A Novel Schiff Base of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran-2-one and 2,2'-(ethylenedioxy)diethylamine as Potential Corrosion Inhibitor for Mild Steel in Acidic Medium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of SB
| IR (KBr cm−1) | 1665 (C=N), 3454 (OH), 1703 (C=O),1254 (C-O), 1358 (C-N) | |
| 1H NMR (CDCl3, δ, ppm) | 2.11 (s, 3H, -C=C-CH3); 2.50 (s, 3H, N=C-CH3); 5.62 (s, 1H, CH3=C-H); 3.27δ (s, 4H); 3.70δ (m, 6H); 13.80δ (s, 1H, enolic OH) | |
| 13C NMR (CDCl3, δ, ppm) | 18.27, 19.58 (-CH3), 183.66 (O-C=O); 176.03 (C-OH); 162.47 (H3C-C-O); 107.49 (C=N); 68.52 (-CH2-O); 44.12 (CH2 –N) | |
| Elemental analysis | Experimental | C=58.32; H=6.24; N=6.25 |
| Calculated | C=58.92; H=5.98; N=6.44 | |
2.2. Electrochemical Measurements
2.2.1. Open Circuit Potential (OCP)
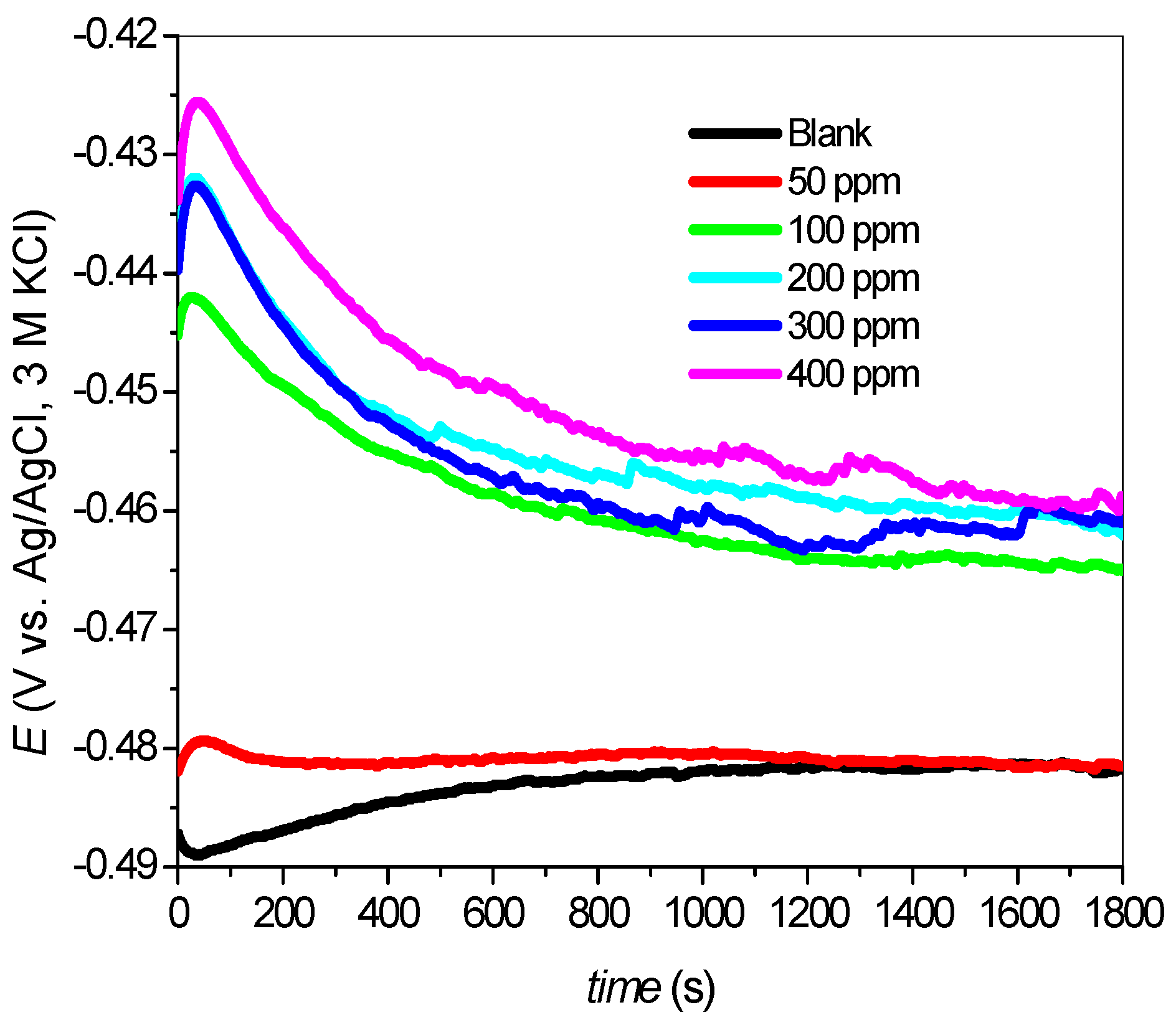
2.2.2. Potentiodynamic Polarization Measurements
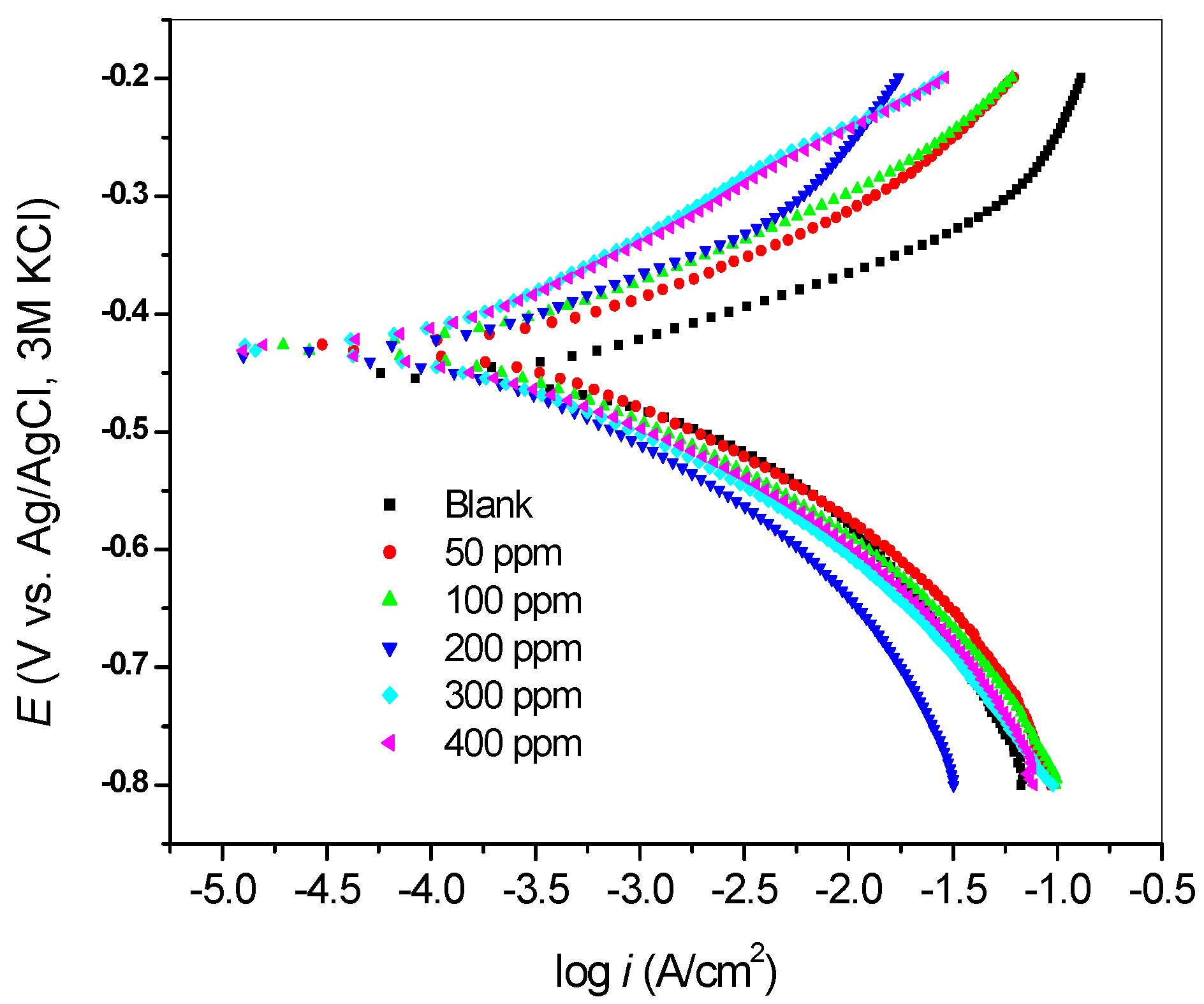
| Inhibitor Concentration (ppm) | −E (mV) | icorr (µA/cm2) | βa (mV/dec) | βc (mV/dec) | Rp (Ω) | %IE |
|---|---|---|---|---|---|---|
| Blank | 452 | 529.7 | 58 | 102 | 4.832 | - |
| 50 | 428 | 310.9 | 71 | 94 | 9.34 | 41.31 |
| 100 | 428 | 214.6 | 72 | 97 | 14.16 | 59.49 |
| 200 | 434 | 189.6 | 82 | 108 | 20.41 | 64.21 |
| 300 | 428 | 172.0 | 107 | 101 | 27.5 | 67.53 |
| 400 | 429 | 102.7 | 86 | 73 | 26.44 | 80.61 |
2.2.3. Electrochemical Impedance Spectroscopy (Eis) Measurements
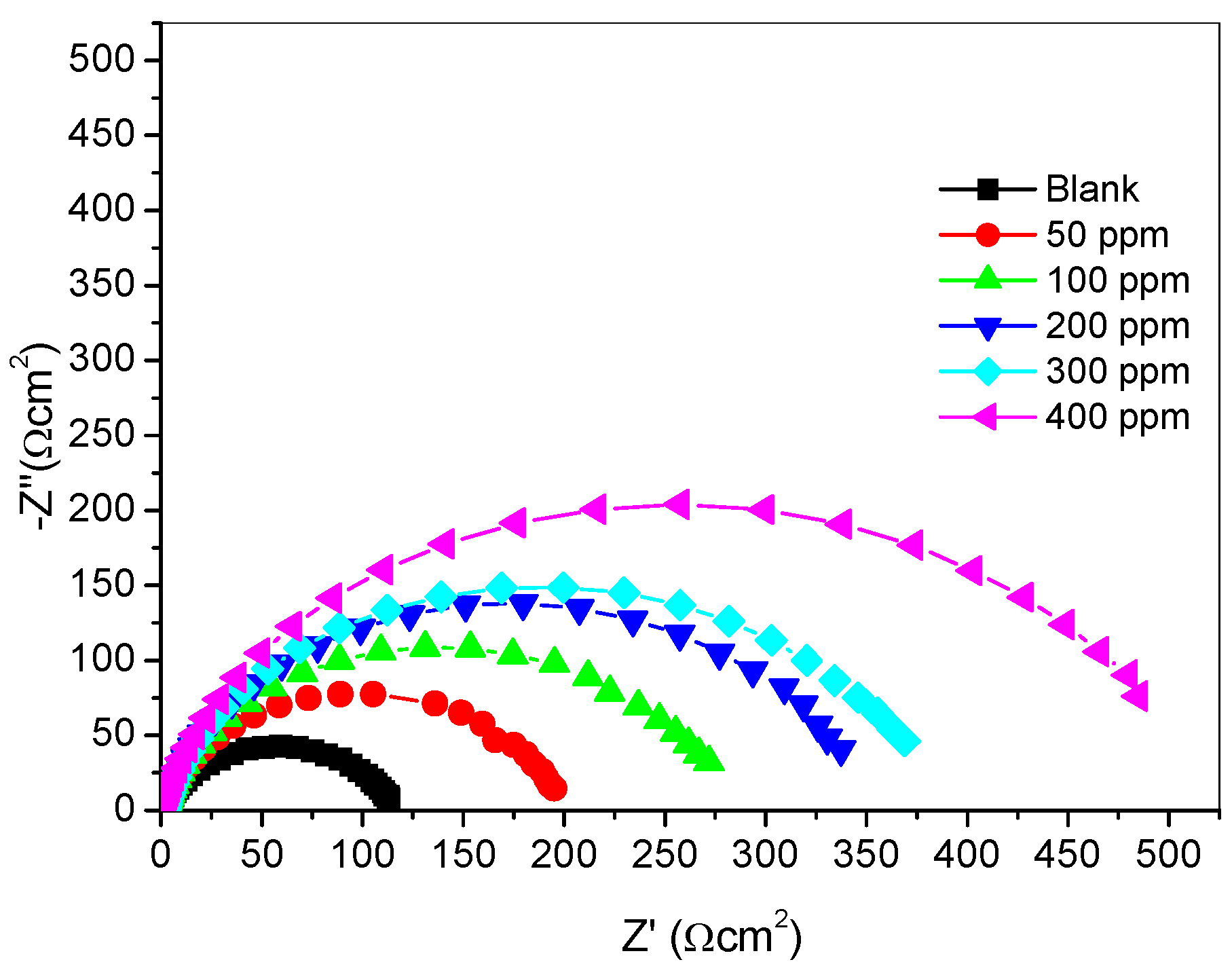
| Inhibitor Concentration (ppm) | Rs (Ωcm−2) | Rct (Ωcm−2) | Q1 (Y0) (µFcm−2) | n | % IEEIS |
|---|---|---|---|---|---|
