Methods for Biomimetic Mineralisation of Human Enamel: A Systematic Review
Abstract
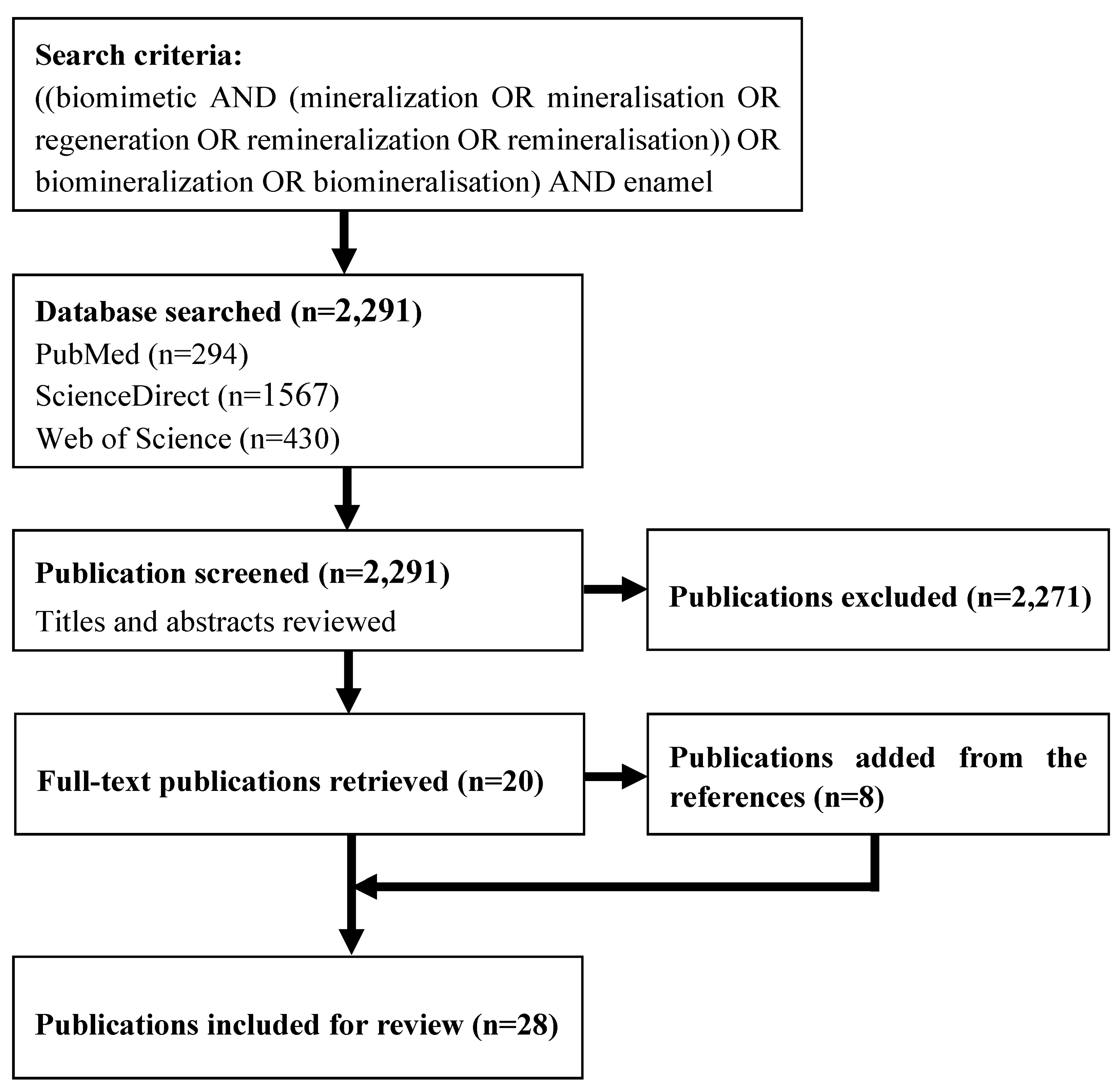
:1. Introduction
2. Results
| Authors, Year [Ref.] | Surface Treatment | Biomineralisation Method | Sources of Ca and P | Main Finding |
|---|---|---|---|---|
| Reynolds, 1997 [22] | Demineralising solution (4d) | CPP-ACP | CaCl2 solution, K3PO4 buffer | The CPP-stabilised CaP solution can remineralise subsurface enamel lesions. |
| Busch, 2004 [20] | 30% PA (30s) | Glycerin-enriched gelatin 620 ppm F | CaCl2 solution, PO4 gelatin | Ordered enamel-like mineral was induced on human teeth. |
| Kirkham et al., 2007 [31] | Acidified gelatine gel (6w) | Peptide | pH cycling | Peptide treatment increased remineralisation and inhibited demineralisation. |
| Kumar et al., 2008 [23] | Demineralising solution (4d) | CPP-ACP 1100 ppm F | pH cycling | CPP-ACP remineralised initial enamel lesions. |
| Fan et al., 2009 [28] | 3% HNO3 solution (50s) | Amelogenin 0.45 ppm F | Calcification solution | The synthetic nanorod crystals formed on etched enamel in the presence of amelogenin. |
| Xie et al., 2011 [21] | 37% PA (30s) | EDTA 950 ppm F | Ca-EDTA complex-phosphate solution | EDTA induced the assembly of hexagonal prism-like FHAP microcrystals. |
| Jayarajan et al., 2011 [24] | Demineralising solution (5h) | CPP-ACP, CPP-ACPF 900 ppm | Artificial saliva | CPP-ACPF showed more amount of remineralisation than CPP-ACP. |
| Fan et al., 2011 [29] | 5% HNO3 solution (30s) | Amelogenin 1–10 ppm F | Remineralisation solution | Densely packed arrays of FHAP nanorods were observed. |
| Hsu et al., 2011 [39] | 35% PA (30s) | Peptide | SBF | The nanomechanical properties of the acid-demineralised enamel were greatly improved. |
| Hsu et al., 2011 [42] | 35% PA (30s) | Peptide | SBF | Peptide promoted the uniform deposition of nano-crystalline CaP over enamel surfaces. |
| Li et al., 2011 [44] | 37% PA (10s) | Glu-apatite nanoparticles | SBF | Nanoparticles turned into rod-like crystals on enamel surface. |
| Zhou et al., 2012 [17] | 37% PA (2m) | Polydopamine 1 ppm F | Calcification solution | No significant difference was observed in the remineralisation of enamel, except in dentine. |
| Fan et al., 2012 [26] | Demineralising solution (3d) | Amelogenin 5 ppm F | Artificial saliva | Application of the amelogenin-hydrogel significantly improved enamel hardness. |
| Chung et al., 2012 [40] | 34% PA (15s) | Peptide | Artificial saliva | Peptide promoted the uniform deposition of apatites with small crystalline size. |
| Li et al., 2013 [18] | 37% PA (15s) | Nacre water-soluble matrix | SBF | Water-soluble matrix facilitated the formation of HAP nanorods. |
| Chung et al., 2013 [27] | 1M citric acid (2m) | Peptide | Artificial saliva | Peptide promoted the formation of nano-HAP crystals. |
| Chen et al., 2013 [30] | 3% HNO3 solution (50s) | PAMAM 1 ppm F | Calcification solution | PAMAM could induce the formation of HAP crystals on demineralised enamel. |
| Ruan et al., 2013 [33] | 30% PA (30s) | Amelogenin 136 ppm F | Amelogenin-CaP hydrogel, artificial saliva | The enamel-like layer was formed on natural enamel. |
| Wu et al., 2013 [36] | 37% PA (45s) | PAMAM | Artificial saliva | PAMAM could induce nanorod-like HAP remineralisation on acid-etched enamel. |
