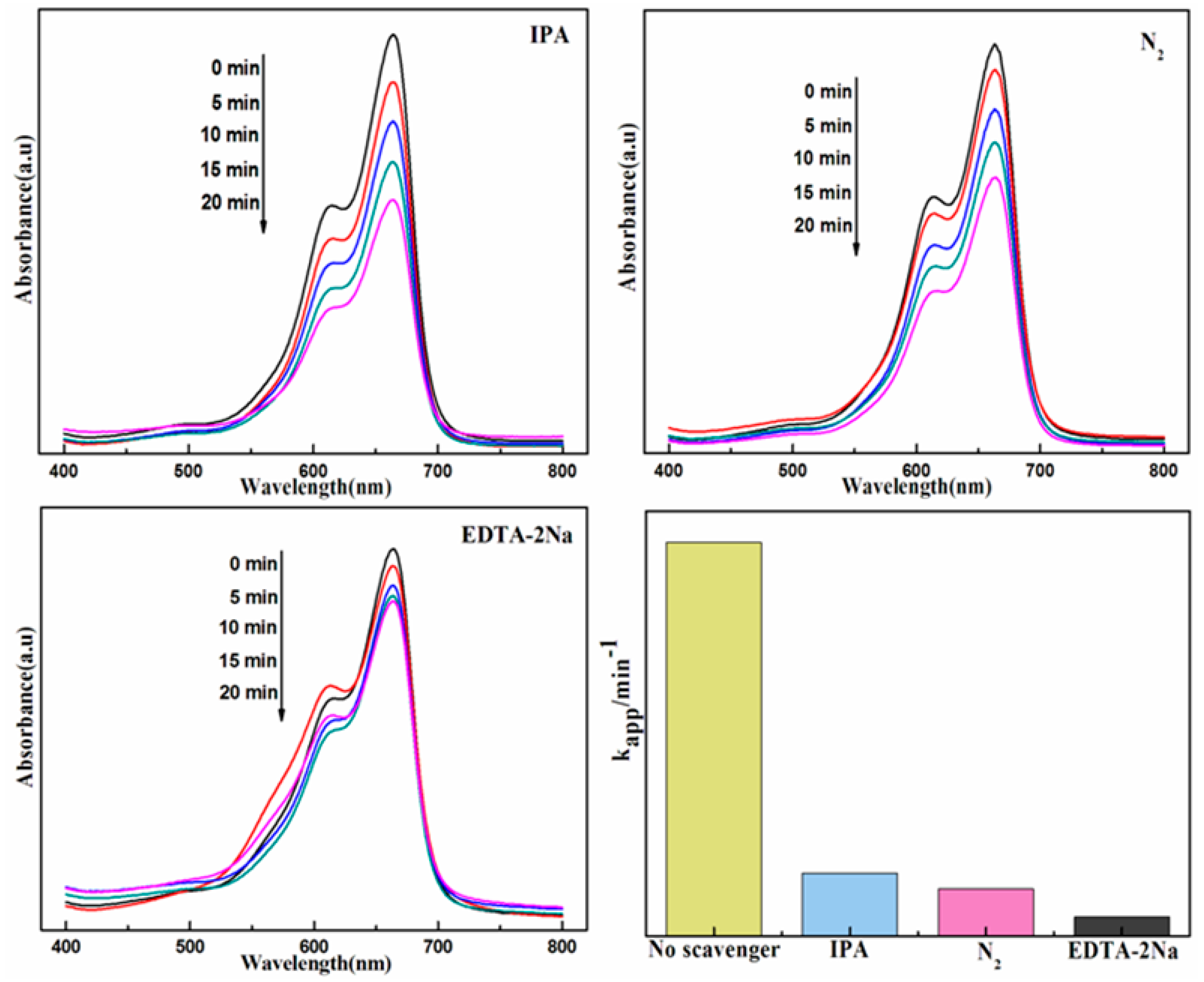
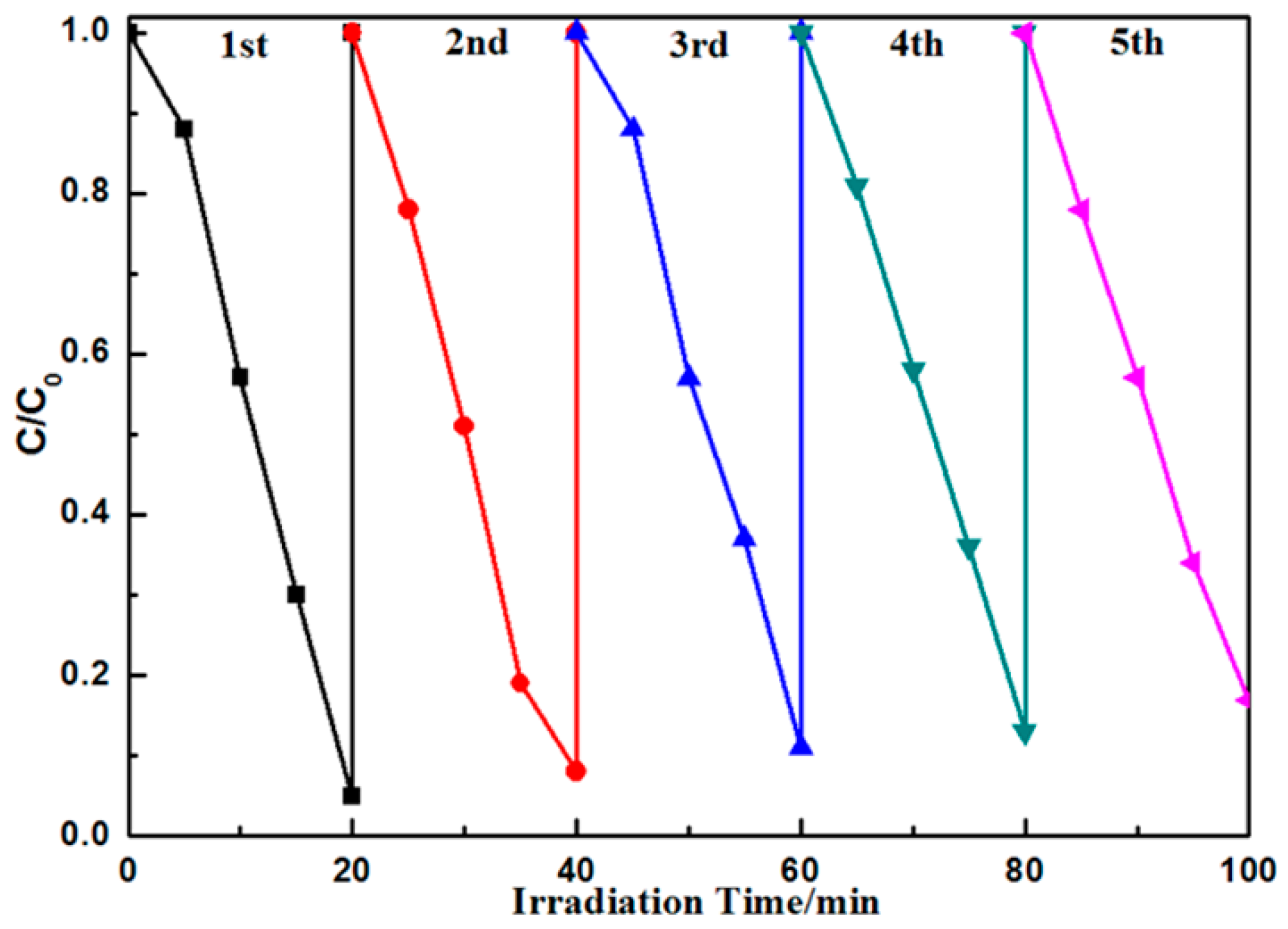
3.2. Photocatalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, P.; Huang, B.B.; Dai, Y.; Whangbo, M.H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. 2012, 14, 9813–9825. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, P.; Dong, S.J. Progress in graphene-based photoactive nanocomposites as a promising class of photocatalyst. Nanoscale 2012, 4, 5814–5825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.H.; Jin, J.; Zhang, J.; Lin, Z.; Huang, F.; Yu, J.G. Efficient Visible Light Photocatalytic Hydrogen Evolution and Enhanced Photostability of Core/Shell CdS/g-C3N4 Nanowires. ACS. Appl. Mater. Interfaces 2013, 5, 10317–10324. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. Z-scheme water splitting using two different semiconductor photocatalysts. ACS Catal. 2013, 3, 1486–1501. [Google Scholar] [CrossRef]
- Shi, R.; Huang, G.; Lin, J.; Zhu, Y.F. Photocatalytic Activity Enhancement for Bi2WO6 by Fluorine Substitution. J. Phys. Chem. C. 2009, 113, 19633–19638. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Sweena, R.; Wu, J.J.; Anandan, S. Synthesis of CuO-ZnO nanophotocatalyst for visible light assisted degradation of a textile dye in aqueous solution. Chem. Eng. J. 2011, 171, 136–140. [Google Scholar] [CrossRef]
- Huang, H.W.; Wang, S.B.; Tian, N.; Zhang, Y.H. One-step hydrothermal preparation strategy for layered BiIO4/Bi2WO6 heterojunctions with enhanced visible light photocatalytic activities. RSC Adv. 2014, 4, 5561–5567. [Google Scholar] [CrossRef]
- Ji, Y.X.; Cao, J.F.; Jiang, L.Q.; Zhang, Y.H.; Yi, Z.G. g-C3N4/BiVO4 composites with enhanced and stable visible light photocatalytic activity. J. Alloys Compd. 2014, 590, 9–14. [Google Scholar] [CrossRef]