| Blank | 1.947 | 115.30 | 145.00 | 0.8027 | - |
| 50 | 2.570 | 193.00 | 85.58 | 0.8756 | 40.26 |
| 100 | 1.808 | 274.10 | 82.21 | 0.8651 | 57.94 |
| 200 | 1.937 | 343.00 | 71.67 | 0.8736 | 66.38 |
| 300 | 1.754 | 374.00 | 64.57 | 0.8707 | 69.17 |
| 400 | 1.504 | 506.00 | 61.56 | 0.8718 | 77.21 |
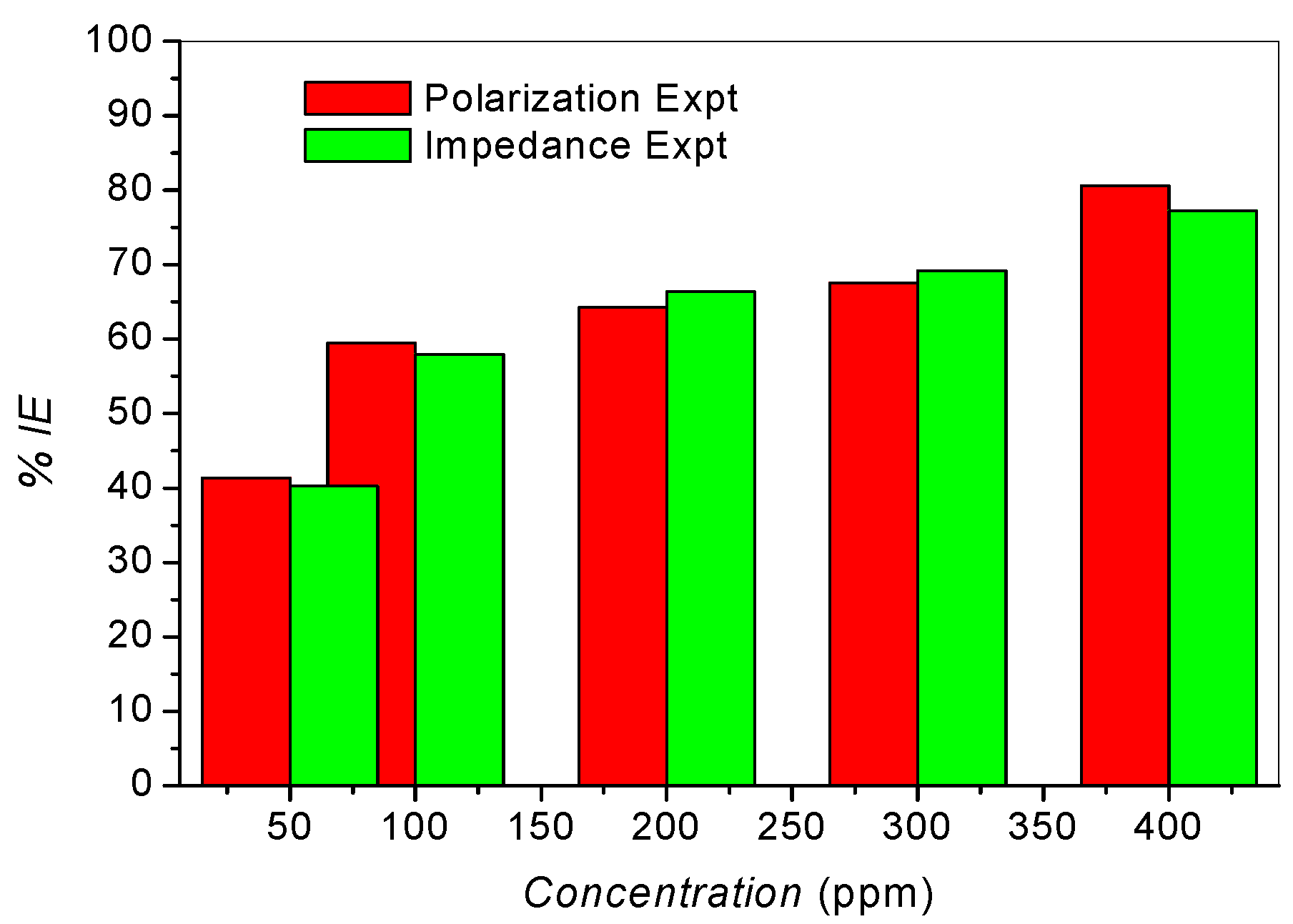
2.3. Adsorption Isotherms
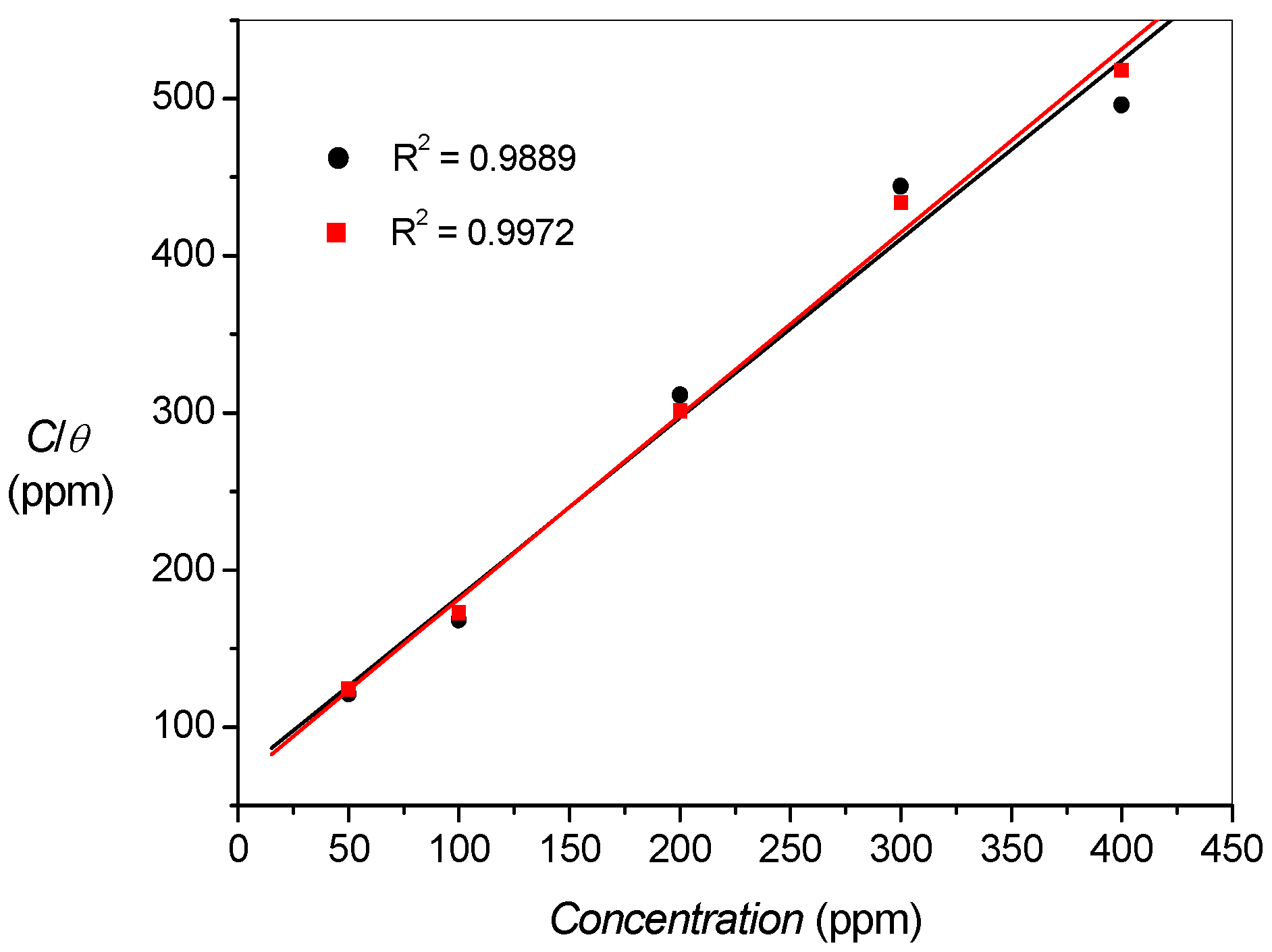
| Title | Kads (×10−3) | − ∆Gads (kJ/mol) |
|---|---|---|
| Polarization Expt. | 6.47 | 31.71 |
| Impedance Expt. | 6.89 | 31.86 |
2.4. Ultraviolet-Visible and Raman Spectroscopic Studies
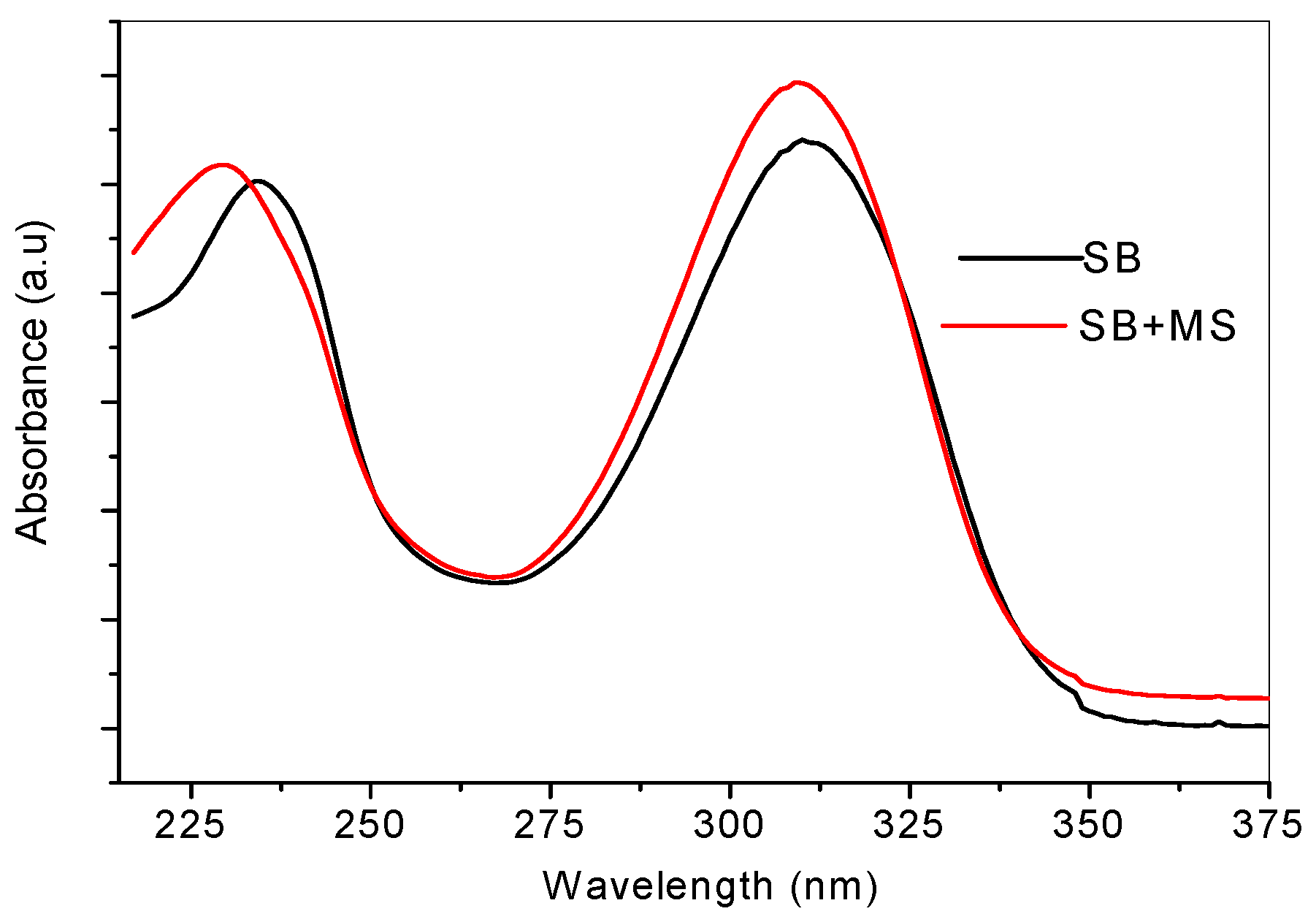
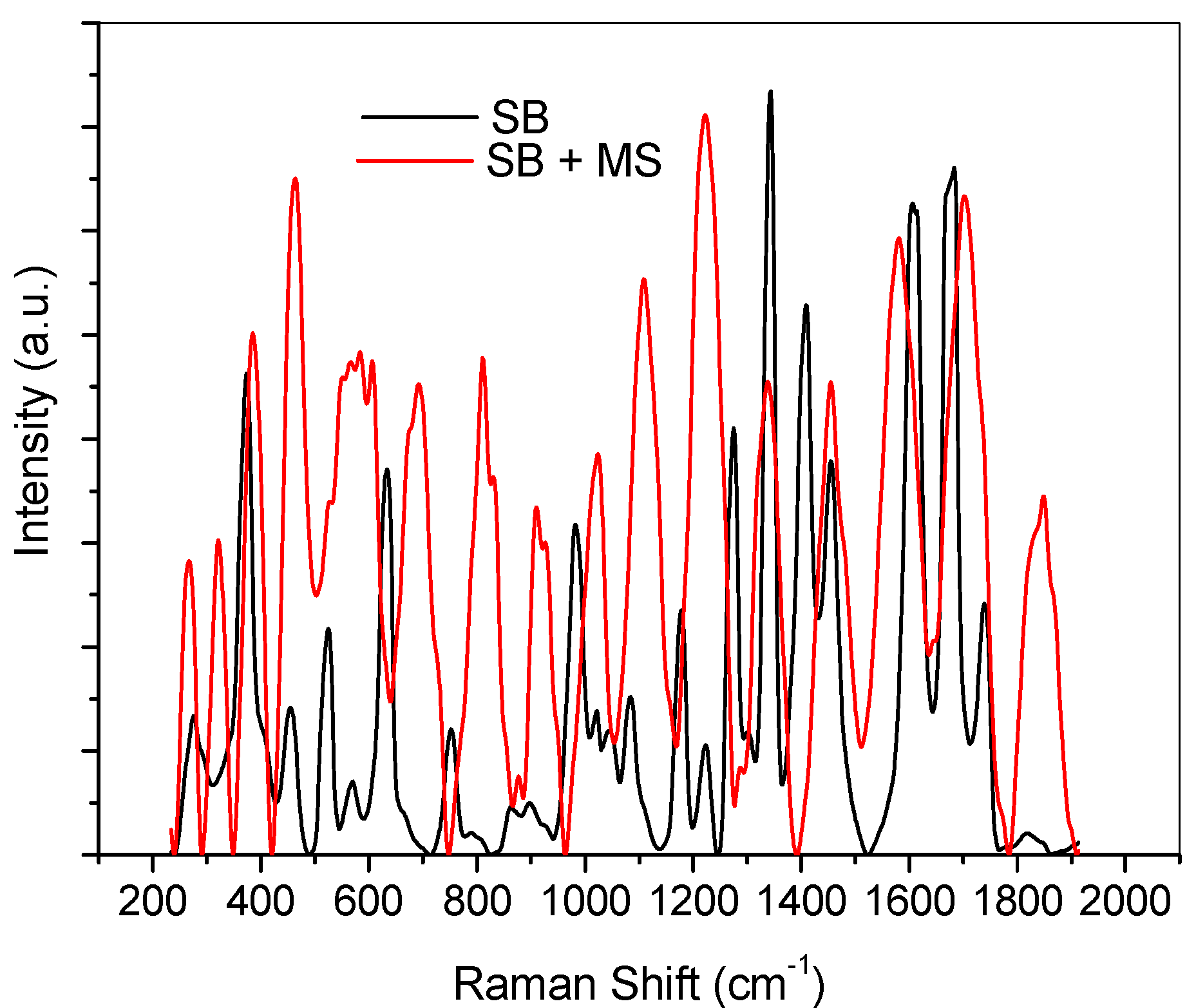
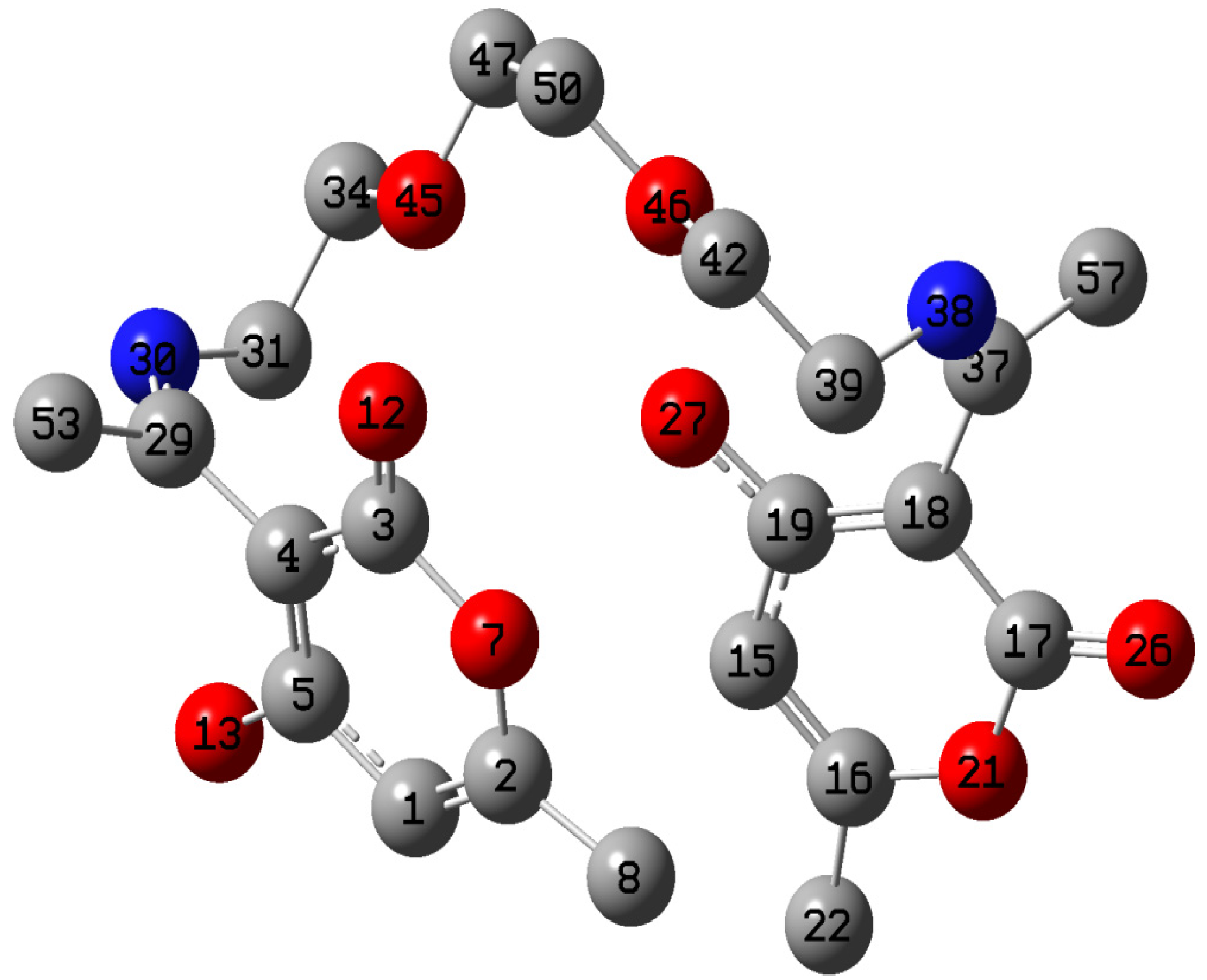
2.5. Quantum Chemical Calculations
| Parameter | Value |
|---|---|
| Total energy (au) | −1566.4527 |
| EHOMO (eV) | −5.99 |
| ELUMO (eV) | −1.46 |
| ∆ELUMO-HOMO | 4.53 |
| I | 5.99 |
| A | 1.46 |
| Dipole moment (Debye) | 5.69 |
| Chemical potential, µ (eV) | −3.72 |
| Hardness, η (eV) | 2.26 |
| Electrophilicity, ω (eV) | 3.06 |
| ∆N | 0.72 |
| ∆E (eV) | -0.57 |
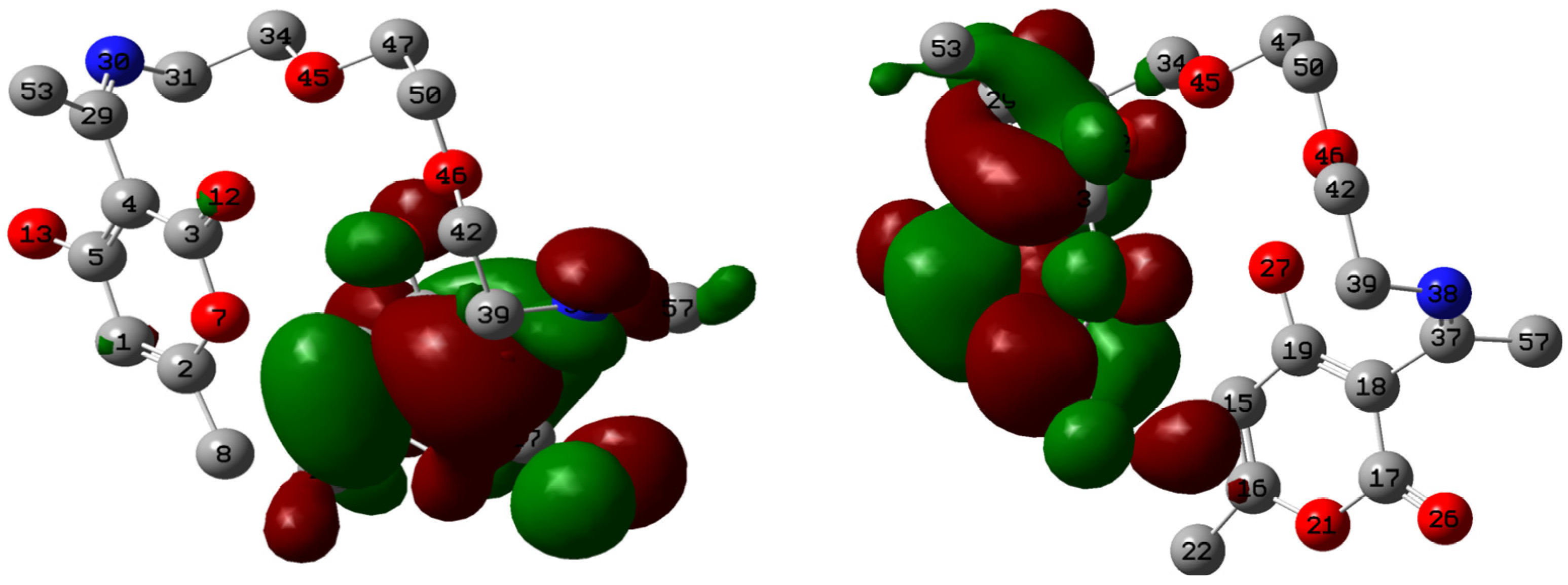
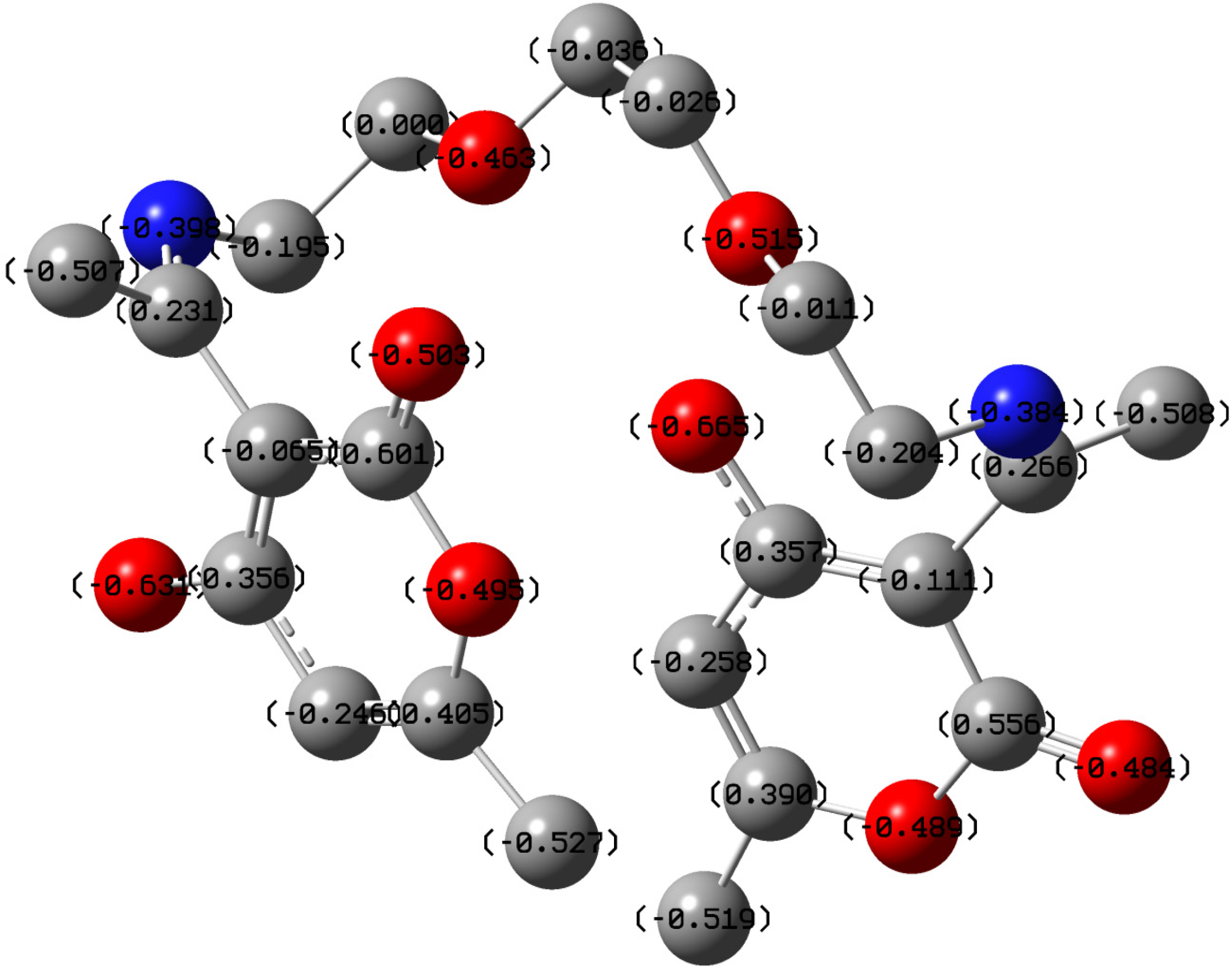
| Atom | f+ | Atom | f− |
|---|---|---|---|
| C1 | 0.0013 | C8 | 0.0022 |
| C8 | 0.0098 | C22 | 0.0085 |
| C15 | 0.0104 | C29 | 0.0054 |
| C22 | 0.0072 | C31 | 0.0102 |
| C31 | 0.0182 | C34 | 0.0097 |
| C34 | 0.0076 | C37 | 0.0005 |
| C39 | 0.0040 | C39 | 0.0163 |
| C42 | 0.0081 | C42 | 0.0077 |
| O45 | 0.0215 | O45 | 0.0135 |
| O46 | 0.0078 | O46 | 0.0088 |
| C47 | 0.0127 | C47 | 0.0114 |
| C50 | 0.0102 | C50 | 0.0136 |