| Chung et al., 2013 [41] | 34% PA (30s) | Peptide | Artificial saliva | Peptide attracted ions from artificial saliva to form HAP crystals and fill enamel caries. |
| Chung et al., 2013 [43] | 34% PA (30s) | Peptide | Artificial saliva | Peptide promoted the deposition of small-grain HAP crystals. |
| Cao et al., 2014 [19] | 37% PA (60s) | Agarose hydrogel 500 ppm F | CaCl2 hydrogel, Phosphate solution | Enamel prism-like tissue was generated on etched enamel surface. |
| Zhang et al., 2014 [25] | Demineralising solution (7d) | Phosphorylated chitosan-ACP | Pchi-ACP solution | Remineralising rate of Pchi-ACP treatment was higher than that of fluoride treatment. |
| Ruan et al., 2014 [34] | 30% PA (30s) | Amelogenin 0.45 ppm F | Amelogenin-CaP hydrogel, artificial saliva | Enamel-like organized apatite crystals can be formed in amelogenin-chitosan system. |
| Milly et al., 2014 [32] | 8% methylcellulose gel, lactic acid (14d) | Polyacrylic acid, Bioactive glass | Bioactive glass slurry | BAG and PAA-BAG treatments enhanced remineralisation of enamel white spot lesions. |
| Cao et al., 2014 [35] | 37% PA (60s) | Enamel matrix derivative 500 ppm F | EMD-CaCl2 hydrogel, Phosphate solution | EMD facilitated enamel prism-like tissue formation on demineralised human enamel. |
| Chen et al., 2014 [37] | 37% PA (45s) | PAMAM | Artificial saliva | PAMAM could produce an enamel prism-like structure on acid-etched enamel. |
| Li et al., 2014 [38] | 37% PA (60s) | Oligopeptide 1 ppm F | Metastable calcium phosphate solution | Oligopeptide induced the formation of HAP crystals on etched enamel. |
| Protein or Its Analogues | Function of Protein or Protein Analogues | Approach [Ref.] |
|---|---|---|
| Amelogenin |
| Amel—Ca/P/F solution: [28,29] Amel—Ca/P hydrogel: [26,33,34] |
| EMD |
| EMD—Ca agarose hydrogel: [35] |
| PAMAM dendrimers: PAMAM-COOH [30] ALN-PAMAM-COOH [36] PAMAM-PO3H2 [37] |
| PAMAM—etched enamel: [30,36,37] |
| Peptide: 3NSS [27,41,43] QQRFEWEFEQQ [31] C18H35O-TKREEVD [38] 8DSS [39,42] 3DSS [40] |
| Peptide—etched enamel: [27,31,39, 40, 41, 42, 43] Peptide—Ca/P/F solution: [38] |
| Glutamic acid |
| Glutamic acid—SBF: [44] |
| Polyacrylic acid |
| Polyacrylic acid—bioactive glass: [32] |
| Phosphorylated chitosan |
| Pchi—CaPO4 solution: [25] |
| Casein phosphopeptide |
| CPP—CaPO4 solution: [22] CPP—ACP Paste: [23,24] |
3. Discussion
4. Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, H.F.; Tang, Z.Y.; Liu, J.; Sun, K.; Chang, S.R.; Peters, M.C.; Mansfield, J.F.; Czajka-Jakubowska, A.; Clarkson, B.H. Acellular synthesis of a human enamel-like microstructure. Adv. Mater. 2006, 18, 1846–1851. [Google Scholar] [CrossRef]
- Veis, A. Materials science. A window on biomineralization. Science 2005, 307, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.Z.; Ge, J. New observations of the hierarchical structure of human enamel, from nanoscale to microscale. J. Tissue Eng. Regen. Med. 2007, 1, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Fincham, A.G.; Moradian-Oldak, J.; Simmer, J.P. The structural biology of the developing dental enamel matrix. J. Struct. Biol. 1999, 126, 270–299. [Google Scholar] [CrossRef] [PubMed]
