Reduction of Adipose Tissue Formation by the Controlled Release of BMP-2 Using a Hydroxyapatite-Coated Collagen Carrier System for Sinus-Augmentation/Extraction-Socket Grafting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the HA-Coated Collagen Carrier
2.2. In Vitro Assay for Cell Differentiation Induced by the rhBMP-2 Released from Its Carriers
2.2.1. Preincubation in the Cell-Growth Medium
- Negative control group: standard culture medium.
- Positive control group: standard culture medium containing ErhBMP-2 (1 μg/mL).
- C/BMP-2 group: standard culture medium in which ErhBMP-2-soaked ACS was placed during the planned releasing periods, after presoaking with ErhBMP-2 (1 μg/mL) for 30 min. (C: absorbable collagen sponge)
- HA/BMP-2 group: standard culture medium with the same conditions as for the C/BMP-2 group, except that HA-coated collagen was used as the carrier instead of ACS.
2.2.2. Induction of Osteogenic and Adipogenic Differentiation
2.3. In Vivo Assay for Tissue Regeneration Using Two Types of Animal Model
2.3.1. Experimental Animals
2.3.2. Study Design and Surgical Procedures
- Sham control group: sham surgery sites with no graft (included only in the extraction-socket grafting experiment).
- DBBM group: experimental sites received DBBM only.
- DBBM/C group: experimental sites received DBBM and ACS.
