TiO2 Nanosols Applied Directly on Textiles Using Different Purification Treatments
Abstract
:1. Introduction
2. Experimental
2.1. Materials
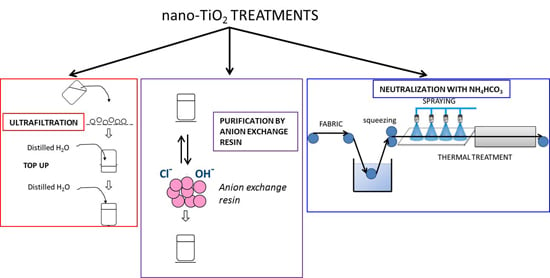
2.2. Methods
- washing by ultrafiltration (TACF);
- purification with an anion exchange resin (TACR);
- neutralization of the TAC-coated textile (TACBIC).
| Sample | Nominal pH | pH * | D50DLS (nm) | Electrical Conductivity (mS/cm) | pHi.e.p. |
|---|---|---|---|---|---|
| TAC | 1.5 | 2.9 | 36 | 1.18 | 7.09 |
| TACF | 4.0 | 3.3 | 42 | 0.25 | 6.92 |
| TACR | 4.5 | 4.2 | 94 | 0.05 | 6.91 |
| TACBIC | – | 5.0 ** | – | – | – |
2.2.1. Washing by Ultrafiltration (TACF)
2.2.2. Purification with an Anion Exchange Resin (TACR)
2.2.3. Neutralization of the TAC-Coated Textile (TACBIC)
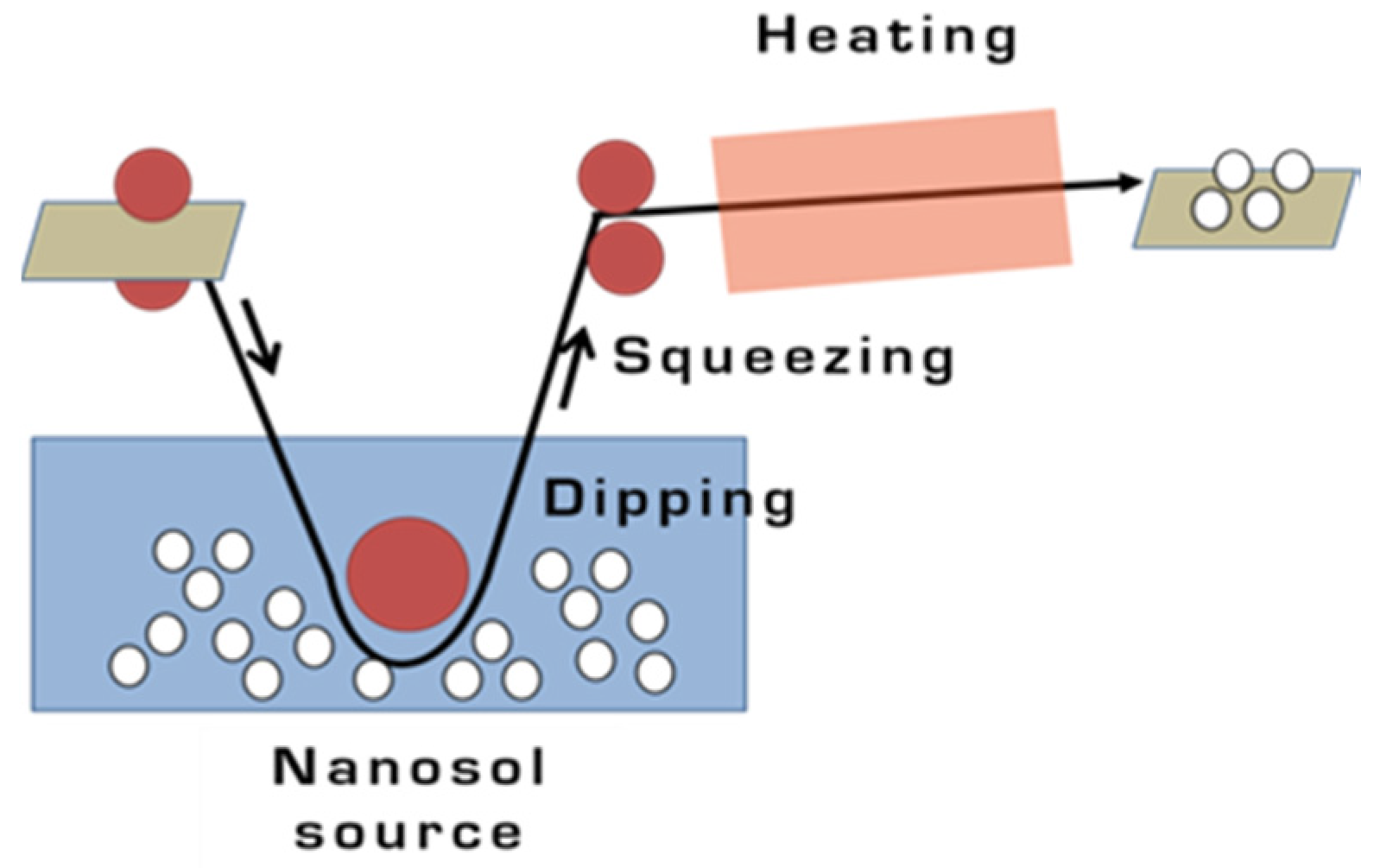
2.3. Dip-Pad-Dry-Cure Method
2.4. Characterization of TiO2 Nanosols
2.5. Textile Characterization
2.6. Photocatalytic Measurements
3. Results and Discussion
3.1. Characterization of TiO2 Nanosols