- Aoba, T. Solubility properties of human tooth mineral and pathogenesis of dental caries. Oral Dis. 2004, 10, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.J.; Yun, S.; Fang, J.S.; Chen, H.F. Chemical regeneration of human tooth enamel under near-physiological conditions. Chem. Commun. 2009, 5892–5894. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, Z.; Moradian-Oldak, J. Effect of fluoride on the morphology of calcium phosphate crystals grown on acid-etched human enamel. Caries Res. 2009, 43, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.E.; Li, M.; Mann, S.; Margolis, H.C. Influence of surfactant assembly on the formation of calcium phosphate materials—A model for dental enamel formation. J. Mater. Chem. 2005, 15, 3317–3325. [Google Scholar] [CrossRef]
- Ye, W.; Wang, X.X. Ribbon-like and rod-like hydroxyapatite crystals deposited on titanium surface with electrochemical method. Mater. Lett. 2007, 61, 4062–4065. [Google Scholar] [CrossRef]
- Yamagishi, K.; Onuma, K.; Suzuki, T.; Okada, F.; Tagami, J.; Otsuki, M.; Senawangse, P. Materials chemistry: A synthetic enamel for rapid tooth repair. Nature 2005, 433, 819. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Clarkson, B.H.; Sun, K.; Mansfield, J.F. Self-assembly of synthetic hydroxyapatite nanorods into an enamel prism-like structure. J. Colloid. Interface Sci. 2005, 288, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Moradian-Oldak, J. Protein-mediated enamel mineralization. Front. Biosci. 2012, 17, 1996–2023. [Google Scholar] [CrossRef]
- Ruan, Q.; Moradian-Oldak, J. Amelogenin and enamel biomimetics. J. Mater. Chem. B 2015, 3, 3112–3129. [Google Scholar] [CrossRef]
- Margolis, H.C.; Beniash, E.; Fowler, C.E. Role of macromolecular assembly of enamel matrix proteins in enamel formation. J. Dent. Res. 2006, 85, 775–793. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, W.; Ning, T.; Mei, M.L.; Li, Q.L.; Lo, E.C.; Chu, C.H. A novel oligopeptide simulating dentine matrix protein 1 for biomimetic mineralization of dentine. Clin. Oral Investig. 2014, 18, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Z.; Cao, Y.; Liu, W.; Chu, C.H.; Li, Q.L. Polydopamine-induced tooth remineralization. ACS Appl. Mater. Interfaces 2012, 4, 6901–6910. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, D.M.; Lin, S.; Zhuang, Z.Y.; Lin, Z. Facile in vitro hydroxyapatite remineralization of human enamel with remarkable hardness. CrystEngComm. 2013, 15, 4351–4356. [Google Scholar] [CrossRef]
- Cao, Y.; Mei, M.L.; Li, Q.L.; Lo, E.C.; Chu, C.H. Agarose hydrogel biomimetic mineralization model for the regeneration of enamel prismlike tissue. ACS Appl. Mater. Interfaces 2014, 6, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Busch, S. Regeneration of human tooth enamel. Angew. Chem. Int. Edit. 2004, 43, 1428–1431. [Google Scholar] [CrossRef]
- Xie, R.Q.; Feng, Z.D.; Li, S.W.; Xu, B.B. EDTA-assisted self-assembly of fluoride-substituted hydroxyapatite coating on enamel substrate. Cryst. Growth. Des. 2011, 11, 5206–5214. [Google Scholar] [CrossRef]
- Reynolds, E.C. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J. Dent. Res. 1997, 76, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.L.N.; Itthagarun, A.; King, N.M. The effect of casein phosphopeptide-amorphous calcium phosphate on remineralization of artificial caries-like lesions: An in vitro study. Aust. Dent. J. 2008, 53, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jayarajan, J.; Janardhanam, P.; Jayakumar, P.; Deepika. Efficacy of CPP-ACP and CPP-ACPF on enamel remineralization—An in vitro study using scanning electron microscope and diagnodent. Indian