Active Nanomaterials to Meet the Challenge of Dental Pulp Regeneration
Abstract
:1. Dental Pulp Vitality
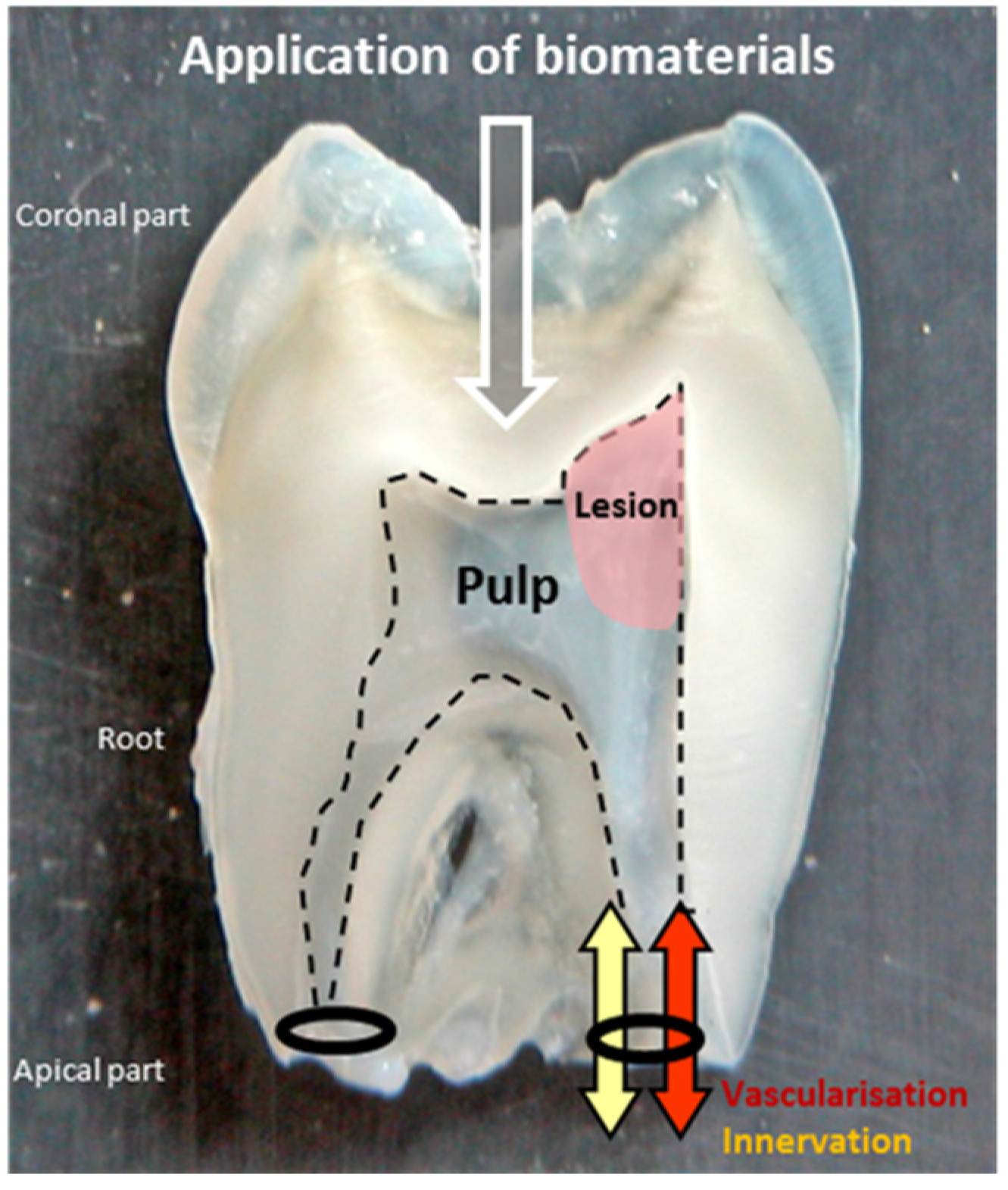
2. Dental Pulp Regeneration

3. Regenerative Medicine for Dental Pulp
4. Nanomaterials for Dental Pulp Regeneration
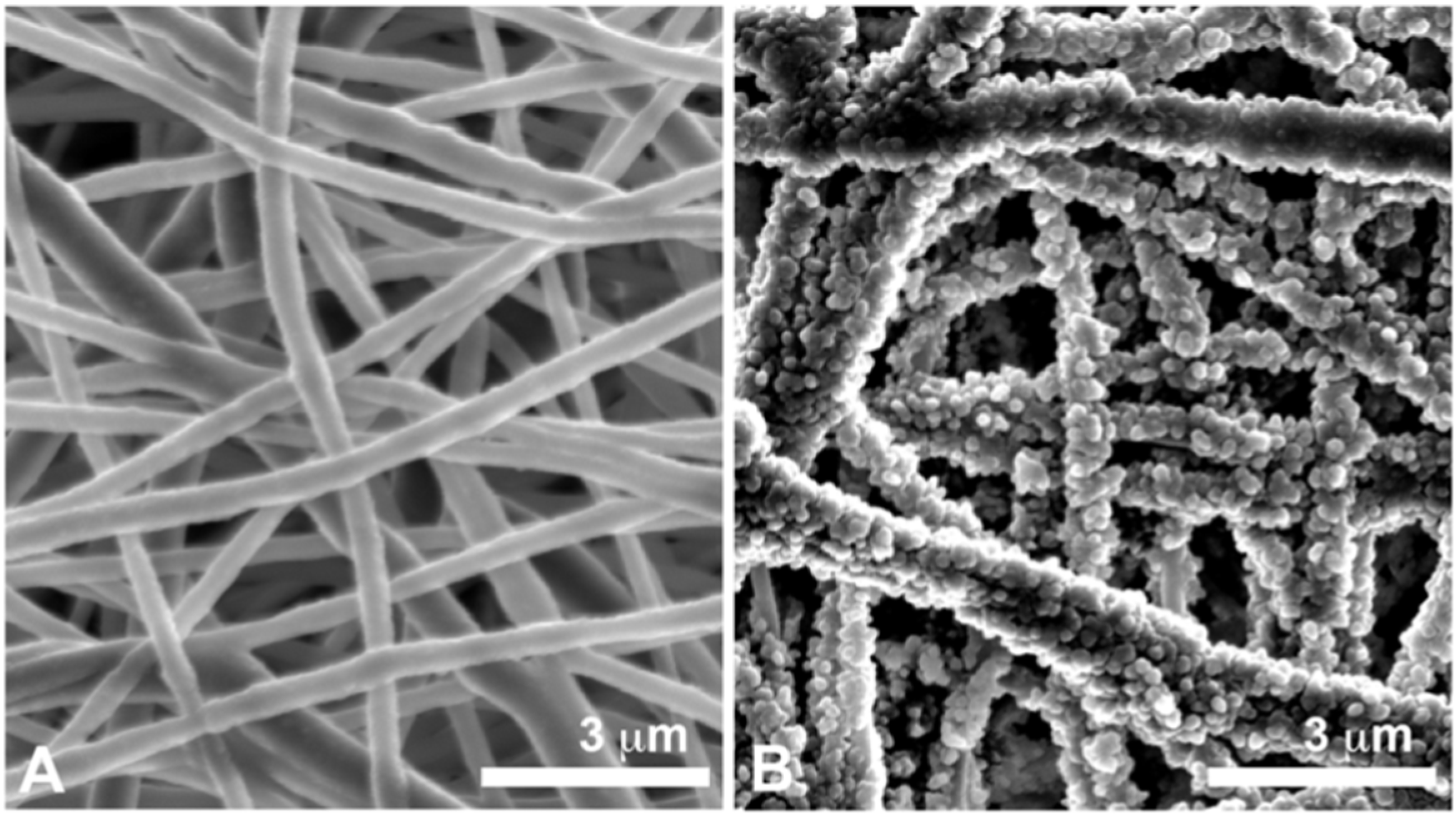
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.F. The microbial challenge to pulp regeneration. Adv. Dent. Res. 2011, 23, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Jin, T.; Yu, Q.; Chen, F.M. Biological approaches toward dental pulp regeneration by tissue engineering. J. Tissue Eng. Reg. Med. 2011, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Zhu, Q. Innovative endodontic therapy for anti-inflammatory direct pulp capping of permanent teeth with a mature apex. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2010, 109, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.M.; Rosenberg, P.A. Repair and regeneration in endodontics. Int. Endo. J. 2011, 44, 889–906. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, S.I.; Ikawa, M.; Kubota, M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent. Traumatol. 2001, 17, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, B.; Teixeira, F.; Yamauchi, M.; Caplan, D.J.; Trope, M. Pulp revascularization of immature dog teeth with apical periodontitis. J. Endod. 2007, 33, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.; Johnson, J.D.; Cohenca, N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: A case report. Int. Endod. J. 2009, 42, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.R.; Tomson, P.L.; Berdal, A. Regenerative endodontics: Regeneration or repair? J. Endod. 2014, 40, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Volponi, A.A.; Pang, Y.; Sharpe, P.T. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010, 20, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Demarco, F.F.; Conde, M.C.; Cavalcanti, B.N.; Casagrande, L.; Sakai, V.T.; Nör, J.E. Dental pulp tissue engineering. Braz. Dent. J. 2011, 22, 3–13. [Google Scholar] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Kerkis, A.; Dozortsev, D.; Stukart-Parsons, G.C.; Gomes Massironi, S.M.; Pereira, L.V.; Caplan, A.I.; Cerruti, H.I. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006, 184, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.-J. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Gotz, W.; Schierholz, J.; Zeilhofer, F.; Kuhn, U.; Mohl, C.; Sipple, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, L.; Cordeiro, M.M.; Nör, S.A.; Nör, J.E. Dental pulp stem cells in regenerative dentistry. Odontology 2011, 99, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Nakashima, M.; Ito, M.; Ishikawa, M.; Nakasima, A.; Akamine, A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J. Dent. Res. 2004, 83, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Sakai, V.T.; Zhang, Z.; Dong, Z.; Neiva, K.G.; Machado, M.A.A.M.; Shi, S.; Santos, C.F.; Nör, J.E. SHED differentiate into functional odontoblasts and endothelium. J. Dent. Res. 2010, 89, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, F.; Mendoza-Palomares, C.; Avoaka-Boni, M.C.; Ramaroson, J.; Bahi, S.; Richert, L.; Granier, F.; Benkirane-Jessel, N.; Haikel, Y. Nano-odontology: Nanostructured assemblies for endodontic regeneration. J. Biomed. Nanotechnol. 2011, 7, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T. A paradigm shift in endodontic management of immature teeth: Conservation of stem cells for regeneration. J. Dent. 2008, 36, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Lee, C.H.; Chen, M.; Zhao, W.; Fu, S.Y.; Qi, J.J.; Chotkowski, G.; Eisig, S.B.; Wong, A.; Mao, J.J. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J. Dent. Res. 2011, 90, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M. Induction of dentine in amputated pulp of dogs by recombinant human bone morphogenetic proteins-2 and -4 with collagen matrix. Arch Oral Biol. 1994, 39, 1085–1089. [Google Scholar] [CrossRef]
- Nakashima, M.; Reddi, A.H. The application of bone morphogenetic proteins to dental tissue engineering. Nat. Biotechnol. 2003, 21, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Decup, F.; Six, N.; Palmier, B.; Buch, D.; Lasfargues, J.J.; Salih, E.; Goldberg, M. Bone sialoprotein-induced reparative dentinogenesis in the pulp of rat's molar. Clin. Oral Investig. 2000, 4, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, L.; Demarco, F.F.; Zhang, Z.; Araujo, F.B.; Shi, S.; Nör, J.E. Dentin-derived BMP-2 and odontoblast differentiation. J. Dent. Res. 2010, 89, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, R.B.; Wahle, J.; Tucker, M.; Rueger, D.; Charette, M. Induction of reparative dentine formation in monkeys by recombinant human osteogenic protein-1. Arch Oral Biol. 1993, 38, 571–576. [Google Scholar] [CrossRef]
- Jepsen, S.; Albers, H.K.; Fleiner, B.; Tucker, M.; Rueger, D. Recombinant human osteogenic protein-1 induces dentin formation: an experimental study in miniature swine. J. Endod. 1997, 23, 378–382. [Google Scholar] [CrossRef]
