Water-Assisted Production of Thermoplastic Nanocomposites: A Review
Abstract
:1. Introduction
- -
- No need for surface modification of the nanofiller. This is especially important for such fillers, which should be rendered organophilic in order to achieve their acceptable dispersions. For suitable anionic and cationic clays ion exchange with suitable bulky surfactants is practiced for this purpose.
- -
- No decomposition/degradation of the surface modifiers of the nanofillers as they are absent. This is a key issue for ammonium (onium) intercalated (surface modified) clays whose thermal decomposition limits the processing temperature and thus the possible range of polymers (they can never be used in high temperature resistant thermoplastics). It was found that the decomposition of alkyl ammonium ions starts as low as T = 180 °C though major thermal decomposition occurs between T = 200 °C and T = 500 °C [6]. It has to be born in mind that commodity and engineering thermoplastics are processed in the ranges of T = 190–250 °C and T = 200–290 °C, respectively.
- -
- Reduced health risk when added in aqueous slurry compared to the dosage in dry powder form. This is a clear advantage when preformed (available ob ovo in nanoscale, such as silica, carbon nanotubes (CNT)) nanoparticles should be incorporated.
- -
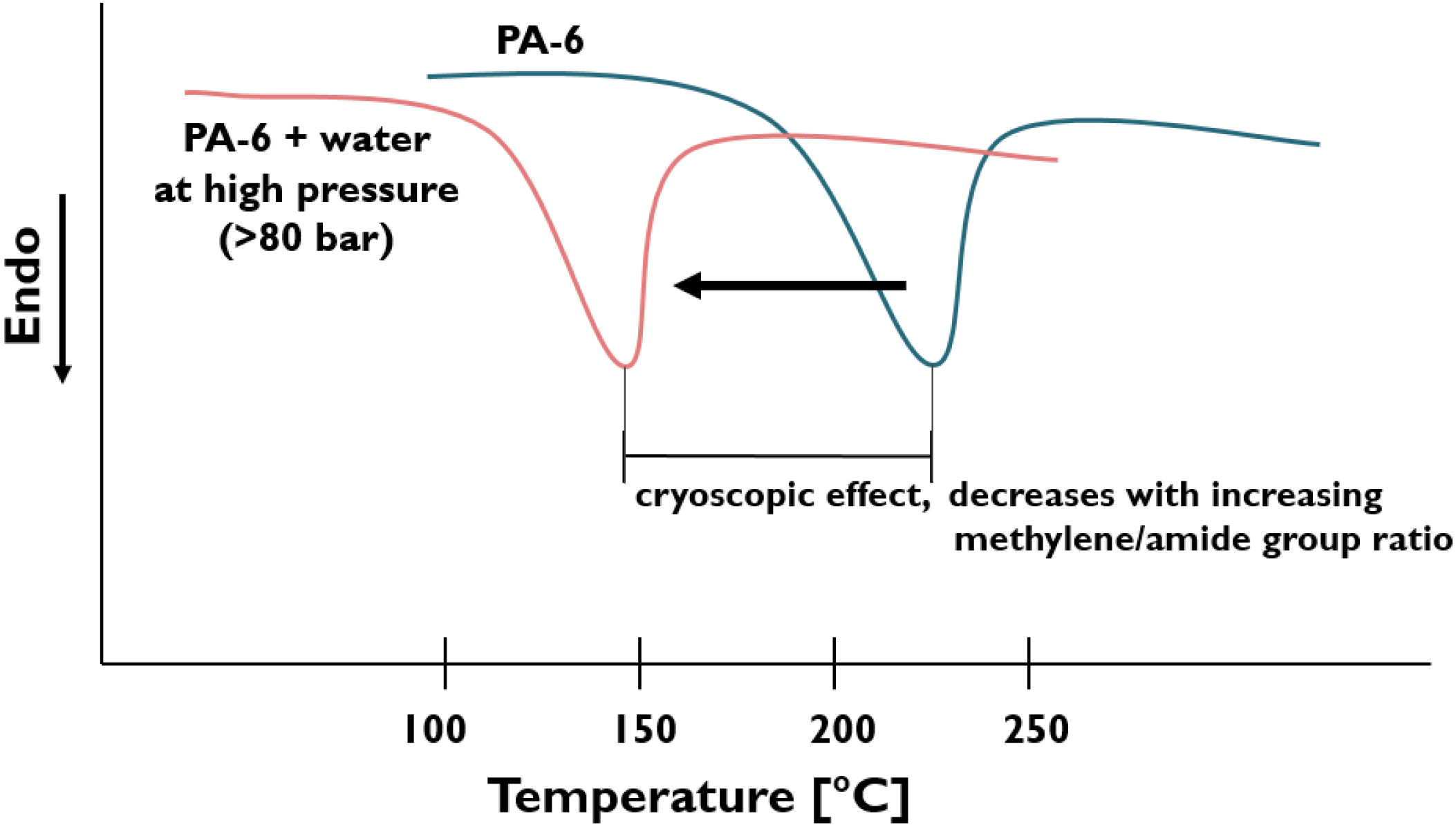
- Improved nanofiller dispersion due to local “blow-up” phenomena when the pressurized liquid evaporates from the melt. This was the basic idea of the early patent [7]. The other fundamental effects, linked with matrix/water (liquid) interactions include cryoscopy (i.e., depression of the melting temperature associated with decreased melt viscosity) and plasticization. Attention should be called to the fact that some polymers are prone to hygrothermal decomposition. This manifests in substantially lower molecular weight (MW) products that should be counterbalanced in a proper way.
- -
- There are further aspects worth mentioning. Water in some cases is an indispensable plasticizer that should not be removed during compounding. This is the case for the production of thermoplastic starch (TPS) from natural starch. So, gelatinization and nanoreinforcement of starch can be performed simultaneously [8]. In other cases, the liquid may work as reactive compound, for example for coupling molecular chains thereby enhancing the MW.
- -
- Polymer and rubber lattices are aqueous dispersions, too. Like nanofiller dispersions these lattices can also be directly incorporated alone or in combination with suitable nanofillers to produce impact-modified (toughened) and nanoreinforced thermoplastic composites. This concept has been patented, as well [9,10].
