2.2. Microstructural Evolution and Mechanical Dehydrogenation during Ball Milling
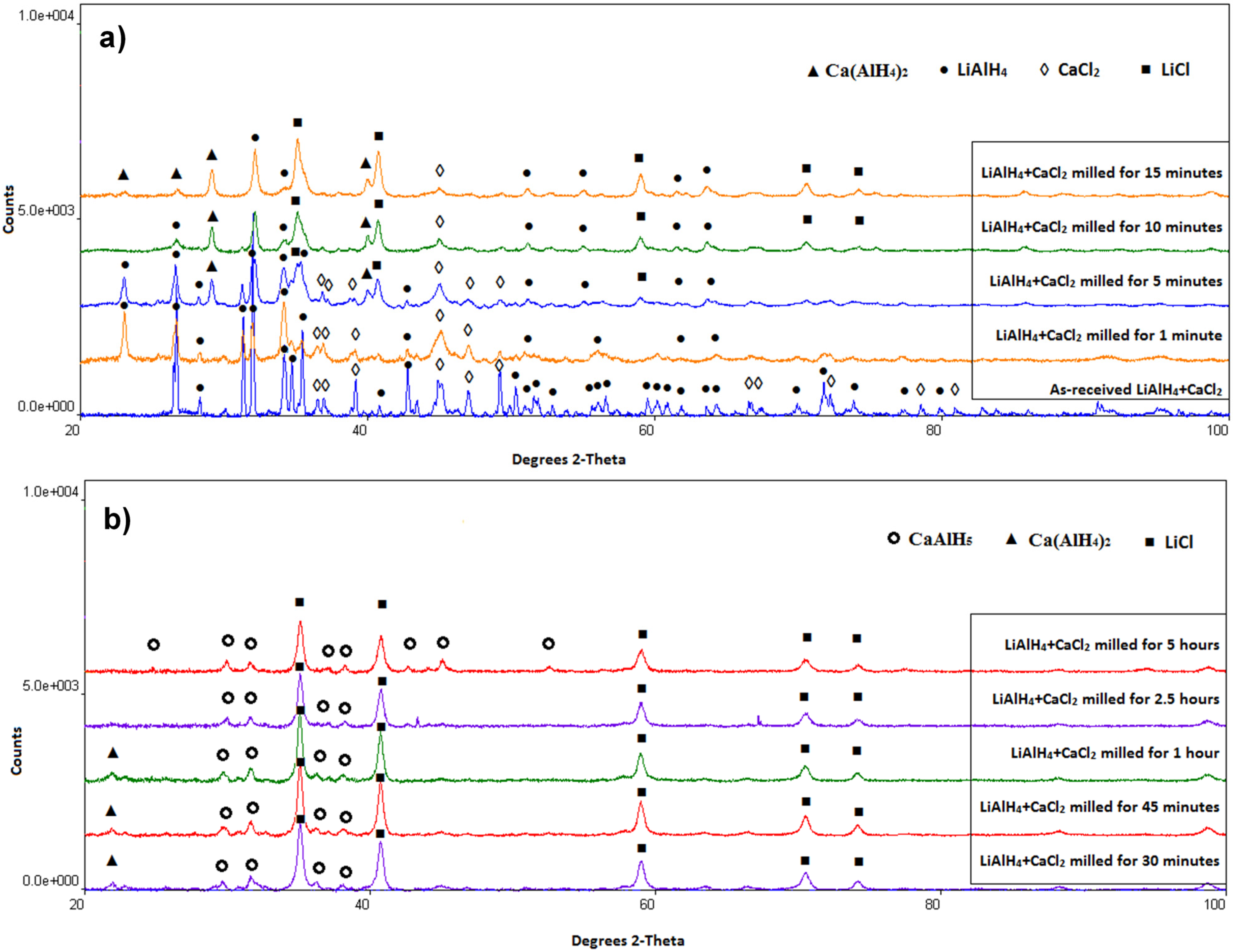
The qualitative results of XRD phase analysis for the (LiAlH
4+CaCl
2) powders after various milling durations are presented in
Figure 3. The XRD patterns for the mixture of as-received reactant powders show the presence of LiAlH
4 and CaCl
2. After barely 5 min of BM, the well-recognizable peaks of Ca(AlH
2)
4 and LiCl appear on the XRD patterns (
Figure 3a). These peaks clearly show that mechano-chemical reaction between these two reactants starts after approximately 5 min of milling. This reaction can described as follows:
Figure 3.
