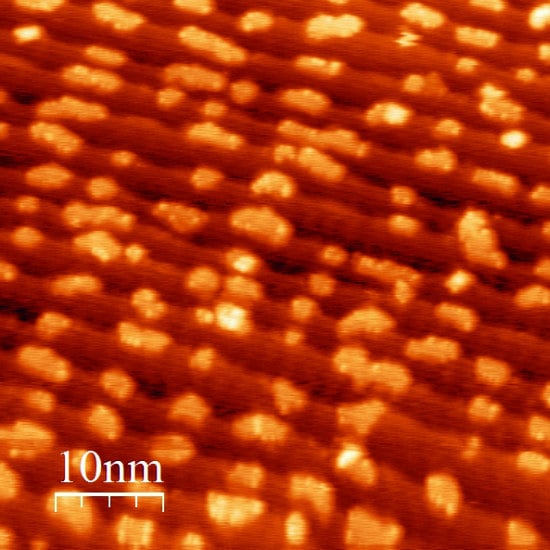
Growth of Ceria Nano-Islands on a Stepped Au(788) Surface
Abstract
:1. Introduction
2. Results and Discussion
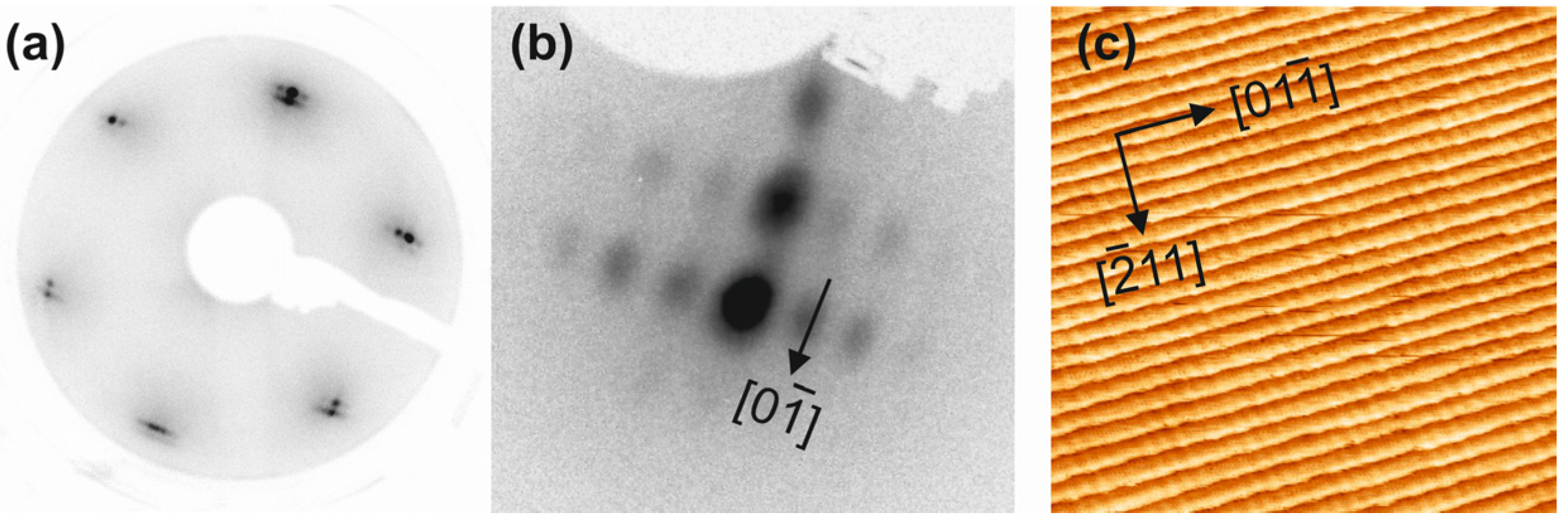
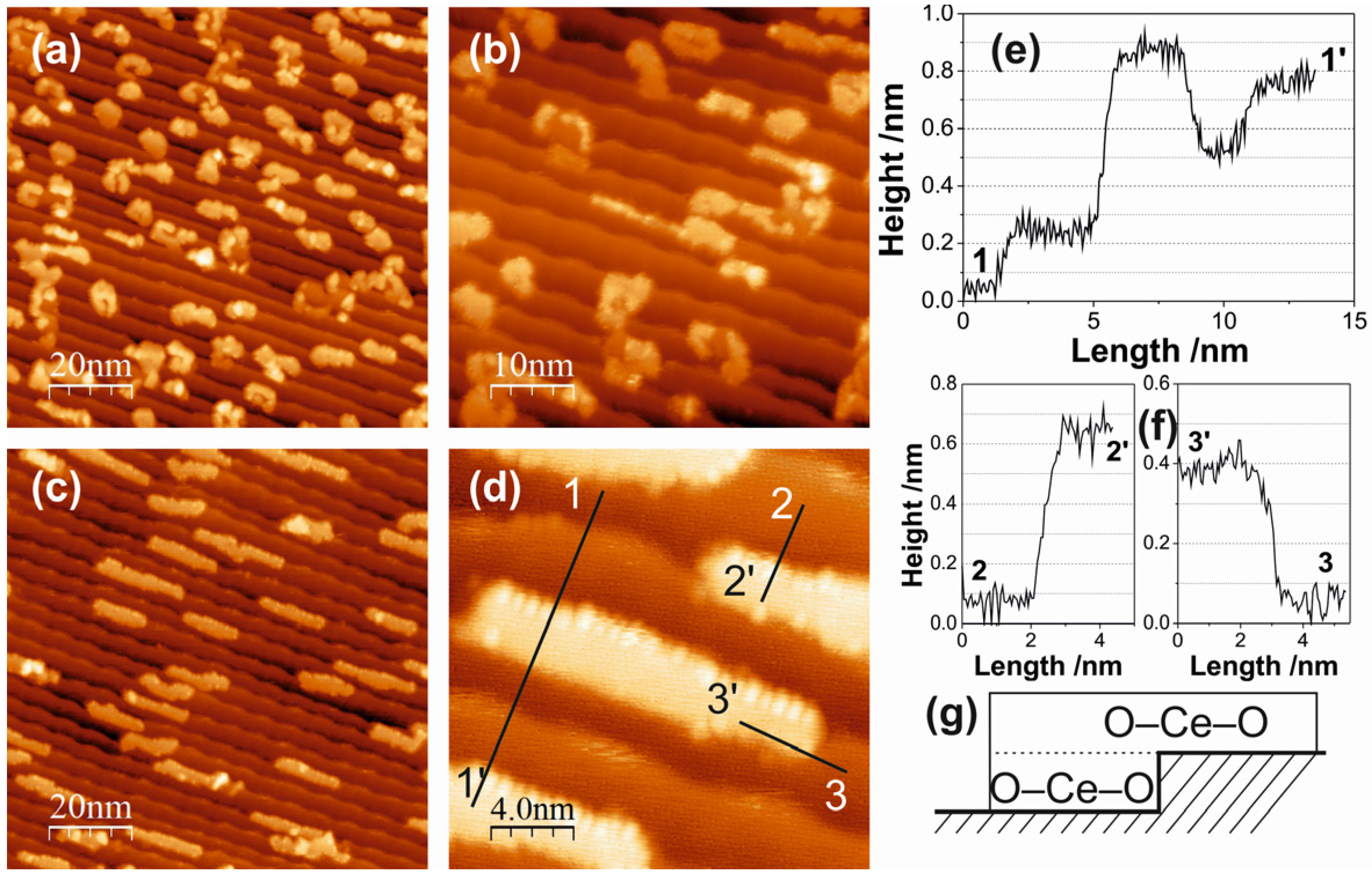
2.1. Ceria on Au(788) by Postoxidation



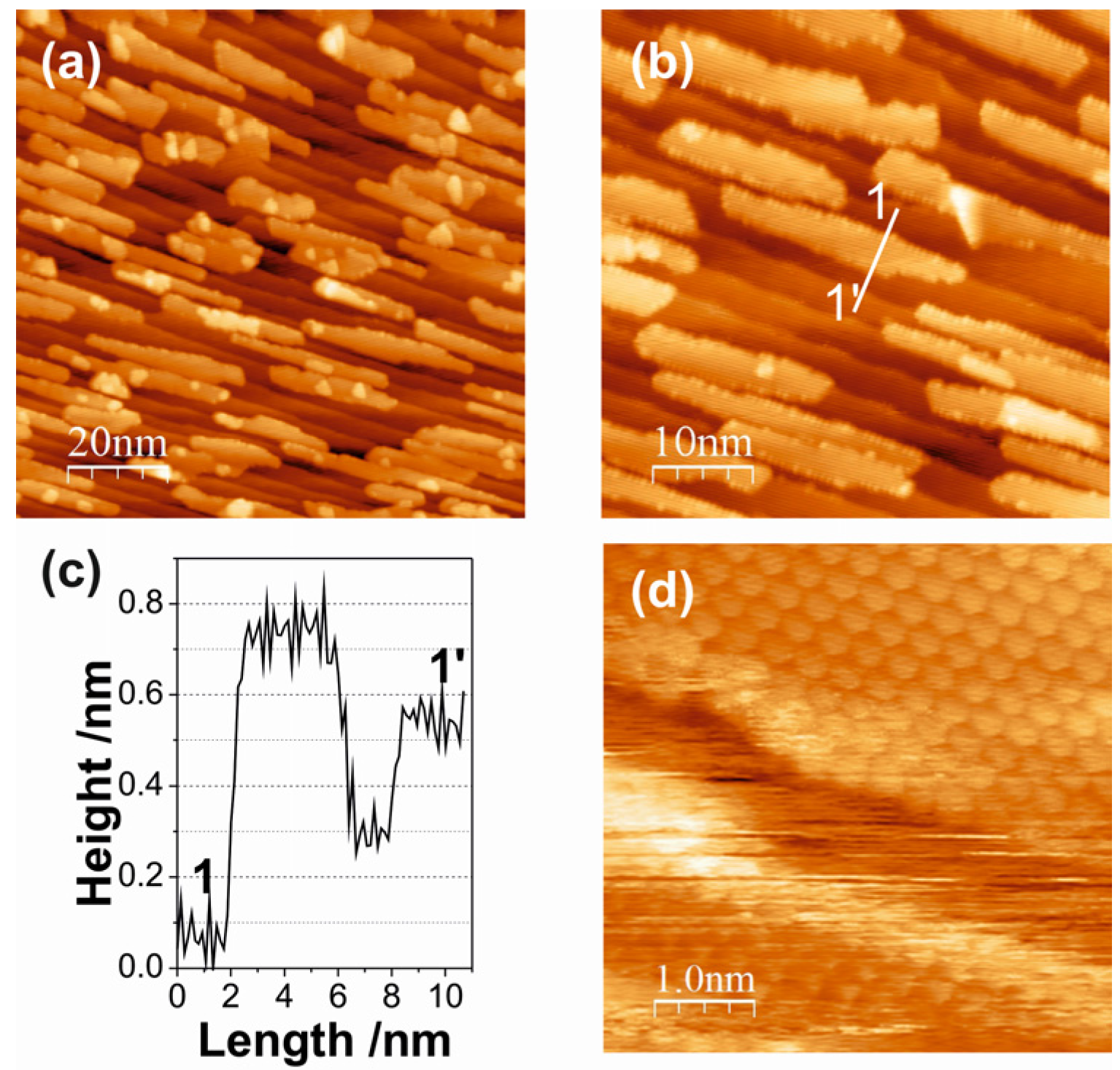
2.2. Ceria on Au(788) by Reactive Evaporation
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trovarelli, A.; Fornasiero, P. Catalysis by Ceria and Related Materials; Imperial College Press: London, UK, 2000. [Google Scholar]
- Twigg, M.V. Catalytic control of emissions from cars. Catal. Today 2011, 163, 33–41. [Google Scholar]
- Aneggi, E.; Wiater, D.; de Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-dependent activity of ceria in soot combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar]
- Satsuma, A.; Yanagihara, M.; Osaki, K.; Saeki, Y.; Liu, H.; Yamamoto, Y.; Arai, S.; Ohyama, J. Promotion of low-temperature oxidation of CO over Pd supported on titania-coated ceria. RSC Adv. 2014, 4, 54187–54193. [Google Scholar]
- Deng, W.; Flytzani-Stephanopoulos, M. On the issue of the deactivation of Au-Ceria and Pt-Ceria water-gas shift catalysts in practical fuel-cell applications. Angew. Chem. Int. Ed. 2006, 45, 2285–2289. [Google Scholar]
- Campbell, C.T.; Peden, C.H.F. Oxygen vacancies and catalysis on ceria surfaces. Science 2005, 309, 713–714. [Google Scholar] [PubMed]
- Saavedra, J.; Doan, H.A.; Pursell, C.J.; Grabow, L.C.; Chandler, B.D. The critical role of water at the gold-titania interface in catalytic CO oxidation. Science 2014, 345, 1599–1602. [Google Scholar] [PubMed]