2. Nanofillers

3. Concept and Realization of WA Melt Compounding
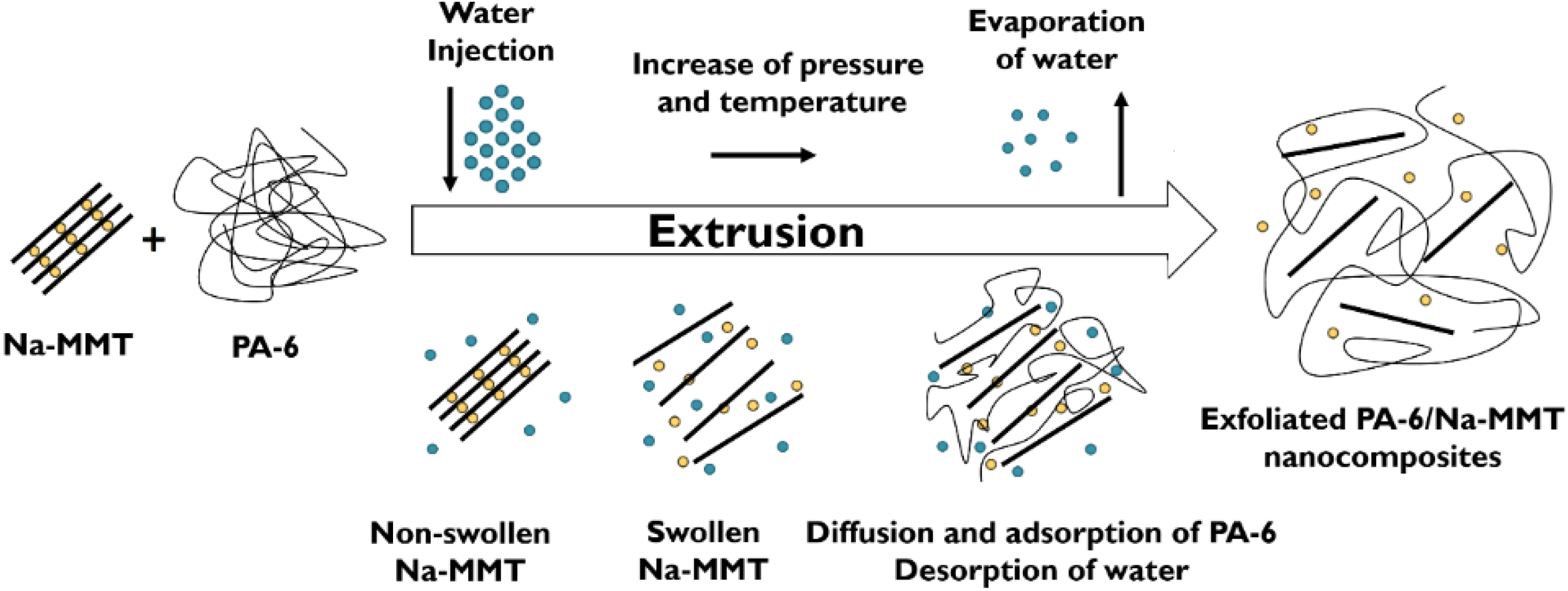
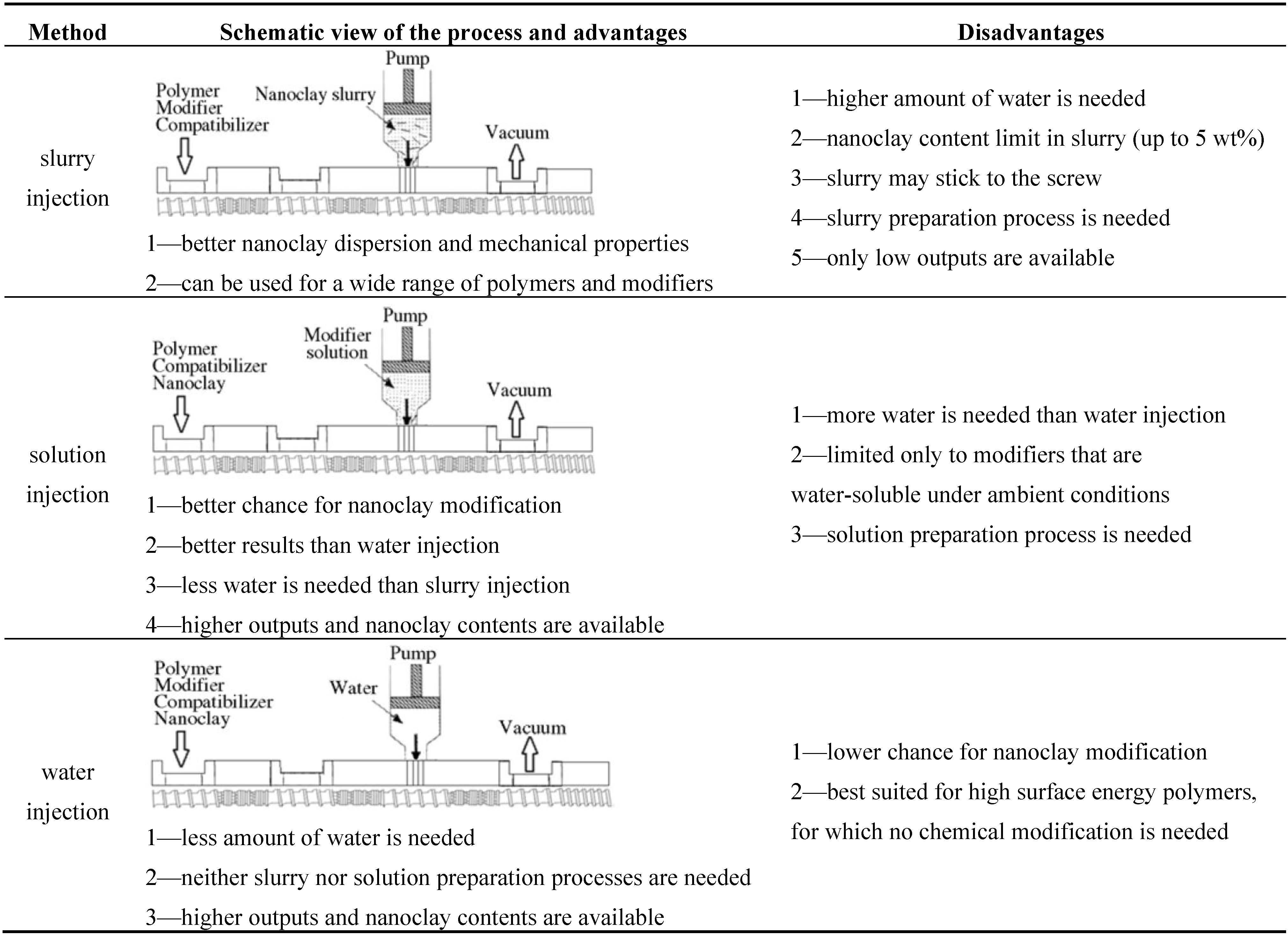

4. WA Melt Compounding of Thermoplastic Nanocomposites
4.1. Commodity Thermoplastics
| Polymer | Filler Type, Amount | Surfactant Type, Amount | Compatibilizer, toughener type, amount | Compounding | Results | Ref. |
|---|---|---|---|---|---|---|
| LDPE granule/powder | Microcrystalline cellulose 0–30 wt% | - | - | Water injected in the high-pressure compression zone (~125 bar) of a corotating twin-screw extruder. | Cellulose could not be fibrillated in nanoscale. WA contributed to a better dispersion of cellulose compared to the reference “dry” process. | [32] |
| LDPE LLDPE | Na-MMT 0–5 wt% | Various quaternary ammonium salts | LDPE-g-MA 0–10 wt% | Water, clay slurry, or aqueous surfactant were injected in the high pressure zone of an intermeshing twin-screw extruder (Figure 5). | Design of experiments used to determine effects of surfactants (type, amount) clay amount and processing conditions on mechanical, rheological and barrier properties. | [30,33] |
| PP 70, 100 part | Na-MMT 0, 5, 10 part | Octadecyl trimethyl ammonium chloride 0, 0.25, 1 part | PP-g-MA 0, 30 part | Corotating intermeshing extruder of very high length-to-diameter ratio (L/D = 77) and special screw design and sealings against high pressure used. Clay slurry injected. | PP/clay nanocomposite by WA melt compounding exhibited similar properties as the reference PP/organoclay. Polymeric compatibilizer (PP-g-MA) required to support MMT exfoliation. | [34] |
| PP PP-g-MA | Na-MMT, organoclay 21 wt% | - | - | Water injected (amount varied) in the high-pressure compression zone of a corotating twin-screw extruder. PP/(organo)clay masterbatches (MB) also processed by WA technique. | Morphological, mechanical, rheological and thermal properties of the nanocomposites studied. The MB process outperformed the “one pot” version. Water improved the dispersion of clay and proved beneficial to support the chemical reaction between PP-g-MA and hydroxyl groups of the organoclay surfactant. | [35] |
| PP | Na-MMT, organoclay <7 wt% | - | PP-g-MA (9–10 wt%), Na-acetate (0, 4 wt%) (to convert PP-g-MA into an ionomer) | Water injected in the high-pressure compression zone of a corotating twin-screw extruder. | Morphological, mechanical, rheological and thermal properties assessed. In situ synthesis of “carboxylate clay” from pristine clay and PP-g-MA ionomer, through trihydrate sodium acetate addition with help of WA compounding proved to be an effective alternative to using organomodified clays and compatibilizers. | [36] |
| TPV (PP-based) | CNF aqueous dispersion (15 g/L) 5 phr | - | EPDM | Crumb EPDM was spray-coated by CNF and melt mixed with TPV. | Morphology, dynamic-mechanical, thermal and tribological properties determined. The fragmented CNF was located in the PP phase. | [37] |