XRD patterns of the (2LiAlH4+CaCl2) powders after varying milling durations. (a) as-received and milling up to 15 min; (b) milling from 30 min to 5 h.
Figure 3.
XRD patterns of the (2LiAlH4+CaCl2) powders after varying milling durations. (a) as-received and milling up to 15 min; (b) milling from 30 min to 5 h.
The theoretical capacity of reaction (1) is 4.32 wt % H
2 and, accordingly, less taking into account of the practical purity of the reactants. It must be pointed out that reaction (1) was also reported to occur during mechano-chemical activation synthesis (MCAS) of Ca(AlH
4)
2 using such reactants as sodium alanate (NaAlH
4) and calcium chloride (CaCl
2) [
13,
14,
15,
16,
17]. So far, the only attempt to use LiAlH
4 as a reactant with CaCl
2 to synthesize Ca(AlH
4)
2 by MCAS was reported in [
13] but without any details.
After 0.5 h of milling (
Figure 3b), CaAlH
5 is also identified which indicates that newly formed Ca(AlH
4)
2 starts gradually decomposing during ball milling as a result of mechanical dehydrogenation [
6]. The decomposition reaction of Ca(AlH
4)
2 could be described as follows:
where the brackets indicate that 2LiCl is not taking part in the reaction being just a dead-weight for the system. The maximum theoretical quantity of H
2 that could be desorbed in reaction (2) is 1.62 wt % H
2 (slightly less if the practical purity of the reactants is taken into consideration). Apparently, the decomposition of Ca(AlH
4)
2 is associated with mechanical dehydrogenation during ball milling. After 0.5 h (30 min) of milling, only remnant peaks of Ca(AlH
4)
2 are discernible in
Figure 3b. After 2.5 and 5 h of ball milling no diffraction peaks of Ca(AlH
4)
2 are visible anymore (
Figure 3b). It must be pointed out that reaction (2) also occurs during
thermal decomposition of Ca(AlH
4)
2 [
13,
14,
15,
16,
17].
Table 1 summarizes the phases present in the microstructure after various milling durations as established from the XRD analysis. It is to be pointed out that the XRD patterns for powders ball milled from 0.5 to 5 h do not exhibit diffraction peaks of Al whose presence is required by reaction (2). Apparently, newly formed Al in reaction (2) must be in an amorphous or highly nanocrystalline state (undetectable by XRD).
Table 1.
Summary of phases present in the microstructure after various milling durations as shown in X-ray diffraction (XRD) patterns.
Table 1.
Summary of phases present in the microstructure after various milling durations as shown in X-ray diffraction (XRD) patterns.
| Milling Time | Phases Present |
|---|
| 1 min | LiAlH4; CaCl2 |
| 5 min | LiAlH4; CaCl2; Ca(AlH4)2; LiCl |
| 10 min | LiAlH4; CaCl2; Ca(AlH4)2; LiCl |
| 15 min | LiAlH4; CaCl2; Ca(AlH4)2; LiCl |
| 30 min | Ca(AlH4)2; CaAlH5; LiCl |
| 45 min | Ca(AlH4)2; CaAlH5; LiCl |
| 1 h | Ca(AlH4)2; CaAlH5; LiCl |
| 2.5 h | CaAlH5; LiCl |
| 5 h | CaAlH5; LiCl |
2.3. Thermal Dehydrogenation
Thermal dehydrogenation process of the (2LiAlH
4+CaCl
2) ball milled powders was investigated by differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA).
