Inactivated Sendai Virus (HVJ-E) Immobilized Electrospun Nanofiber for Cancer Therapy
Abstract
:1. Introduction
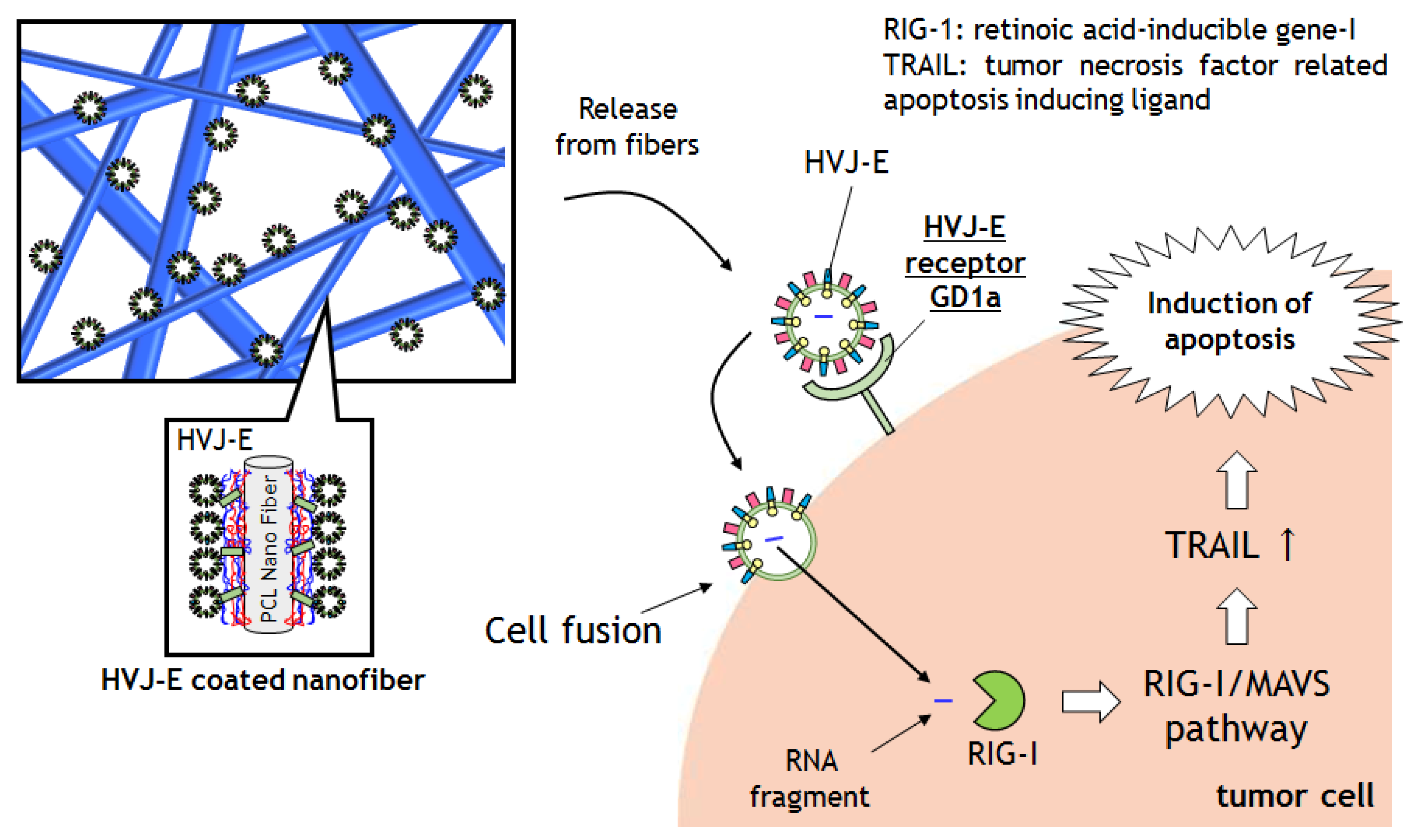
2. Results and Discussion
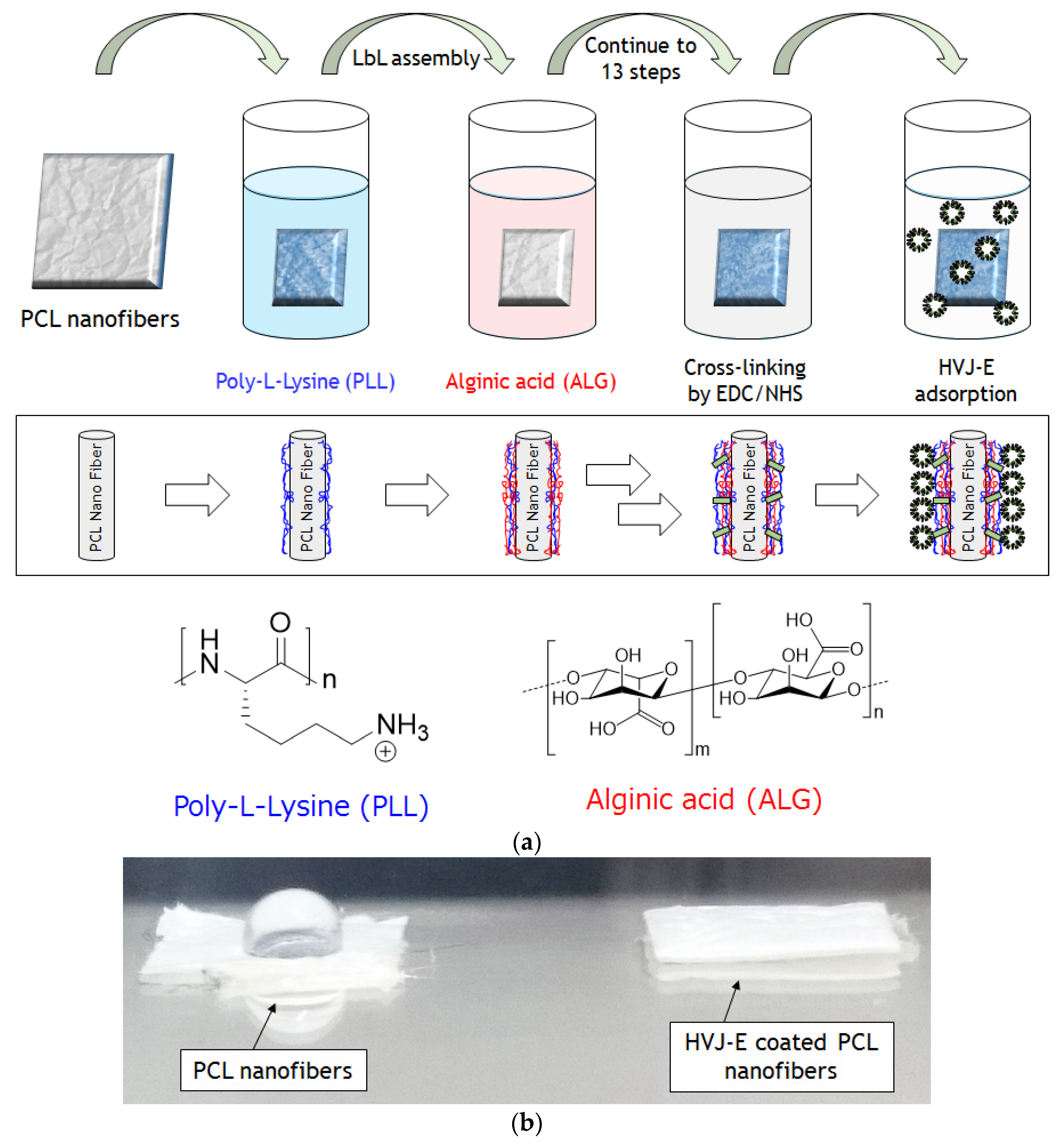
2.1. Preparations of HVJ-E Immobilized PCL Nanofibers via LbL Method