| TPV (PP-based) | BA (particle size in water 300 nm) 5 wt% | - | - | BA added dry or via WA technique using a corotating twin-screw extruder. | Tensile, thermal, DMA, creep and stress relaxation tests performed. BA located in the PP-phase. WA produced better dispersion than the traditional dry dosage. The better dispersion was best reflected in the creep and stress relaxation results. | [38] |
| PS | Na-fluorohectorite 0–7 wt% | - | - | Micro- and nanocomposites produced batchwise in a kneader. For nanocomposite preparation Na-fluorohectorite was mixed first with a PS latex which after drying was used as a MB for dilution with molten PS. Dry melt mixing, resulted in microcomposite. | Nanocomposites outperformed the microcomposites with respect to stiffness and resistance to creep. Dispersion in nanoscale affected, however, mostly the initial creep compliance. | [39,40] |
| PS | BA (particle size in water 25 and 220 nm, respectively) 4.5 wt% | - | - | Nanocomposites produced batchwise in a kneader; dry or through WA technique (latex-mediated). In the latter case PS latex was compounded with BA followed by drying and dilution with molten PS. | Latex-mediated nanocomposites exhibited higher stiffness, resistance to creep, to thermal deflection than the reference composite produced by traditional “dry” melt compounding. | [41] |
| PS | BA (particle size in water 25 and 220 nm, respectively) 3 wt% | - | SBR from latex 10 wt% | Binary (PS/BA, PS/SBR) and ternary systems (PS/BA/SBR) were produced via WA in a twin-screw extruder | Morphology, DMA, tensile mechanical, impact and short term creep and stress relaxation behaviors studied. BA acted as efficient nanoreinforcement while SBR as toughening agent in the binary systems. BA was mostly embedded in the SBR phase in the ternary blends. Modifiers’ effects best manifested in tensile and stress relaxation tests. | [42] |
4.2. Engineering Thermoplastics
- -
- Pristine MMT (Na-MMT) and similar cationic clays can be well dispersed in different PAs, including PA-based elastomers. The hydrolytic degradation of the PAs is small to negligible with increasing methylene groups in the structural unit. The water-induced cryoscopic effect lowers the actual viscosity and thus enhances the molecular mobility of the PA. This, along with the improved hydrophilic character of the PA chains strongly support the intercalation, and even result in full exfoliation of the clay.
- -
- WA is less efficient for apolar polymers in absence of suitable compatibilizers. Recall that this is the learning from studies performed on polyolefins, as well.
- -
- In case of thermoplastic polyesters, precautions are needed to avoid/compensate the prominent hygrothermal degradation manifesting in highly reduced average MW.
- -
- The promise of WA technique is not yet explored for carbonaceous nanofillers and polymer blends. For the latter it seems to be very promising to disperse these carbonaceous nanofillers in water-soluble polymers and incorporate the related concentrated MBs in engineering polymers.