- Rodriguez, J.A.; Ma, S.; Liu, P.; Hrbek, J.; Evans, J.; Perez, M. Activity of CeOx and TiOx nanoparticles grown on Au(111) in the water-gas shift reaction. Science 2007, 318, 1757–1760. [Google Scholar] [PubMed]
- Grinter, D.C.; Muryn, C.; Santos, B.; Shaw, B.J.; Menteş, T.O.; Locatelli, A.; Thronton, G. Spectromicroscopy of a model water-gas shift catalyst: Gold nanoparticles supported on ceria. J. Phys. Chem. C 2014, 118, 19194–19204. [Google Scholar]
- Cargnello, M.; Doan-Nguyen, V.V.T.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 2013, 341, 771–773. [Google Scholar] [PubMed]
- Leisenberger, F.P.; Surnev, S.; Koller, G.; Ramsey, M.G.; Netzer, F.P. Probing the metal sites of a vanadium oxide–Pd(111) ‘inverse catalyst’: Adsorption of CO. Surf. Sci. 2000, 444, 211–220. [Google Scholar]
- Hayek, K.; Fuchs, M.; Klötzer, B.; Reichl, W.; Rupprechter, G. Studies of metal-support interactions with “real” and “inverted” model systems: Reactions of CO and small hydrocarbons with hydrogen on noble metals in contact with oxides. Top. Catal. 2000, 13, 55–66. [Google Scholar]
- Schoiswohl, J.; Surnev, S.; Netzer, F.P. Reactions on inverse model catalyst surfaces: Atomic views by STM. Top. Catal. 2005, 36, 91–105. [Google Scholar]
- Netzer, F.P.; Allegretti, F.; Surnev, S. Low-dimensional oxide nanostructures on metals: Hybrid systems with novel properties. J. Vac. Sci. Technol. 2010, 28, 1–16. [Google Scholar]
- Alexandrou, M.; Nix, R.M. The growth, structure and stability of ceria overlayers on Pd(111). Surf. Sci. 1994, 321, 47–57. [Google Scholar]
- Mullins, D.R.; Radulovic, P.V.; Overbury, S.H. Ordered cerium oxide thin films grown on Ru(0001) and Ni(111). Surf. Sci. 1999, 429, 186–198. [Google Scholar]
