Nanosized Hydroxyapatite Coating on PEEK Implants Enhances Early Bone Formation: A Histological and Three-Dimensional Investigation in Rabbit Bone
Abstract
:1. Introduction
2. Results
2.1. Overall
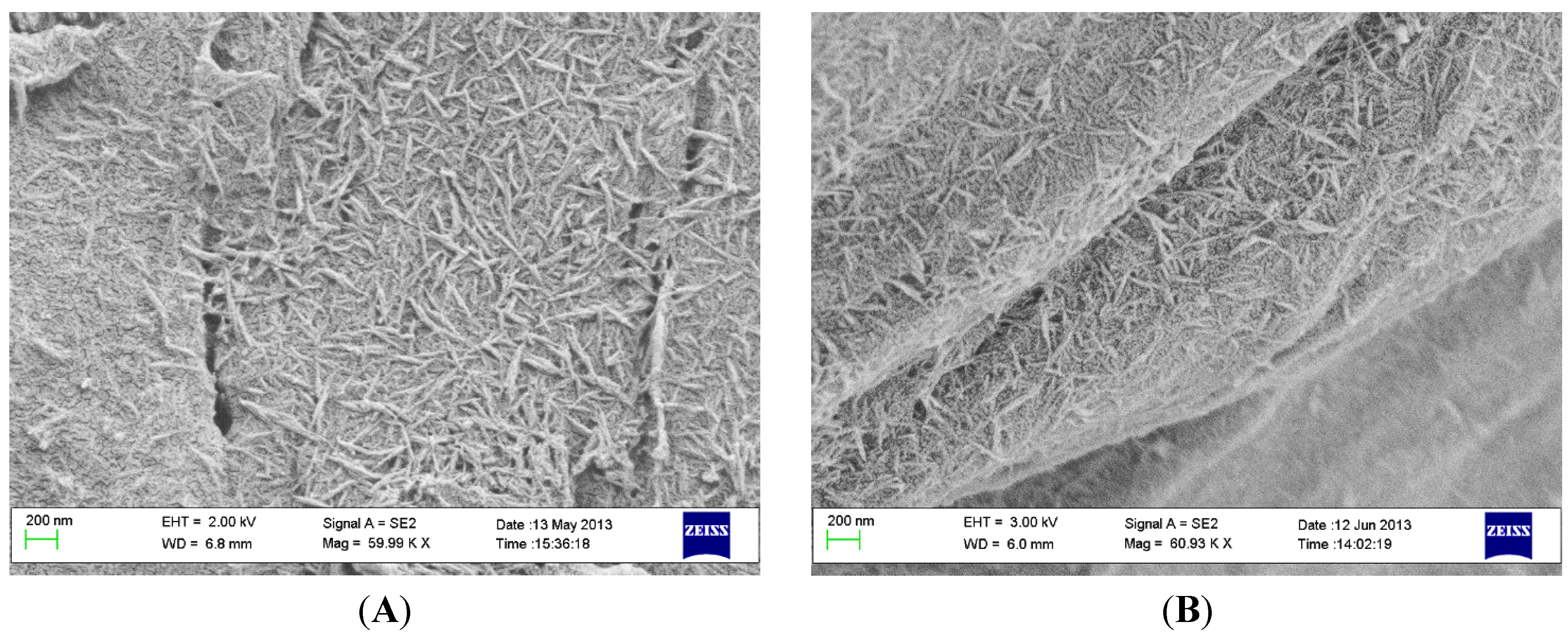
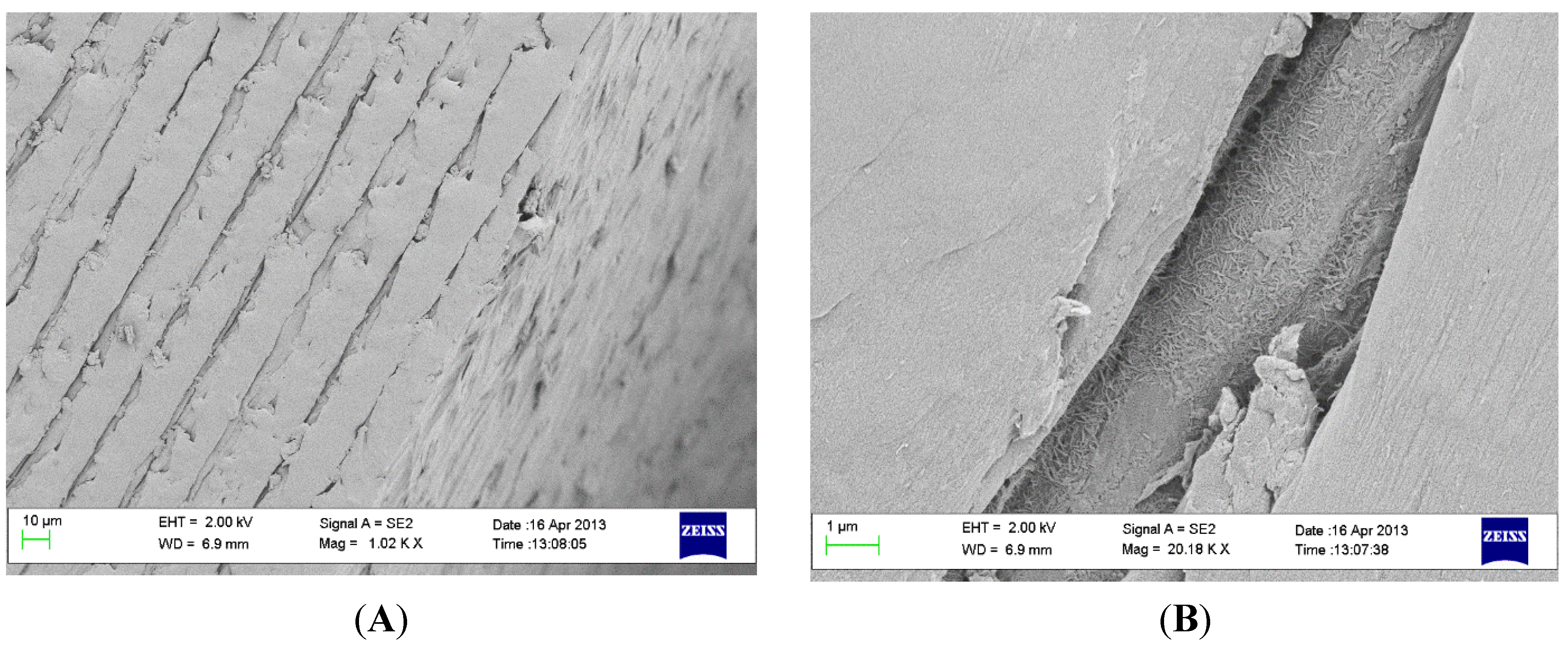
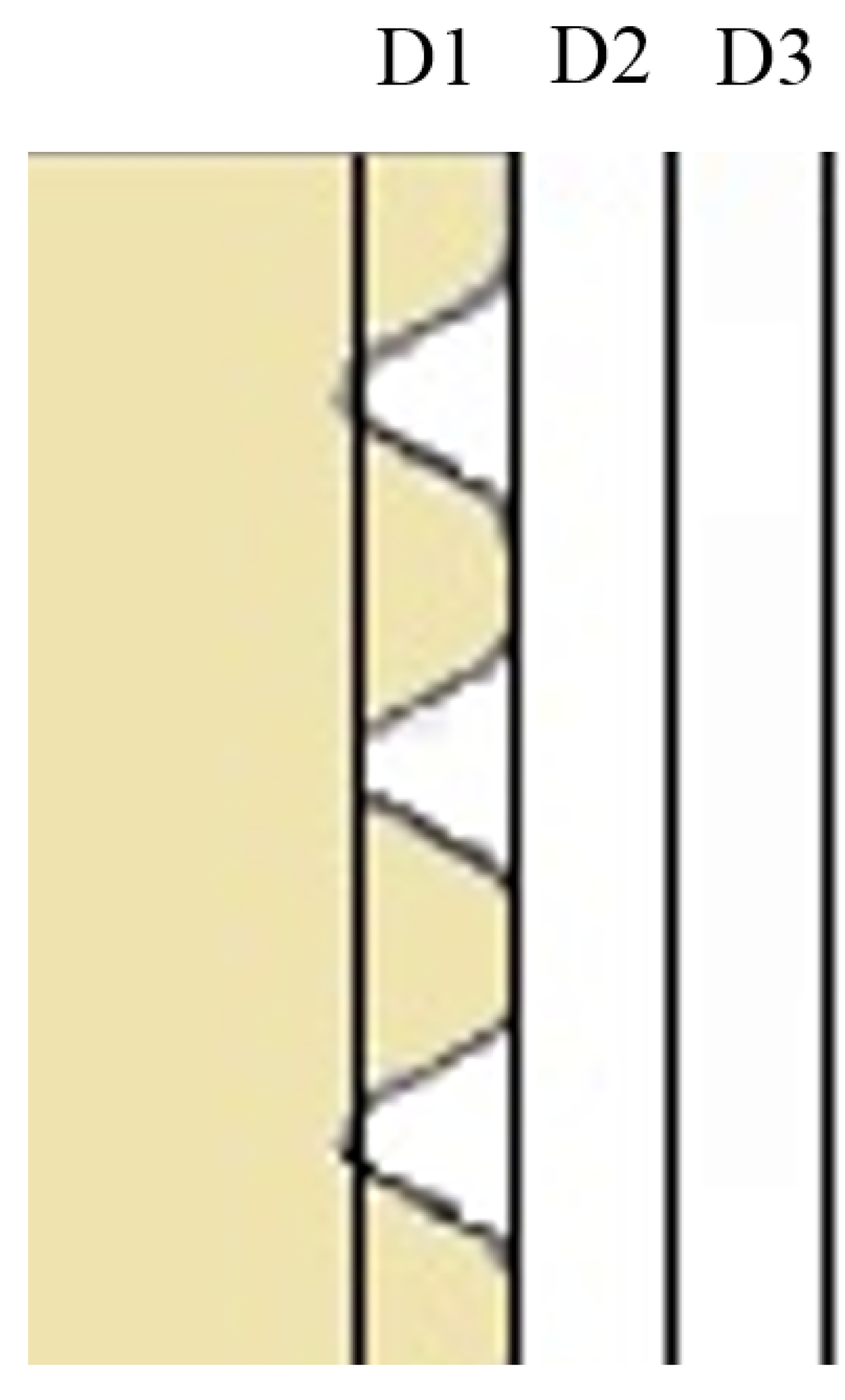
2.2. Coating Stability
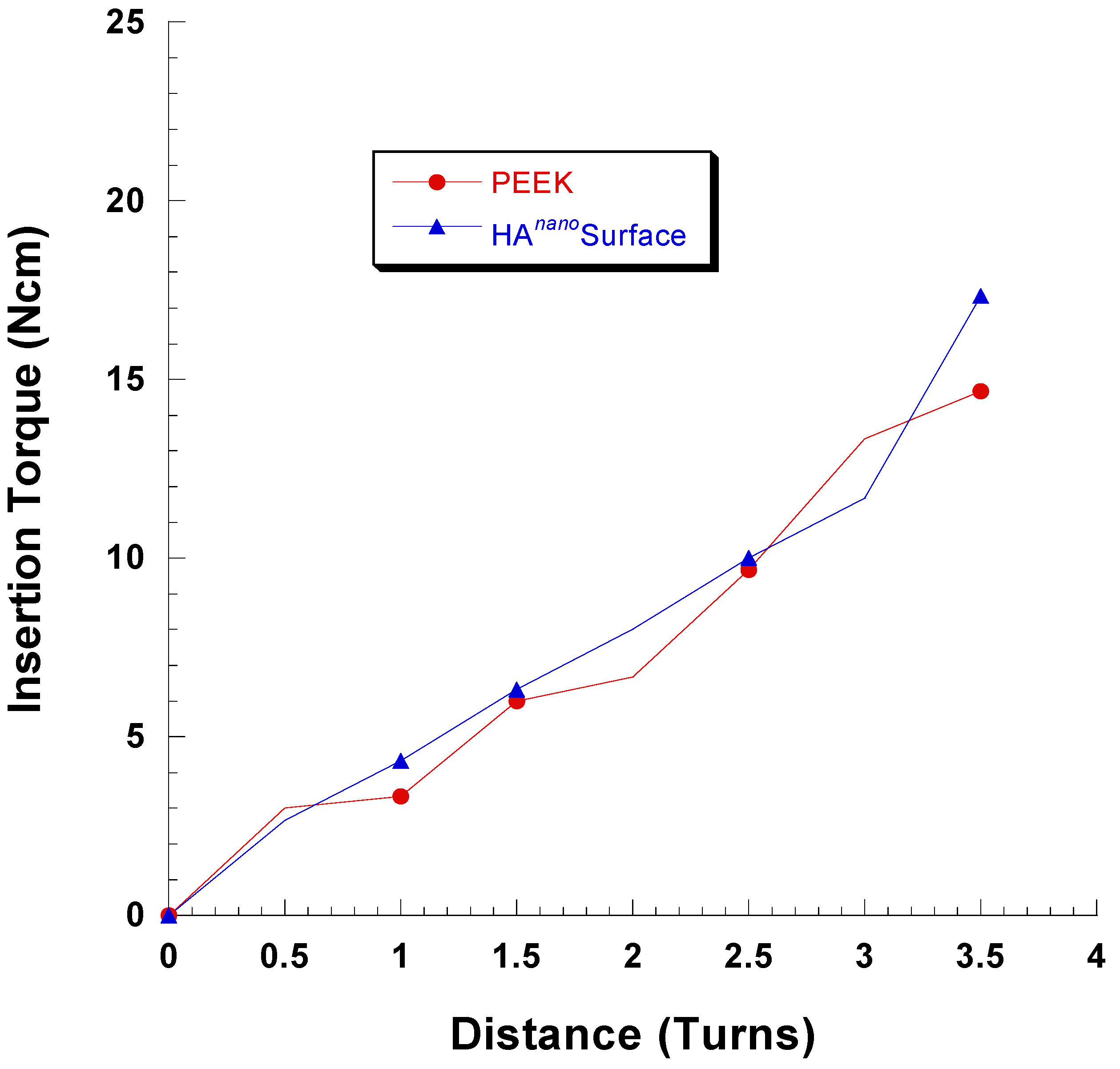
2.3. Micro-CT Evaluation
2.4. Histomorphometry
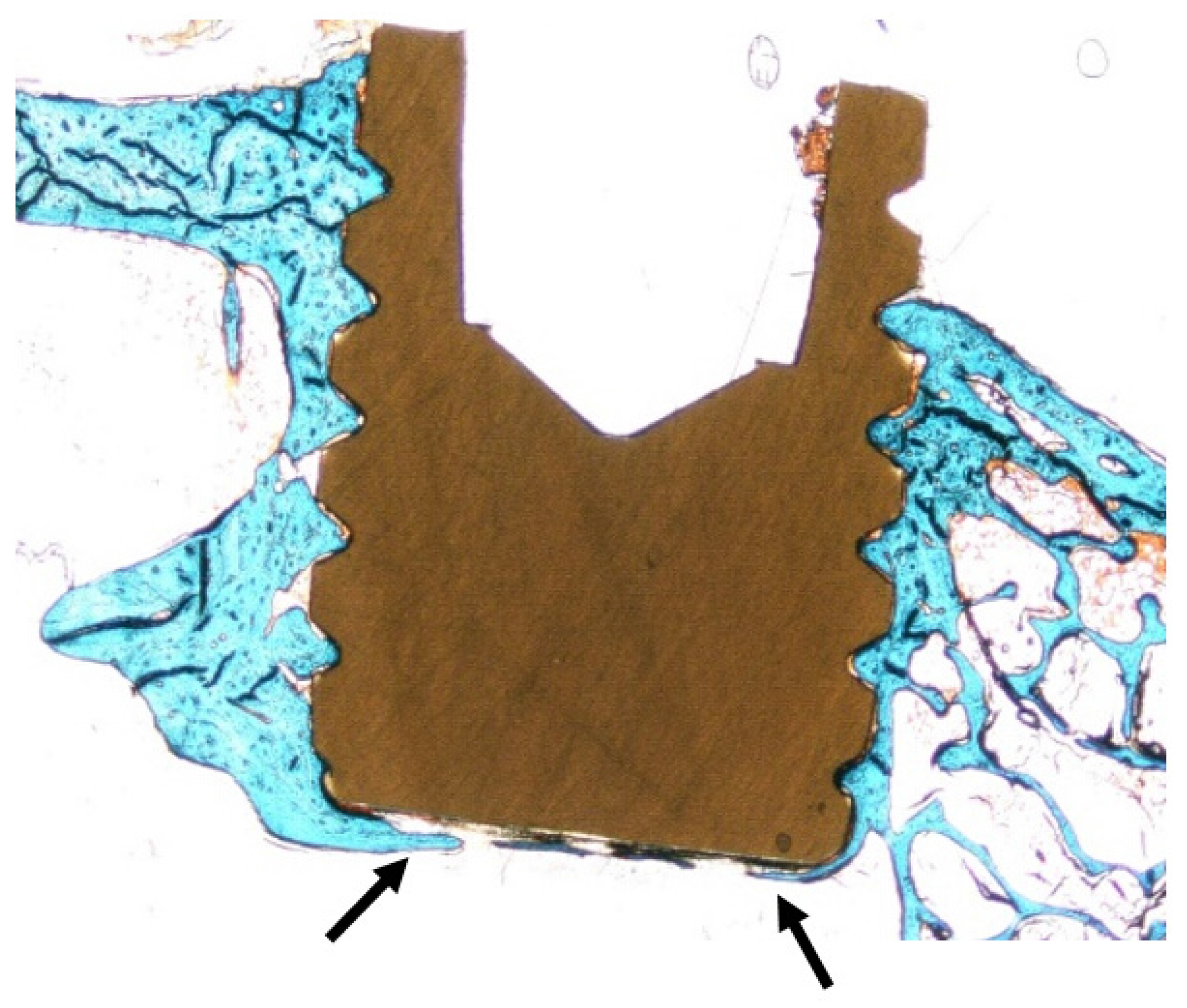
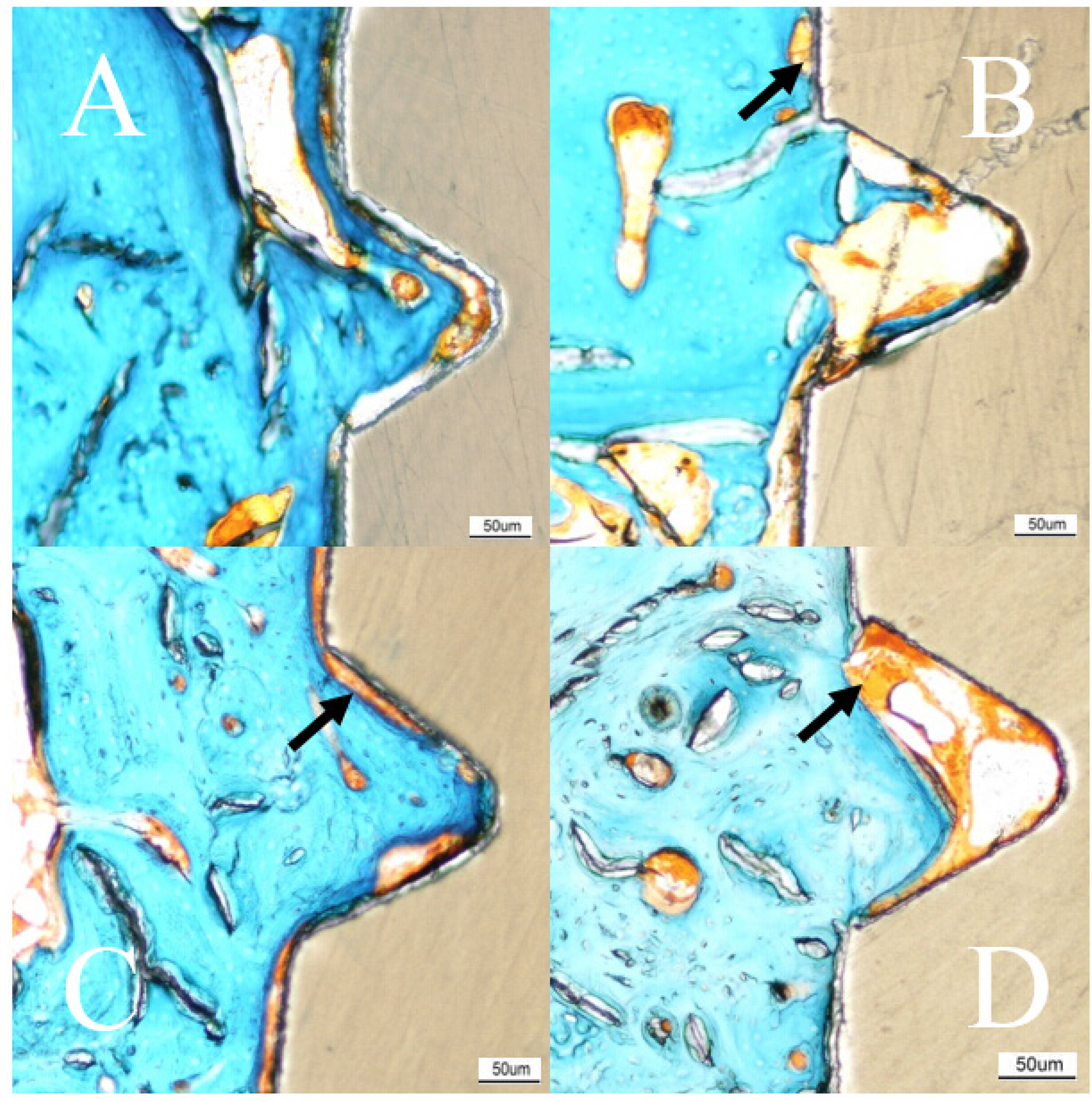
| n = 24 | Average (SD) | ||
|---|---|---|---|
| Bone-Implant Contact (BIC, %) | Bone Area (BA, %) (in Thread) | ||
| 3 weeks | Test | 7.94 (6.94) | 27.49 (10.63) |
| Control | 2.91 (1.87) | 17.91 (5.78) | |
| p-value | 0.016 | 0.027 | |
| 12 weeks | Test | 6.75 (6.52) | 48.72 (11.12) |
| Control | 4.34 (3.28) | 38.27 (9.43) | |
| p-value | 0.622 | 0.02 | |

3. Discussion
4. Experimental Section
4.1. Implant Manufacturing

4.2. Coating Preparation
4.3. Coating Adhesion
4.4. Morphological Characterization
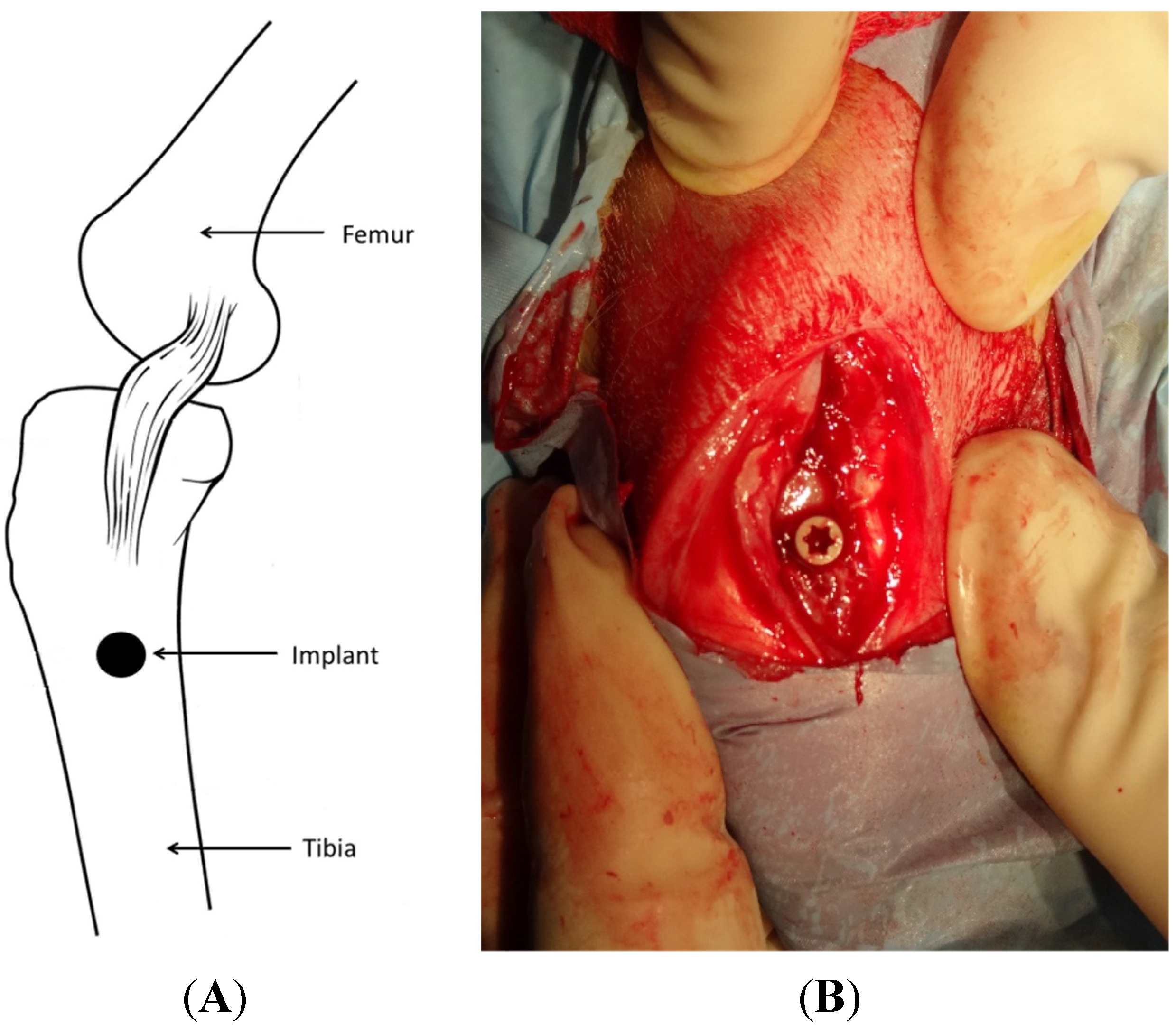
4.5. Surgical Procedure and Implantation
4.6. Micro-CT Evaluation
4.7. Histomorphometry
4.8. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Branemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindstrom, J.; Hallen, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar] [PubMed]
- Head, W.C.; Bauk, D.J.; Emerson, R.H., Jr. Titanium as the material of choice for cementless femoral components in total hip arthroplasty. Clin. Orthop. Relat. Res. 1995, 85–90. [Google Scholar]
