Preparation and Characterization of Microencapsulated Phase Change Materials for Use in Building Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Shell: Methyl methacrylate (MMA) (99%, contains ≤ 30 ppm monomethyl ether hydroquinone (MEHQ) as inhibitor, Sigma Aldrich, Auckland, New Zealand) and pentaerythritol tetraacrylate (PETRA) (contains 350 ppm (MEHQ), Sigma Aldrich, Auckland, New Zealand) were used as a monomer and cross-linking agent respectively in order to obtain proper shells for MPCM.
- Free radical thermal initiator: Luperox® A75, Benzoyl peroxide (BPO) (75%, contains 25% water, Sigma Aldrich, Auckland, New Zealand) was used as free radical thermal initiator.
- Surfactants: Polyvinyl alcohol (PVA) (Mw 85,000–124,000, Sigma Aldrich, Auckland, New Zealand) and sodium dodecyl sulfate (SDS) (BioXtra, 99%, Sigma Aldrich, Auckland, New Zealand) were used as a non-ionic and ionic surfactant, respectively.
- PCM: a commercial paraffinic PCM, Rubitherm® RT 21 (Tm = 21 °C, ΔHm = 135 J·g−1, Rubitherm® Technologies GmbH, Berlin, Germany) was used.
2.2. Synthesis of PCMs Microcapsules
2.2.1. Emulsification
2.2.2. Polymerization
3. Characterization of Microcapsules
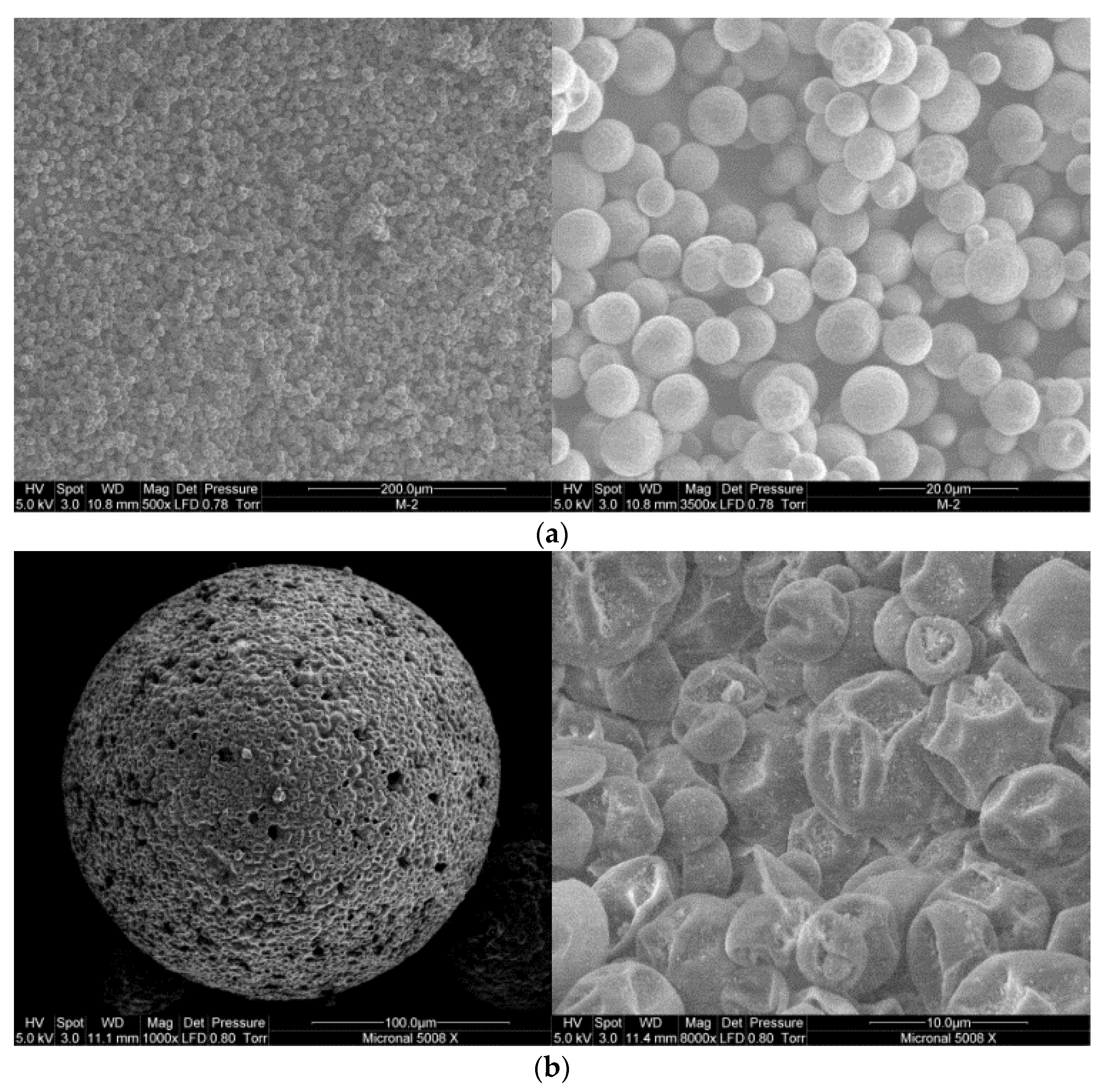
3.1. Scanning Electron Microscopy (SEM)
3.2. Differential Scanning Calorimetry (DSC)
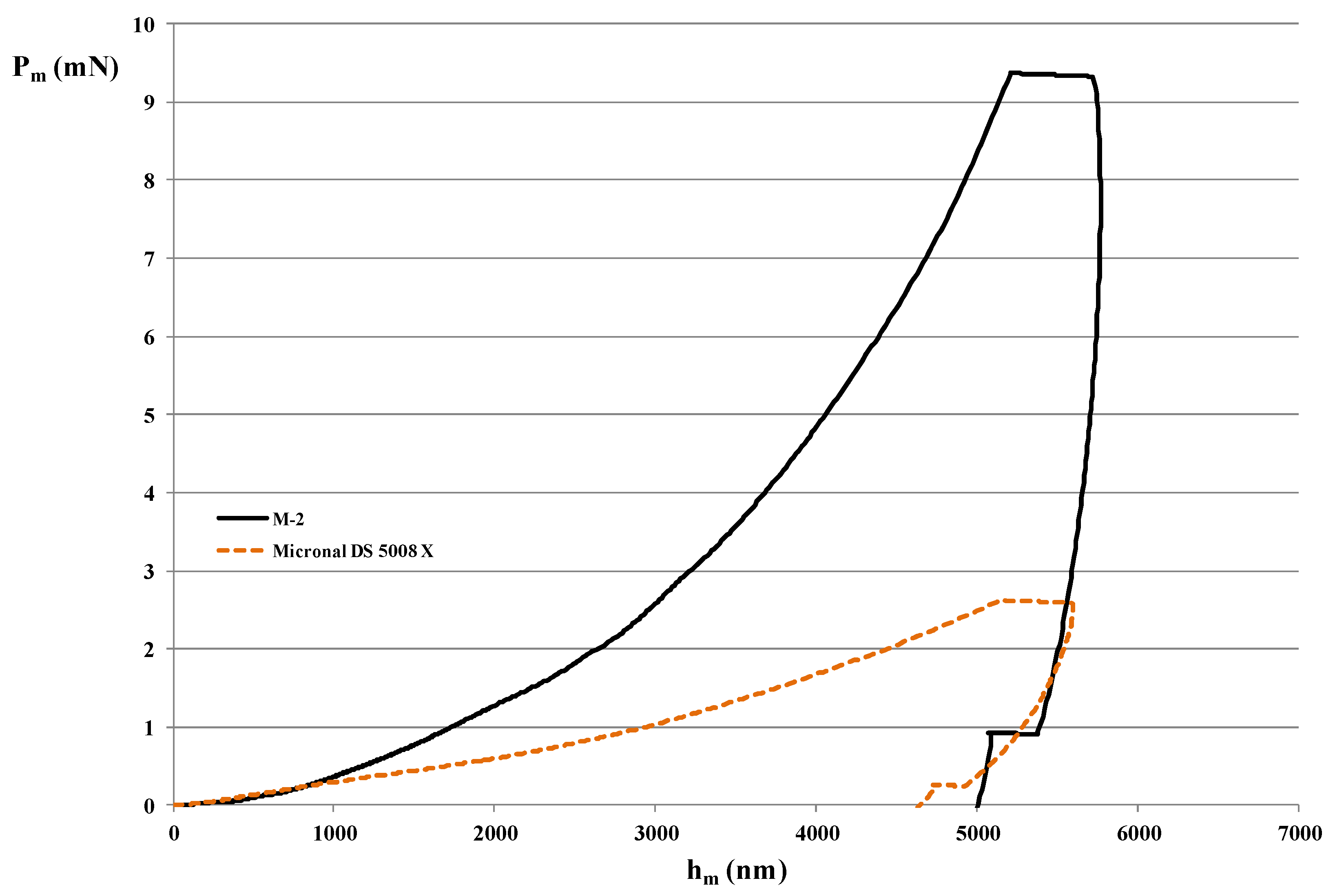
3.3. Nano-Indentation Technique
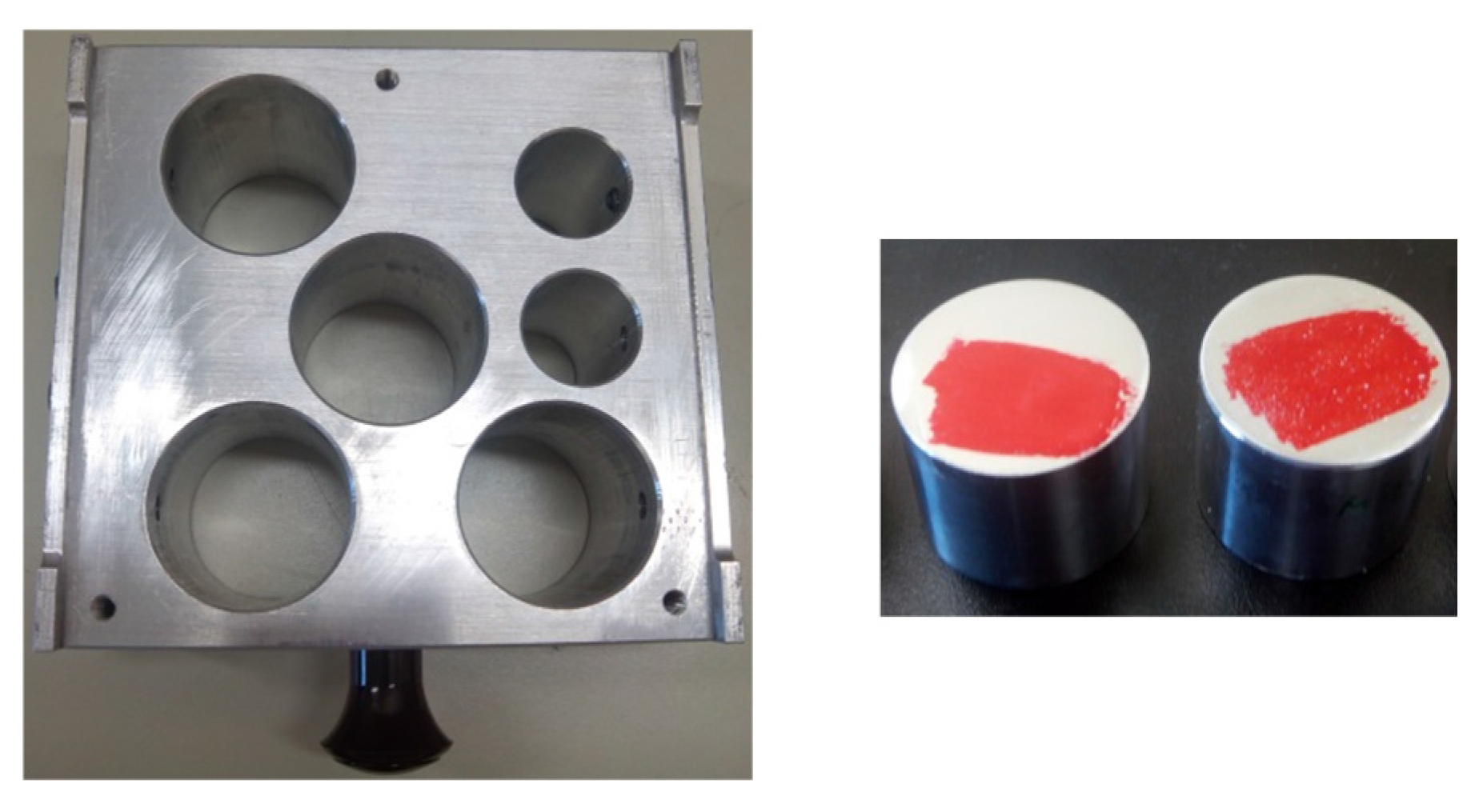
- Hardness (H) is defined as the maximum indentation load divided by the cross-sectional area of the indenter specified at the maximum indentation depth (A(hm)).
- Load (Pm) at maximum displacement (mN): It is the load recorded at the maximum load, which occurs when sample fails.
- Elastic modulus (E) is evaluated following Equations (2) and (3) from the nano-indentation test using the maximum indentation load (Pm) and the depth sensing indentation. Hardness (H), elastic work (We), and total work (W) can be calculated by integrating the areas under the indentation unloading. We and W are the elastic work and total work, which are equal to the areas under the unloading and loading curves, respectively which is correlated with E and H through the function ψ described in [71]. value is independent of the degree of work-hardening behavior [66].
- Displacement (hm) at maximum load (nm) is a measure of the extent the tip penetrates into the material.