- Lu, J.L.; Gao, H.J.; Shaikhutdinov, S.; Freund, H.J. Morphology and defect structure of the CeO2(111) films grown on Ru(0001) as studied by scanning tunneling microscopy. Surf. Sci. 2006, 600, 5004–5010. [Google Scholar]
- Kaemena, B.; Senanayake, S.D.; Meyer, A.; Sadowski, J.T.; Falta, J.; Flege, J.I. Growth and morphology of ceria on ruthenium (0001). J. Phys. Chem. C 2013, 117, 221–232. [Google Scholar] [CrossRef]
- Berner, U.; Schierbaum, K. Cerium oxide layers on Pt(111): A scanning tunneling microscopy study. Thin Solid Films 2001, 400, 46–49. [Google Scholar] [CrossRef]
- Breinlich, C.; Essen, J.M.; Barletta, E.; Wandelt, K. Growth, structure and electronic properties of ultrathin cerium oxide films grown on Pt(111). Thin Solid Films 2011, 519, 3752–3755. [Google Scholar] [CrossRef]
- Eck, S.; Castellarin-Cudia, C.; Surnev, S.; Ramsey, M.G.; Netzer, F.P. Growth and thermal properties of ultrathin cerium oxide layers on Rh(111). Surf. Sci. 2002, 520, 173–185. [Google Scholar] [CrossRef]
- Ma, S.; Rodriguez, J.; Hrbek, J. STM study of the growth of cerium oxide nanoparticles on Au(111). Surf. Sci. 2008, 602, 3272–3278. [Google Scholar] [CrossRef]
- Mašek, K.; Beran, J.; Matolin, V. RHEED study of the growth of cerium oxide on Cu(111). Appl. Surf. Sci. 2012, 259, 34–38. [Google Scholar] [CrossRef]
- Stetsovych, O.; Beran, J.; Dvořák, F.; Mašek, K.; Mysliveček, J.; Matolín, V. Polarity driven morphology of CeO2(100) islands on Cu(111). Appl. Surf. Sci. 2013, 285, 766–771. [Google Scholar] [CrossRef]