- Treves, C.; Martinesi, M.; Stio, M.; Gutierrez, A.; Jimenez, J.A.; Lopez, M.F. In vitro biocompatibility evaluation of surface-modified titanium alloys. J. Biomed. Mater. Res. Part A 2010, 92, 1623–1634. [Google Scholar]
- Anandjiwala, J.; Seo, J.Y.; Ha, K.Y.; Oh, I.S.; Shin, D.C. Adjacent segment degeneration after instrumented posterolateral lumbar fusion: A prospective cohort study with a minimum five-year follow-up. Eur. Spine J. 2011, 20, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Garton, H.J.; Gala, V.C.; Hoff, J.T.; McGillicuddy, J.E. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine 2004, 29, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.H.; Chen, W.M.; Lee, K.Y.; Park, K.W.; Lee, S.J. Comparison of the load-sharing characteristics between pedicle-based dynamic and rigid rod devices. Biomed. Mater. 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Mantripragada, V.P.; Lecka-Czernik, B.; Ebraheim, N.A.; Jayasuriya, A.C. An overview of recent advances in designing orthopedic and craniofacial implants. J. Biomed. Mater. Res. Part A 2013, 101, 3349–3364. [Google Scholar] [CrossRef] [PubMed]
- Sagomonyants, K.B.; Jarman-Smith, M.L.; Devine, J.N.; Aronow, M.S.; Gronowicz, G.A. The in vitro response of human osteoblasts to polyetheretherketone (peek) substrates compared to commercially pure titanium. Biomaterials 2008, 29, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Devine, J.N. Peek biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Katzer, A.; Marquardt, H.; Westendorf, J.; Wening, J.V.; von Foerster, G. Polyetheretherketone—cytotoxicity and mutagenicity in vitro. Biomaterials 2002, 23, 1749–1759. [Google Scholar] [CrossRef]