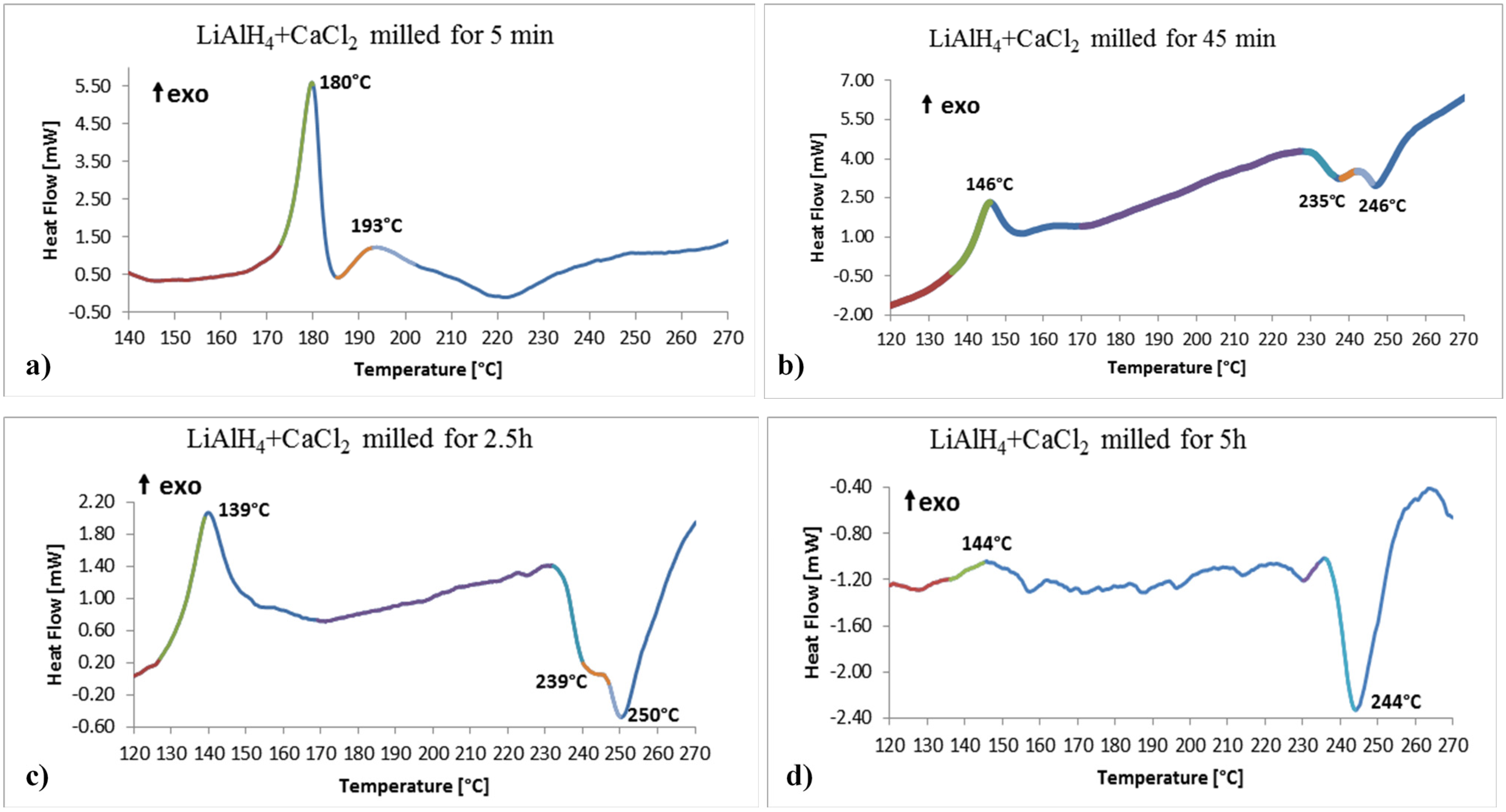
Figure 4 shows the selected examples of DSC curves as a function of ball milling time. For the powder milled for barely 5 min, the first thermal peak is exothermic at around 180 °C (
Figure 4a).