2.2. Characterizations of HVJ-E Coated PCL Nanofibers
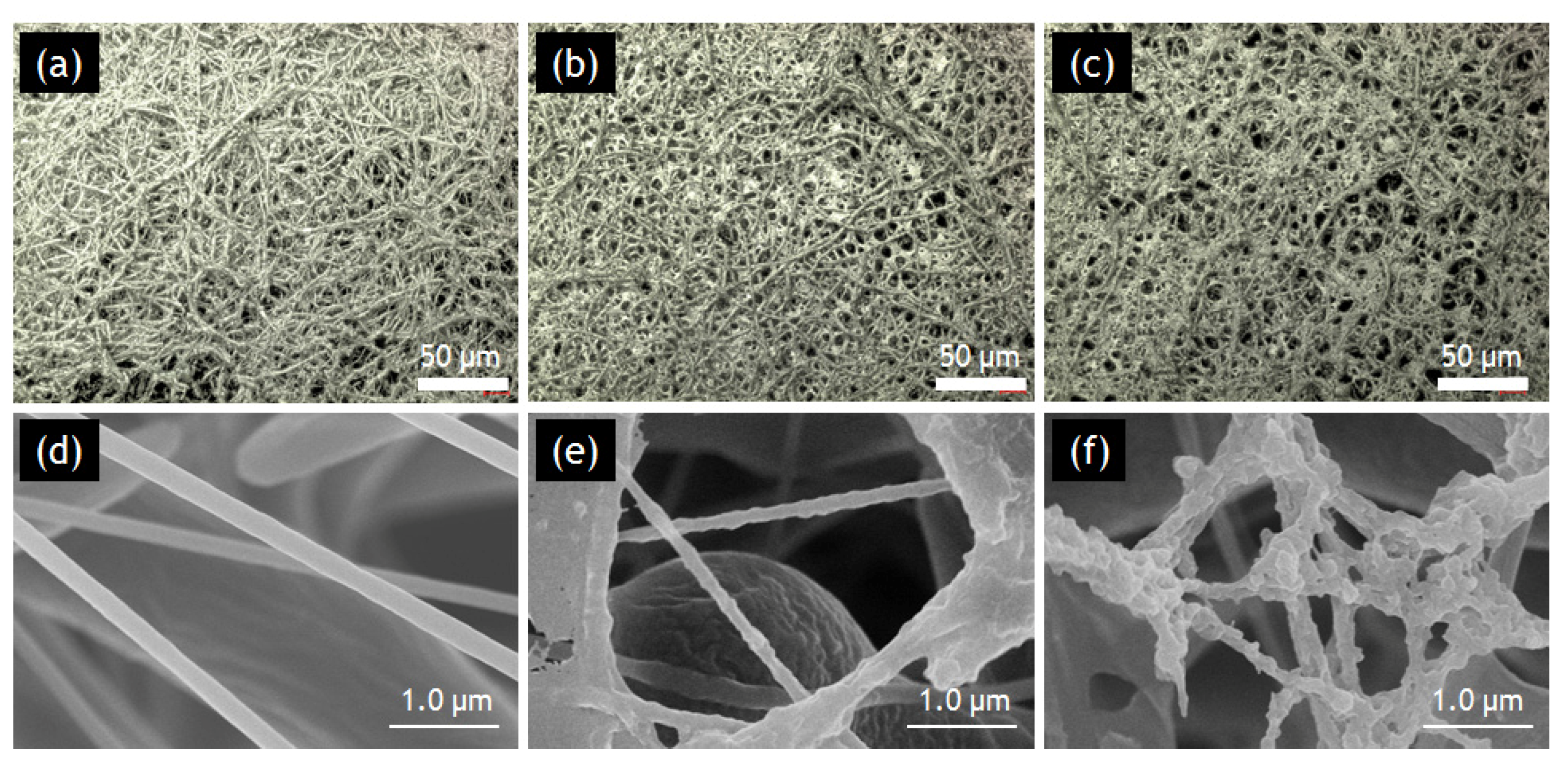
2.2.1. SLM and SEM Observations.

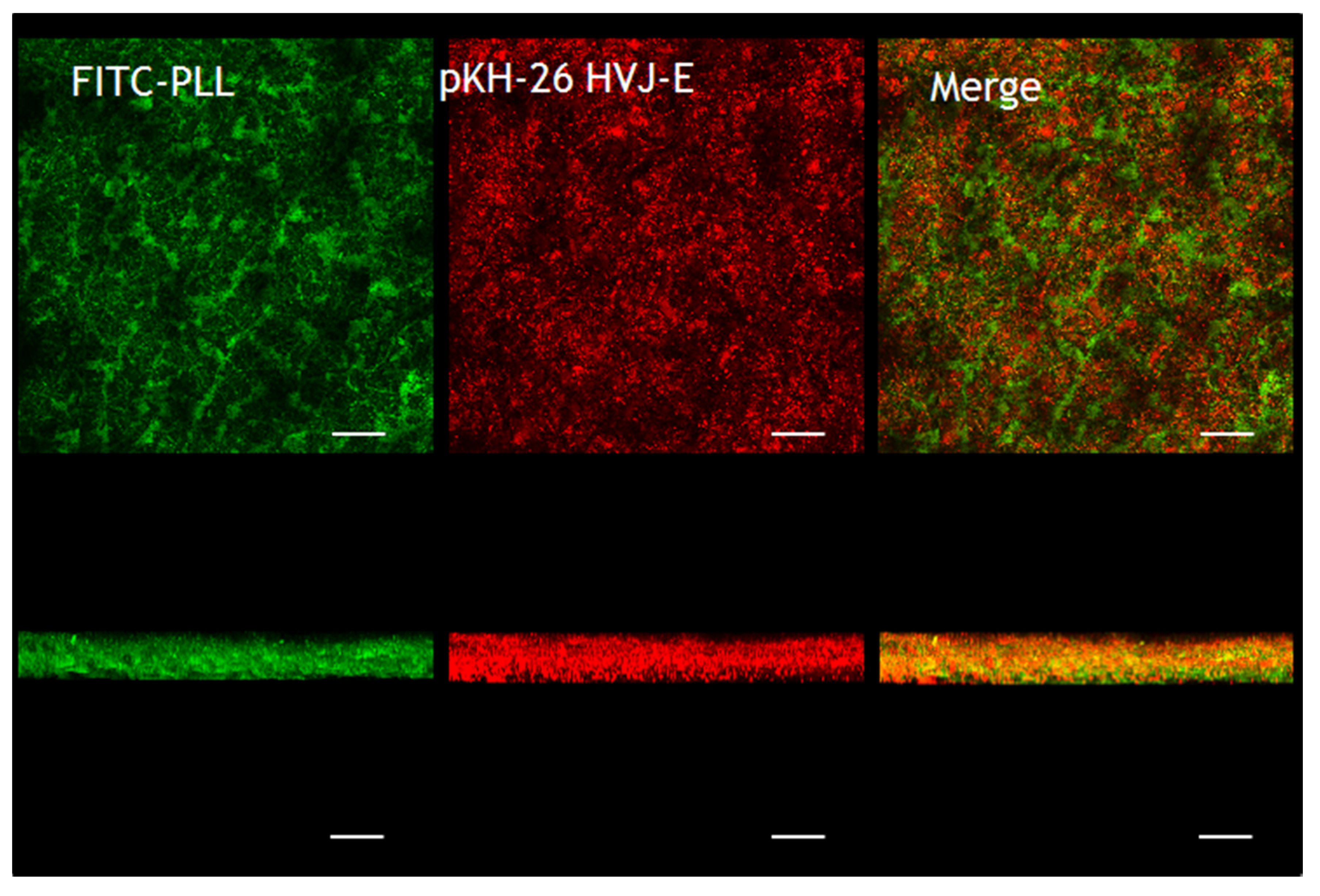
2.2.2. CLM Observations
2.3. HVJ-E Releasing from the Nanofibers