- Nieminen, T.; Kallela, I.; Wuolijoki, E.; Kainulainen, H.; Hiidenheimo, I.; Rantala, I. Amorphous and crystalline polyetheretherketone: Mechanical properties and tissue reactions during a 3-year follow-up. J. Biomed. Mater. Res. Part A 2008, 84, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B., 3rd; Turner, A.S. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. Polyetheretherketone for long-term implantable devices. Med. Device Technol. 2008, 19, 10–11. [Google Scholar]
- Rabiei, A.; Sandukas, S. Processing and evaluation of bioactive coatings on polymeric implants. J. Biomed. Mater. Res. Part A 2013. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.S.; Cheng, M.H.; Tang, S.M.; Yu, S.C.; Liao, K.; Tan, C.T.; Khor, K.A.; Cheang, P. Tensile properties, tension-tension fatigue and biological response of polyetheretherketone-hydroxyapatite composites for load-bearing orthopedic implants. Biomaterials 2003, 24, 2245–2250. [Google Scholar] [CrossRef]
- Converse, G.L.; Yue, W.; Roeder, R.K. Processing and tensile properties of hydroxyapatite-whisker-reinforced polyetheretherketone. Biomaterials 2007, 28, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.P.; Tsui, C.P.; Tang, C.Y.; Chow, C.L. Influence of interphase layer on the overall elasto-plastic behaviors of ha/peek biocomposite. Biomaterials 2004, 25, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Wong, C.T.; Liu, W.C.; Pan, H.B.; Fong, M.K.; Lam, W.M.; Cheung, W.L.; Tang, W.M.; Chiu, K.Y.; Luk, K.D.; et al. Mechanical properties and in vitro response of strontium-containing hydroxyapatite/polyetheretherketone composites. Biomaterials 2009, 30, 3810–3817. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hariram, K.P.; Kumar, R.; Cheang, P.; Aik, K.K. In vitro apatite formation and its growth kinetics on hydroxyapatite/polyetheretherketone biocomposites. Biomaterials 2005, 26, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Peraire, C.; Arias, J.L.; Bernal, D.; Pou, J.; Leon, B.; Arano, A.; Roth, W. Biological stability and osteoconductivity in rabbit tibia of pulsed laser deposited hydroxylapatite coatings. J. Biomed. Mater. Res. Part A 2006, 77, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Barkarmo, S.; Wennerberg, A.; Hoffman, M.; Kjellin, P.; Breding, K.; Handa, P.; Stenport, V. Nano-hydroxyapatite-coated peek implants: A pilot study in rabbit bone. J. Biomed. Mater. Res. Part A 2013, 101, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.; Arvidsson, A.; Andersson, M.; Kjellin, P.; Albrektsson, T.; Wennerberg, A. Nano hydroxyapatite structures influence early bone formation. J. Biomed. Mater. Res. Part A 2008, 87, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Weiner, B.K.; Fraser, R.D. Spine update lumbar interbody cages. Spine 1998, 23, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Linder, E.; Larsson, L.; Berglundh, T. Deposition of nanometer scaled calcium-phosphate crystals to implants with a dual acid-etched surface does not improve early tissue integration. Clin. Oral Implants Res. 2013, 24, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Rocchietta, I.; Addis, A.; Schupbach, P.; Zanotti, G.; Simion, M. Effects