- Wang, T.; Jiao, Z.B.; Chen, T.; Li, Y.W.; Ren, W.; Lin, S.L.; Lu, G.X.; Ye, J.H.; Bi, Y.P. Vertically aligned ZnO nanowire arrays tip-grafted with silver nanoparticles for photoelectrochemical applications. Nanoscale 2013, 5, 7552–7557. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; So, M.H.; Yang, J.; Deng, F.; Che, C.M.; Sun, H.Z. Fabrication of bismuth subcarbonate nanotube arrays from bismuth citrate. Chem. Commun. 2006, 226, 2265–2267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Huang, B.B.; Yang, K.S.; Wang, Z.Y.; Qin, X.Y.; Zhang, X.Y.; Dai, Y. Facile template-free synthesis of Bi2O2CO3 hierarchical microflowers and their associated photocatalytic activity. Chem. Phys. Chem. 2010, 11, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, Y.; Sugimoto, W.; Sugahara, Y. Intercalation behavior of n-alkylamines into a protonated from of a layered perovskite derived from aurivilius phase Bi2SrTa2O9. Chem. Mater. 2003, 15, 632–635. [Google Scholar] [CrossRef]
- Huang, H.W.; Wang, J.J.; Dong, F.; Guo, Y.X.; Tian, N.; Zhang, Y.H.; Zhang, T.R. Highly Efficient Bi2O2CO3 Single-Crystal Lamellas with Dominantly Exposed {001} Facets. Cryst. Growth Des. 2015, 15, 534–537. [Google Scholar] [CrossRef]
- Huang, H.W.; Tian, N.; Jin, S.F.; Zhang, Y.H.; Wang, S.B. Syntheses, characterization and nonlinear optical properties of a bismuth subcarbonate Bi2O2CO3. Solid State Sci. 2014, 30, 1–5. [Google Scholar] [CrossRef]
- Fan, X.Y.; Zang, L.; Zhang, M.; Qiu, H.S.; Wang, Z.; Yin, J.; Jia, H.Z.; Pan, S.L.; Wang, C.Y. A Bulk Boron-Based Photocatalyst for Efficient Dechlorination: K3B6O10Br. Chem. Mater. 2014, 26, 3169–3174. [Google Scholar] [CrossRef]