J. Dent. Res. 2011, 22, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Sun, X.; Kishen, A.; Deng, X.; Yang, X.; Wang, H.; Cong, C.; Wang, Y.; Wu, M. Biomimetic remineralization of demineralized enamel with nano-complexes of phosphorylated chitosan and amorphous calcium phosphate. J. Mater. Sci. Mater. Med. 2014, 25, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wen, Z.T.; Liao, S.; Lallier, T.; Hagan, J.L.; Twomley, J.T.; Zhang, J.F.; Sun, Z.; Xu, X. Novel amelogenin-releasing hydrogel for remineralization of enamel artificial caries. J. Bioact. Compat. Polym. 2012, 27, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Huang, K.C. Effects of peptide concentration on remineralization of eroded enamel. J. Mech. Behav. Biomed. 2013, 28, 213–221. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, Z.; Moradian-Oldak, J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials 2009, 30, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Nelson, J.R.; Alvarez, J.R.; Hagan, J.; Berrier, A.; Xu, X. Amelogenin-assisted ex vivo remineralization of human enamel: Effects of supersaturation degree and fluoride concentration. Acta Biomater. 2011, 7, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liang, K.N.; Li, J.S.; Wu, D.; Zhou, X.D.; Li, J.Y. Regeneration of biomimetic hydroxyapatite on etched human enamel by anionic PAMAM template in vitro. Arch. Oral Biol. 2013, 58, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.C.; Brookes, S.J.; Aggeli, A. Self-assembling peptide scaffolds promote enamel remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Milly, H.; Festy, F.; Watson, T.F.; Thompson, I.; Banerjee, A. Enamel white spot lesions can remineralise using bio-active glass and polyacrylic acid-modified bio-active glass powders. J. Dent. 2014, 42, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Zhang, Y.; Yang, X.; Nutt, S.; Moradian-Oldak, J. An amelogenin-chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013, 9, 7289–7297. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Siddiqah, N.; Li, X.; Nutt, S.; Moradian-Oldak, J. Amelogenin-chitosan matrix for human enamel regrowth: Effects of viscosity and supersaturation degree. Connect. Tissue Res. 2014, 55, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mei, M.L.; Li, Q.L.; Lo, E.C.M.; Chu, C.H. Enamel prism-like tissue regeneration using enamel matrix derivative. J. Dent. 2014, 42, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, J.; Li, J.; Chen, L.; Tang, B.; Chen, X.; Wu, W.; Li, J. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 2013, 34, 5036–5047. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, J.; Li, J.; Liang, K.; He, L.; Lin, Z.; Chen, X.; Ren, X.; Li, J. Modulated regeneration of acid-etched human tooth enamel by a functionalized dendrimer that is an analog of amelogenin. Acta Biomater. 2014, 10, 4437–4446. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Ning, T.Y.; Cao, Y.; Zhang, W.B.; Mei, M.L.; Chu, C.H. A novel self-assembled oligopeptide amphiphile for biomimetic mineralization of enamel. BMC Biotechnol. 2014, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.C.; Chung, H.Y.; Yang, J.M.; Shi, W.; Wu, B. Influences of ionic concentration on nanomechanical behaviors for remineralized enamel. J. Mech. Behav. Biomed. Mater. 2011, 4, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Li, C.C.; Hsu, C.C. Characterization of the effects of 3DSS peptide on remineralized enamel in artificial saliva. J. Mech. Behav. Biomed. Mater. 2012, 6, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Li, C.C. Microstructure and nanomechanical properties of enamel remineralized with asparagine-serine-serine peptide. Mate. Sci. Eng. C. Mater. Biol. Appl. 2013, 33, 969–973. [Google Scholar] [CrossRef]
