Decomposition of Cyclohexane on Ni3Al Thin Foil Intermetallic Catalyst
Abstract
:1. Introduction
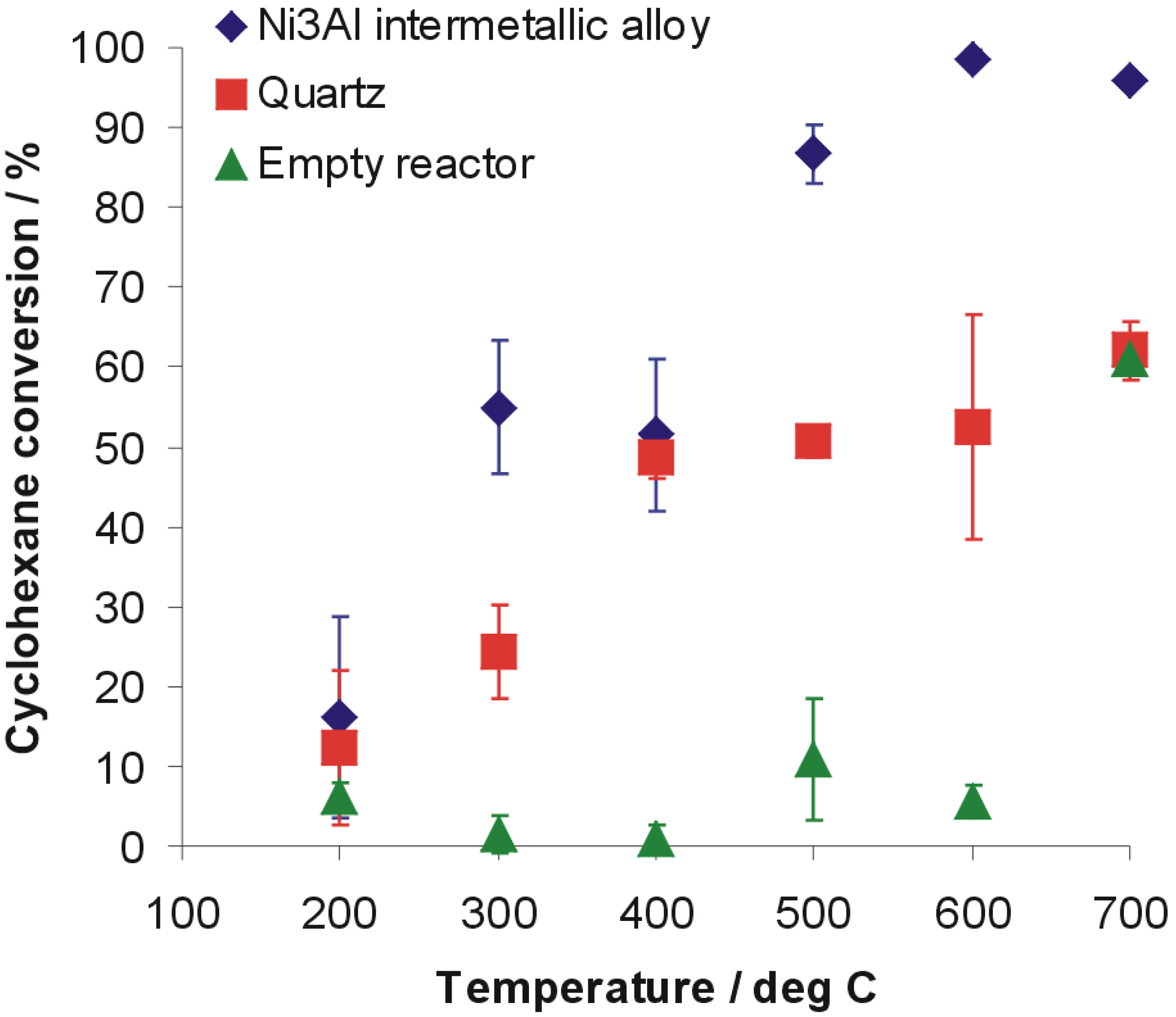
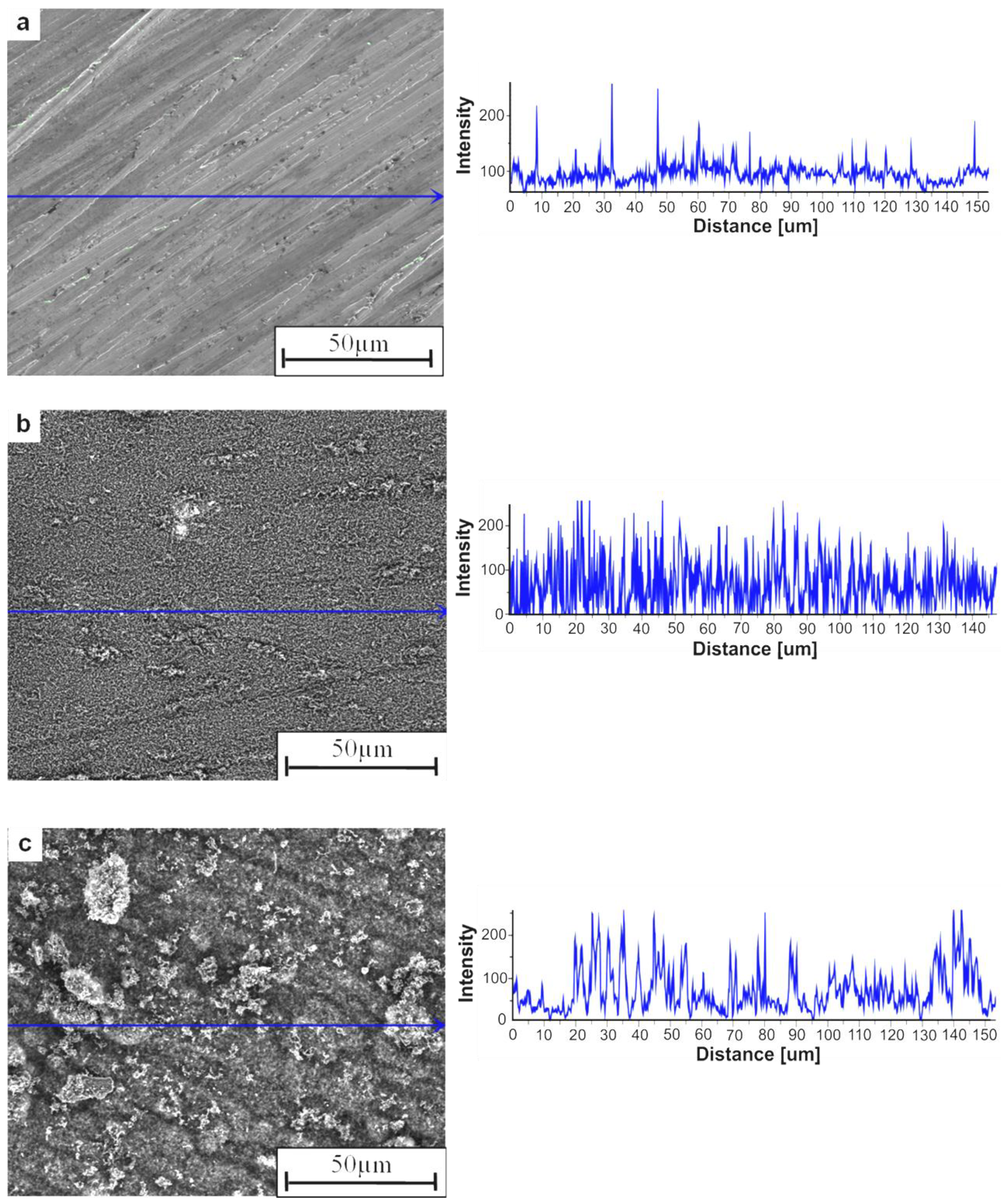