| Polymer | Filler type, amount | Surfactant Type, Amount | Compatibilizer, toughener type, amount | Compounding | Results | Ref. |
|---|---|---|---|---|---|---|
| PA-6 | Na-MMT 1.6 wt% | - | - | Aqueous clay slurry injected in the high-pressure zone of a corotating extruder equipped with a sealing zone. | Clay dispersion, mechanical and barrier properties determined and compared with the effect of an organoclay (stearyl ammonium ion) melt compounded “dry”. The properties were practically the same. WA only slightly reduced the MW of PA-6. | [26] |
| PA-6 | Na-MMT nano-ZnO | octadecyl ammonium salt | epoxy resin (EP) | Compounding in a twin-screw extruder but not disclosing how water and other additives were introduced. | EP supposed to enter into the galleries and react with the terminal groups of PA-6. Incorporation of ZnO contributed to better intercalation of MMT, the reason of which was unknown. According to our feeling, this may be linked with coordination complexing between the Zn2+ and amid groups of PA-6 [43]. | [44] |
| PA-6 | Na-MMT organoMMT (dioctadecyl dime-thylammonium ion), 5 wt% | - | - | Compounding on a twin-screw extruder water injected into the extruder barrel downstream at various flow rates. | Morphology, mechanical, tribological and thermal properties determined. The hydrolysis of PA-6 was negligible. Unlike to organoMMT, WA compounding strongly improved the dispersion and reinforcing effectiveness of Na-MMT. | [45,46] |
| PA-6 | Na-MMT | - | - | Water is pumped into the high-pressure compression zone of the twin-screw extruder with special screw design. | Morphology studied, cryoscopic effect of water emphasized (cf. Figure 6). Model proposed for the exfoliation of pristine clay, cf. Figure 2. | [47,48] |
| PA-6 | Na-fluorohectorite BA (mean size in water dispersion 220 nm) 2.5 wt% | - | HNBR from latex 9 wt% | Binary (PA-6/nanofiller) and ternary systems (PA-6/nanofiller/HNBR) were produced in a kneader via WA. In the aqueous HNBR latex were also the nanofillers dispersed. | Morphology, tensile, impact, DMA and creep properties determined. Na-fluorohectorite was embedded in the PA-6 matrix, whereas BA into the dispersed HNBR domains. HNBR acted as efficient impact modifier. Na- fluorohectorite outperformed BA with respect to the properties tested. This was traced to its preferred dispersion in the PA-6 matrix. | [49,50] |
| PA-6 | Na-MMT 1.5, 3 wt% | - | - | Two-step extrusion process used. First step: MB production with and without WA. Second step: dilution of MB with and without WA. Water injected at >26 bar in the mixing zone of the extruder. | High level of MMT, reflected in the mechanical properties, achieved with longer contact time between water and PA-6 melt. Accordingly, the WA process is controlled by diffusion mechanism. | [51] |
| PA-6/PP blend (PP major phase) | Na-MMT | - | SEBS-g-MA | PA/clay (60/20 wt%) nanocomposite produced by WA melt compounding and it was used as MB to dilute with PP. | The compatibilizer (SEBS-g-MA) was located in the interphase between PA-6 (in submicron nodules) and PP matrix. | [48] |
| PA-11 | Na-MMT 0–20 wt% | - | - | Water pumped into the high-pressure compression zone of a twin-screw extruder of special design. | Exfoliated morphology demonstrated up to 10 wt% clay. Stiffness and thermal stability of PA-11 are drastically enhanced, ductility decreased. Based on WAXS the crystal axis was parallel to the clay surface. Strong effect of screw rotation speed concluded. | [52] |
| PA-12 | Halloysite 0–16 wt% | - | - | Water injected into the high-pressure compression zone (~125 bar) of the twin-screw extruder. | Fracture, tensile, thermal and flammability properties determined. Stiffness, strength markedly improved at cost of elongation at break. Water was an efficient dispersing aid for halloysite. Improved dispersion ascribed to potential H-bond formation between PA-12 and surface hydroxyl groups of halloysite. | [53] |
| PEBA | raw MMT (non-purified bentonite) Na-MMT organoMMT | - | - | Water injected into the high-pressure zone (70–100 bar) of the twin-screw extruder. Pressure of the injected water higher than the water vapor pressure at the processing temperature. | PEBA degradation checked by GPC and no hydrolytic degradation found. Clay dispersion, morphological, tensile properties determined. Stiffness, strength strongly enhanced at cost of ductility with increasing MMT content. Properties comparable with compounds with organoMMT. | [54] |
| PET | MMT centrifuged MMT 0–2 wt% | - | - | Clay slurry (through a peristaltic pump) and PET granules fed into a corotating twin-screw extruder. | Morphology and melt viscosity determined. Centrifuged clay (having no large agglomerates) yielded better dispersion than MMT. | [55] |
| PET | Na-MMT organoMMT (different surfactants) 0–6 wt% | - | - | PET with dry mixed clay was fed into the extruder. Water steam (160 °C saturated or not) was introduced in the second zone of a corotating twin-screw extruder with special screw design. | MW degradation determined by measuring the intrinsic viscosity. MW markedly decreased by WA compounding, reflected by a large drop in the ductility. WA method resulted in better stiffness, strength than traditional “dry” one. To compensate MW degradation, solid-state polymerization (SSP) was performed. No improvement of SSP was found at high organoMMT contents. Rheological results proved to be useful indicators of the clay dispersion. | [56,57,58] |