- Cao, X.F. Persimmon-like (BiO)2CO3 microstructures: Hydrothermal preparation, photocatalytic properties and their conversion into Bi2S3. CrystEngComm 2011, 13, 1939–1945. [Google Scholar] [CrossRef]
- Liu, S.Q.; Tu, Y.Q.; Dai, G.P. The effects of citrate ion on morphology and photocatalytic activity of flower-like Bi2O2CO3. Ceram. Int. 2014, 40, 2343–2348. [Google Scholar] [CrossRef]
- Madhusudan, P.; Ran, J.R.; Zhang, J.; Yu, J.G.; Liu, G. Novel urea assisted hydrothermal synthesis of hierarchical BiVO4/Bi2O2CO3 nanocomposites with enhanced visible-light photocatalytic activity. Appl. Catal. B 2011, 110, 286–295. [Google Scholar] [CrossRef]
- Liang, N.; Wang, M.; Jin, L.; Huang, S.S.; Chen, W.L.; Xu, M.; He, Q.Q.; Zai, J.T.; Fang, N.H.; Qian, X.F. Highly efficient Ag2O/Bi2O2CO3 p-n heterojunction photocatalysts with improved visible-light responsive activity. ACS Appl. Mater. Interfaces 2014, 6, 11698–11705. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Zai, J.T.; Xu, M.; Zhu, Q.; Wei, X.; Qian, X.F. Novel Bi2S3/Bi2O2CO3 heterojunction photocatalysts with enhanced visible light responsive activity and wastewater treatment. J. Mater. Chem. A 2014, 2, 4208–4216. [Google Scholar] [CrossRef]
- Zhang, W.D.; Sun, Y.J.; Dong, F.; Zhang, W.; Duan, S.; Zhang, Q. Facile synthesis of organic-inorganic layered nanojunctions of g-C3N4/(BiO)2CO3 as efficient visible light photocatalyst. Dalton Trans. 2014, 43, 12026–12036. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Huang, H.W.; Guo, Y.X.; He, Y.; Zhang, Y.H.A. A g-C3N4/Bi2O2CO3 composite with high visible-light-driven photocatalytic activity for rhodamine B degradation. Appl. Surf. Sci. 2014, 322, 249–254. [Google Scholar] [CrossRef]