- DBBM/C/BMP-2 group: experimental sites received DBBM and ErhBMP-2-loaded ACS.
- DBBM/HA group: experimental sites received DBBM and HA-coated collagen.
- DBBM/HA/BMP-2 group: experimental sites received DBBM and ErhBMP-2-loaded HA-coated collagen.
2.3.3. Radiographic Analysis Using Micro-Computed Tomographic Images
2.3.4. Histologic/Histomorphometric Analysis
2.4. Statistical Analysis
3. Results
3.1. In Vitro Cell Differentiation Induced by rhBMP-2 Released from Its Carrier System
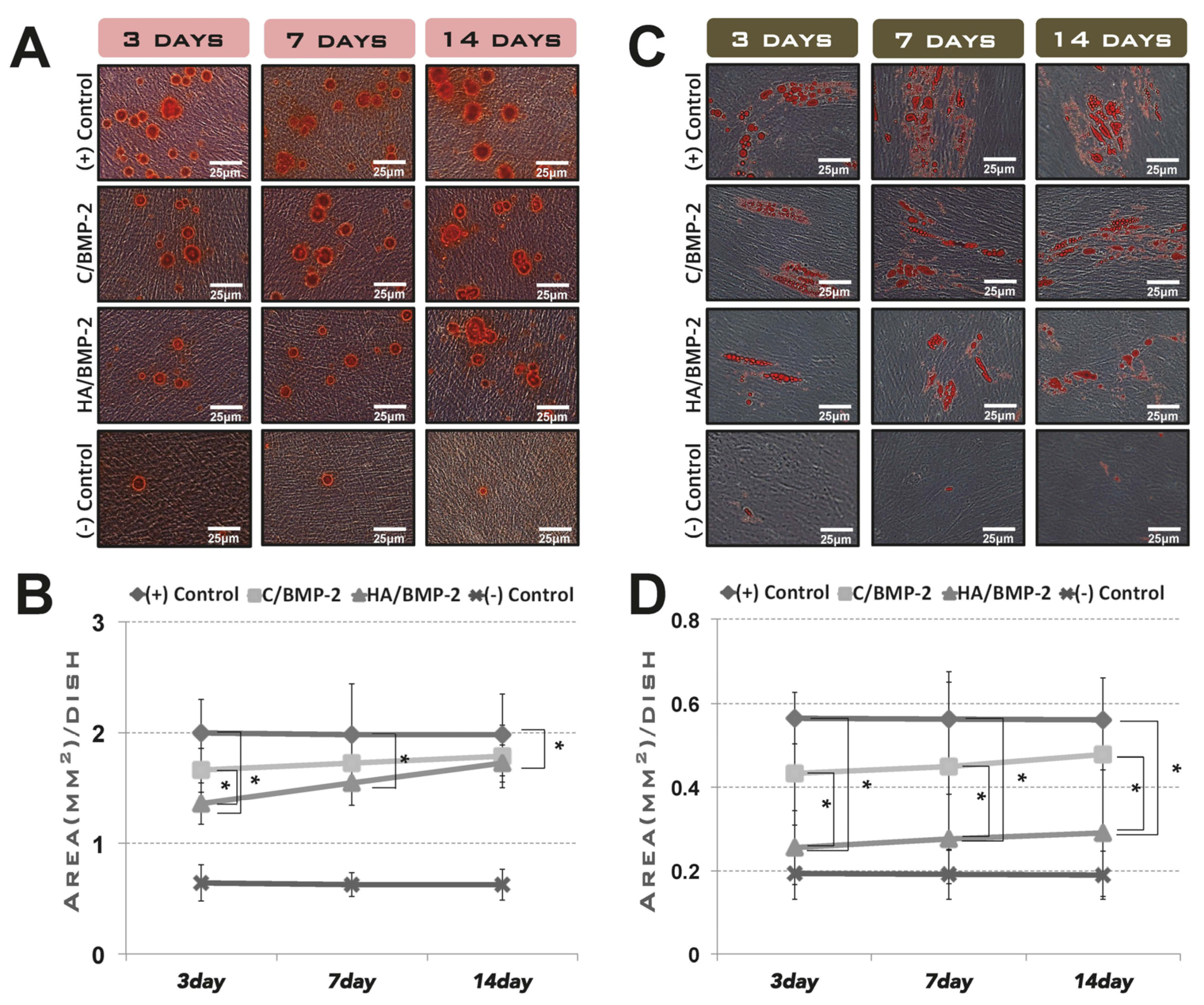
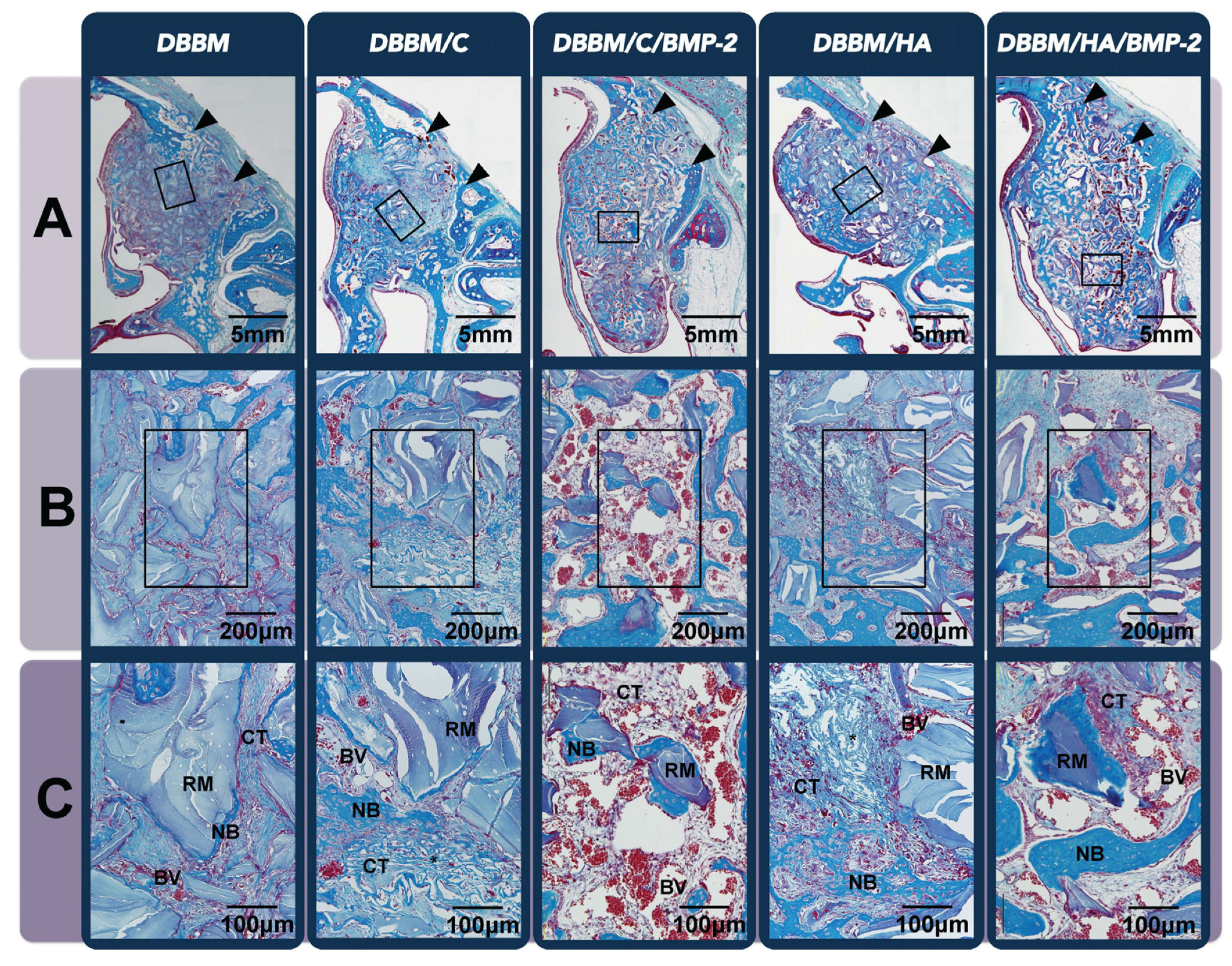
3.2. In Vivo Tissue Regeneration in the Rabbit Sinus-Augmentation Model
3.2.1. Radiographic Analysis

3.2.2. Histologic Analysis
| Group | DBBM | DBBM/C | DBBM/C/BMP-2 | DBBM/H | DBBM/H/BMP-2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 weeks | 8 weeks | 2 weeks | 8 weeks | 2 weeks | 8 weeks | 2 weeks | 8 weeks | 2 weeks | 8 weeks | |
| NB (%) | 10.42 ± 0.43 | 25.29 ± 4.16 * | 10.58 ± 2.30 | 24.87 ± 4.69 * | 12.09 ± 2.14 | 28.42 ± 2.51 * | 10.97 ± 2.04 | 23.01 ± 3.50 * | 13.29 ± 6.02 | 30.80 ± 5.43 * |
| RM (%) | 29.10 ± 4.33 | 19.71 ± 3.89 | 17.52 ± 2.01 | 16.09 ± 3.01 | 13.27 ± 2.32 | 13.95 ± 3.13 | 18.68 ± 2.90 | 15.83 ± 3.63 | 15.26 ± 0.39 | 11.63 ± 1.05 |
| AT (%) | 0.15 ± 0.08 | 0.65 ± 0.61 | 0.18 ± 0.06 | 1.55 ± 1.00 | 0.12 ± 0.02 | 12.92 ± 2.91 *†§ | 0.21 ± 0.10 | 1.88 ± 0.92 | 0.11 ± 0.03 | 5.96 ± 2.05 *# |