| SAN | Na-MMT organoMMT 0–3 wt% | - | - | Water pumped into the high-pressure compression zone (~125 bar). | Changes in MW and morphology determined. Dynamic-mechanical analysis, mechanical and flammability tests conducted. WA improved the dispersion of organoMMT and Na-MMT. According to XRD no intercalation was observed for Na-MMT. | [59] |
| POM | BA in different nanodimensions 3 wt% | - | - | Aqueous BA slurry introduced in the low-pressure feeding zone of a twin-screw extruder. | BA dispersion with its effects on DMA and creep properties studied. BA of smaller size resulted in better property improvement than the coarser one. | [60] |
| POM | BA 0, 3 wt% | - | PU (from latex) 0, 10 wt% | Binary (POM/PU, POM/BA) and ternary (POM/PU/BA) systems produced by WA method. PU latex and aqueous BA slurry introduced in the low pressure feeding zone of a twin-screw extruder. | Morphology, DMA, creep, tensile and impact properties determined. Good dispersion in the binary system, BA embedded in the PU in the ternary nanocomposite, cf. Figure 7. | [28] |
| POM | CNF 0.1 wt% | - | PU (from latex) 0, 10 wt% | Binary (POM/PU, POM/CNF) and ternary (POM/PU/CNF) systems produced by WA in a kneader (inner mixer) | Morphology, crystallinity, DMA, creep, stress relaxation and dielectrical properties studied. CNF worked as nanoreinforcement. | [61] |
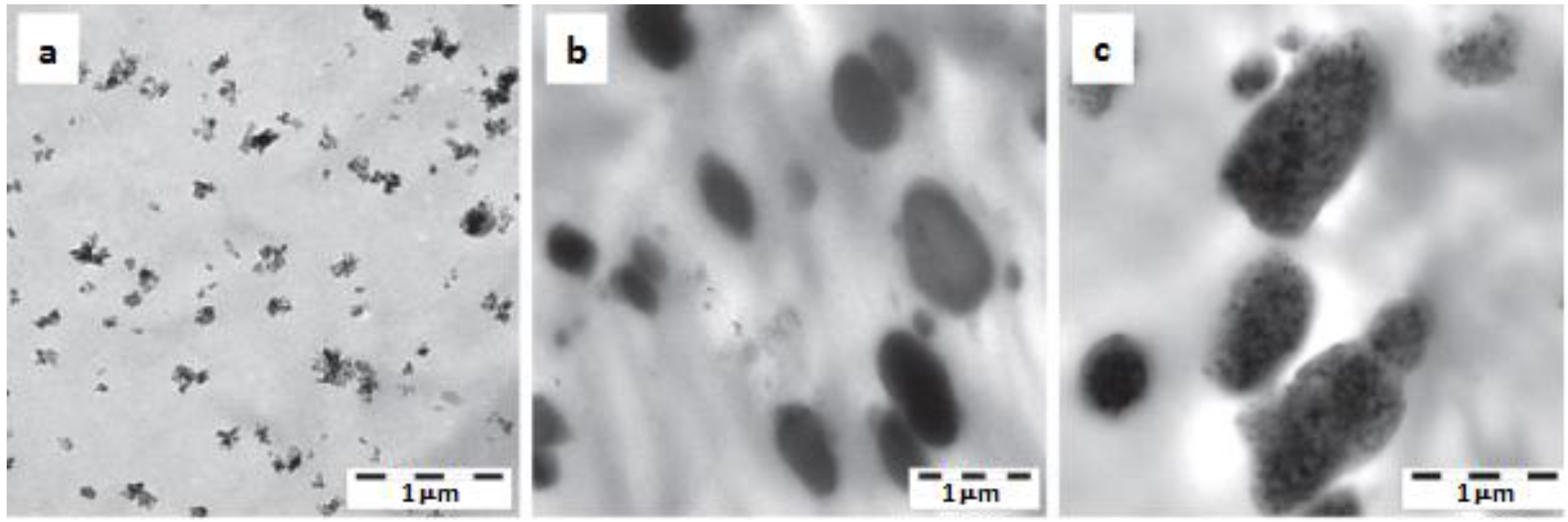
5. WA Melt Compounding of Plasticized Thermoplastic Nanocomposites
| Microcellulose content (wt%) | NR Latex content (wt%) | Young’s modulus (GPa) | Yield strength (MPa) | Elongation at yield (%) |
|---|---|---|---|---|
| 5 | - | 0.15 ± 0.02 | 4.7 ± 0.7 | 39.3 ± 5.8 |
| 10 | - | 0.16 ± 0.02 | 4.9 ± 0.3 | 26.9 ± 2.7 |
| 10 | 10 | 0.31 ± 0.03 | 3.1 ± 0.4 | 2.2 ± 0.1 |
| 15 | - | 0.17 ± 0.01 | 5.3 ± 0.3 | 19.6 ± 1.8 |
| 20 | - | 0.23 ± 0.04 | 5.6 ± 0.7 | 15.3 ± 0.9 |
| 20 | 10 | 0.53 ± 0.05 | 3.1 ± 0.4 | 1.0 ± 0.3 |
6. Conclusions and Outlook
Acknowledgments
List of Abbreviations
| 0D | zero-dimensional |
| 1D | one-dimensional |
| 2D | two-dimensional |
| 3D | three-dimensional |
| ALAMS | alkyl ammonium salt |
| Al2O3 | aluminum oxide |
| AlO(OH) | aluminum oxide hydroxide |
| BA | boehmite alumina |
| DW-CNT | double-walled carbon nanotube |
| Ca | calcium |
| CNF | carbon nanofiber |
| CNT | carbon nanotube |
| DMA | dynamic mechanical analysis |
| DSC | differential scanning calorimetry |
| EPDM | ethylene propylene diene rubber |
| EVA | ethylene vinylacetate copolymer |
| GPC | gel permeation chromatography |
| H | hydrogen |
| HDPE | high density polyethylene |
| HNBR | hydrogenated nitrile (acrylonitrile butadiene) rubber |
| LDPE | low density polyethylene |
| LDPE-g-MA | maleated low-density polyethylene (grafted by maleic anhydride) |
| LLDPE | linear low density polyethylene |
| K | potassium |
| MA | maleic anhydride |
| MB | masterbatch |
| MMT | montmorillonite |
| MW | molecular weight |
| MW-CNT | multi-walled carbon nanotube |
| Na | sodium |
| Na-MMT | sodium montmorillonite |
| NR | natural rubber |
| organoMMT | organophilic modified montmorillonite |
| OTAC | octadecyl trimethyl ammonium chloride |
| PA | polyamide |
| PE | polyethylene |
| PEBA | polyether-block-amide |
| PEMA | polyethyl methacrylate |
| PET | polyethylene terephthalate |
| PLA | polylactic acid |
| PLA-g-MA | maleated polylactic acid (grafted by maleic anhydride) |
| POM | polyoxymethylene |
| PP | polypropylene |
| PP-g-MA | maleated polypropylene (grafted by maleic anhydride) |
| PS | polystyrene |
| PU | polyurethane |
| SAN | styrene acrylonitril copolymer |
| SBR | styrene butadiene rubber |
| SEBS-g-MA | maleated styrene-ethylene butylene-styrene block copolymer |
| SEM | scanning electron microscopy |
| SSP | solid state polymerization |
| SW-CNT | single-walled carbon nanotube |
| TEM | transmission electron microscopy |
| TPS | thermoplastic starch |
| TPV | thermoplastic vulcanizate |
| TiO2 | titanium dioxide |
| WA | water-assisted |
| XRD | X-ray diffraction |
| ZnO | zinc oxide |
Conflicts of Interest
References
- Utracki, L.A. Clay-Containing Polymeric Nanocomposites; Rapra Technology Limited: Shawbury, Shropshire, UK, 2004; Volume 1. [Google Scholar]
- Kalia, S.; Kaith, B.S.; Kaur, I. Cellulose Fibers: Bio- and Nano-Polymer Composites; Springer: Berlin, Germany, 2011. [Google Scholar]
- Michler, G.H.; Baltá-Calleja, F.J. Nano- and Micromechanics of Polymers; Hanser: Munich, Germany, 2012. [Google Scholar]
- Karger-Kocsis, J.; Fakirov, S. Nano- and Micromechanics of Polymer Blends and Composites; Carl Hanser Verlag GmbH & Co. KG: Munich, Germany, 2009. [Google Scholar]
- Karger-Kocsis, J. On the toughness of “nanomodified” polymers and their traditional polymer composites. In Nano- and Mircro- Mechanics of Polymer Blends and Composites; Karger-Kocsis, J., Fakirov, S., Eds.; Hanser: Munich, Germany, 2009; pp. 425–470. [Google Scholar]
- Heinz, H. Alkylammonium chains on layered clay mineral surfaces. In Rubber-Clay Nanocomposites; Galimberti, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 101–125. [Google Scholar]
- Korbee, R.A.; van Geenen, A.A. Process for the Preparation of a Polyamide Nanocomposite Composition. WO 1999029767 A1, 17 June 2002. [Google Scholar]
- Shanks, R.; Kong, I. Thermoplastic starch. In Thermoplastic Elastomers; El-Sonbati, A.Z., Ed.; InTech: Rijeka, Croatia, 2012; pp. 95–116. [Google Scholar]
- Karger-Kocsis, J.; Harmia, T. Extrusionsverfahren zur Herstellung von zähmodifizierten und schichtsilikatverstärkten thermoplastischen Systemen. WO 2005040254 A1, 6 May 2005. [Google Scholar]
- Karger-Kocsis, J. Extrusion Method for the Production of Strength-Modified and Phyllosilicate Reinforced Thermoplastic Systems. U.S. Patent 20060264553 A1, 23 November 2006. [Google Scholar]