| C53 | 0.0076 | C53 | 0.0042 |
| C57 | 0.0052 | C57 | 0.0072 |
3. Experimental Section
3.1. Materials
3.2. Chemicals and Instrumentation
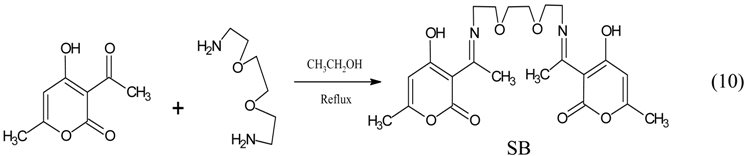
3.3. Synthesis of 3-acetyl-4-hydroxy-6-methyl-(2H) pyran-2-one Schiff base (SB)
3.4. Electrochemical Measurements
3.5. Quantum Chemical Calculations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De la Fuente, D.; Diaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Ekpe, U.J.; Chidiebere, M.A.; Oguzie, E.E. Corrosion inhibition of mild steel in hydrochloric acid by acid extracts of Eichhornia crassipes. Int. J. Mat. Chem. 2012, 2, 158–164. [Google Scholar]
- Guzman-Lucero, D.; Olivares-Xometl, O.; Martinez-Palou, R.; Likhanova, N.V.; Dominguez-Aguilar, M.A.; Garibay-Febles, V. Synthesis of selected vinylimidazolium ionic liquids and their effectiveness as corrosion inhibitors for carbon steel in aqueous sulfuric acid. Ind. Eng. Chem. Res. 2011, 50, 7129–7140. [Google Scholar] [CrossRef]
- Sastri, V.S. Corrosion Inhibitors Principles and Applications; Jonh Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Mishra, M.; Tiwari, K.; Singh, A.K.; Singh, V.P. Synthesis, structural and corrosion inhibition studies on Mn(II), Cu(II) and Zn(II) complexes with a Schiff base derived from 2-hydroxypropiophenone. Polyhedron 2014, 77, 57–65. [Google Scholar]
- Quan, Z.; Chen, S.; Li, S. Protection of copper corrosion by modification of self-assembled films of Schiff bases with alkanethiol. Corros. Sci. 2001, 43, 1071–1080. [Google Scholar] [CrossRef]