of a calcium phosphate coating on the osseointegration of endosseous implants in a rabbit model. Clin. Oral Implants Res. 2011, 22, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Gobbato, L.; Arguello, E.; Martin, I.S.; Hawley, C.E.; Griffin, T.J. Early bone healing around 2 different experimental, ha grit-blasted, and dual acid-etched titanium implant surfaces. A pilot study in rabbits. Implant Dent. 2012, 21, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P. Biomechanical evaluation and surface characterization of a nano modified surface on peek implants: A study in the rabbit tibia. Int. J. Nanomed. 2014. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.G.; Jeon, G.R.; Jeong, C.M.; Kim, Y.K.; Kim, S.G.; Cho, I.H.; Cho, Y.S.; Oh, J.S. Experimental study of bone response to hydroxyapatite coating implants: Bone-implant contact and removal torque test. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Xue, Y.; Hayashi, M.; Schwartz-Filho, H.O.; Andersson, M.; Mustafa, K.; Wennerberg, A. Genetic responses to nanostructured calcium-phosphate-coated implants. J. Dent. Res. 2011, 90, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, O.; Johansson, C.; Stenport, V.; Wennerberg, A.; Reigstad, A.; Rokkum, M. Different patterns of bone fixation with hydroxyapatite and resorbable cap coatings in the rabbit tibia at 6, 12, and 52 weeks. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. Quantitative comparison of novel cao-sio2-p2o5-b2o3 glass-ceramics (bgs-7) with hydroxyapatite as bone graft extender in rabbit ilium. Tissue Eng. Regen. Med. 2010, 7, 540–547. [Google Scholar]