- Siengchin, S. Polyethylene and polypropylene hybrid composites based on nano silicon dioxide and different flax structures. J. Thermoplast. Compos. Mater. 2014, 27, 1428–1447. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mahmood, H.; Pegoretti, A. Recent advances in fiber/matrix interphase engineering for polymer composites. Prog. Mater. Sci. 2014, in press. [Google Scholar]
- Thomas, S.; Paul, S.A.; Pothan, L.A.; Deepa, B. Natural fibres: Structure, properties and applications. In Cellulose Fibers: Bio- and Nano-Polymer Composites; Kalia, S., Kaith, B.S., Kaur, I., Eds.; Springer: Berlin, Germany, 2011; pp. 3–42. [Google Scholar]
- Ratna, D.; Abraham, T.N.; Siengchin, S.; Karger-Kocsis, J. Novel Method for dispersion of multiwall carbon nanotubes in poly(ethylene oxide) matrix using dicarboxylic acid salts. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 1156–1165. [Google Scholar] [CrossRef]
- Grossiord, N.; Hermant, M.C.; Koning, C. Polymer Carbon Nanotube Composites: The Polymer Latex Concept; Pan Stanford Publishing: Singapore, 2012. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Guo, B.; Chen, F.; Lei, Y.; Liu, X.; Wan, J.; Jia, D. Styrene-butadiene rubber/halloysite nanotubes nanocomposites modified by sorbic acid. Appl. Surf. Sci. 2009, 255, 7329–7336. [Google Scholar] [CrossRef]
- Alateyah, A.I.; Dhakal, H.N.; Zhang, Z.Y. Processing, properties, and applications of polymer nanocomposites based on layer silicates: A review. Adv. Polym. Technol. 2013, 32, 21368. [Google Scholar] [CrossRef]
- Utracki, L.A.; Sepehr, M.; Boccaleri, E. Synthetic, layered nanoparticles for polymeric nanocomposites (PNCs). Polym. Adv. Technol. 2007, 18, 1–37. [Google Scholar] [CrossRef]
- Ulibarri, M.A.; Pavlovic, I.; Barriga, C.; Hermosín, M.C.; Cornejo, J. Adsorption of anionic species on hydrotalcite-like compounds: Effect of interlayer anion and crystallinity. Appl. Clay Sci. 2001, 18, 17–27. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Stöckelhuber, K.W.; Wagenknecht, U.; Heinrich, G. Advances in layered double hydroxide (LDH)-based elastomer composites. Prog. Polym. Sci. 2014, 39, 594–626. [Google Scholar] [CrossRef]
- Greco, A.; Corcione, C.E.; Strafella, A.; Maffezzoli, A. Analysis of the structure and mass transport properties of clay nanocomposites based on amorphous PET. J. Appl. Polym. Sci. 2010, 118, 3666–3672. [Google Scholar] [CrossRef]
- Corcione, C.E.; Cavallo, A.; Pesce, E.; Greco, A.; Maffezzoli, A. Evaluation of the degree of dispersion of nanofillers by mechanical, rheological, and permeability analysis. Polym. Eng. Sci. 2011, 51, 1280–1285. [Google Scholar] [CrossRef]
- Wevers, M.G.M.; Pijpers, T.F.J.; Mathot, V.B.F. The way to measure quantitatively full dissolution and crystallization of polyamides in water up to 200 °C and above by DSC. Thermochim. Acta 2007, 453, 67–71. [Google Scholar] [CrossRef]
- Charlet, K.; Mathot, V.; Devaux, J. Crystallization and dissolution behaviour of polyamide 6–water systems under pressure. Polym. Int. 2011, 60, 119–125. [Google Scholar] [CrossRef]
- Hasegawa, N.; Okamoto, H.; Kato, M.; Usuki, A.; Sato, N. Nylon 6/Na–montmorillonite nanocomposites prepared by compounding Nylon 6 with Na–montmorillonite slurry. Polymer 2003, 44, 2933–2937. [Google Scholar] [CrossRef]
- Alexandre, M.; Beyer, G.; Henrist, C.; Cloots, R.; Rulmont, A.; Jerome, R.; Dubois, P. “One-pot” preparation of polymer/clay nanocomposites starting from Na+ montmorillonite. 1. Melt intercalation of ethylene-vinyl acetate copolymer. Chem. Mater. 2001, 13, 3830–3832. [Google Scholar] [CrossRef]
- Siengchin, S.; Karger-Kocsis, J.; Thomann, R. Nanofilled and/or toughened POM composites produced by water-mediated melt compounding: Structure and mechanical properties. Express Polym. Lett. 2008, 2, 746–756. [Google Scholar] [CrossRef]
- Hassinger, I.; Becker, T.; Walter, R.; Burkhart, T.; Kopnarski, M.; Brodyanski, A. Innovative direct nanoparticle dispersion injection into injection molding processing. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Shahabadi, S.I.S.; Garmabi, H. Response surface analysis of structural, mechanical, and permeability properties of polyethylene/Na+-montmorillonite composites, prepared by slurry-fed melt intercalation. Express Polym. Lett. 2012, 6, 657–671. [Google Scholar] [CrossRef]
- Taguet, A.; Cassagnau, P.; Lopez-Cuesta, J.M. Structuration, selective dispersion and compatibilizing effect of (nano)fillers in polymer blends. Prog. Polym. Sci. 2014, 39, 1526–1563. [Google Scholar] [CrossRef]
- Soulestin, J.; Quiévy, N.; Sclavons, M.; Devaux, J. Polyolefins–biofibre composites: A new way for an industrial production. Polym. Eng. Sci. 2007, 47, 467–476. [Google Scholar] [CrossRef]
- Shahabadi, S.I.S.; Garmabi, H. Polyethylene/Na+-montmorillonite composites prepared by slurry-fed melt intercalation: Response surface analysis of rheological behavior. J. Reinf. Plast. Compos. 2012, 31, 977–988. [Google Scholar] [CrossRef]
- Kato, M.; Matsushita, M.; Fukumori, K. Development of a new production method for a polypropylene-clay nanocomposite. Poly. Eng. Sci. 2004, 44, 1205–1211. [Google Scholar] [CrossRef]
- Rousseaux, D.D.J.; Sallem-Idrissi, N.; Baudouin, A.C.; Devaux, J.; Godard, P.; Marchand-Brynaert, J.; Sclavons, M. Water-assisted extrusion of polypropylene/clay nanocomposites: A comprehensive study. Polymer 2011, 52, 443–451. [Google Scholar] [CrossRef]