This peak temperature correlates very well with temperature for the first exothermic decomposition peak of LiAlH
4 according to the well-known reaction [
3,
4]:
In order to confirm additionally the origin of the first exothermic peak in
Figure 4a, we conducted a DSC run for as-received LiAlH
4 which exhibited a strong exothermic peak with a temperature maximum at 172 °C (a DSC curve not shown here). This confirms well that the first peak in
Figure 4a is indeed related to the decomposition process of LiAlH
4 according to reaction (3). Theoretical capacity of this reaction is 5.3 wt % H
2 and correspondingly less in the present case because LiAlH
4 coexists with other phases like CaCl
2, Ca(AlH
4)
2 and LiCl (
Table 1), although LiAlH
4 is still a majority phase in the mixture, which agrees with a high intensity of its DSC peak in
Figure 4a. The shape of the LiAlH
4 decomposition peak suggests that reaction occurs in solid state instead of going through endothermic melting before decomposition as occurs for as-received LiAlH
4 without additives [
18]. The small exothermic peak with the temperature maximum at 193 °C is most likely due to the decomposition of newly formed Ca(AlH
4)
2, co-existing in the mixture with the other phases as shown in
Table 1. Since Ca(AlH
4)
2 is formed in a small quantity after barely 5 min BM, its DSC thermal peak is small, too. It is well known that the first decomposition reaction of Ca(AlH
4)
2 according to reaction (2) is exothermic [
13,
14,
15,
16,
17]. Experimentally observed and reported in the literature DSC peaks for thermal reaction (2) are centered around 150 °C, although on occasions the peak is broad, covering a wide temperature range of 100–160 °C [
16,
17]. The present peak temperature at 193 °C is slightly higher, perhaps, because it overlaps with the beginning of thermolysis of Li
3AlH
6 as discussed below.
Figure 4.
Selected DSC traces for the (2LiAlH4+CaCl2) powders after varying milling durations. (a) 5 min; (b) 45 min; (c) 2.5 h and (d) 5 h. Heating rate 5 °C/min.
Figure 4.
Selected DSC traces for the (2LiAlH4+CaCl2) powders after varying milling durations. (a) 5 min; (b) 45 min; (c) 2.5 h and (d) 5 h. Heating rate 5 °C/min.
The third DSC peak in
Figure 4a is
endothermic, very broad, with the shallow maximum at around 220 °C. It can be assigned to the simultaneous decomposition of both Li
3AlH
6 and CaAlH
5 according to the following reactions [
3,
4,
13,
14,
15,
16,
17]:
Experimentally observed DSC temperature ranges for reactions (4) and (5) are within 180–260 °C [
3,
4,
18] and 220–270 °C [
15,
16,
17], respectively, which agrees well with thermal events depicted in
Figure 4a.
Figure 3a shows that the diffraction intensity of LiAH
4 peaks decrease after milling for 10 and 15 min because more of them are consumed in reaction (1) forming Ca(AlH
4)
2. Accordingly, the obtained DSC curves (not shown here) exhibited a principal exothermic peak having a peak maximum temperature slightly shifted to 172 °C and 158 °C for 10 and 15 min ball milled powders, respectively, which corresponds to the decomposition of retained LiAlH
4 according to reaction (3). Starting from 30 min milling, there is no LiAlH
4 in the microstructure (
Figure 3b and
Table 1) and the exothermic peak of the decomposition of retained Ca(AlH
4)
2 in the powder ball milled for 45 min, according to reaction (4), is shifted to ~146 °C (
Figure 4b) which is in good agreement with the values reported in the literature being in the range of 100–160 °C [
16,
17]. The most interesting is a DSC curve in
Figure 4c for a powder milled for 2.5 h. It shows pretty strong exothermic peak with the maximum at 139 °C. Apparently, its temperature corresponds very well to the decomposition temperature range of Ca(AlH
4)
2. However, the corresponding XRD pattern in
Figure 3b shows no peak corresponding to Ca(AlH
4)
2. This apparent discrepancy can possibly be explained by proposing a hypothesis that a fraction of Ca(AlH
4)
2 mechanically dehydrogenates during ball milling and the other fraction becomes disordered/amorphous, which is undetected by XRD but it still decomposes during DSC measurements. This hypothesis is supported by the shape of a DSC curve for a powder ball milled for 5 h in
Figure 4d which shows a small hump at 144 °C most likely corresponding to the decomposition of retained disordered/amorphous Ca(AlH
4)
2. Finally, the endothermic DSC peaks in
Figure 4b,c, correspond to the decomposition of CaAlH
5 according to reaction (5).