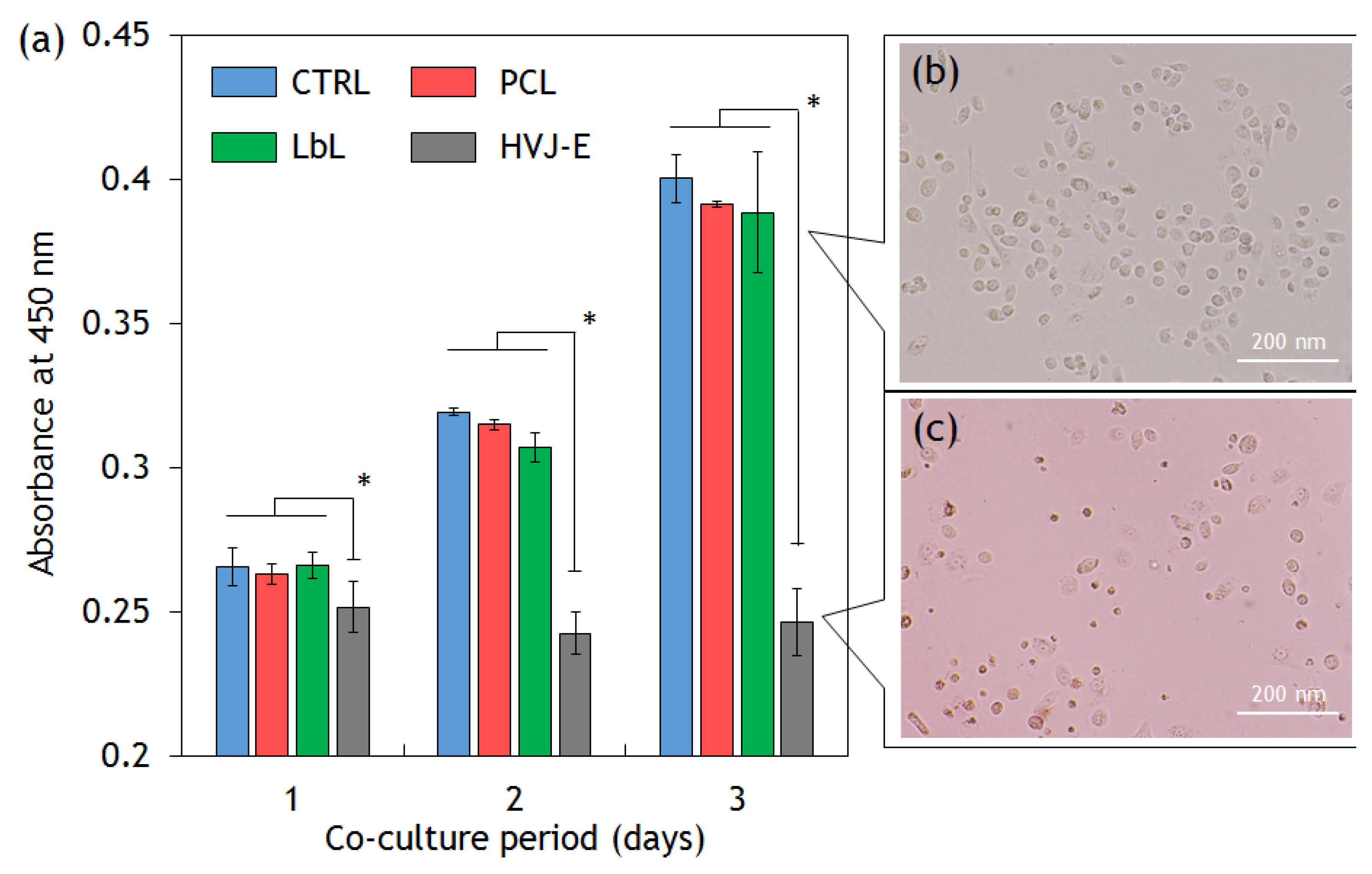
2.4. Cytotoxic Assay
3. Experimental Section
3.1. Materials
3.2. Fabrication of Nanofibers
3.3. Preparation of Crosslinked Layer-by-Layer Assembled Films on PCL Nanofibers
3.4. HVJ-E Adsorption on the Crosslinked Layer-by-Layer Film on PCL Nanofibers
3.5. Characterization of PCL Nanofibers with/without HVJ-E Coating
3.6. HVJ-E Release from HVJ-E Immobilized LbL Coated Nanofibers
3.7. Cytotoxic Ability of HVJ-E Coated PCL Nanofibers
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Latest World Cancer Statistics Global Cancer Burden Rises to 14.1 Million New Cases in 2012: Marked Increase in Breast Cancers Must Be Addressed. Available online: http://www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdf (accessed on 30 October 2015).