- Rousseaux, D.D.J.; Sclavons, M.; Godard, P.; Marchand-Brynaert, J. Polypropylene/clay nanocomposites: An innovative one-pot process. Polym. Compos. 2014, 1–7. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Felhös, D.; Thomann, R. Tribological behavior of a carbon-nanofiber-modified santoprene thermoplastic elastomer under dry sliding and fretting conditions against steel. J. Appl. Polym. Sci. 2008, 108, 724–730. [Google Scholar] [CrossRef]
- Siengchin, S.; Karger-Kocsis, J. Mechanical and stress relaxation behavior of Santoprene® thermoplastic elastomer/boehmite alumina nanocomposites produced by water-mediated and direct melt compounding. Compos. Part A Appl. Sci. Manuf. 2010, 41, 768–773. [Google Scholar] [CrossRef]
- Siengchin, S.; Karger-Kocsis, J. Creep behavior of polystyrene/fluorohectorite micro- and nanocomposites. Macromol. Rapid Commun. 2006, 27, 2090–2094. [Google Scholar] [CrossRef]
- Siengchin, S.; Karger-Kocsis, J.; Apostolov, A.A.; Thomann, R. Polystyrene–fluorohectorite nanocomposites prepared by melt mixing with and without latex precompounding: Structure and mechanical properties. J. Appl. Polym. Sci. 2007, 106, 248–254. [Google Scholar] [CrossRef]
- Siengchin, S.; Karger-Kocsis, J.; Thomann, R. Alumina-filled polystyrene micro- and nanocomposites prepared by melt mixing with and without latex precompounding: Structure and properties. J. Appl. Polym. Sci. 2007, 105, 2963–2972. [Google Scholar] [CrossRef]
- Siengchin, S.; Karger-Kocsis, J. Binary and ternary composites of polystyrene, styrene–butadiene rubber and boehmite produced by water-mediated melt compounding: Morphology and mechanical properties. Compos. Part B Eng. 2013, 45, 1458–1463. [Google Scholar] [CrossRef]
- Szafner, A.; Karger-Kocsis, J. Increase of the melt viscosity of polycaproamide by chromium (III) ions. Polymer 1975, 16, 879–880. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Yu, J. Direct melt extrusion processing of nylon 6/clay nanocomposites based on pristine montmorillonite. Macromol. Mater. Eng. 2005, 290, 649–652. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Hu, G.H.; Varlet, J.; Dasari, A.; Mai, Y.W. Water-assisted melt compounding of nylon-6/pristine montmorillonite nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 1100–1112. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Mai, Y.W.; Hu, G.H.; Varlet, J. Clay exfoliation and organic modification on wear of nylon 6 nanocomposites processed by different routes. Compos. Sci. Technol. 2005, 65, 2314–2328. [Google Scholar] [CrossRef]
- Fedullo, N.; Sclavons, M.; Bailly, C.; Lefebvre, J.M.; Devaux, J. Nanocomposites from untreated clay: A myth? Macromol. Symp. 2006, 233, 235–245. [Google Scholar] [CrossRef]
- Fedullo, N.; Sorlier, E.; Sclavons, M.; Bailly, C.; Lefebvre, J.M.; Devaux, J. Polymer-based nanocomposites: Overview, applications and perspectives. Prog. Org. Coat. 2007, 58, 87–95. [Google Scholar] [CrossRef]
- Siengchin, S.; Karger-Kocsis, J. Structure and creep response of toughened and nanoreinforced polyamides produced via the latex route: Effect of nanofiller type. Compos. Sci. Technol. 2009, 69, 677–683. [Google Scholar] [CrossRef]
- Siengchin, S.; Karger-Kocsis, J. Structure, mechanical, and fracture properties of nanoreinforced and HNBR-toughened polyamide-6. J. Appl. Polym. Sci. 2012, 123, 897–902. [Google Scholar] [CrossRef]
- Molajavadi, V.; Garmabi, H. Water assisted exfoliation of PA6/clay nanocomposites using a twin screw extruder: Effect of water contact time. J. Appl. Polym. Sci. 2011, 119, 736–743. [Google Scholar] [CrossRef]
- Stoclet, G.; Sclavons, M.; Devaux, J. Relations between structure and property of polyamide 11 nanocomposites based on raw clays elaborated by water-assisted extrusion. J. Appl. Polym. Sci. 2012, 127, 4809–4824. [Google Scholar] [CrossRef]
- Lecouvet, B.; Sclavons, M.; Bourbigot, S.; Bailly, C. Towards scalable production of polyamide 12/halloysite nanocomposites via water-assisted extrusion: Mechanical modeling, thermal and fire properties. Polym. Adv. Technol. 2014, 25, 137–151. [Google Scholar] [CrossRef]
- Touchaleaume, F.; Soulestin, J.; Sclavons, M.; Devaux, J.; Cordenier, F.; Van Velthem, P.; Flat, J.J.; Lacrampe, M.F.; Krawczak, P. Efficient one-step melt-compounding of copolyetheramide/pristine clay nanocomposites using water-injection as intercalating/exfoliating aid. Express Polym. Lett. 2011, 5, 1085–1101. [Google Scholar] [CrossRef]
- Majdzadeh-Ardakani, K.; Lofgren, E.A.; Jabarin, S.A. The effect of particle size distribution on the dispersion of nanoclays in poly(ethylene terephthalate)/clay nanocomposites. J. Reinf. Plast. Compos. 2014, 33, 358–368. [Google Scholar] [CrossRef]
- Dini, M.; Mousavand, T.; Carreau, P.J.; Kamal, M.R.; Ton-That, M.T. Microstructure and properties of poly(ethylene terephthalate)/organoclay nanocomposites prepared by water-assisted extrusion: Effect of organoclay concentration. Polym. Eng. Sci. 2014, 54, 1879–1892. [Google Scholar] [CrossRef]
- Dini, M.; Mousavand, T.; Carreau, P.J.; Kamal, M.R.; Ton-That, M.T. Effect of water-assisted extrusion and solid-state polymerization on the microstructure of PET/Clay nanocomposites. Polym. Eng. Sci. 2014, 54, 1723–1736. [Google Scholar] [CrossRef]
- Dini, M.; Mousavand, T.; Carreau, P.J.; Kamal, M.R.; Ton-That, M.T. Water-assisted melt mixing and solid-state polymerization of PET/clay nanocomposites. Soc. Plast. Eng. Plast. Res. Online 2013. [Google Scholar] [CrossRef]
- Mainil, M.; Urbanczyk, L.; Calberg, C.; Germain, A.; Jerome, C.; Bourbigot, S.; Devaux, J.; Sclavons, M. Morphology and properties of SAN-clay nanocomposites prepared principally by water-assisted extrusion. Polym. Eng. Sci. 2010, 50, 10–21. [Google Scholar] [CrossRef]