- Six, N.; Decup, F.; Lasfargues, J.J.; Salih, E.; Goldberg, M. Osteogenic proteins (bone sialoprotein and bone morphogenetic protein-7) and dental pulp mineralization. J. Mater. Sci. Mater. Med. 2002, 13, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Six, N.; Lasfargues, J.J.; Goldberg, M. Differential repair responses in the coronal and radicular areas of the exposed rat molar pulp induced by recombinant human bone morphogenetic protein 7 (osteogenic protein 1). Arch Oral Biol. 2002, 47, 177–187. [Google Scholar] [CrossRef]
- Jazayeri, M.; Allameh, A.; Soleimani, M.; Jazayeri, S.H.; Piryaei, A.; Kazemnejad, S. Molecular and ultrastructural characterization of endothelial cells differentiated from human bone marrow mesenchymal stem cells. Cell Biol. Int. 2008, 32, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Marchionni, C.; Bonsi, L.; Alviano, F.; Lanzoni, G.; Di Tullio, A.; Costa, R.; Montanari, M.; Tazzzri, P.L.; Ricci, F.; Pasquinelli, G.; et al. Angiogenic potential of human dental pulp stromal (stem) cells. Int. J. Immunopathol. Pharmacol. 2009, 22, 699–706. [Google Scholar] [PubMed]
- Torabinejad, M.; Turman, M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: A case report. J. Endod. 2011, 37, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R. Regenerative endodontics: A state of the art. Indian J. Dent. Res. 2011, 22, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Zheng, L.; Ito, M.; Ishizaka, R.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. Regeneration of dental pulp after pulpotomy by transplantation of CD31−/CD146− side population cells from a canine tooth. Regen. Med. 2009, 4, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shoichet, M.S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater. 2004, 3, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Cavender, A.; Yuwono, V.; Dong, H.; Shi, S.; Schmalz, G.; Hartgerink, J.D.; D'Souza, R.N. Self-assembling peptide amphiphile nanofibers as a scaffold for dental stem cells. Tissue Eng. Part A. 2008, 14, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Misawa, H.; Kobayashi, N.; Soto-Gutierrez, A.; Chen, Y.; Yoshida, A.; Rivas-Carrillo, J.D.; Navarro-Alvarez, N.; Tanaka, K.; Miki, A.; Takei, J.; et al. PuraMatrixTM facilitates bone regeneration in bone defects of calvaria in mice. Cell Transplant. 2006, 15, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Narmoneva, D.A.; Oni, O.; Sieminski, A.L.; Zhang, S.; Gertler, J.P.; Kamm, R.D.; Lee, R.T. Self-assembling short oligopeptides and the promotion of angiogenesis. Biomaterials 2005, 26, 4837–4846. [Google Scholar] [CrossRef] [PubMed]