3.4. Emission of Volatile Organic Compounds (VOC’s)
4. Results and Discussion
4.1. Characterization of MPCM Shape, Size, and Morphology
4.2. Thermophysical Properties of MPCM
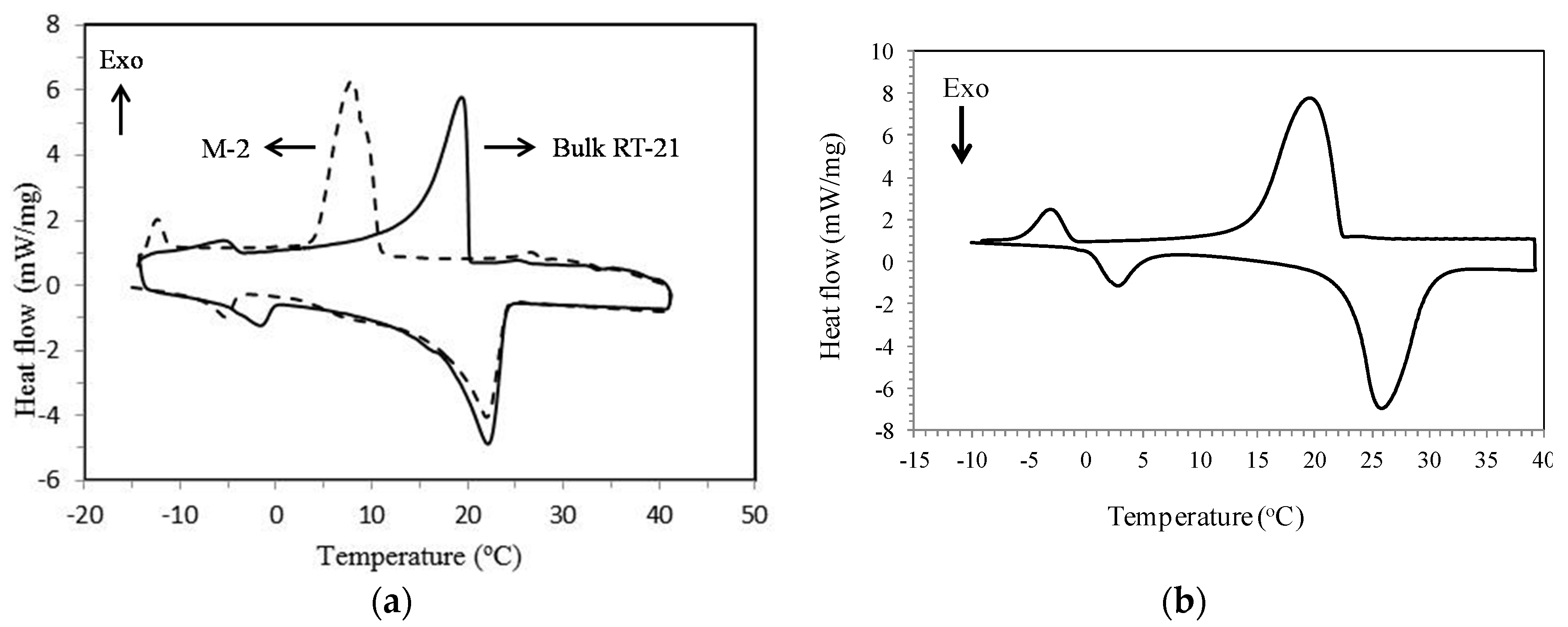

| Status | Transition Temperatures and Heat of Fusion | M-2 | RT-21 | Micronal® DS 5008 X | M-2 Containing RT-58 |
|---|---|---|---|---|---|
| Heating | Tonset (°C) | 16.7 | 16.5 | 22.1 | 16.1 |
| Tpeak (°C) | 22.0 | 22.1 | 20.3 | 22.3 | |
| Tendset (°C) | 24.0 | 23.9 | 26.3 | 24.3 | |
| ΔH (J·g−1) | 113.9 | 132.0 | 99.9 | 110.4 | |
| Cooling | Tonset (°C) | 10.9 | 20.2 | 22.5 | 19.8 |
| Tpeak (°C) | 7.9 | 19.4 | 24.2 | 17.5 | |
| Tendset (°C) | 4.2 | 14.5 | 17.5 | 11.4 | |
| ΔH (J·g−1) | 111.9 | 132.5 | 103.5 | 108.3 |
4.3. Mechanical Properties of MPCM
| Mechanical Property | M-2 | Micronal® DS 5008 X |
|---|---|---|
| E (GPa) | 1.89 | 0.15 |
| 1.04 | 0.19 | |
| 1.16 | 0.17 | |
| 1.68 | 0.24 | |
| 1.38 | 0.22 | |
| 1.9 | 0.28 | |
| Mean | 1.51 | 0.21 |
| Standard Deviation | 0.37 | 0.05 |
4.4. Characterization of Volatile Organic Compounds (VOCs)
| Temperature | M-2 | Micronal® DS 5008 X | ||
|---|---|---|---|---|
| tr (min) | Compound | tr (min) | Compound | |
| 25 °C | No signal | No signal | ||
| 35 °C | No signal | No signal | ||
| 45 °C | No signal | 11.88 | C17H36 | |
| 12.66 | C18H38 | |||
| 55 °C | No signal | 11.88 | C17H36 | |
| 12.65 | C18H38 | |||
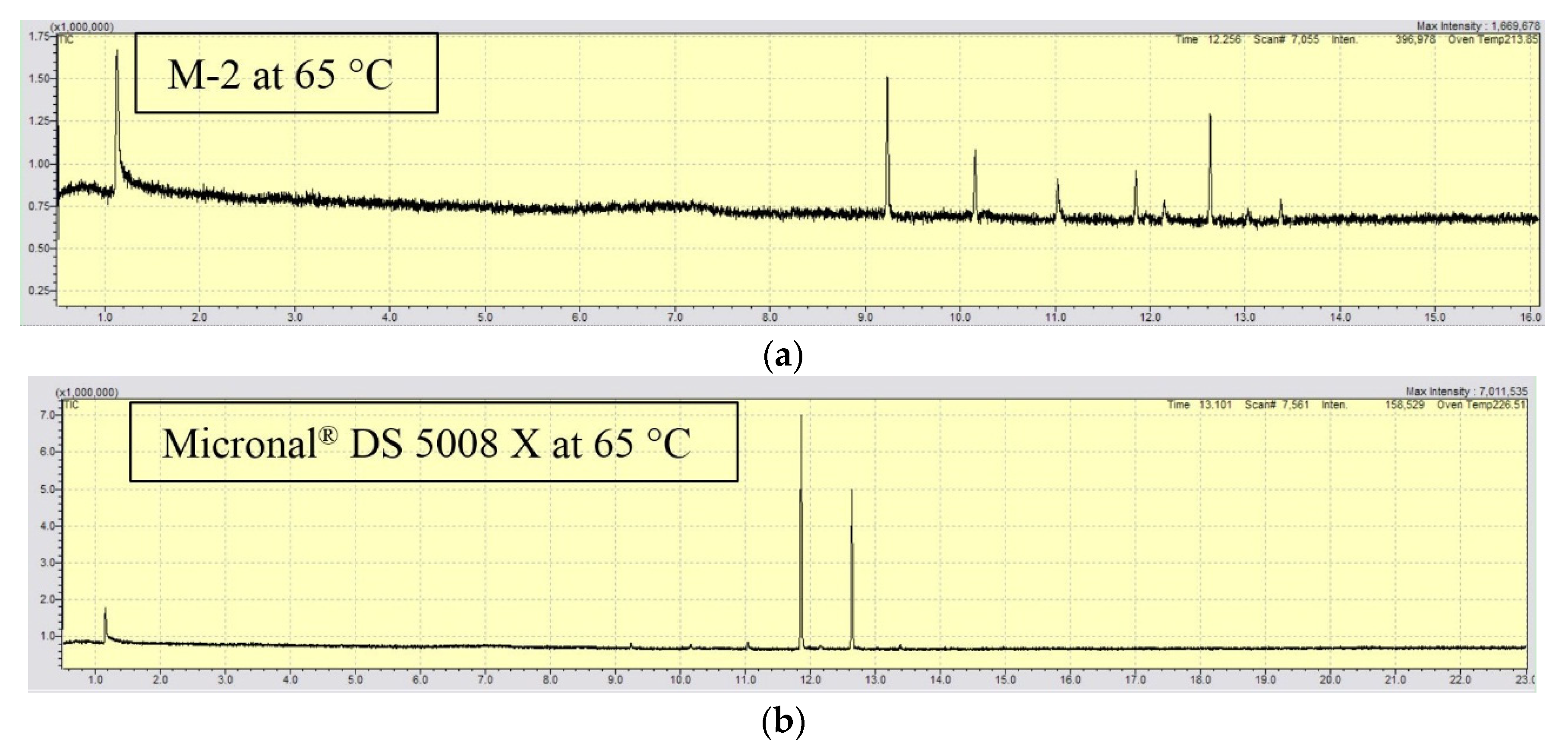
| 65 °C | 9.25 | C14H30 | - | - |
| 10.18 | C15H32 | - | - | |
| 11.06 | C16H34 | - | - | |
| 11.91 | C17H36 | 11.85 | C17H36 | |
| 12.68 | C18H38 | 12.63 | C18H38 | |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.-J. A review on phase change materials integrated in building walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Soares, N.; Costa, J.J.; Gaspar, A.R.; Santos, P. Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency. Energy Build. 2013, 59, 82–103. [Google Scholar] [CrossRef]
- Griffiths, P.W.; Eames, P.C. Performance of chilled ceiling panels using phase change material slurries as the heat transport medium. Appl. Therm. Eng. 2007, 27, 1756–1760. [Google Scholar] [CrossRef]
- Huang, L.; Petermann, M.; Doetsch, C. Evaluation of paraffin/water emulsion as a phase change slurry for cooling applications. Energy 2009, 34, 1145–1155. [Google Scholar] [CrossRef]
- Youssef, Z.; Delahaye, A.; Huang, L.; Trinquet, F.; Fournaison, L.; Pollerberg, C.; Doetsch, C. State of the art on phase change material slurries. Energy Convers. Manag. 2013, 65, 120–132. [Google Scholar] [CrossRef]
- Pendyala, S. Macroencapsulation of Phase Change Materials for Thermal Energy Storage. Master Thesis, University of South Florida, Tampa, FL, USA, 22 June 2012. [Google Scholar]