- Surnev, S.; Fortunelli, A.; Netzer, F.P. Structure-property relationship and chemical aspects of oxide-metal hybrid nanostructures. Chem. Rev. 2013, 113, 4314–4372. [Google Scholar] [CrossRef] [PubMed]
- Castellarin-Cudia, C.; Surnev, S.; Schneider, G.; Podlucky, R.; Ramsey, M.G.; Netzer, F.P. Strain-induced formation of arrays of catalytically active sites at the metal-oxide interface. Surf. Sci. 2004, 554, L120–L126. [Google Scholar] [CrossRef]
- Gragnaniello, L.; Ma, T.; Barcaro, G.; Sementa, L.; Negreiros, F.R.; Fortunelli, A.; Surnev, S.; Netzer, F.P. Ordered arrays of size-selected oxide nanoparticles. Phys. Rev. Lett. 2012, 108. [Google Scholar] [CrossRef]
- Rousset, S.; Repain, V.; Baudot, G.; Garreau, Y.; Lecoeur, J. Self-ordering of Au(111) vicinal surfaces and application to nanostructure organized growth. J. Phys. Condens. Matter 2003, 15. [Google Scholar] [CrossRef]
- Repain, V.; Baudot, G.; Ellmer, H.; Rousset, S. Two-dimensional long-range–ordered growth of uniform cobalt nanostructures on a Au(111) vicinal template. Europhys. Lett. 2002, 58. [Google Scholar] [CrossRef]
- Baudot, G.; Rohart, S.; Repain, V.; Ellmer, H.; Girard, Y.; Rousset, S. Temperature dependence of ordered cobalt nanodots growth on Au (788). Appl. Surf. Sci. 2003, 212–213, 360–366. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.M.; Larson, A.M.; Miller, G.P.; Pohl, K. Sharp organic interface of molecular C60 chains and a pentacene derivative SAM on Au(788): A combined STM & DFT study. Surf. Sci. 2013, 618, 78–82. [Google Scholar]