| BV (%) | 1.37 ± 0.54 | 2.42 ± 1.04 | 1.45 ± 0.53 | 3.04 ± 1.30 | 6.26 ± 1.73 *†§ | 2.83 ± 0.50 | 1.34 ± 0.48 | 3.10 ± 1.58 | 3.87 ± 0.77 † | 2.48 ± 0.50 |
| CT (%) | 58.96 ± 4.15 | 51.93 ± 7.17 | 70.28 ± 3.04 | 54.46 ± 4.11 | 68.25 ± 3.63 *# | 41.88 ± 5.22 | 68.80 ± 3.86 | 56.19 ± 2.71 | 67.47 ± 6.29 # | 49.13 ± 9.00 |
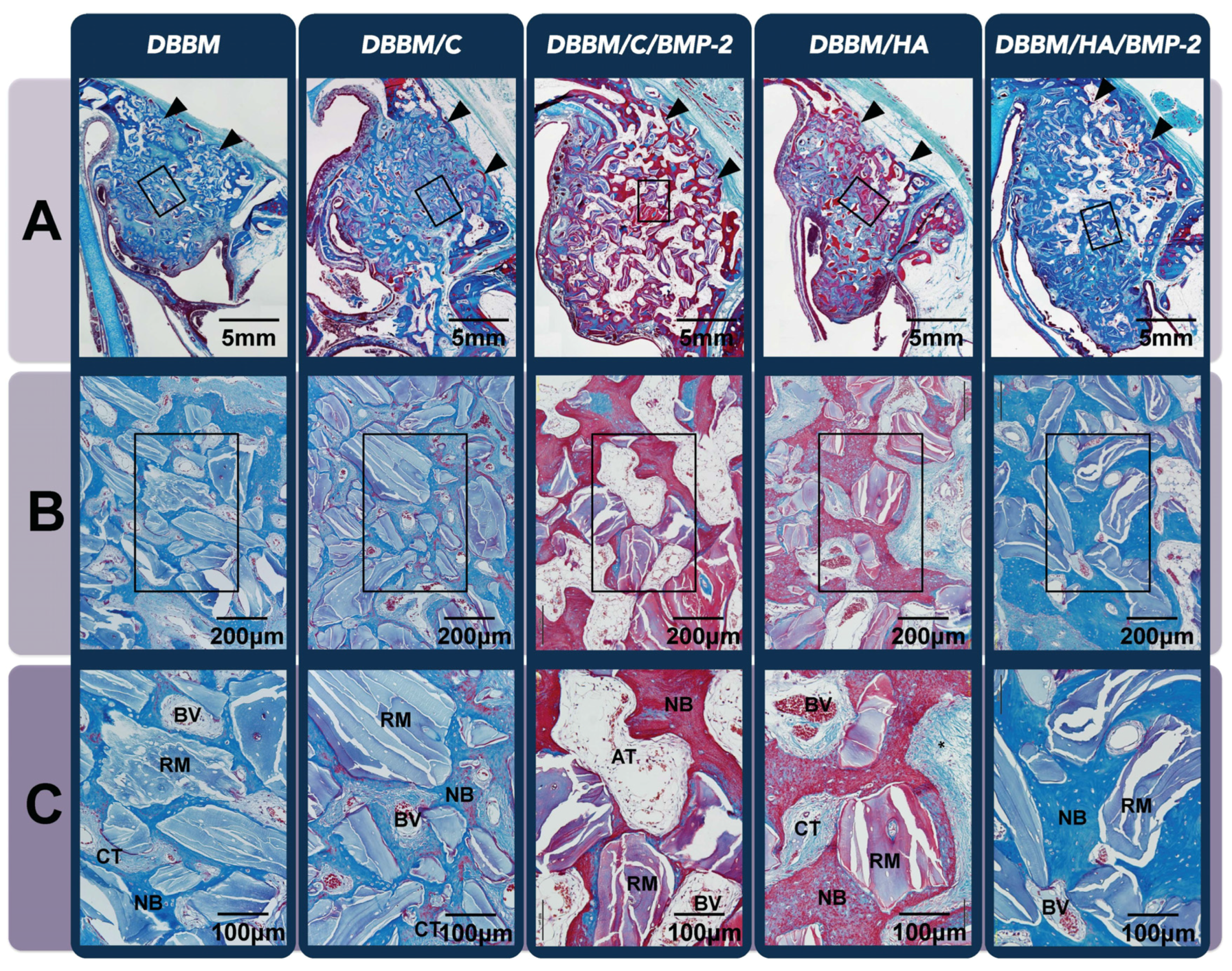
3.3. In Vivo Tissue Regeneration in the Dog Extraction-Socket Model
3.3.1. Radiographic Analysis
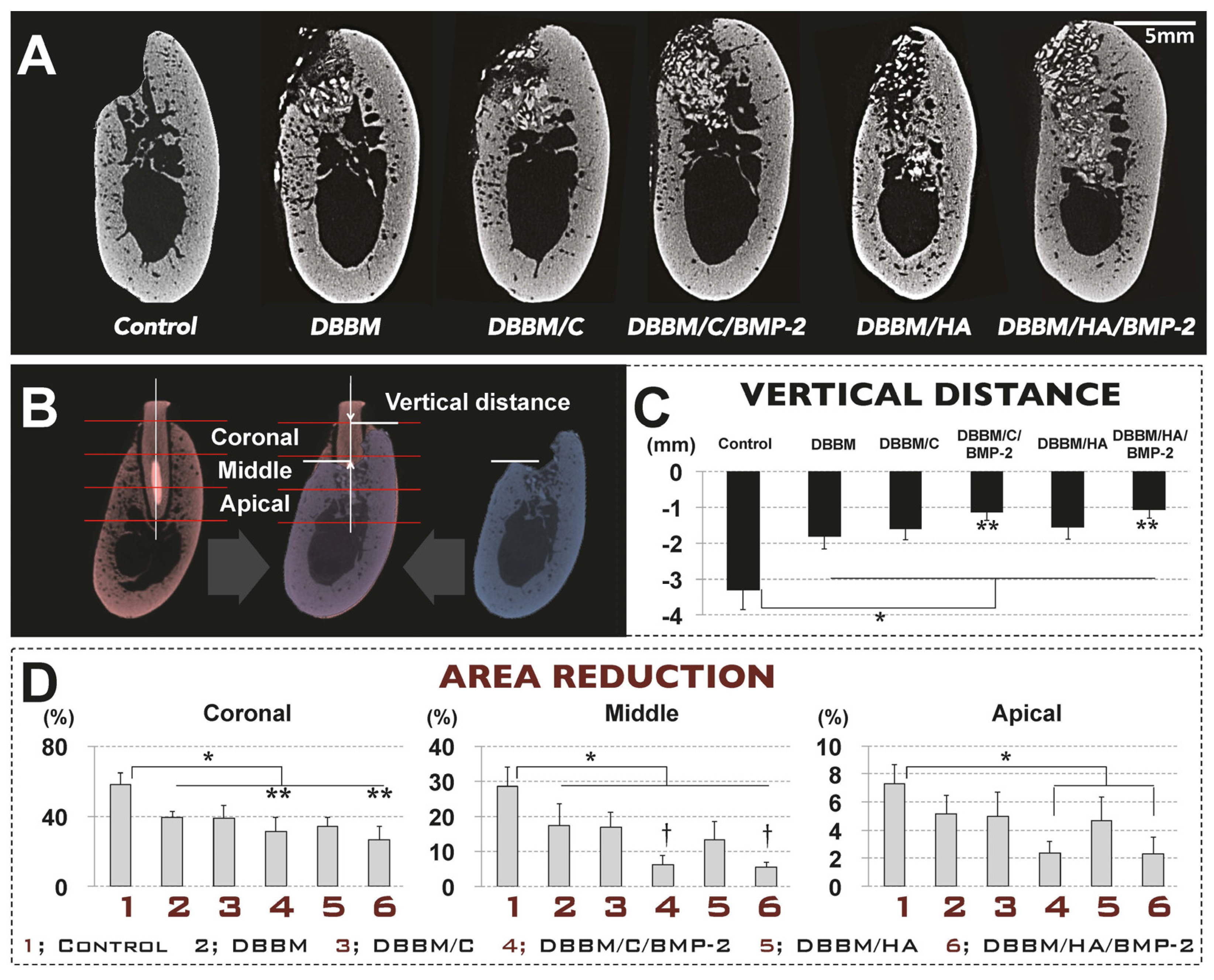
3.3.2. Histologic Analysis
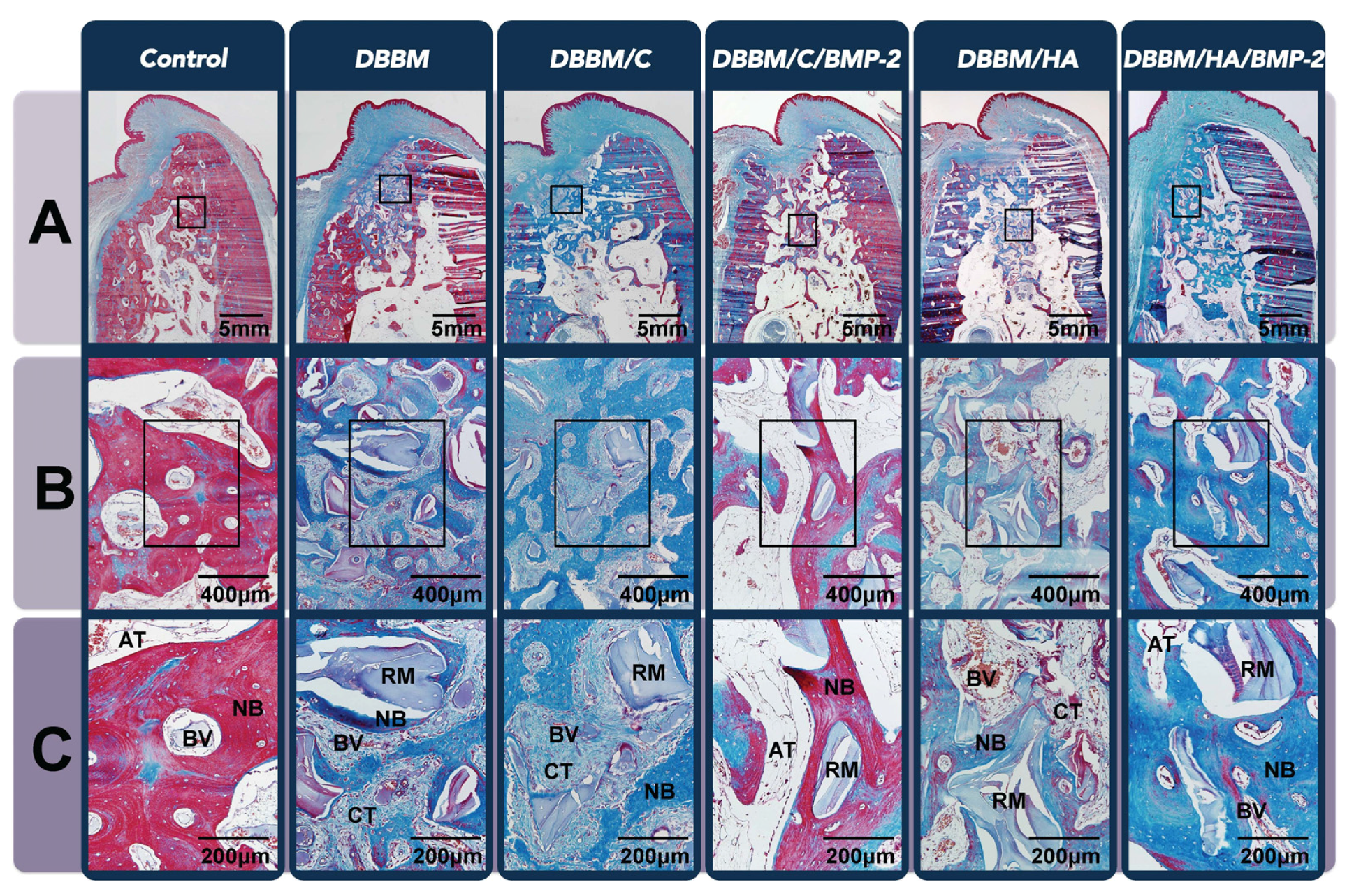
| Group | Control | DBBM | DBBM/C | DBBM/C/BMP-2 | DBBM/H | DBBM/H/BMP-2 |
|---|---|---|---|---|---|---|
| NB (%) | 39.72 ± 12.85 | 31.58 ±5.92 | 28.44 ± 8.37 | 42.26 ± 8.46 | 26.11 ± 4.93 | 52.92 ± 7.33 † |
| RM (%) | 0.00 | 19.78 ± 3.50 | 20.22 ± 8.13 | 8.96 ± 3.93 † | 17.73 ± 5.81 | 4.71 ± 1.19 † |
| AT (%) | 26.07 ± 8.08 † | 10.19 ± 1.20 | 12.27 ± 0.88 | 27.20 ± 8.43 †§ | 12.70 ± 2.31 | 16.16 ± 6.12 † |
| BV (%) | 6.88 ± 2.43 †§ | 2.74 ± 1.07 | 1.60 ± 0.91 | 4.46 ± 1.24 | 3.24 ± 0.59 | 3.61 ± 2.04 |
| CT (%) | 27.33 ± 5.69 | 35.71 ± 8.99 | 37.47 ± 8.86 | 17.13 ± 5.61 † | 40.23 ± 6.68 #§ | 22.60 ± 6.29 † |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Katagiri, T.; Ikeda, T.; Wozney, J.M.; Rosen, V.; Wang, E.A.; Kahn, A.J.; Suda, T.; Yoshiki, S. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J. Cell Biol. 1991, 113, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Sellers, R.S.; Zhang, R.; Glasson, S.S.; Kim, H.D.; Peluso, D.; D’Augusta, D.A.; Beckwith, K.; Morris, E.A. Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2). J. Bone Joint Surg. 2000, 82, 151–160. [Google Scholar] [PubMed]
- Riew, K.D.; Wright, N.M.; Cheng, S.; Avioli, L.V.; Lou, J. Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model. Calcif. Tissue Int. 1998, 63, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Wikesjo, U.M.; Guglielmoni, P.; Promsudthi, A.; Cho, K.S.; Trombelli, L.; Selvig, K.A.; Jin, L.; Wozney, J.M. Periodontal repair in dogs: Effect of rhBMP-2 concentration on regeneration of alveolar bone and periodontal attachment. J. Clin. Periodontol. 1999, 26, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H. Bone morphogenetic proteins: From basic science to clinical applications. J. Bone Joint Surg. 2001, 83, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Kanakaris, N.K.; Giannoudis, P.V. Clinical applications of bone morphogenetic proteins: Current evidence. J. Surg. Orthop. Adv. 2008, 17, 133–146. [Google Scholar] [PubMed]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Kao, D.W.; Kubota, A.; Nevins, M.; Fiorellini, J.P. The negative effect of combining rhBMP-2 and Bio-Oss on bone formation for maxillary sinus augmentation. Int. J. Periodontics Restor. Dent. 2012, 32, 61–67. [Google Scholar]