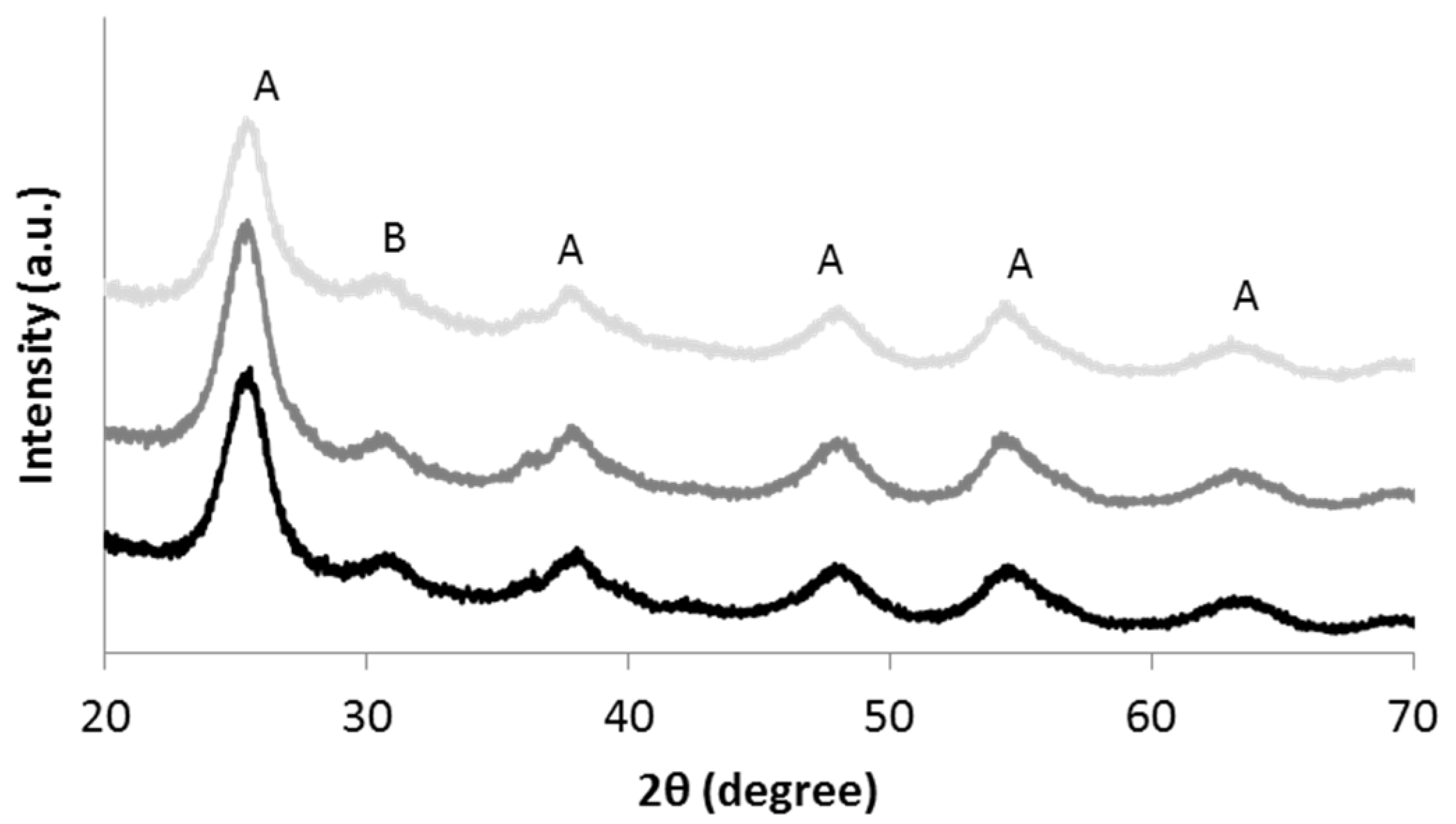
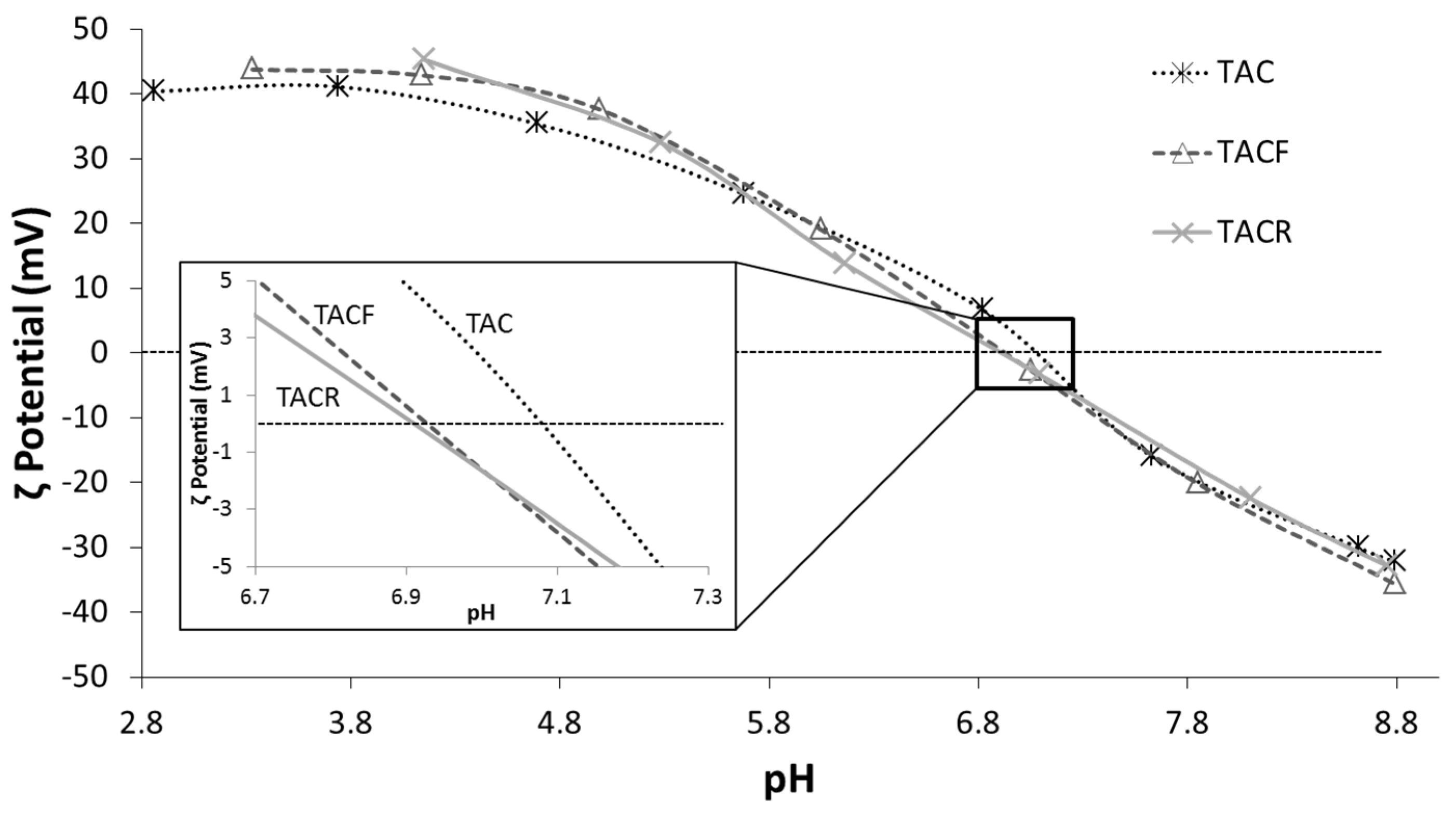
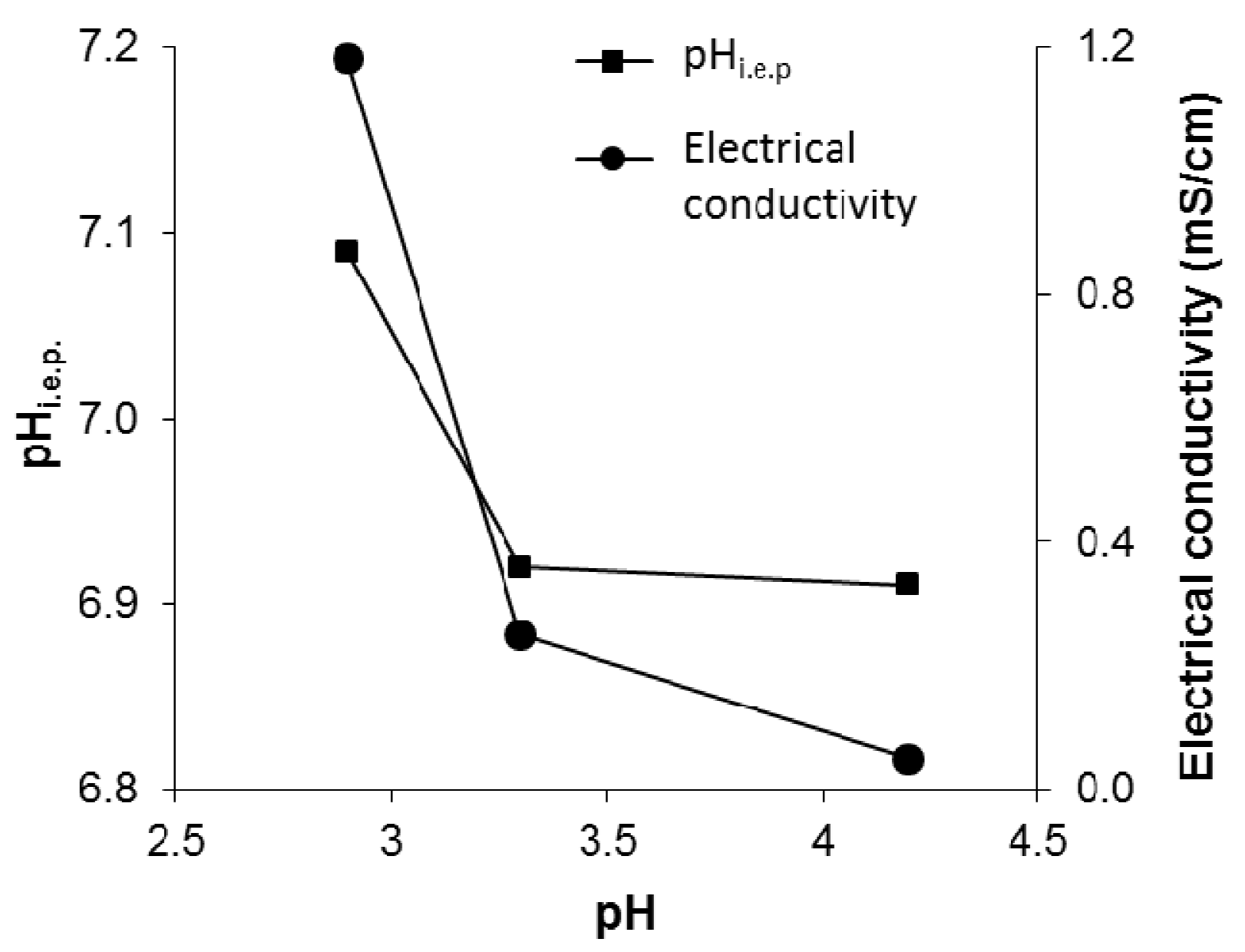
3.2. Characterization of Textiles
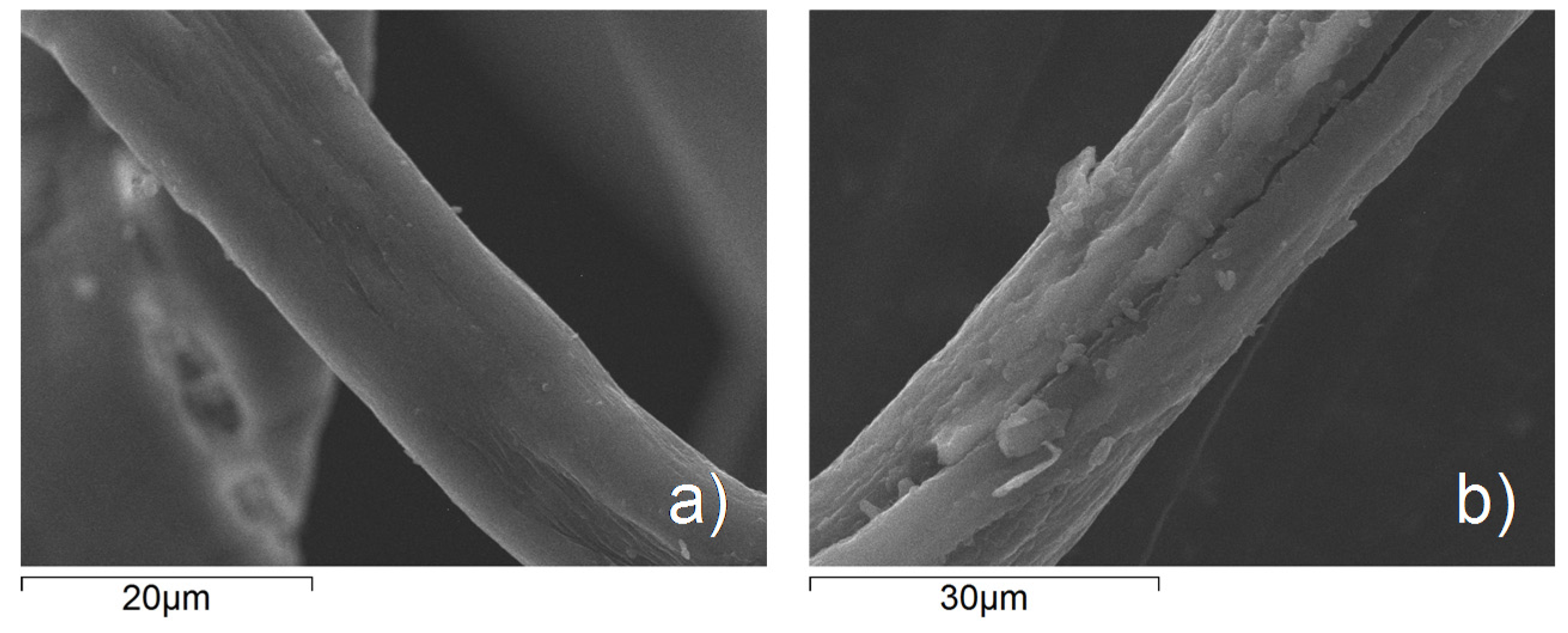
3.3. Photocatalytic Measurements
| Sample | Photocatalytic Efficiency (%) | Increase in Photocatalytic Efficiency * |
|---|---|---|
| TAC | 68.5 | 1.54 |
| TACF | 84.3 | 1.90 |
| TACR | 92.5 | 2.08 |
| TACBIC | 73.2 | 1.65 |
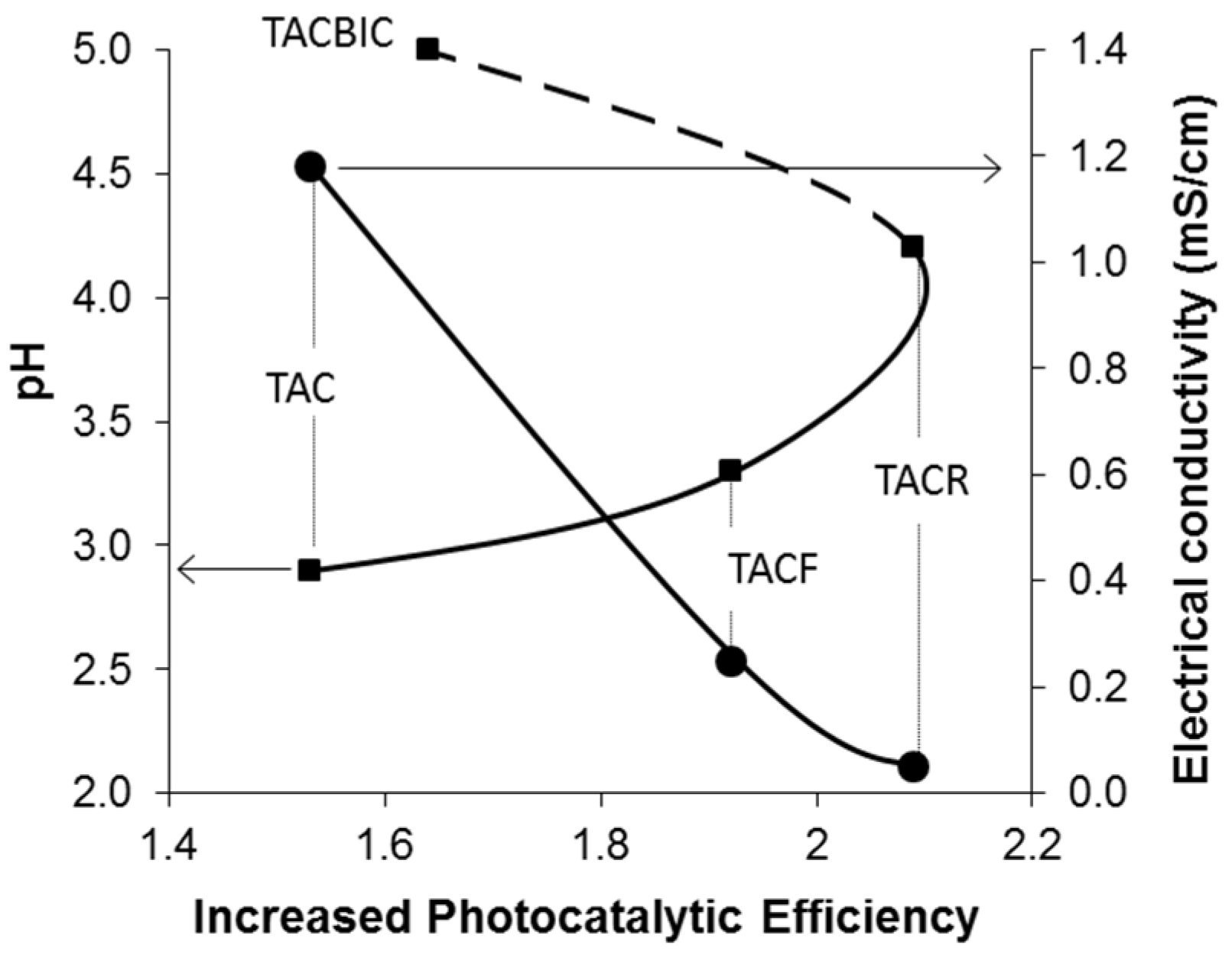
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thorat, S.; Diaspro, A.; Scarpellini, A.; Povia, M.; Salerno, M. Comparative study of loading of anodic porous alumina with silver nanoparticles using different methods. Materials 2013, 6, 206–216. [Google Scholar] [CrossRef]
- Shi, Z.; Wymana, I.; Liua, G.; Hua, H.; Zoub, H.; Hub, J. Preparation of water-repellent cotton fabrics from fluorinated diblock copolymers and evaluation of their durability. Polymer 2013, 54, 6406–6414. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Wei, Q.; Liu, H.; Deng, B. Structures and properties of the polyester nonwovens coated with titanium dioxide by reactive sputtering. J. Coat. Technol. Res. 2010, 7, 637–642. [Google Scholar] [CrossRef]
- Chattopadhyay, D.P.; Patel, B.H. Modification of cotton textiles with nanostructural zinc particles. J. Nat. Fiber 2011, 8, 39–47. [Google Scholar] [CrossRef]
- Bozzi, A.; Yuranova, T.; Guasaquillo, I.; Laub, D.; Kiwi, J. Self-cleaning of modified cotton textiles by TiO2 at low temperatures under daylight irradiation. J. Photochem. Photobiol. A Chem. 2005, 174, 156–164. [Google Scholar] [CrossRef]
- Yuranova, T.; Mosteo, R.; Bandara, J.; Laub, D.; Kiwi, J. Self-cleaning cotton textiles surfaces modified by photoactive SiO2/TiO2 coating. J. Mol. Catal. A Chem. 2006, 244, 160–167. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cesano, F.; Scarano, D.; Bonino, F.; Agostini, G.; Spoto, G.; Bordiga, S.; Zecchina, A. Cotton textile fibres coated by Au/TiO2 films: Synthesis, characterization and self-cleaning properties. J. Photochem. Photobiol. A Chem. 2008, 199, 64–72. [Google Scholar] [CrossRef]
- Yuranova, T.; Laub, D.; Kiwi, J. Synthesis, activity and characterization of textiles showing self-cleaning activity under daylight irradiation. Catal. Today 2007, 122, 109–117. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Hequet, E. Functionalization of a cotton fabric surface with titania nanosols: Applications for self-cleaning and UV-protection properties. ACS Appl. Mater. Interfaces 2009, 1, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Kiwi, J.; Pulgarin, C. Innovative self-cleaning and bactericide textiles. Catal. Today 2010, 151, 2–7. [Google Scholar] [CrossRef]
- Lin, L. Method of Making Fabric with Photo-Catalyst. U.S. 2005/0227557 A1, 13 October 2005. [Google Scholar]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of surface area, primary particle size and crystal phase on titanium dioxide particle dispersion properties. Nanoscale Res. Lett. 2011, 6. [Google Scholar] [CrossRef]