- Shiraki, S.; Fujisawa, H.; Nantoh, M.; Kawai, M. One-dimensional Fe nanostructures formed on vicinal Au(111) surfaces. J. Phys. Soc. Jpn. 2005, 74, 2033–2044. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, S.; Hrbek, J.; Rodriguez, J.A. Reaction of water with Ce–Au(111) and CeOx/Au(111) surfaces: Photoemission and STM studies. Surf. Sci. 2007, 601, 2445–2452. [Google Scholar] [CrossRef]
- Grinter, D.C.; Ithnin, R.; Pang, C.L.; Thornton, G. Defect structure of ultrathin ceria films on Pt(111): Atomic views from scanning tunnelling microscopy. J. Phys. Chem. C 2010, 114, 17036–17041. [Google Scholar] [CrossRef]
- Dvorak, F.; Stetsovych, O.; Steger, M.; Cherradi, E.; Matolínová, I.; Tsud, N.; Skoda, M.; Skala, T.; Myslivecek, J.; Matolin, V. Adjusting morphology and surface reduction of CeO2(111) thin films on Cu(111). J. Phys. Chem. C 2011, 115, 7496–7503. [Google Scholar] [CrossRef]
- Luches, P.; Pagliuca, F.; Valeri, S. Morphology, stoichiometry, and interface structure of CeO2 ultrathin films on Pt(111). J. Phys. Chem. C 2011, 115, 10718–10726. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, X.; Rodriguez, J.A.; Hrbek, J. STM and XPS study of growth of Ce on Au(111). J. Phys. Chem. C 2007, 111, 3685–3691. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Surnev, S.; Netzer, F.P. Growth of Ceria Nano-Islands on a Stepped Au(788) Surface. Materials 2015, 8, 5205-5215. https://doi.org/10.3390/ma8085205
Ma T, Surnev S, Netzer FP. Growth of Ceria Nano-Islands on a Stepped Au(788) Surface. Materials. 2015; 8(8):5205-5215. https://doi.org/10.3390/ma8085205
Chicago/Turabian StyleMa, Teng, Svetlozar Surnev, and Falko P. Netzer. 2015. "Growth of Ceria Nano-Islands on a Stepped Au(788) Surface" Materials 8, no. 8: 5205-5215. https://doi.org/10.3390/ma8085205