- Zhang, X.C.; Guo, T.Y.; Wang, X.W.; Wang, Y.W.; Fan, C.M.; Zhang, H. Facile composition-controlled preparation and photocatalytic application of BiOCl/Bi2O2CO3 nanosheets. Appl. Catal. B 2014, 150–151, 486–495. [Google Scholar] [CrossRef]
- Hu, D.D.; Zhang, K.Y.; Yang, Q.; Wang, M.J.; Xi, Y.; Hu, C.G. Super-high photocatalytic activity of Fe2O3 nanoparticles anchored on Bi2O2CO3 nanosheets with exposed {001} active facets. Appl. Surf. Sci. 2014, 316, 93–101. [Google Scholar] [CrossRef]
- Lu, H.J.; Xu, L.L.; Wei, B.; Zhang, M.Y.; Gao, H.; Sun, W.J. Enhanced photosensitization process induced by the p–n junction of Bi2O2CO3/BiOCl heterojunctions on the degradation of rhodamine B. Appl. Surf. Sci. 2013, 303, 360–366. [Google Scholar] [CrossRef]
- Song, P.Y.; Xu, M.; Zhang, W.D. Sodium citrate-assisted anion exchange strategy for construction of Bi2O2CO3/BiOI photocatalysts. Mater. Res. Bull. 2014, 62, 88–95. [Google Scholar] [CrossRef]
- Xu, Y.S.; Zhang, W.D. Anion exchange strategy for construction of sesame-biscuit-like Bi2O2CO3/Bi2MoO6 nanocomposites with enhanced photocatalytic activity. Appl. Catal. B 2013, 140, 306–316. [Google Scholar] [CrossRef]
- Madhusudan, P.; Yu, J.G.; Wang, W.G.; Cheng, B.; Liu, G. Facile synthesis of novel hierarchical graphene-Bi2O2CO3 composites with enhanced photocatalytic performance under visible light. Dalton Trans. 2012, 41, 14345–14353. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Sun, Y.J.; Fu, M.; Ho, W.K.; Lee, S.C.; Wu, Z.B. Novel in Situ N-Doped (BiO)2CO3 Hierarchical Microspheres Self-Assembled by Nanosheets as Efficient and Durable Visible Light Driven Photocatalyst. Langmuir 2012, 28, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xiong, T.; Wang, R.; Sun, Y.; Jiang, Y. Growth mechanism and photocatalytic activity of self-organized N-doped (BiO)2CO3 hierarchical nanosheet microspheres from bismuth citrate and urea. Dalton Trans. 