- Battaini, G.; Monzani, E.; Casella, L.; Santagostini, L.; Pagliarin, R. Inhibition of the catecholase activity of biomimetic dinuclear copper complexes by kojic acid. J. Biol. Inorg. Chem. 2000, 5, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Puerta, D.T.; Cohen, S.M. Examination of novel zinc-binding groups for use in matrix metalloproteinase inhibitors. Inorg. Chem. 2003, 42, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Narasaiah, A.V. Synthesis, characterization and biological studies of oxovanadium(IV), manganese(II), iron(II), cobalt(II), nickle(II) and copper(II) complexes derived from a quadridentate ligand. Indian J. Chem. A 2003, 42, 1896–1899. [Google Scholar]
- Chalaca, M.Z.; Figueroa-Villar, J.D.; Ellena, J.A.; Castellano, E.E. Synthesis and structure of cadmium and zinc complexes of dehydroacetic acid. Inorg. Chem. Acta 2002, 328, 45–52. [Google Scholar] [CrossRef]
- Fouad, D.M.; Bayoumi, A.; El-Gahami, M.A.; Ibrahim, S.A.; Hammam, A.M. Synthesis and thermal studies of mixed ligand complexes of Cu(II), Co(II), Ni(II) and Cd(II) with mercaptotriazoles and dehydroacetic acid. Natural Science 2010, 2, 817–827. [Google Scholar]
- Chitrapriya, N.; Mahalingam, V.; Zeller, M.; Jayabalan, R.; Swaminathan, K.; Natarajan, K. Synthesis, crystal structure and biological activities of dehydroacetic acid complexes of Ru(II) and Ru(III) containing PPh3/AsPh3. Polyhedron 2008, 27, 939–946. [Google Scholar]
- Asegbeloyin, J.N.; Ujam, O.T.; Ngige, C.M.; Onwukeme, V.I.; Groutso, T. Crystal structure of N'-[(1E)-1-(6-methyl-2,4-dioxo-3,4-dihydro-2H-pyran-3-ylidene)ethyl]benzenesulfonohydr-azide. Acta Cryst. 2014, E70, 01179–01180. [Google Scholar]