- Choi, Y.; Lee, J.S.; Kim, Y.J.; Kim, M.S.; Choi, S.H.; Cho, K.S.; Jung, U.W. Recombinant human bone morphogenetic protein-2 stimulates the osteogenic potential of the Schneiderian membrane: A histometric analysis in rabbits. Tissue Eng. Part A 2013, 19, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, S.K.; Kim, B.S.; Im, G.I.; Cho, K.S.; Kim, C.S. Controlled release of BMP-2 using a heparin-conjugated carrier system reduces in vivo adipose tissue formation. J. Biomed. Mater. Res. Part A 2015, 103, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Yamaguchi, S.; Kusunose, T.; Toyonaga, T.; Hamada, Y.; Takahashi, J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 2004, 25, 3807–3812. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; La, W.G.; Bhang, S.H.; Lee, T.J.; Lee, M.; Kim, B.S. Apatite-coated collagen scaffold for bone morphogenetic protein-2 delivery. Tissue Eng. Part A 2011, 17, 2153–2164. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.S.; Choi, K.H.; Jung, U.W.; Yun, J.H.; Choi, S.H.; Cho, K.S. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials 2010, 31, 3512–3519. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.M. The bone morphogenetic protein family and osteogenesis. Mol. Reprod. Dev. 1992, 32, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Boerckel, J.D.; Kolambkar, Y.M.; Dupont, K.M.; Uhrig, B.A.; Phelps, E.A.; Stevens, H.Y.; Garcia, A.J.; Guldberg, R.E. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials 2011, 32, 5241–5251. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; La, W.G.; Bhang, S.H.; Jeon, J.Y.; Lee, J.H.; Kim, B.S. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng. Part A 2010, 16, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; La, W.G.; Cho, Y.M.; Shin, W.; Yeo, G.D.; Kim, B.S. Comparison between heparin-conjugated fibrin and collagen sponge as bone morphogenetic protein-2 carriers for bone regeneration. Exp. Mol. Med. 2012, 44, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Susin, C.; Lee, J.; Fiorini, T.; Bisch, F.C.; Dixon, D.R.; McPherson III, J.C.; Buxton, A.N.; Wikesjo, U.M. Effect of rhBMP-2 dose on bone formation/maturation in a rat critical-size calvarial defect model. J. Clin. Periodontol. 2014, 41, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Leknes, K.N.; Yang, J.; Qahash, M.; Polimeni, G.; Susin, C.; Wikesjo, U.M. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: Radiographic observations. Clin. Oral Implants Res. 2008, 19, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Sciadini, M.F.; Johnson, K.D. Evaluation of recombinant human bone morphogenetic protein-2 as a bone-graft substitute in a canine segmental defect model. J. Orthop. Res. 2000, 18, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Mohyeldin, A.; Garzon-Muvdi, T.; Quinones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontal. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, G.; Araujo, M.; Hayacibara, R.; Sukekava, F.; Lindhe, J. Healing of extraction sockets and surgically produced—Augmented and non-augmented—Defects in the alveolar ridge. An experimental study in the dog. J. Clin. Periodontal. 2005, 32, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Wikesjo, U.M.; Qahash, M.; Polimeni, G.; Susin, C.; Shanaman, R.H.; Rohrer, M.D.; Wozney, J.M.; Hall, J. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: Histologic observations. J. Clin. Periodontal. 2008, 35, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.M.; Aghaloo, T.; Chou, Y.F.; Walder, B.; Zhang, X.; Soo, C.; Ting, K.; Wu, B. MicroCT evaluation of three-dimensional mineralization in response to BMP-2 doses in vitro and in critical sized rat calvarial defects. Tissue Eng. 2007, 13, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, H.S.; Kanim, L.E.; Kabo, J.M.; Toth, J.M.; Zeegen, E.N.; Liu, D.; Delamarter, R.B.; Dawson, E.G. Effective doses of recombinant human bone morphogenetic protein-2 in experimental spinal fusion. Spine 1996, 21, 2115–2122. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-S.; Kim, T.-W.; Park, S.; Kim, B.-S.; Im, G.-I.; Cho, K.-S.; Kim, C.-S. Reduction of Adipose Tissue Formation by the Controlled Release of BMP-2 Using a Hydroxyapatite-Coated Collagen Carrier System for Sinus-Augmentation/Extraction-Socket Grafting. Materials 2015, 8, 7634-7649. https://doi.org/10.3390/ma8115411
Lee J-S, Kim T-W, Park S, Kim B-S, Im G-I, Cho K-S, Kim C-S. Reduction of Adipose Tissue Formation by the Controlled Release of BMP-2 Using a Hydroxyapatite-Coated Collagen Carrier System for Sinus-Augmentation/Extraction-Socket Grafting. Materials. 2015; 8(11):7634-7649. https://doi.org/10.3390/ma8115411
Chicago/Turabian StyleLee, Jung-Seok, Tae-Wan Kim, Soyon Park, Byung-Soo Kim, Gun-Il Im, Kyoo-Sung Cho, and Chang-Sung Kim. 2015. "Reduction of Adipose Tissue Formation by the Controlled Release of BMP-2 Using a Hydroxyapatite-Coated Collagen Carrier System for Sinus-Augmentation/Extraction-Socket Grafting" Materials 8, no. 11: 7634-7649. https://doi.org/10.3390/ma8115411
APA StyleLee, J.-S., Kim, T.-W., Park, S., Kim, B.-S., Im, G.-I., Cho, K.-S., & Kim, C.-S. (2015). Reduction of Adipose Tissue Formation by the Controlled Release of BMP-2 Using a Hydroxyapatite-Coated Collagen Carrier System for Sinus-Augmentation/Extraction-Socket Grafting. Materials, 8(11), 7634-7649. https://doi.org/10.3390/ma8115411