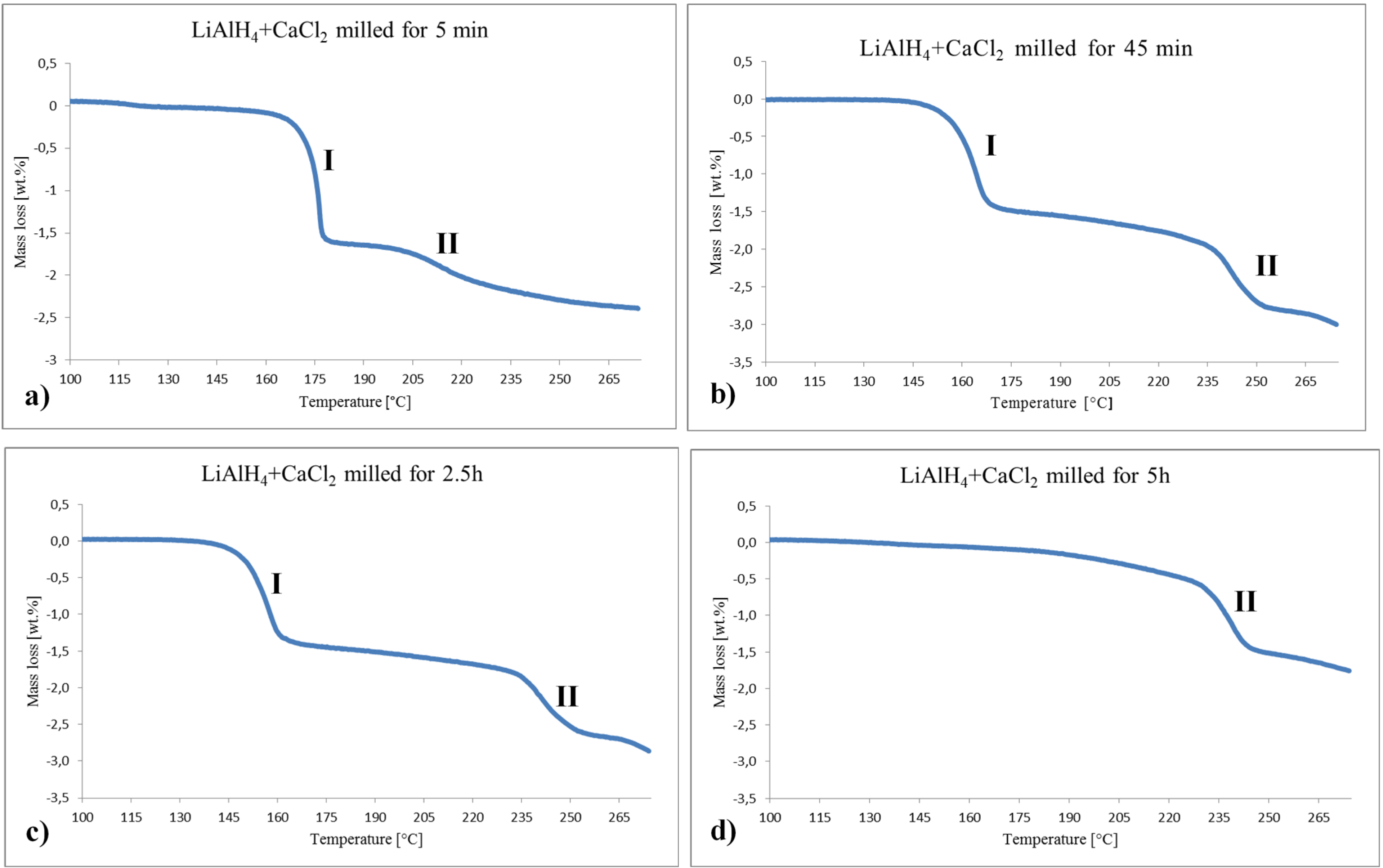
Figure 5 shows selected curves of TGA, exactly corresponding to the DSC curves in
Figure 4. The curves for the powders milled for 5 min, 45 min and 2.5 h exhibit Stage I and II of mass loss. Apparently, Stage I in
Figure 5a corresponds to the decomposition of LiAlH
4 (reaction (3)) and that in
Figure 5b,c corresponds to the decomposition of both the crystalline and disordered/amorphous Ca(AlH
4)
2 fractions (reaction (2)). There is no clearly visible Stage I in
Figure 5d after ball milling for 5 h which confirms that the retained quantity of likely amorphous Ca(AlH
4)
2 is nearly negligible. Stage II in
Figure 5a corresponds to the decomposition of Li
3AlH
6 (reaction (4)) and in
Figure 5b–d to the decomposition of CaAlH
5.
Table 2 summarizes the mass losses in both Stage I and II measured between the inflection points on the TGA curves for each stage and the temperature ranges for both stages. For the 5, 10 and 15 min samples, the values of mass loss are between 1.2 and 1.5 wt %. The observed mass losses correspond to thermolysis of both LiAlH
4 and Ca(AlH
4)
2 (
Figure 3a and
Table 1), so it is hard to estimate how much of the mass loss is related to the mechanical dehydrogenation of Ca(AlH
4)
2 during BM assuming that the observed mass loss in Stage I is fully due to thermal dehydrogenation. However, samples ball milled for 30 min, 45 min and 1 h exhibit thermal mass loss of 1.1, 1.3 and 1.4 wt %, respectively. Assuming that this mass loss directly corresponds to thermal dehydrogenation and the maximum theoretical quantity of mechanically dehydrogenated H