- Hsu, C.C.; Chung, H.Y.; Yang, J.M.; Shi, W.; Wu, B. Influence of 8DSS peptide on nano-mechanical behavior of human enamel. J. Dent. Res. 2011, 90, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Li, C.C. Asparagine-serine-serine peptide regulates enamel remineralization in vitro. J. Mater. Res. 2013, 28, 2890–2896. [Google Scholar] [CrossRef]
- Li, L.; Mao, C.; Wang, J.; Xu, X.; Pan, H.; Deng, Y.; Gu, X.; Tang, R. Bio-inspired enamel repair via Glu-directed assembly of apatite nanoparticles: An approach to biomaterials with optimal characteristics. Adv. Mater. 2011, 23, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, R.M.; Moradian-Oldak, J.; Fincham, A.G. Tyrosyl motif in amelogenins binds N-acetyl-D-glucosamine. J. Biol. Chem. 1999, 274, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Moradian-Oldak, J. Control of apatite crystal growth in a fluoride containing amelogenin-rich matrix. Biomaterials 2005, 26, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Banaszak Holl, M.; Orr, B.G.; Majoros, I.; Clarkson, B.H. Interaction of dendrimers (artificial proteins) with biological hydroxyapatite crystals. J. Dent. Res. 2003, 82, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.H.; Chang, J.A.; Zhou, Y.; Lin, K.L. In vitro remineralization of human dental enamel by bioactive glasses. J. Mater. Sci. 2011, 46, 1591–1596. [Google Scholar] [CrossRef]
- Beniash, E.; Metzler, R.A.; Lam, R.S.; Gilbert, P.U. Transient amorphous calcium phosphate in forming enamel. J. Struct. Biol. 2009, 166, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mei, M.L.; Xu, J.; Lo, E.C.; Li, Q.; Chu, C.H. Biomimetic mineralisation of phosphorylated dentine by CPP-ACP. J. Dent. 2013, 41, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. The science and practice of caries prevention. J. Am. Dent. Assoc. 2000, 131, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Tohda, H.; Suzuki, H.; Yanagisawa, T.; Moriwaki, Y. Effects of F- on apatite-octacalcium phosphate intergrowth and crystal morphology in a model system of tooth enamel formation. Calcif. Tissue Int. 1992, 50, 357–361. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, C.Y.; Mei, M.L.; Li, Q.-l.; Lo, E.C.M.; Chu, C.H. Methods for Biomimetic Mineralisation of Human Enamel: A Systematic Review. Materials 2015, 8, 2873-2886. https://doi.org/10.3390/ma8062873
Cao CY, Mei ML, Li Q-l, Lo ECM, Chu CH. Methods for Biomimetic Mineralisation of Human Enamel: A Systematic Review. Materials. 2015; 8(6):2873-2886. https://doi.org/10.3390/ma8062873
Chicago/Turabian StyleCao, Chris Ying, May Lei Mei, Quan-li Li, Edward Chin Man Lo, and Chun Hung Chu. 2015. "Methods for Biomimetic Mineralisation of Human Enamel: A Systematic Review" Materials 8, no. 6: 2873-2886. https://doi.org/10.3390/ma8062873
APA StyleCao, C. Y., Mei, M. L., Li, Q.-l., Lo, E. C. M., & Chu, C. H. (2015). Methods for Biomimetic Mineralisation of Human Enamel: A Systematic Review. Materials, 8(6), 2873-2886. https://doi.org/10.3390/ma8062873