- Thonhoff, J.R.; Lou, D.I.; Jordan, P.M.; Zhao, X.; Wu, P. Compatibility of human fetal neural stem cells with hydrogel biomaterials in vitro. Brain Res. 2008, 1187, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Wadajkar, A.; Santimano, S.; Ahn, C.; Zhu, Q.; Opperman, L.A.; Bellinger, L.L.; Yang, J.; Nguyen, K.T. Preliminary study of light-cured hydrogel for endodontic drug delivery vehicle. J. Invest. Clin. Dent. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, C.; Nishihara, T.; Terashita, M.; Tabata, Y.; Washio, A. Local regeneration of dentin-pulp complex using controlled release of FGF-2 and naturally derived sponge-like Scaffolds. Inter. J. Dent. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Conde, C.M.; Demarco, F.F.; Casagrande, L.; Alcazar, J.C.; Nör, J.E.; Tarquinio, S.B. Influence of poly-L-lactic acid scaffold's pore size on the proliferation and differentiation of dental pulp stem cells. Braz. Dent. J. 2015, 26, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.W.; Yeatts, A.B.; Richbourg, W.J.; Caccamese, J.F.; Coletti, D.P.; Falco, E.E.; Fisher, J.P. Macroporous hydrogels upregulate osteogenic signal expression and promote bone regeneration. Biomacromolecules 2010, 11, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nano-structured polymer scaffolds for tissue engineering and regenerative medicine. Nanomed. Nanobiotechnol. 2009, 1, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Fioretti, F.; Mendoza-Palomares, C.; Helms, M.; Al Alam, D.; Richert, L.; Arntz, Y.; Rinckenbach, S.; Garnier, F.; Haïkel, Y.; Gangloff, S.C.; et al. Nanostructured assemblies for dental application. ACS Nano 2010, 4, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, N.; Yamauchi, S.; Nagaoka, H.; Duggan, D.; Zhong, S.; Lee, S.M.; Teixeira, F.B.; Yamauchi, M. Tissue engineering strategies for immature teeth with apical periodontitis. J. Endod. 2011, 37, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.T.; Valera, M.C.; Nakashima, M.; Nör, J.E.; Bottino, M.C. Tissue-engineering-based strategies for regenerative endodontics. J. Dent. Res. 2014, 93, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Palomares, C.; Ferrand, A.; Facca, S.; Fioretti, F.; Ladam, G.; Kuchler-Bopp, S.; Regnier, T.; Mainard, D.; Benkirane-Jessel, N. Smart hybrid materials equipped by nanoreservoirs of therapeutics. ACS Nano 2012, 6, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Eap, S.; Morand, D.; Clauss, F.; Huck, O.; Stoltz, J.F.; Lutz, J.C.; Benkirane-Jessel, N.; Keller, L.; Fioretti, F. Nanostructured thick 3D nanofibrous scaffold can induce bone. Biomed. Mater. Eng. 2015, 25, 79–85. [Google Scholar] [PubMed]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; He, C.L.; Yang, A.; Zhang, Y.; Han, X.J.; Yin, J.; Wu, Q. Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J. Biomed. Mater. Res. Part A 2006, 77, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, K.H.; Lee, J.C.; Lee, J. In-situ nanofabrication via electrohydrodynamic jetting of countercharged nozzles. Polym. Bull. 2008, 61, 521–528. [Google Scholar] [CrossRef]
- Ferrand, A.; Eap, S.; Richert, L.; Lemoine, S.; Kalaskar, D.; Demoustier-Champagne, S.; Atmani, H.; Mély, Y.; Fioretti, F.; Schlatter, G.; et al. Osteogenetic properties of electrospun nanofibrous PCL scaffolds equipped with chitosan-based nanoreservoirs of growth factors. Macromol. Biosci. 2014, 14, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, T.; Li, M.; Fu, N.; Fu, Y.; Ba, K.; Deng, S.; Jiang, Y.; Hu, J.; Peng, Q.; et al. Electrospun fibers for dental and craniofacial applications. Curr. Stem Cell. Res. Ther. 2014, 9, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Eap, S.; Keller, L.; Schiavi, J.; Huck, O.; Jacomine, L.; Fioretti, F.; Gauthier, C.; Sebastian, V.; Schwinté, P.; Benkirane-Jessel, N. A Living thick nanofibrous implant bi-functionalized with active growth factor and stem cells for bone regeneration. Int. J. Nanomed. 2015, 10, 1061–1075. [Google Scholar]
- Yang, X.; Yang, F.; Walboomers, X.F.; Bian, Z.; Fan, M.; Jansen, J.A. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J. Biomed. Mater. Res. A 2010, 93, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Jin, X.; Ma, H.; Hu, J.; Ni, L.; Ma, P.X. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010, 6, 3856–3863. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, H.; Jin, X.; Hu, J.; Liu, X.; Ni, L.; Ma, P.X. The effect of scaffold architecture on odontogenic differentiation of human dental pulp stem cells. Biomaterials 2011, 32, 7822–7830. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Bae, W.J.; Kim, J.M.; Kim, J.J.; Lee, E.J.; Kim, H.W.; Kim, E.C. Mineralized polycaprolactone nanofibrous matrix for odontogenesis of human dental pulp cells. J. Biomater. Appl. 2014, 28, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Park, Y.D.; Lee, S.Y.; El-Fiqi, A.; Kim, J.J.; Lee, E.J.; Kim, H.W.; Kim, E.C. Odontogenic stimulation of human dental pulp cells with bioactive nanocomposite fiber. J. Biomater. Appl. 2015, 29, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Jing, J.; Jiang, Y.; Taylor, R.J.; Feng, J.Q.; Geiger, B.; Liu, X. Magnesium-containing nanostructured hybrid scaffolds for enhanced dentin regeneration. Tissue Eng. Part A 2014, 20, 2422–2433. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Kuchler-Bopp, S.; Mendoza, S.A.; Poliard, A.; Lesot, H. Tooth engineering: Searching for dental mesenchymal cells sources. Front. Physiol 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Kuchler-Bopp, S.; Bécavin, T.; Kökten, T.; Fioretti, F.; Benkirane-Jessel, N.; Keller, L. Nanostructured hybrid materials for bone-tooth unit regeneration. Open J. Reg. Med. 2013, 2, 47–52. [Google Scholar] [CrossRef]
- Eap, S.; Bécavin, T.; Keller, L.; Kökten, T.; Fioretti, F.; Weickert, J.L.; Deveaux, E.; Benkirane-Jessel, N.; Kuchler-Bopp, S. Nanofibers implant functionalized by neural growth factor as a strategy to innervate a bioengineered tooth. Adv. Healthc. Mater. 2014, 3, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Palasuk, J.; Kamocki, K.; Hippenmeyer, L.; Platt, J.A.; Spolnik, K.J.; Gregory, R.L.; Bottino, M.C. Bimix antimicrobial scaffolds for regenerative endodontics. J. Endod. 2014, 40, 1879–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottino, M.C.; Kamocki, K.; Yassen, G.H.; Platt, J.A.; Vail, M.M.; Ehrlich, Y.; Spolnik, K.J.; Gregory, R.L. Bioactive nanofibrous scaffolds for regenerative endodontics. J. Dent. Res. 2013, 92, 963–969. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, L.; Offner, D.; Schwinté, P.; Morand, D.; Wagner, Q.; Gros, C.; Bornert, F.; Bahi, S.; Musset, A.-M.; Benkirane-Jessel, N.; et al. Active Nanomaterials to Meet the Challenge of Dental Pulp Regeneration. Materials 2015, 8, 7461-7471. https://doi.org/10.3390/ma8115387
Keller L, Offner D, Schwinté P, Morand D, Wagner Q, Gros C, Bornert F, Bahi S, Musset A-M, Benkirane-Jessel N, et al. Active Nanomaterials to Meet the Challenge of Dental Pulp Regeneration. Materials. 2015; 8(11):7461-7471. https://doi.org/10.3390/ma8115387
Chicago/Turabian StyleKeller, Laetitia, Damien Offner, Pascale Schwinté, David Morand, Quentin Wagner, Catherine Gros, Fabien Bornert, Sophie Bahi, Anne-Marie Musset, Nadia Benkirane-Jessel, and et al. 2015. "Active Nanomaterials to Meet the Challenge of Dental Pulp Regeneration" Materials 8, no. 11: 7461-7471. https://doi.org/10.3390/ma8115387
APA StyleKeller, L., Offner, D., Schwinté, P., Morand, D., Wagner, Q., Gros, C., Bornert, F., Bahi, S., Musset, A.-M., Benkirane-Jessel, N., & Fioretti, F. (2015). Active Nanomaterials to Meet the Challenge of Dental Pulp Regeneration. Materials, 8(11), 7461-7471. https://doi.org/10.3390/ma8115387