- Lee, J.H.; Ryu, H.S.; Lee, D.S.; Hong, K.S.; Chang, B.S.; Lee, C.K. Biomechanical and histomorphometric study on the bone-screw interface of bioactive ceramic-coated titanium screws. Biomaterials 2005, 26, 3249–3257. [Google Scholar] [PubMed]
- Kim, D.S.; Kim, D.G.; Park, C.J.; Cho, L.R. Histomorphometry and stability analysis of early loaded implants with two different surface conditions in beagle dogs. J. Adv. Prosthodont. 2009, 1, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T.; Andersson, B.; Krol, J.J. A histomorphometric and removal torque study of screw-shaped titanium implants with three different surface topographies. Clin. Oral Implants Res. 1995, 6, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Chung, H.T.; Cho, J.L.; Kim, D.J.; Chung, N.S. Subsidence of polyetheretherketone cage after minimally invasive transforaminal lumbar interbody fusion. J. Spinal Disord. Tech. 2013, 26, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Meirelles, L.; Currie, F.; Jacobsson, M.; Albrektsson, T.; Wennerberg, A. The effect of chemical and nanotopographical modifications on the early stages of osseointegration. Int. J. Oral Maxillofac. Implants 2008, 23, 641–647. [Google Scholar] [PubMed]
- Soballe, K.; Overgaard, S.; Hansen, E.S.; Brokstedt-Rasmussen, H.; Lind, M.; Bunger, C. A review of ceramic coatings for implant fixation. J. Long-Term Effects Med. Implants 1999, 9, 131–151. [Google Scholar]