- Sameut Bouhaika, I.; Leroya, P.; Ollivier, P.; Azaroual, M.; Mercury, L. Influence of surface conductivity on the apparent zeta potential of TiO2 nanoparticles: Application to the modeling of their aggregation kinetics. J. Colloid Interface Sci. 2013, 406, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Springer, F.; Laborie, S.; Guigui, C. Removal of SiO2 nanoparticles from industry wastewaters and subsurface waters by ultrafiltration: Investigation of process efficiency, deposit properties and fouling mechanism. Sep. Purif. Technol. 2013, 108, 6–14. [Google Scholar] [CrossRef]
- Iritani, E.; Katagiri, N.; Tsukamoto, M. Determination of cake properties in ultrafiltration of nanocolloids based on single step-up pressure filtration test. AIChE J. 2014, 60, 289–299. [Google Scholar] [CrossRef]
- Costa, A.L.; Ortelli, S.; Blosi, M.; Albonetti, S.; Vaccari, A.; Dondi, M. TiO2 based photocatalytic coatings: From nanostructure to functional properties. Chem. Eng. J. 2013, 225, 880–886. [Google Scholar] [CrossRef]
- Ortelli, S.; Blosi, M.; Albonetti, S.; Vaccari, A.; Dondi, M.; Costa, A.L. TiO2 based nano-photocatalysis immobilized on cellulose substrates. J. Photochem. Photobiol. A Chem. 2013, 276, 58–64. [Google Scholar] [CrossRef]
- Ortelli, S.; Blosi, M.; Delpivo, C.; Gardini, D.; Dondi, M.; Gualandi, I.; Tonelli, D.; Aina, V.; Fenoglio, I.; Gandhi, A.A.; et al. Multiple approach to test nano TiO2 photo-activity. J. Photochem. Photobiol. A Chem. 2014, 292, 26–33. [Google Scholar] [CrossRef]
- He, B.; Ren, Y.; Chen, Y.; Li, J. Deactivation and in situ regeneration of anion exchange resin in the continuous transesterification for biodiesel production. Energy Fuels 2012, 26, 3897–3902. [Google Scholar] [CrossRef]