2014, 43, 6631–6642. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Huang, H.W.; Sun, Y.J.; Dong, F.J. In situ synthesis of a C-doped (BiO)2CO3 hierarchical self-assembly effectively promoting visible light photocatalysis. Mater. Chem. A 2015, 3, 6118–6127. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.B.; Zhang, X.Y.; Qin, X.Y.; Jin, H.; Dai, Y.; Wang, Z.Y.; Wei, J.Y.; Zhan, J.; Wang, S.Y.; et al. Highly Efficient Visible Light Plasmonic Photocatalyst Ag@Ag(Br,I). Chemistry 2009, 15, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
- An, C.H.; Peng, S.; Sun, Y.G. Facile Synthesis of Sunlight-Driven AgCl:Ag Plasmonic Nanophotocatalyst. Adv. Mater. 2010, 22, 2570–2574. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.B.; Qin, X.Y.; Zhang, X.Y.; Dai, Y.; Wei, J.Y.; Whangbo, M.-H. Ag@AgCl: A Highly Efficient and Stable Photocatalyst Active under Visible Light. Angew. Chem. Int. Ed. 2008, 47, 7931–7933. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.S.; Chen, P.L.; Liu, M.H. Ag/AgBr/Graphene Oxide Nanocomposite Synthesized via Oil/Water and Water/Oil Microemulsions: A Comparison of Sunlight Energized Plasmonic Photocatalytic Activity. Langmuir 2012, 28, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zuo, F.; Ma, Q.; Wang, C.; Bartels, L.; Feng, P. Ag3PO4 Oxygen Evolution Photocatalyst Employing Synergistic Action of Ag/AgBr Nanoparticles and Graphene Sheets. J. Phys. Chem. C 2012, 116, 20132–20139. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Zhao, Q.; Chen, G.; Raston, C.L. Role of hydroxyl radicals and mechanism of Escherichia coli inactivation on Ag/AgBr/TiO2 nanotube array electrode under visible light irradiation. Environ. Sci. Technol. 