2 in reaction (2) is 1.62 wt % H
2, then one can estimate that about 0.5, 0.3 and 0.2 wt % H
2 was mechanically desorbed during ball milling from samples ball milled for 30 min, 45 min and 1 h, respectively. Therefore, it seems that the mechanical dehydrogenation phenomenon is not quite predominant in the (Ca(AlH
4)
2+LiCl) hydride nanocomposite studied in the present work. Furthermore, the sample milled for 2.5 h shows 1.2 wt % mass loss corresponding to 1.2 wt % H
2 desorbed from this sample in which, most likely, Ca(AlH
4)
2 exists as a disordered/amorphous hydride. Accordingly, it shows that about 0.4 wt % H
2 was mechanically dehydrogenated during ball milling for 5 h, confirming a rather modest occurrence of the mechanical dehydrogenation phenomenon in this study. It is to be pointed out that the mechano-chemical synthesis was carried out in the present work using a Fritsch Pulverisette 7 planetary mill. The milling energy generated by this type of mill is relatively modest by comparison with the magneto-mill Uni-Ball Mill 5 used for inducing a profound mechanical dehydrogenation reported in [
3,
4,
6,
7,
8,
9,
10,
11,
12]. In addition, the milling time up to 5 h used in the present work might have been too short to provide a sufficient milling energy input per gram of powder (kJ/g) to induce more pronounced mechanical dehydrogenation in a Fritsch Pulverisette 7 planetary mill. It would be of interest to repeat mechanical dehydrogenation studies of the present system in the magneto-mill Uni-Ball Mill 5 and compare the results.
Figure 5.