- Calvet, N.; Py, X.; Olivès, R.; Bédécarrats, J.-P.; Dumas, J.-P.; Jay, F. Enhanced performances of macro-encapsulated phase change materials (PCMs) by intensification of the internal effective thermal conductivity. Energy 2013, 55, 956–964. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zhang, G.H. Review on microencapsulated phase change materials (MEPCMs): Fabrication, characterization and applications. Renew. Sustain. Energy Rev. 2011, 15, 3813–3832. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Oncins, G.; Barreneche, C.; Martínez, M.; Fernández, A.I.; Cabeza, L.F. Physico-chemical and mechanical properties of microencapsulated phase change material. Appl. Energy 2013, 109, 441–448. [Google Scholar] [CrossRef]
- Al Shannaq, R.; Farid, M.M. Microencapsulation of phase change materials (PCMs) for thermal energy storage systems. In Advances in Thermal Energy Storage Systems; Cabeza, L.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 247–284. [Google Scholar]
- Alvarado, J.L.; Marsh, C.; Sohn, C.; Phetteplace, G.; Newell, T. Thermal performance of microencapsulated phase change material slurry in turbulent flow under constant heat flux. Int. J. Heat Mass Transf. 2007, 50, 1938–1952. [Google Scholar] [CrossRef]
- Diaconu, B.M.; Varga, S.; Oliveira, A.C. Experimental study of natural convection heat transfer in a microencapsulated phase change material slurry. Energy 2010, 35, 2688–2693. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.W.; Wang, R.Z. An overview of phase change material slurries: MPCS and CHS. Renew. Sustain. Energy Rev. 2010, 14, 598–614. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.W. An overview of fundamental studies and applications of phase change material slurries to secondary loop refrigeration and air conditioning systems. Renew. Sustain. Energy Rev. 2012, 16, 5021–5058. [Google Scholar] [CrossRef]
- Medrano, M.; Yilmaz, M.O.; Nogués, M.; Martorell, I.; Roca, J.; Cabeza, L.F. Experimental evaluation of commercial heat exchangers for use as PCM thermal storage systems. Appl. Energy 2009, 86, 2047–2055. [Google Scholar] [CrossRef]
- Su, J.-F.; Wang, L.-X.; Ren, L. Preparation and characterization of double-MF shell microPCMs used in building materials. J. Appl. Polym. Sci. 2005, 97, 1755–1762. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Farid, M.M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Alawadhi, E.M. Thermal analysis of a building brick containing phase change material. Energy Build. 2008, 40, 351–357. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Karaipekli, A.; Uzun, O. Microencapsulated n-octacosane as phase change material for thermal energy storage. Sol. Energy 2009, 83, 1757–1763. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castellón, C.; Nogués, M.; Medrano, M.; Leppers, R.; Zubillaga, O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007, 39, 113–119. [Google Scholar] [CrossRef]
- Castellón, C.; Medrano, M.; Roca, J.; Cabeza, L.F.; Navarro, M.E.; Fernández, A.I.; Lázaro, A.; Zalba, B. Effect of microencapsulated phase change material in sandwich panels. Renew. Energy 2010, 35, 2370–2374. [Google Scholar] [CrossRef]
- Toppi, T.; Mazzarella, L. Gypsum based composite materials with micro-encapsulated PCM: Experimental correlations for thermal properties estimation on the basis of the composition. Energy Build. 2013, 57, 227–236. [Google Scholar] [CrossRef]