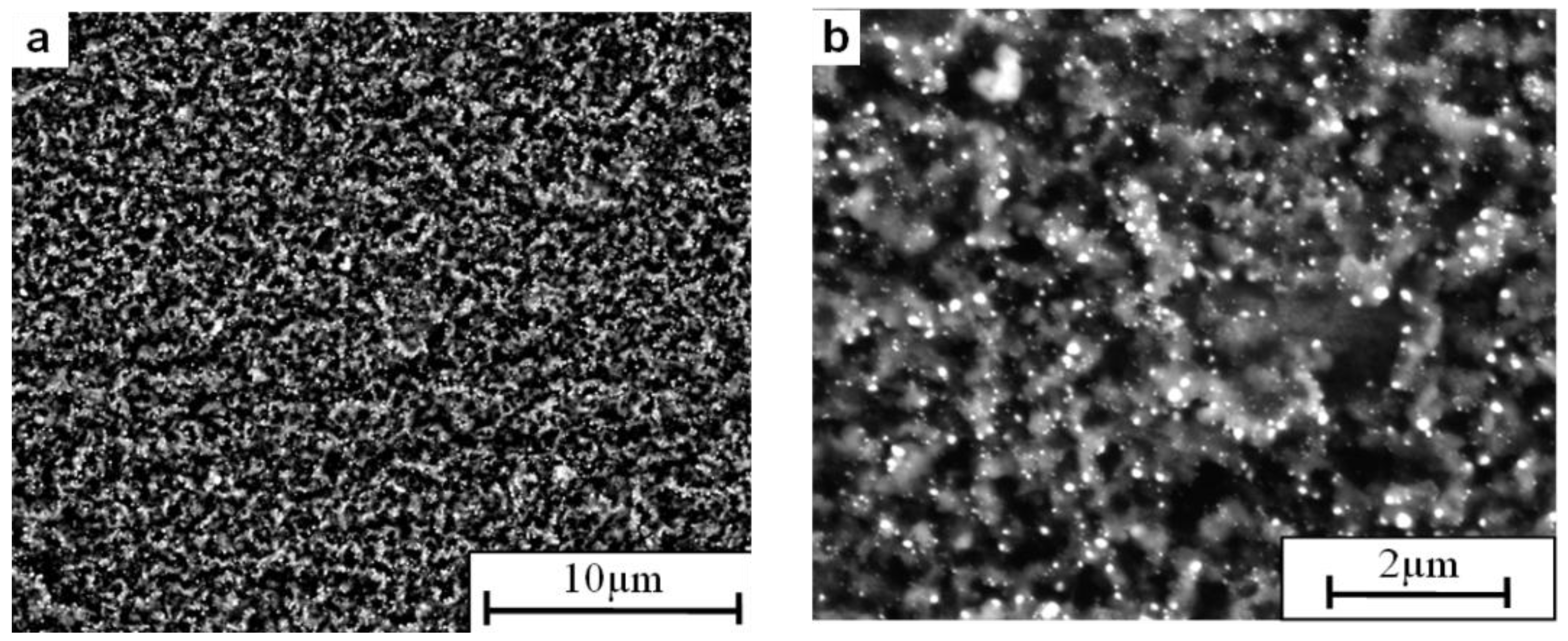
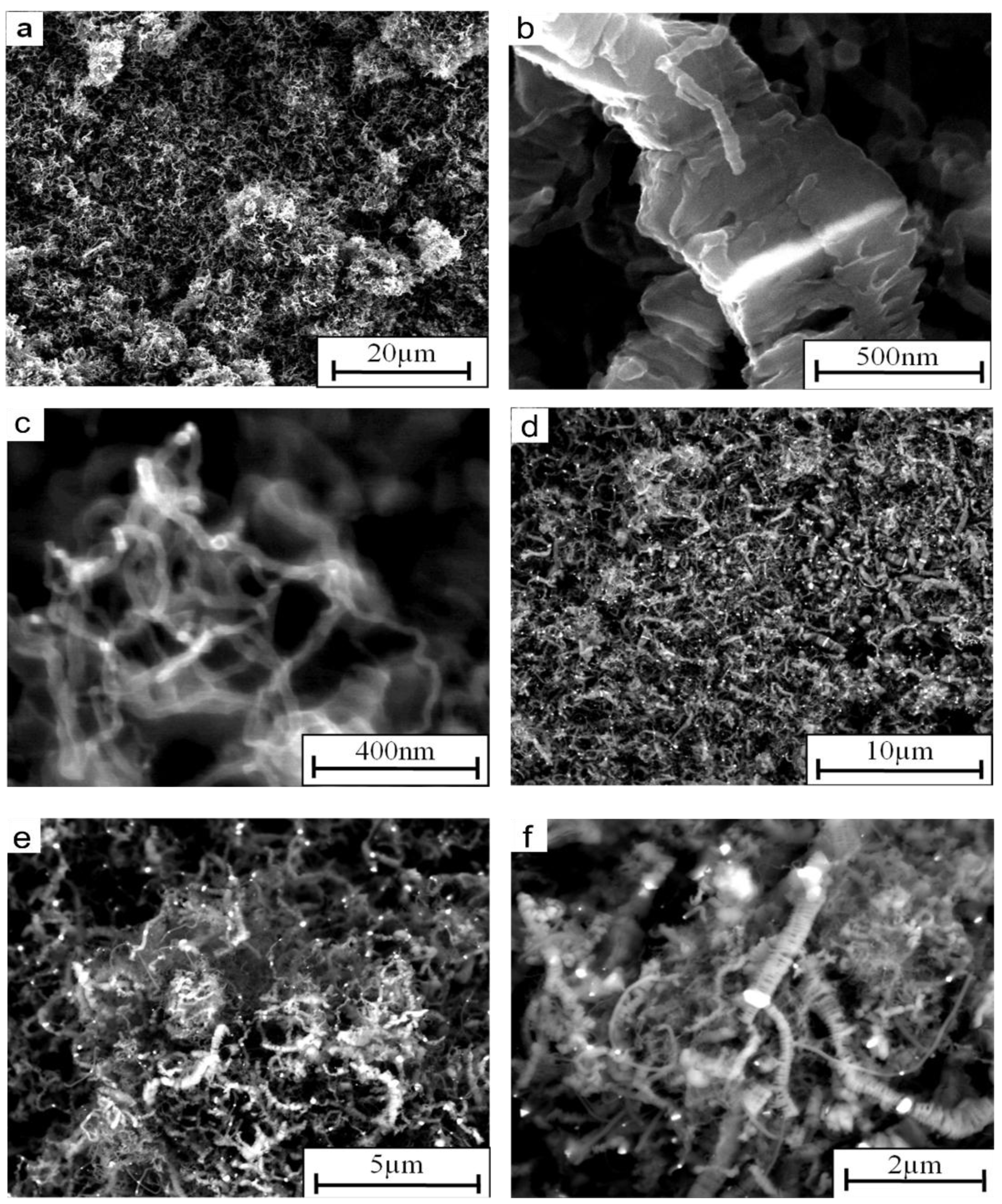