2012, 46, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Lan, Y.; Qu, J.; Hu, X.; Wang, A. Ag/AgBr/TiO2 Visible Light Photocatalyst for Destruction of Azodyes and Bacteria. J. Phys. Chem. B. 2006, 110, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Wong, K.H.; Yip, H.Y.; Hu, C.; Yu, J.C.; Chan, C.Y.; Wong, P.K. Effective Photocatalytic Disinfection of E. coli K-12 Using AgBr-Ag-Bi2WO6 Nanojunction System Irradiated by Visible Light: The Role of Diffusing Hydroxyl Radicals. Environ. Sci. Technol. 2010, 44, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.S.; Chen, P.L.; Liu, M.H. Graphene oxide enwrapped Ag/AgX (X = Br, Cl) nanocomposite as a highly efficient visible-light plasmonic photocatalyst. ACS Nano 2011, 5, 4529–4536. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Liu, L.; Liang, Y.H.; Cui, W.Q.; Zhang, Z.S. Oil-in-Water Self-Assembled Synthesis of Ag@AgCl Nano-Particles on Flower-like Bi2O2CO3 with Enhanced Visible-Light-Driven Photocatalytic Activity. Materials 2016, 9, 486. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Yun, G.X.; Bai, Y.; An, N.; Lian, J.H.; Huang, H.H.; Su, B.T. Photodegradation of rhodamine B with MoS2/Bi2O2CO3 composites under UV light irradiation. Appl. Surf. Sci. 2014, 313, 537–544. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, H.G.; Cheng, B.; Zhao, X.J.; Yu, J.C.; Ho, W.K. The effects of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Zhuang, J.D.; Dai, W.X.; Tian, Q.F.; Li, Z.H.; Xie, L.Y.; Wang, J.X.; Liu, P. Photocatalytic degradation of RhB over TiO2 bilayer films: Effect of defects and their location. Langmuir 2010, 26, 9686–9694. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B Environ. 