- Entrop, A.G.; Brouwers, H.J.H.; Reinders, A.H.M.E. Experimental research on the use of micro-encapsulated Phase Change Materials to store solar energy in concrete floors and to save energy in Dutch houses. Sol. Energy 2011, 85, 1007–1020. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Alkan, C.; Sari, A. Fatty acid/poly(methyl methacrylate) (PMMA) blends as form-stable phase change materials for latent heat thermal energy storage. Sol. Energy 2008, 82, 118–124. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Q.; Zhao, Z.; Zhang, J.; Sun, Y.; Sun, L.; Xu, F.; Sawada, Y. Preparation and thermal performance of gypsum boards incorporated with microencapsulated phase change materials for thermal regulation. Sol. Energy Mater. Sol. Cells 2012, 102, 93–102. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Garrido, I.; Valverde, J.L.; Rodríguez, J.F.; Carmona, M. Development of smart gypsum composites by incorporating thermoregulating microcapsules. Energy Build. 2014, 76, 631–639. [Google Scholar] [CrossRef]
- Karkri, M.; Lachheb, M.; Albouchi, F.; Nasrallah, S.B.; Krupa, I. Thermal properties of smart microencapsulated paraffin/plaster composites for the thermal regulation of buildings. Energy Build. 2015, 88, 183–192. [Google Scholar] [CrossRef]
- Eddhahak-Ouni, A.; Drissi, S.; Colin, J.; Neji, J.; Care, S. Experimental and multi-scale analysis of the thermal properties of Portland cement concretes embedded with microencapsulated Phase Change Materials (PCMs). Appl. Therm. Eng. 2014, 64, 32–39. [Google Scholar] [CrossRef]
- Schlink, U.; Rehwagen, M.; Damm, M.; Richter, M.; Borte, M.; Herbarth, O. Seasonal cycle of indoor-VOCs: Comparison of apartments and cities. Atmos. Environ. 2004, 38, 1181–1190. [Google Scholar] [CrossRef]
- Ayoko, G.A. Volatile organic compounds in indoor environments. In The Handbook of Environmental Chemistry; Pluschke, P., Ed.; Springer-Verlag: Heidelberg, Germany, 2004; pp. 1–35. [Google Scholar]
- Gallego, E.; Roca, X.; Perales, J.F.; Guardino, X. Determining indoor air quality and identifying the origin of odour episodes in indoor environments. J. Environ. Sci. 2009, 21, 333–339. [Google Scholar] [CrossRef]
- Missia, D.A.; Demetriou, E.; Michael, N.; Tolis, E.I.; Bartzis, J.G. Indoor exposure from building materials: A field study. Atmos. Environ. 2010, 44, 4388–4395. [Google Scholar] [CrossRef]
- Shin, S.H.; Jo, W.K. Volatile organic compound concentrations, emission rates, and source apportionment in newly-built apartments at pre-occupancy stage. Chemosphere 2012, 89, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Haghighat, F. Building materials VOC emissions—A systematic parametric study. Build. Environ. 2003, 38, 995–1005. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y. Characteristics and correlations of VOC emissions from building materials. Int. J. Heat Mass Transf. 2003, 46, 4877–4883. [Google Scholar] [CrossRef]
- Kim, S.; Choi, Y.-K.; Park, K.-W.; Kim, J.T. Test methods and reduction of organic pollutant compound emissions from wood-based building and furniture materials. Bioresour. Technol. 2010, 101, 6562–6568. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Haghighat, F.; Ghaly, W. Conjugate mass transfer modeling for VOC source and sink behavior of porous building materials: When to apply it? J. Build. Phys. 2006, 30, 91–111. [Google Scholar] [CrossRef]