- Stein, I.C.; Than, K.D.; Chen, K.S.; Wang, A.C.; Park, P. Failure of a polyether-ether-ketone expandable interbody cage following transforaminal lumbar interbody fusion. Eur. Spine J. 2014, 24, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.D.; Baron, E.M.; Levesque, M. Extrusion of expandable stacked interbody device for lumbar fusion: Case report of a complication. Spine 2012, 37, E1155–E1158. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.K.; Chien, A.; Wang, J.L. Biomechanical analysis between peek and titanium screw-rods spinal construct subjected to fatigue loading. J. Spinal Disord. Tech. 2014, 28, E121–E125. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.J.; Pelletier, M.H.; Walsh, W.R.; Mobbs, R.J. Spine interbody implants: Material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop. Surg. 2014, 6, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, O.; Asazuma, T.; Yato, Y.; Imabayashi, H.; Yasuoka, H.; Fujikawa, A. Comparison of fusion rates following transforaminal lumbar interbody fusion using polyetheretherketone cages or titanium cages with transpedicular instrumentation. Eur. Spine J. 2014, 23, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.; Alter, C.; Reichel, H.; Birke, A.; Hein, W.; Spielmann, R.P. Possibilities of follow-up imaging after implantation of a carbon fiber-reinforced hip prosthesis. Aktuelle Radiol. 1998, 8, 81–86. [Google Scholar] [PubMed]
- Numata, Y.; Sakae, T.; Nakada, H.; Suwa, T.; LeGeros, R.Z.; Okazaki, Y.; Kobayashi, K. Micro-CT analysis of rabbit cancellous bone around implants. J. Hard Tissue Biol. 2007, 16, 91–93. [Google Scholar] [CrossRef]