Selected TGA traces for the (2LiAlH4+CaCl2) powders after various milling durations. (a) 5 min; (b) 45 min; (c) 2.5 h and (d) 5 h. Heating rate 5 °C/min.
Figure 5.
Selected TGA traces for the (2LiAlH4+CaCl2) powders after various milling durations. (a) 5 min; (b) 45 min; (c) 2.5 h and (d) 5 h. Heating rate 5 °C/min.
Stage II of TGA is related to the decomposition of CaAlH
5 according to reaction (5) when it is a single phase. However, in the mixture with LiCl reaction (5) is modified to:
The theoretical capacity of reaction (6) is 1.65 wt % H
2. Assuming that mass losses for Stage II in
Table 2 are due to the hydrogen release; then, approximately only about 50% of the total H
2 capacity of reaction (6) is dehydrogenated in Stage II. Apparently, CaAlH
5 seems to be rather stable at a quite elevated temperature range 230–250 °C under a heating rate of 5 °C/min.
Table 2.
Summary of thermal gravimetric analysis (TGA) mass loss (wt %) at Stage I and II of decomposition of hydride nanocomposites after varying milling time and the temperature ranges of both stages.
Table 2.
Summary of thermal gravimetric analysis (TGA) mass loss (wt %) at Stage I and II of decomposition of hydride nanocomposites after varying milling time and the temperature ranges of both stages.
| Milling Time | Mass loss Stage I (wt %) | Mass Loss Stage II (wt %) | Stage I Range (°C) | Stage II Range (°C) |
|---|
| 5 min | 1.5 | 0.4 | 160–175 | 205–235 |
| 10 min | 1.3 | 0.8 | 160–175 | 205–235 |
| 15 min | 1.2 | 0.9 | 145–160 | 230–250 |
| 30 min | 1.1 | 0.7 | 145–160 | 230–250 |
| 45 min | 1.3 | 0.9 | 150–170 | 235–250 |
| 1 h | 1.4 | 0.9 | 147–165 | 235–250 |
| 2.5 h | 1.2 | 0.8 | 140–160 | 235–250 |
| 5 h | ~0.0 | 1.0 | - | 230–250 |
2.4. Apparent Activation Energy
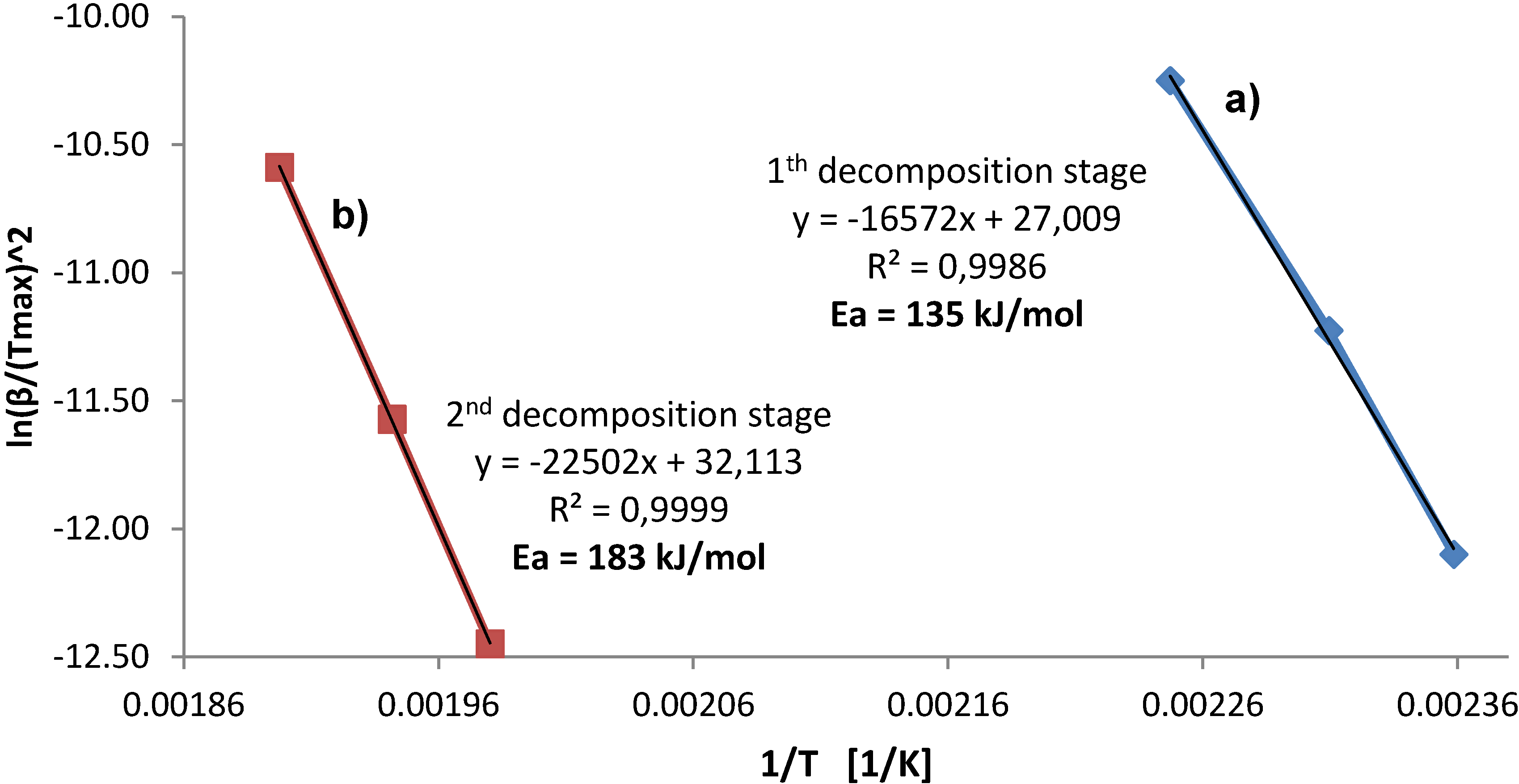
The apparent activation energy,
Ea, of decomposition processes occurring in DSC in
Figure 4, was estimated in