- Kubaisi, A.A.; Ismail, K.Z. Nickel(II) and palladium(II) chelates of dehydroacetic acid Schiff bases derived from thiosemicarbazide and hydrazinecarbodithioate. Can. J. Chem. 1994, 72, 1785–1788. [Google Scholar] [CrossRef]
- Asegbeloyin, J.N.; Babahan, I.; Ukwueze, N.N.; Oruma, U.S.; Poyrazoglu, E.C.; Eze, U.F. Synthesis, characterization and antimicrobial activity of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran-2-one Schiff base with 2,2'-(ethylenedioxy)diethylamine and its Co(II), Ni(II) and Cu(II) complexes. Asian J. Chemistry 2015, 27. in press. [Google Scholar]
- Sudheer; Quraishi, M.A. 2-Amino-3,5-dicarbonitrile-6-thio-pyridines: New and effective corrosion inhibitors for mild steel in 1 M HCl. Ind. Eng. Chem. Res. 2014, 53, 2851–2859. [Google Scholar] [CrossRef]
- Rammelt, U.; Koehler, S.; Reinhard, G. Electrochemical characterization of the ability of dicarboxylic acid salts to the corrosion inhibition of mild steel in aqueous solutions. Corros. Sci. 2011, 53, 3515–3520. [Google Scholar] [CrossRef]
- Sheriff, E.M.; Erasmus, R.M.; Comins, J.D. In situ Raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions Electrochimica. Acta 2010, 55, 3657–3663. [Google Scholar]
- El Adnani, Z.; Mcharfi, M.; Sfaira, M.; Benzakour, M.; Benjelloun, A.T.; Ebn Touhami, M.; Hammouti, B.; Taleb, M. DFT study of 7-R-3methylquinoxalin-2(1H)-ones (R=H; CH3; Cl) as corrosion inhibitors in hydrochloric acid. Int. J. Electrochem. Sci. 2012, 7, 6738–6751. [Google Scholar]