- Haghighat, F.; Huang, H. Integrated IAQ model for prediction of VOC emissions from building material. Build. Environ. 2003, 38, 1007–1017. [Google Scholar] [CrossRef]
- Lee, C.-S.; Haghighat, F.; Ghaly, W.S. A study on VOC source and sink behavior in porous building materials—Analytical model development and assessment. Indoor Air 2005, 15, 183–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.-J.; Moon, S.-J. Evaluation of VOC emissions from building finishing materials using a small chamber and VOC analyser. Indoor Built Environ. 2006, 15, 511–523. [Google Scholar] [CrossRef]
- Magee, R.J.; Bodalal, A.; Biesenthal, T.A.; Lusztyk, E.; Brouzes, M.; Shaw, C.Y. Prediction of VOC Concentration Profiles in a Newly Constructed House Using Small Chamber Data and an IAQ Simulation Program. In Proceedings of the 9th International Conference on IAQ and Climate, Monterey, CA, USA, 30 June–5 July 2002; pp. 298–303.
- Jia, C.; Batterman, S.A.; Relyea, G.E. Variability of indoor and outdoor VOC measurements: An analysis using variance components. Environ. Pollut. 2012, 169, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Norbäck, D.; Torgén, M.; Edling, C. Volatile organic compounds, respirable dust, and personal factors related to prevalence and incidence of sick building syndrome in primary schools. Br. J. Ind. Med. 1990, 47, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Redlich, C.A.; Sparer, J.; Cullen, M.R. Sick-building syndrome. Lancet 1997, 349, 1013–1016. [Google Scholar] [CrossRef]
- Apte, M.G.; Daisey, J.M. VOCs and “Sick Building Syndrome”: Application of a New Statistical Approach for SBS Research to US EPA BASE Study Data. In Proceedings of the Indoor Air 99, Edinburgh, Scotland, 30 June–5 July 1999; pp. 2–7.
- Rufford, T.E.; Zhu, J.; Hulicova-Jurcakova, D. Green Carbon Materials: Advances and Applications; Pan Stanford: Boca Raton, FL, USA, 2014; pp. 1–288. [Google Scholar]
- Standard Guide for Small-Scale Environmental Chamber Determinations of Organic Emissions from Indoor Material/Products; ASTM Standard D5116-97; ASTM International: West Conshohocken, PA, USA, 1997.
- Standard Test Method for Determining Formaldehyde Concentrations in Air from Wood Products Using a Small Scale Chamber; ASTM Standard D6007-96; ASTM International: West Conshohocken, PA, USA, 1996.
- Won, D.; Magee, R.J.; Lusztyk, E.; Nong, G.; Zhu, J.P.; Zhang, J.S.; Reardon, J.T.; Shaw, C.Y. A Comprehensive VOC Emission Database for Commonly-Used Building Materials. In Proceedings of the 7th International Conference of Healthy Buildings, Singapore, 7–11 December 2003; pp. 1–6.
- Ouazia, B.; Reardon, J.; Sander, D. Making the Case for Reducing Ventilation Requirements through Selection of Low-Emission Materials. In Proceedings of the 10th International Conference on Indoor Air Quality and Climate, Beijing, China, 4–9 September 2005.