- Reight, A.; Come, S.E.; Henderson, C.; Gelman, R.S.; Silver, B.; Hayes, D.F.; Shulman, L.N.; Harris, J.R. The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N. Engl. J. Med. 1996, 334, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, M.D.; Hansen, J.L.; Prokopios-Davos, S.; Manola, J.; Wang, Q.; Bornstein, B.A.; Hynynen, K.; Kaplan, I.D. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer. Cancer 2011, 117, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liao, B.; Chiang, C.; Chen, P.; Chen, I.; Chen, S. Core-shell nanocapsules stabilized by single-component polymer and nanoparticles for magneto-chemotherapy/hyperthermia with multiple drugs. 2012, 24, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. A smart hyperthermia nanofiber with switchable drug release for inducing cancer apoptosis. Adv. Funct. Mater. 2013, 23, 5753–5761. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(n-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Hoffman, J.M.; Hoffman, A.S.; Stayton, P.S.; Lai, J.J. A photoinduced nanoparticle separation in microchannels via ph-sensitive surface traps. Langmuir 2013, 29, 5388–5393. [Google Scholar] [CrossRef] [PubMed]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Blattman, J.N.; Greenberg, P.D. Cancer immunotherapy: A treatment for the masses. Science 2004, 305, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, Y.; Nakajima, T.; Nishikawa, T.; Yamamoto, S.; Ikegami, H.; Suzuki, N.; Nakamura, H.; Morishita, R.; Kotani, H. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol. Ther. 2002, 6, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Mercado, N.; Barnes, P.J.; Ito, K. Defects of protein phosphatase 2A causes corticosteroid insensitivity in severe asthma. PLOS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Okada, N.; Kodama, T.; Honda, T.; Iida, T. A cytotoxic type III secretion effector of vibrio parahaemolyticus targets vacuolar H+-ATPase subunit c and ruptures host cell lysosomes. PLOS Pathog. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Salker, M.S.; Christian, M.; Steel, J.H.; Nautiyal, J.; Lavery, S.; Trew, G.; Webster, Z.; Al-Sabbagh, M.; Puchchakayala, G.; Föller, M.; et al. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat. Med. 2011, 17, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Kurooka, M.; Kaneda, Y. Inactivated Sendai virus particles eradicate tumors by inducing immune responses through blocking regulatory T cells. Cancer Res. 2007, 67, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kurooka, M.; Hiroaki, Y.; Fujiyoshi, Y.; Kaneda, Y. Sendai virus F glycoprotein induces IL-6 production in dendritic cells in a fusion-independent manner. FEBS Lett. 2008, 582, 1325–1329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawaguchi, Y.; Miyamoto, Y.; Inoue, T.; Kaneda, Y. Efficient eradication of hormone-resistant human prostate cancers by inactivated Sendai virus particle. Int. J. Cancer 2009, 124, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Coombes, A.G.A.; Rizzi, S.C.; Williamson, M.; Barralet, J.E.; Downes, S.; Wallace, W.A. Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials 2004, 25, 315–325. [Google Scholar] [CrossRef]
- Rai, B.; Teoh, S.H.; Hutmacher, D.W.; Cao, T.; Ho, K.H. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials 2005, 26, 3739–3748. [Google Scholar] [CrossRef] [PubMed]