- Satapathy, A.K.; Gunasekaran, G.; Sahoo, S.C.; Kumar, A.; Rodrigues, P.V. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci. 2009, 51, 2848–2856. [Google Scholar] [CrossRef]
- Abdallah, M.; Asghar, B.H.; Zaafarany, I.; Fouda, A.S. The inhibition of carbon steel corrosion in hydrochloric acid solution using some phenolic compounds. Int. J. Electrochem. Sci. 2012, 7, 282–304. [Google Scholar]
- Daoud, D.; Douadi, T.; Issaadi, S.; Chafaa, S. Adsorption and corrosion inhibition of new synthesized thiophene Schiff base on mild steel X52 in HCl and H2SO4 solutions. Corros. Sci. 2014, 79, 50–58. [Google Scholar] [CrossRef]
- Benabdellah, M.; Ousslim, A.; Hammouti, B.; Elidrissi, A.; Aouniti, A.; Dafali, A.; Bekkouch, K.; Benkaddour, M.J. The effect of poly(vinyl caprolactone-co-vinyl pyridine) and poly(vinyl imidazol-co-vinyl pyridine) on the corrosion of steel in H3PO4 media. Appl. Electrochem. 2007, 37, 819–826. [Google Scholar] [CrossRef]
- Chakravarthy, M.P.; Mohana, K.N.; Kumar, C.B.P. Study of adsorption properties and inhibition of mild steel corrosion in hydrochloric acid media by water soluble composite poly (vinyl alcohol-omethoxy aniline). J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 16, 74–82. [Google Scholar]
- Chakravarthy, M.P.; Mohana, K.N.; Kumar, C.B.P. Corrosion inhibition effect and adsorption behaviour of nicotinamide derivatives on mild steel in hydrochloric acid solution. Int. J. Ind. Chem. 2014, 5, 1–21. [Google Scholar] [CrossRef]