- Chularueangaksorn, P.; Tanka, S.; Fujii, S.; Kunacheva, C. Regeneration and reusability of anion exchange resin used in perfluoro octane sulfonate removal by batch experiments. J. Appl. Polym. Sci. 2013, 130, 884–889. [Google Scholar] [CrossRef]
- Di Girolamo, M.; Marchionna, M. Acidic and basic ion exchange resins for industrial applications. J. Mol. Catal. A Chem. 2001, 177, 33–40. [Google Scholar] [CrossRef]
- Levison, P.R. Large-scale ion-exchange column chromatography of proteins Comparison of different formats. J. Chromatogr. B 2003, 790, 17–33. [Google Scholar] [CrossRef]
- Dloczik, L.; Koenenkamp, R. Nanostructured metal sulfide surfaces by ion exchange processes. J. Solid State Eletrochem. 2004, 8, 142–146. [Google Scholar] [CrossRef]
- Shinkazh, O.; Kanani, D.; Barth, M.; Long, M.; Hussain, D.; Zydney, A.L. Countercurrent tangential chromatography for large-scale protein purification. Biotechnol. Bioeng. 2011, 108, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Porat, A.; Winters, D.; Cai, L.; Smith, S.; Abroson, F.; Tony Tam, L.-T.; Shen, Z.; Hecht, R. A novel anion-exchange resin suitable for both discovery research and clinical manufacturing purpose. Prep. Biochem. Biotechnol. 2012, 42, 304–321. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortelli, S.; Costa, A.L.; Dondi, M. TiO2 Nanosols Applied Directly on Textiles Using Different Purification Treatments. Materials 2015, 8, 7988-7996. https://doi.org/10.3390/ma8115437
Ortelli S, Costa AL, Dondi M. TiO2 Nanosols Applied Directly on Textiles Using Different Purification Treatments. Materials. 2015; 8(11):7988-7996. https://doi.org/10.3390/ma8115437
Chicago/Turabian StyleOrtelli, Simona, Anna Luisa Costa, and Michele Dondi. 2015. "TiO2 Nanosols Applied Directly on Textiles Using Different Purification Treatments" Materials 8, no. 11: 7988-7996. https://doi.org/10.3390/ma8115437
APA StyleOrtelli, S., Costa, A. L., & Dondi, M. (2015). TiO2 Nanosols Applied Directly on Textiles Using Different Purification Treatments. Materials, 8(11), 7988-7996. https://doi.org/10.3390/ma8115437