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Mayer, M.T.; Du, C.; Wang, D. Hematite/Si Nanowire Dual-Absorber System for Photoelectrochemical Water Splitting at Low Applied Potentials. J. Am. Chem. Soc. 2012, 134, 12406–12409. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, Y.; Mayer, M.T.; Simpson, Z.I.; McMahon, G.; Zhou, S.; Wang, D. Growth of p-Type Hematite by Atomic Layer Deposition and Its Utilization for Improved Solar Water Splitting. J. Am. Chem. Soc. 2012, 134, 5508–5511. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.J.; Wang, Y.; Zhang, Q.H.; Deng, W.P.; Wang, Y. MgO- and Pt-Promoted TiO2 as an Efficient Photocatalyst for the Preferential Reduction of Carbon Dioxide in the Presence of Water. ACS Catal. 2014, 4, 3644–3653. [Google Scholar] [CrossRef]
- Zhai, Q.G.; Xie, S.J.; Fan, W.Q.; Zhang, Q.H.; Wang, Y.; Deng, W.P.; Wang, Y. Photocatalytic Conversion of Carbon Dioxide with Water into Methane: Platinum and Copper(I) Oxide Co-catalysts with a Core–Shell Structure. Angew. Chem. Int. Ed. 2013, 125, 5888–5891. [Google Scholar] [CrossRef]
- Li, G.; Wang, F.; Jiang, Q.; Gao, X.; Shen, P. Carbon Nanotubes with Titanium Nitride as a Low-Cost Counter-Electrode Material for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2010, 49, 3653–3656. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.X.; Bi, Y.P.; Umezawa, N.; Oshikiri, M.; Ye, J.H. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.H.; Lin, S.L.; Liu, L.; Hu, J.S.; Cui, W.Q. Synthesis and photocatalytic performance of an efficient Ag@AgBr/K2Ti4O9 composite photocatalyst under visible light. Mater. Res. Bull. 2014, 56, 25–33. [Google Scholar] [CrossRef]