- Abd-Elaal, A.A.; Aiad, I.; Shaban, S.M.; Tawfik, S.M.; Atef, S. Synthesis and Evaluation of Some Triazole Derivativesas Corrosion Inhibitors and Biocides. J. Surfact. Deterg. 2014, 17, 483–491. [Google Scholar] [CrossRef]
- John, S.; Kuruvilla, M.; Joseph, A. Adsorption and inhibition effect of methyl carbamate on copper metal in 1 N HNO3: An experimental and theoretical study. RSC Adv. 2013, 3, 8929–8938. [Google Scholar] [CrossRef]
- Murulana, L.C.; Singh, A.K.; Shukla, S.K.; Kabanda, M.M.; Ebenso, E.E. Experimental and quantum chemical studies of some bis(trifluoromethyl-sulfonyl) imide imidazolium-based ionic liquids as corrosion inhibitors for mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res. 2012, 51, 13282–13299. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Thermodynamic, electrochemical and quantum chemical investigation of some Schiff bases as corrosion inhibitors for mild steel in hydrochloric acid solutions. Corros. Sci. 2010, 52, 933–942. [Google Scholar] [CrossRef]
- Gomez, B.; Likhanova, N.V.; Dominguez-Aguilar, M.A.; Martinez-Palou, R.; Vela, A.; Gazquez, J.L. Quantum chemical study of the inhibitive properties of 2-pyridyl-azoles. J. Phys. Chem. B. 2006, 110, 8928–8934. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.; Zhang, Y.; Wang, P.; Zhang, J. Theoretical evaluation of inhibition performance of purine corrosion inhibitors. Mol. Sim. 2013, 39, 1034–1041. [Google Scholar] [CrossRef]
- Echegaray, E.; Cárdenas, C.; Rabi, S.; Rabi, N.; Lee, S.; Zadeh, F.H.; Toro-Labbe, A.; Anderson, J.S.M.; Ayers, P.W. In pursuit of negative Fukui functions: Examples where the highest occupied molecular orbital fails to dominate the chemical reactivity. J. Mol. Model. 