- Siengchin, S. Dynamic mechanic and creep behaviors of polyoxymethylene/boehmite alumina nanocomposites produced by water-mediated compounding: Effect of particle size. J. Thermoplast. Compos. Mater. 2013, 26, 863–877. [Google Scholar] [CrossRef]
- Siengchin, S.; Psarras, G.C.; Karger-Kocsis, J. POM/PU/carbon nanofiber composites produced by water-mediated melt compounding: Structure, thermomechanical and dielectrical properties. J. Appl. Polym. Sci. 2010, 117, 1804–1812. [Google Scholar]
- Rodriguez-Gonzalez, F.J.; Ramsay, B.A.; Favis, B.D. High performance LDPE/thermoplastic starch blends: A sustainable alternative to pure polyethylene. Polymer 2003, 44, 1517–1526. [Google Scholar] [CrossRef]
- Taguet, A.; Bureau, M.N.; Huneault, M.A.; Favis, B.D. Toughening mechanisms in interfacially modified HDPE/thermoplastic starch blends. Carbohydr. Polym. 2014, 114, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Huneault, M.A.; Li, H. Morphology and properties of compatibilized polylactide/thermoplastic starch blends. Polymer 2007, 48, 270–280. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Comparison of sorbitol and glycerol as plasticizers for thermoplastic starch in TPS/PLA blends. J. Appl. Polym. Sci. 2011, 119, 2439–2448. [Google Scholar] [CrossRef]
- Mikus, P.Y.; Alix, S.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P.; Coqueret, X.; Dole, P. Deformation mechanisms of plasticized starch materials. Carbohydr. Polym. 2014, 114, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Cyras, V.P.; Manfredi, L.B.; Ton-That, M.T.; Vázquez, A. Physical and mechanical properties of thermoplastic starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2008, 73, 55–63. [Google Scholar] [CrossRef]
- Arroyo, O.H.; Huneault, M.A.; Favis, B.D.; Bureau, M.N. Processing and properties of PLA/thermoplastic starch/montmorillonite nanocomposites. Polym. Compos. 2010, 31, 114–127. [Google Scholar]
- Courgneau, C.; Rusu, D.; Henneuse, C.; Ducruet, V.; Lacrampe, M.F.; Krawczak, P. Characterisation of low-odour emissive polylactide/cellulose fibre biocomposites for car interior. Express Polym. Lett. 2013, 7, 787–804. [Google Scholar] [CrossRef]
- Hietala, M.; Mathew, A.P.; Oksman, K. Bionanocomposites of thermoplastic starch and cellulose nanofibers manufactured using twin-screw extrusion. Eur. Polym. J. 2013, 49, 950–956. [Google Scholar] [CrossRef]
- Ayana, B.; Suin, S.; Khatua, B.B. Highly exfoliated eco-friendly thermoplastic starch (TPS)/poly (lactic acid)(PLA)/clay nanocomposites using unmodified nanoclay. Carbohydr. Polym. 2014, 110, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Karger-Kocsis, J.; Keki, S. Biodegradable polyester-based shape memory polymers: Concepts of (supra)molecular architecturing. Express Polym. Lett. 2014, 8, 397–412. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Chen, H.; Yang, G.; Zheng, X.; Zhou, S. Water-induced shape-memory poly(d,l-lactide)/microcrystalline cellulose composites. Carbohydr. Polym. 2014, 104, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Dufresne, A.; Tahir, P.; Karimi, A.; Abdulkhani, A. Biodegradable starch-based composites: Effect of micro and nanoreinforcements on composite properties. J. Mater. Sci. 2014, 49, 4513–4521. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Torre, L.; Jimenez, A.; Kenny, J.M. Effects of modified cellulose nanocrystals on the barrier and migration properties of PLA nano-biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, I.; Fambri, L.; Pegoretti, A.; Ferrara, G. Organically modified hydrotalcite for compounding and spinning of polyethylene nanocomposites. Express Polym. Lett. 2013, 7, 936–949. [Google Scholar] [CrossRef]
- Schmitt, H.; Creton, N.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Preparation and characterization of plasticized starch/halloysite porous nanocomposites possibly suitable for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41341. [Google Scholar] [CrossRef]
- Karger-Kocsis, J. Water-mediated dispersion of “nanofillers” in thermoplastics: Is it the right way? Express Polym. Lett. 2008, 2, 312–312. [Google Scholar] [CrossRef]
- Takahashi, C.; Shirai, T.; Fuji, M. Selective intercalation of ionic liquid in montmorillonite and influence of water molecules. Solid State Ion. 2014, 267, 16–21. [Google Scholar] [CrossRef]
- Annamalia, P.K.; Singh, R.P. Biopolymeric nanocomposites as environment benign materials. In Cellulose Fibers: Bio- and Nano-Polymer Composites; Kalia, S., Kaith, B.S., Kaur, I., Eds.; Springer: Berlin, Germany, 2011; pp. 523–535. [Google Scholar]
- Schmitt, H.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Preparation and properties of novel melt-blended halloysite nanotubes/wheat starch nanocomposites. Carbohydr. Polym. 2012, 89, 920–927. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karger-Kocsis, J.; Kmetty, Á.; Lendvai, L.; Drakopoulos, S.X.; Bárány, T. Water-Assisted Production of Thermoplastic Nanocomposites: A Review. Materials 2015, 8, 72-95. https://doi.org/10.3390/ma8010072
Karger-Kocsis J, Kmetty Á, Lendvai L, Drakopoulos SX, Bárány T. Water-Assisted Production of Thermoplastic Nanocomposites: A Review. Materials. 2015; 8(1):72-95. https://doi.org/10.3390/ma8010072
Chicago/Turabian StyleKarger-Kocsis, József, Ákos Kmetty, László Lendvai, Stavros X. Drakopoulos, and Tamás Bárány. 2015. "Water-Assisted Production of Thermoplastic Nanocomposites: A Review" Materials 8, no. 1: 72-95. https://doi.org/10.3390/ma8010072
APA StyleKarger-Kocsis, J., Kmetty, Á., Lendvai, L., Drakopoulos, S. X., & Bárány, T. (2015). Water-Assisted Production of Thermoplastic Nanocomposites: A Review. Materials, 8(1), 72-95. https://doi.org/10.3390/ma8010072