Figure 6 for the sample ball milled for 45 min, by the Kissinger method [
19] plotting the maximum reaction rate ln(β/(
T2max) as a function of (1/
T), where β is the heating rate (K/min) and
Tmax is the peak temperature in K, carried out with varying heating rates of 1, 2.5 and 7 °C/min for DSC measurements. The reaction rates were obtained from the slopes of the tangent lines at the inflection points of DSC peaks corresponding to H
2 desorption.
Figure 6.
Kissinger plots for (
a) first and (
b) second stage of calcium alanate decomposition for the sample milled for 45 min (
Figure 4b).
Figure 6.
Kissinger plots for (
a) first and (
b) second stage of calcium alanate decomposition for the sample milled for 45 min (
Figure 4b).
Apparently, a good linearity between ln(β/(
T2max) and 1/
T is obtained with
R2 = 0.99. The apparent activation energy equals 135 kJ/mol and 183 kJ/mol for the first decomposition of Ca(AlH
4)
2 and the second decomposition of CaAlH
5, respectively. Unfortunately, we were unable to find in the literature a value of the apparent activation energy for the Ca(AlH
4)
2 decomposition stage obtained in other studies. In contrast, the literature reported values of the apparent activation energy for the CaAlH
5 decomposition are reported as 161 kJ/mol [
20] and 153.4 kJ/mol [
16,
17]. The value of the apparent activation energy of 183 kJ/mol is close to the reported range although slightly higher. This relatively high value explains the fact that only about 50% of the total H
2 capacity of reaction (6) is desorbed in Stage II, as discussed earlier, pointing towards an enhanced thermal stability of CaAlH
5. It is hypothesized that a disordered/amorphous fraction of Ca(AlH
4)
2, which likely coexists with CaAlH
5 after ball milling for 2.5 and 5 h, could be responsible for a sluggishness of CaAlH
5 thermal decomposition as mentioned earlier.