- Uto, K.; Ebara, M.; Aoyagi, T. Temperature-responsive Poly(ε-Caprolactone) cell culture platform with dynamically tunable nano-roughness and elasticity for control of myoblast morphology. Int. J. Mol. Sci. 2014, 15, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Uto, K.; Idota, N.; Hoffman, J.M.; Aoyagi, T. Shape-memory surface with dynamically tunable nanogeometry activated by body heat. Adv. Mater. 2012, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Garrett, R.; Niiyama, E.; Kotsuchibashi, Y.; Uto, K.; Ebara, M. Biodegradable nanofiber for delivery of immunomodulating agent in the treatment of basal cell carcinoma. Fibers 2015, 3, 478–490. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Mansouri, S.; Merhi, Y.; Winnik, F.M.; Tabrizian, M. Investigation of Layer-by-Layer assembly of polyelectrolytes on fully functional human red blood cells in suspension for attenuated immune response. Biomacromolecules 2011, 12, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Trau, D.; Möhwald, H.; Renneberg, R. Enzyme encapsulation in Layer-by-Layer engineered polymer multilayer capsules. Langmuir 2000, 16, 1485–1488. [Google Scholar] [CrossRef]
- Lvov, Y.; Ariga, K.; Ichinose, I.; Kunitake, T. Assembly of multicomponent protein films by means of electrostatic Layer-by-Layer adsorption. J. Am. Chem. Soc. 1995, 117, 6117–6123. [Google Scholar] [CrossRef]
- Guillot, R.; Gilde, F.; Becquart, P.; Sailhan, F.; Lapeyrere, A.; Logeart-Avramoglou, D.; Picart, C. The stability of BMP loaded polyelectrolyte multilayer coatings on titanium. Biomaterials 2013, 34, 5737–5746. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Niiyama, E.; Uto, K.; Aoyagi, T.; Ebara, M. A biomimetic approach to hormone resistant prostate cancer cell isolation using inactivated Sendai virus (HVJ-E). Biomater. Sci. 2016, 4, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kunjukunju, S.; Roy, A.; Ramanathan, M.; Lee, B.; Candiello, J.E.; Kumta, P.N. Acta biomaterialia a Layer-by-Layer approach to natural polymer-derived bioactive coatings on magnesium alloys Q. Acta Biomater. 2013, 9, 8690–8703. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Uto, K.; Sasai, M.; Lee, C.M.; Ebara, M.; Aoyagi, T. Nano-decoration of the hemagglutinating virus of Japan Envelope (HVJ-E) using a Layer-by-Layer assembly technique. Langmuir 2013, 7384–7392. [Google Scholar] [CrossRef] [PubMed]
- Communie, G.; Crépin, T.; Maurin, D.; Jensen, M.R.; Blackledge, M.; Ruigrok, R.W.H. Structure of the tetramerization domain of measles virus phosphoprotein. J. Virol. 2013, 87, 7166–7169. [Google Scholar] [CrossRef] [PubMed]
- Namekawa, K.; Schreiber, M.T.; Aoyagi, T.; Ebara, M. Fabrication of zeolite–polymer composite nanofibers for removal of uremic toxins from kidney failure patients. Biomater. Sci. 2014, 2, 674–679. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Matsushima-Miyagi, T.; Hatano, K.; Nomura, M.; Li-Wen, L.; Nishikawa, T.; Saga, K.; Shimbo, T.; Kaneda, Y. Trail and noxa are selectively upregulated in prostate cancer cells downstream of the RIG-I/MAVS signaling pathway by nonreplicating Sendai virus particles. Clin. Cancer Res. 2012, 18, 6271–6283. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, T.; Niiyama, E.; Uto, K.; Aoyagi, T.; Ebara, M. Inactivated Sendai Virus (HVJ-E) Immobilized Electrospun Nanofiber for Cancer Therapy. Materials 2016, 9, 12. https://doi.org/10.3390/ma9010012
Okada T, Niiyama E, Uto K, Aoyagi T, Ebara M. Inactivated Sendai Virus (HVJ-E) Immobilized Electrospun Nanofiber for Cancer Therapy. Materials. 2016; 9(1):12. https://doi.org/10.3390/ma9010012
Chicago/Turabian StyleOkada, Takaharu, Eri Niiyama, Koichiro Uto, Takao Aoyagi, and Mitsuhiro Ebara. 2016. "Inactivated Sendai Virus (HVJ-E) Immobilized Electrospun Nanofiber for Cancer Therapy" Materials 9, no. 1: 12. https://doi.org/10.3390/ma9010012
APA StyleOkada, T., Niiyama, E., Uto, K., Aoyagi, T., & Ebara, M. (2016). Inactivated Sendai Virus (HVJ-E) Immobilized Electrospun Nanofiber for Cancer Therapy. Materials, 9(1), 12. https://doi.org/10.3390/ma9010012