- Xiong, J.; Zhang, Y.; Huang, S. Characterization of VOC and formaldehyde emission from building materials in static environmental chamber: Model development and application. Indoor Built Environ. 2011, 20, 217–225. [Google Scholar] [CrossRef]
- Knöppel, H.; Schauenburg, H. Screening of household products for the emission of volatile organic compounds. Environ. Int. 1989, 15, 413–418. [Google Scholar] [CrossRef]
- Lin, C.-C.; Yu, K.-P.; Zhao, P.; Lee, G. Evaluation of impact factors on VOC emissions and concentrations from wooden flooring based on chamber tests. Build. Environ. 2009, 44, 525–533. [Google Scholar] [CrossRef]
- Safari, V.; Barreneche, C.; Castell, A.; Basatni, A.; Navarro, L.; Cabeza, L.; Haghighat, F. Volatile Organic Emission from PCM Building Materials. In Proceedings of the Innostock 2012—The 12th International Conference on Energy Storage, Lleida, Spain, 16–18 May 2012.
- Sánchez, L.; Sánchez, P.; de Lucas, A.; Carmona, M.; Rodríguez, J.F. Microencapsulation of PCMs with a polystyrene shell. Colloid Polym. Sci. 2007, 285, 1377–1385. [Google Scholar] [CrossRef]
- Sánchez-Silva, L.; Rodríguez, J.F.; Romero, A.; Borreguero, A.M.; Carmona, M.; Sánchez, P. Microencapsulation of PCMs with a styrene-methyl methacrylate copolymer shell by suspension-like polymerisation. Chem. Eng. J. 2010, 157, 216–222. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.; Dickinson, M.; Mohammed, F. Supercooling elimination of phase change materials (PCMs) microcapsules. Energy 2015, 87, 654–662. [Google Scholar] [CrossRef]
- Rahman, A.; Dickinson, M.E.; Farid, M.M. Microencapsulation of a PCM through membrane emulsification and nanocompression-based determination of microcapsule strength. Mater. Renew. Sustain. Energy 2012, 1. [Google Scholar] [CrossRef]
- Troyon, M.; Huang, L. Correction factor for contact area in nanoindentation measurements. J. Mater. Res. 2011, 20, 610–617. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1580. [Google Scholar] [CrossRef]
- Hochstetter, G.; Jimenez, A.; Loubet, J.L. Strain-rate effects on hardness of glassy polymers in the nanoscale range. Comparison between quasi-static and continuous stiffness measurements. J. Macromol. Sci. Part B 2006, 38, 681–692. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Roa, J.J.; Díez-Pascual, A.M.; Rayón, E.; Flores, A.; Martínez, M.; Chimenos, J.M.; Fernández, A.I. Depth-sensing indentation applied to polymers: A comparison between standard methods of analysis in relation to the nature of the materials. Eur. Polym. J. 2013, 49, 4047–4053. [Google Scholar] [CrossRef]
- Fischer-Cripps, A.C. Introduction to Contact Mechanics, 2nd ed.; Springer: New York, NY, USA, 2007; p. 221. [Google Scholar]
- Ma, D. New relationship between Young’s modulus and nonideally sharp indentation parameters. J. Mater. Res. 2004, 19, 2144–2151. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Farid, M.; Al-Muhtaseb, S.; Kurdi, J. Emulsion stability and cross-linking of PMMA microcapsules containing phase change materials. Sol. Energy Mater. Sol. Cells 2015, 132, 311–318. [Google Scholar] [CrossRef]
- Qiu, X.; Li, W.; Song, G.; Chu, X.; Tang, G. Fabrication and characterization of microencapsulated n-octadecane with different crosslinked methylmethacrylate-based polymer shells. Sol. Energy Mater. Sol. Cells 2012, 98, 283–293. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, Y.; Tao, X.; Yick, K. Crystallization and prevention of supercooling of microencapsulated n-alkanes. J. Colloid Interface Sci. 2005, 281, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasat, P.; Ogino, Y.; Suzuki, T.; Okubo, M. Influence of water domain formed in hexadecane core inside cross-linked capsule particle on thermal properties for heat storage application. Colloid Polym. Sci. 2008, 286, 753–759. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giro-Paloma, J.; Al-Shannaq, R.; Fernández, A.I.; Farid, M.M. Preparation and Characterization of Microencapsulated Phase Change Materials for Use in Building Applications. Materials 2016, 9, 11. https://doi.org/10.3390/ma9010011
Giro-Paloma J, Al-Shannaq R, Fernández AI, Farid MM. Preparation and Characterization of Microencapsulated Phase Change Materials for Use in Building Applications. Materials. 2016; 9(1):11. https://doi.org/10.3390/ma9010011
Chicago/Turabian StyleGiro-Paloma, Jessica, Refat Al-Shannaq, Ana Inés Fernández, and Mohammed M. Farid. 2016. "Preparation and Characterization of Microencapsulated Phase Change Materials for Use in Building Applications" Materials 9, no. 1: 11. https://doi.org/10.3390/ma9010011
APA StyleGiro-Paloma, J., Al-Shannaq, R., Fernández, A. I., & Farid, M. M. (2016). Preparation and Characterization of Microencapsulated Phase Change Materials for Use in Building Applications. Materials, 9(1), 11. https://doi.org/10.3390/ma9010011