- Voor, M.J.; Yang, S.; Burden, R.L.; Waddell, S.W. In vivo micro-ct scanning of a rabbit distal femur: Repeatability and reproducibility. J. Biomech. 2008, 41, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kjellin, P.; Andersson, M. Synthetic Nano-Sized Crystalline Calcium Phosphate and Method of Production. US Pantent 8206813 B2, 26 June 2012. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johansson, P.; Jimbo, R.; Kozai, Y.; Sakurai, T.; Kjellin, P.; Currie, F.; Wennerberg, A. Nanosized Hydroxyapatite Coating on PEEK Implants Enhances Early Bone Formation: A Histological and Three-Dimensional Investigation in Rabbit Bone. Materials 2015, 8, 3815-3830. https://doi.org/10.3390/ma8073815
Johansson P, Jimbo R, Kozai Y, Sakurai T, Kjellin P, Currie F, Wennerberg A. Nanosized Hydroxyapatite Coating on PEEK Implants Enhances Early Bone Formation: A Histological and Three-Dimensional Investigation in Rabbit Bone. Materials. 2015; 8(7):3815-3830. https://doi.org/10.3390/ma8073815
Chicago/Turabian StyleJohansson, Pär, Ryo Jimbo, Yusuke Kozai, Takashi Sakurai, Per Kjellin, Fredrik Currie, and Ann Wennerberg. 2015. "Nanosized Hydroxyapatite Coating on PEEK Implants Enhances Early Bone Formation: A Histological and Three-Dimensional Investigation in Rabbit Bone" Materials 8, no. 7: 3815-3830. https://doi.org/10.3390/ma8073815
APA StyleJohansson, P., Jimbo, R., Kozai, Y., Sakurai, T., Kjellin, P., Currie, F., & Wennerberg, A. (2015). Nanosized Hydroxyapatite Coating on PEEK Implants Enhances Early Bone Formation: A Histological and Three-Dimensional Investigation in Rabbit Bone. Materials, 8(7), 3815-3830. https://doi.org/10.3390/ma8073815