2013, 19, 2779–2783. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Mortier, W.J. The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asegbeloyin, J.N.; Ejikeme, P.M.; Olasunkanmi, L.O.; Adekunle, A.S.; Ebenso, E.E. A Novel Schiff Base of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran-2-one and 2,2'-(ethylenedioxy)diethylamine as Potential Corrosion Inhibitor for Mild Steel in Acidic Medium. Materials 2015, 8, 2918-2934. https://doi.org/10.3390/ma8062918
Asegbeloyin JN, Ejikeme PM, Olasunkanmi LO, Adekunle AS, Ebenso EE. A Novel Schiff Base of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran-2-one and 2,2'-(ethylenedioxy)diethylamine as Potential Corrosion Inhibitor for Mild Steel in Acidic Medium. Materials. 2015; 8(6):2918-2934. https://doi.org/10.3390/ma8062918
Chicago/Turabian StyleAsegbeloyin, Jonnie N., Paul M. Ejikeme, Lukman O. Olasunkanmi, Abolanle S. Adekunle, and Eno E. Ebenso. 2015. "A Novel Schiff Base of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran-2-one and 2,2'-(ethylenedioxy)diethylamine as Potential Corrosion Inhibitor for Mild Steel in Acidic Medium" Materials 8, no. 6: 2918-2934. https://doi.org/10.3390/ma8062918
APA StyleAsegbeloyin, J. N., Ejikeme, P. M., Olasunkanmi, L. O., Adekunle, A. S., & Ebenso, E. E. (2015). A Novel Schiff Base of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran-2-one and 2,2'-(ethylenedioxy)diethylamine as Potential Corrosion Inhibitor for Mild Steel in Acidic Medium. Materials, 8(6), 2918-2934. https://doi.org/10.3390/ma8062918