- Jin, L.; Zhu, G.Q.; Hojamberdiev, M.; Luo, X.C.; Tan, C.W.; Peng, J.H.; Wei, X.M.; Li, J.P.; Liu, P. A Plasmonic Ag-AgBr/Bi2O2CO3 Composite Photocatalyst with Enhanced Visible-Light Photocatalytic Activity. Ind. Eng. Chem. Res. 2014, 53, 13718–13727. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Bai, H.W.; Wang, Y.J.; Liu, Z.Y.; Zhang, X.W.; Sun, D.D. Self-assembling TiO2 nanorods on large graphene oxide sheets at a two-phase interface and their anti-recombination in photocatalytic applications. Adv. Funct. Mater. 2010, 20, 4175–4181. [Google Scholar] [CrossRef]


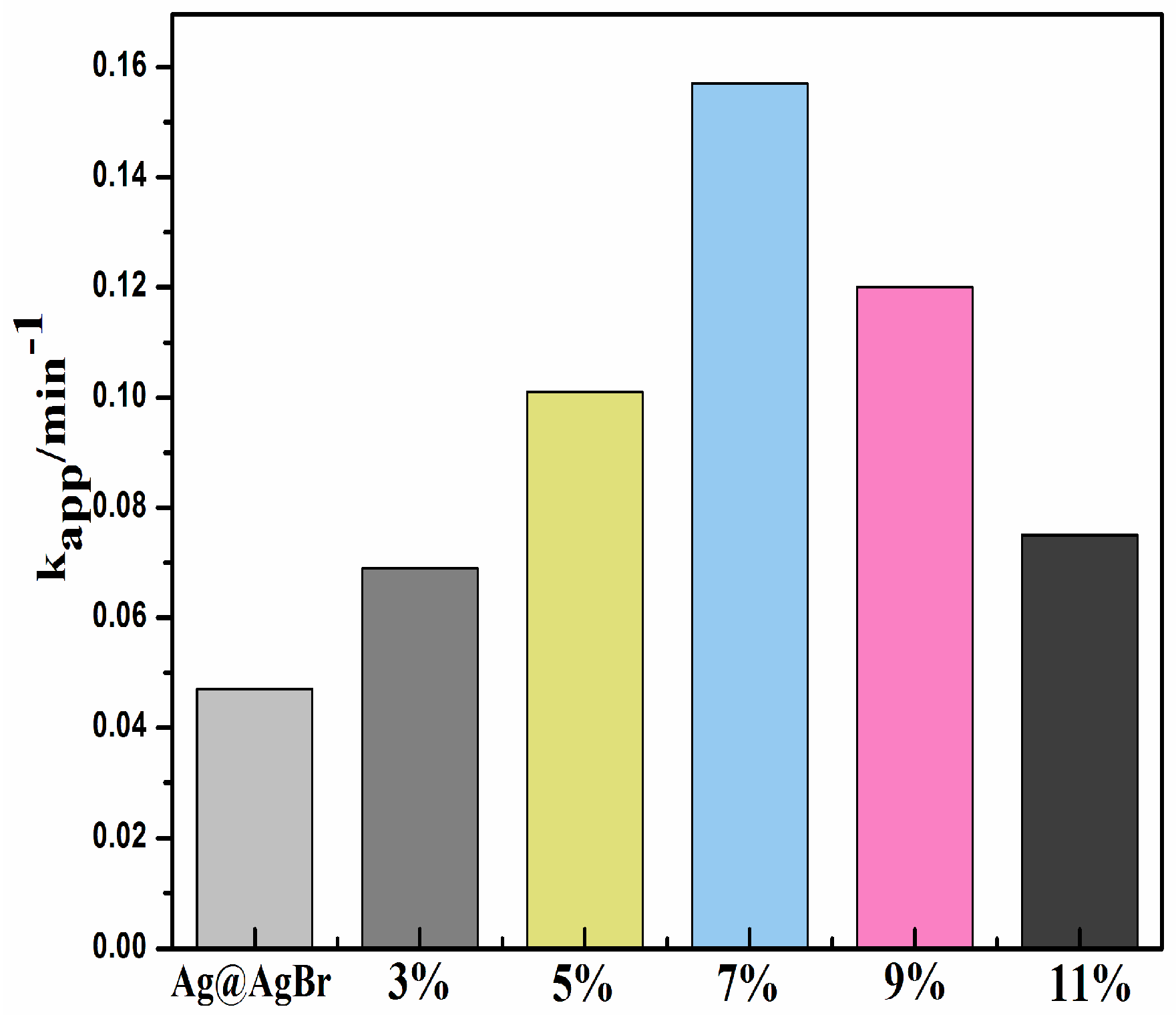
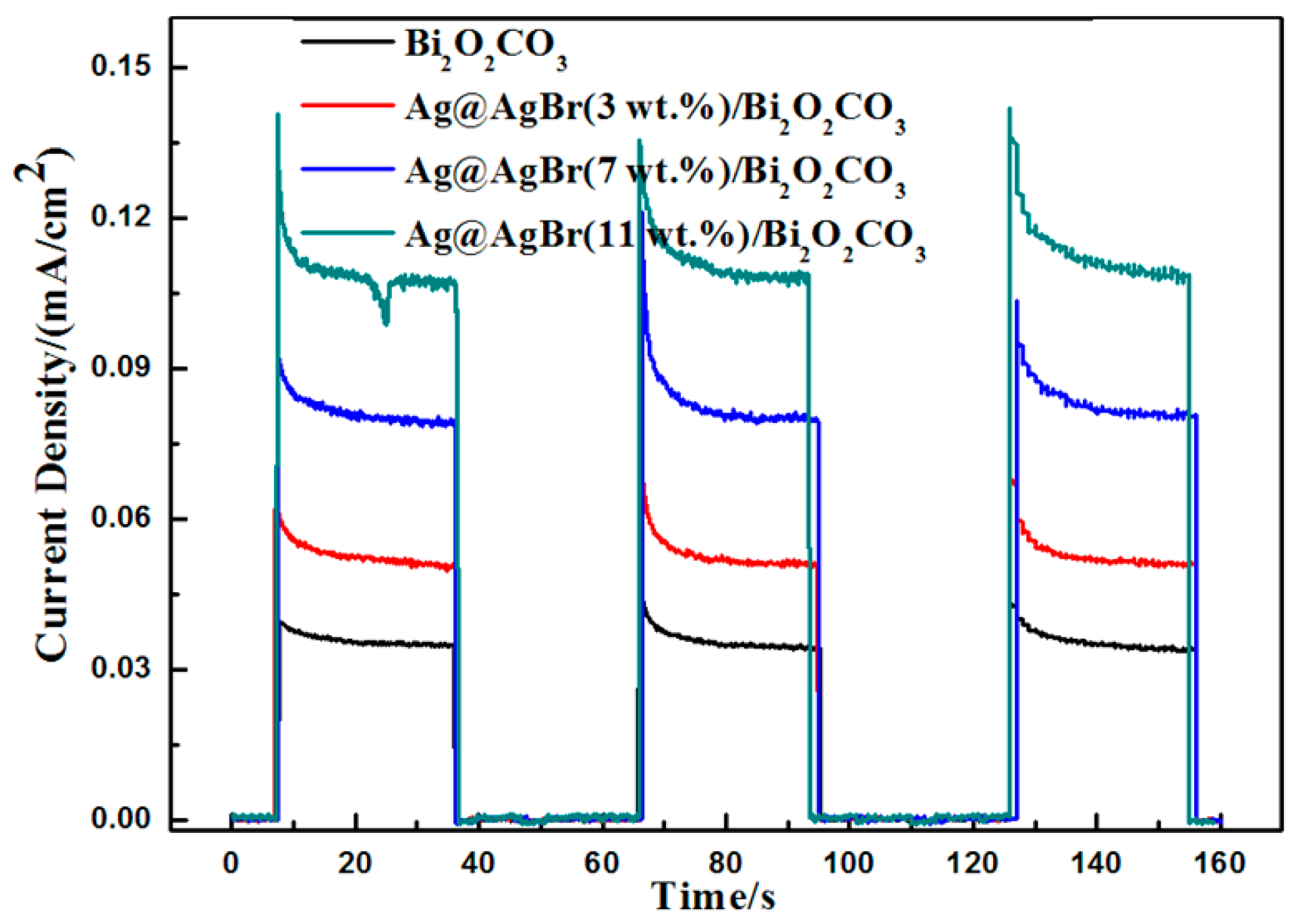
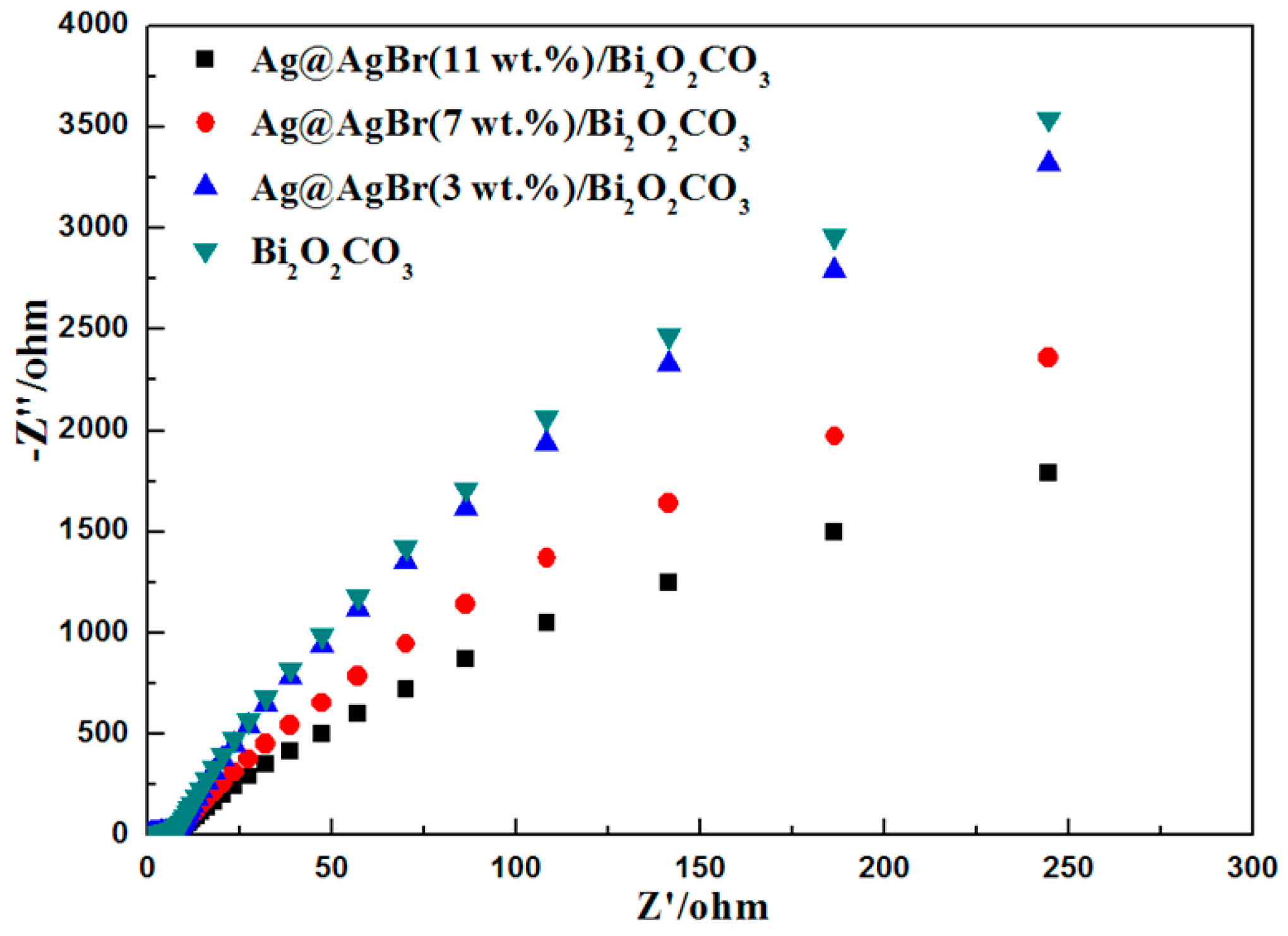

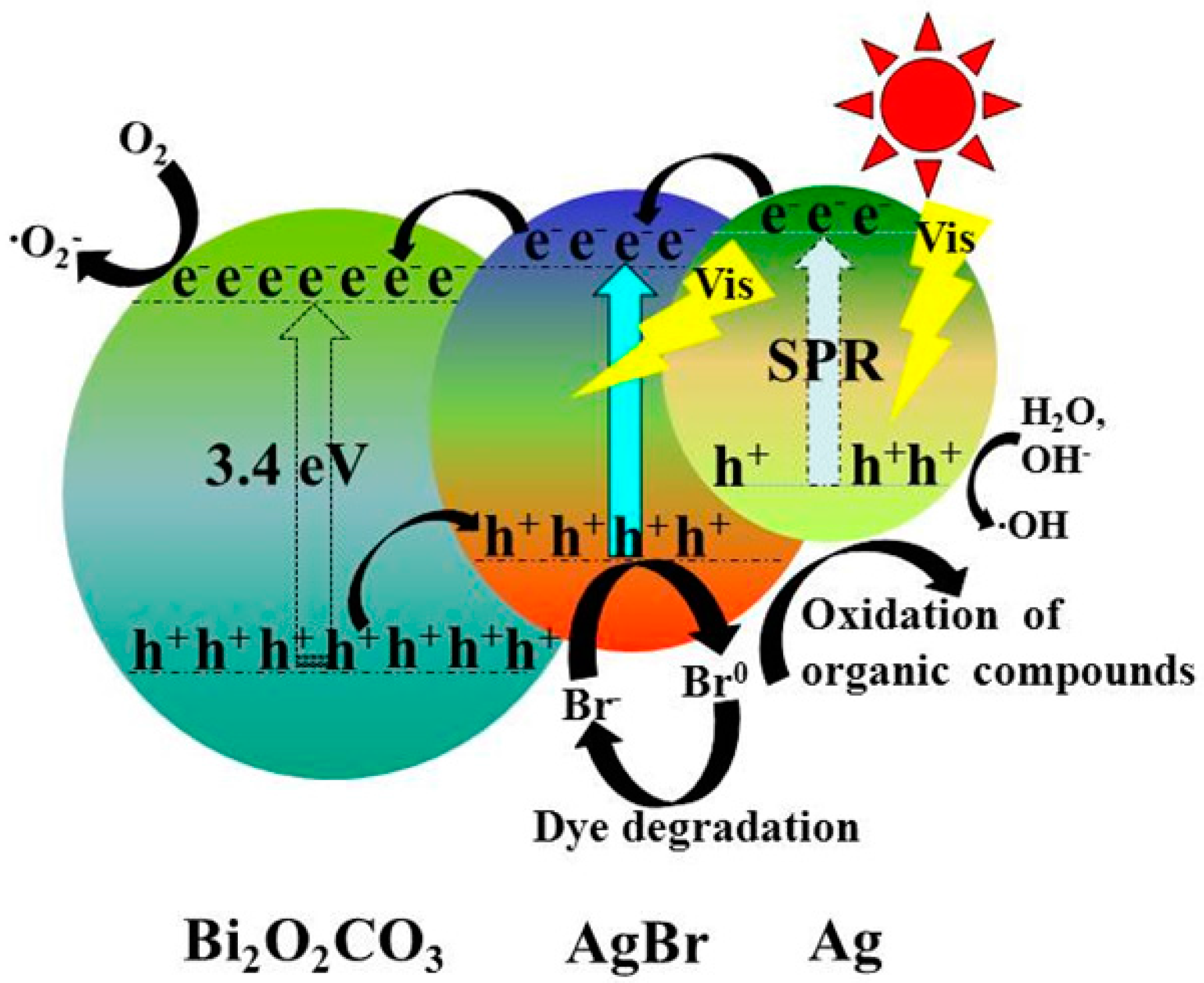
| Photocatalyst | Bi2O2CO3 | Ag@AgBr (3 wt %)/Bi2O2CO3 | Ag@AgBr (5 wt %)/Bi2O2CO3 | Ag@AgBr (7 wt %)/Bi2O2CO3 | Ag@AgBr (9 wt %)/Bi2O2CO3 | Ag@AgBr (11 wt %)/Bi2O2CO3 |
|---|---|---|---|---|---|---|
| Surface area/m2·g−1 | 12.59 | 15.15 | 18.1 | 22.33 | 25.16 | 28.91 |
| Average pore size/nm | 21.15 | 21.43 | 21.64 | 22.02 | 21.75 | 21.25 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Wang, M.; Liu, L.; Liang, Y.; Cui, W.; Zhang, Z.; Yun, N. Enhanced Visible Light Photocatalytic Degradation of Organic Pollutants over Flower-Like Bi2O2CO3 Dotted with Ag@AgBr. Materials 2016, 9, 882. https://doi.org/10.3390/ma9110882
Lin S, Wang M, Liu L, Liang Y, Cui W, Zhang Z, Yun N. Enhanced Visible Light Photocatalytic Degradation of Organic Pollutants over Flower-Like Bi2O2CO3 Dotted with Ag@AgBr. Materials. 2016; 9(11):882. https://doi.org/10.3390/ma9110882
Chicago/Turabian StyleLin, Shuanglong, Miao Wang, Li Liu, Yinghua Liang, Wenquan Cui, Zisheng Zhang, and Nan Yun. 2016. "Enhanced Visible Light Photocatalytic Degradation of Organic Pollutants over Flower-Like Bi2O2CO3 Dotted with Ag@AgBr" Materials 9, no. 11: 882. https://doi.org/10.3390/ma9110882