2. Results and Discussion
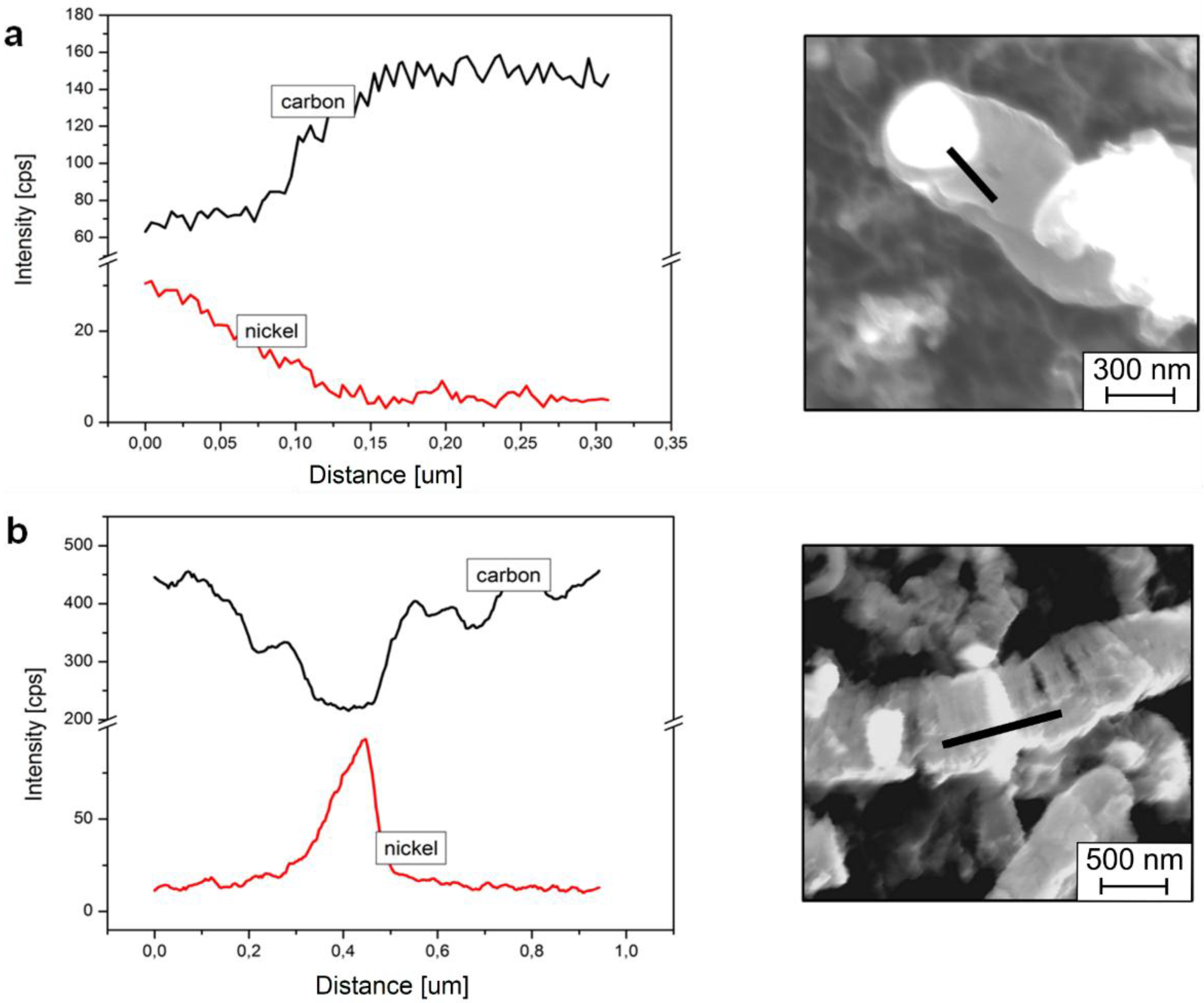
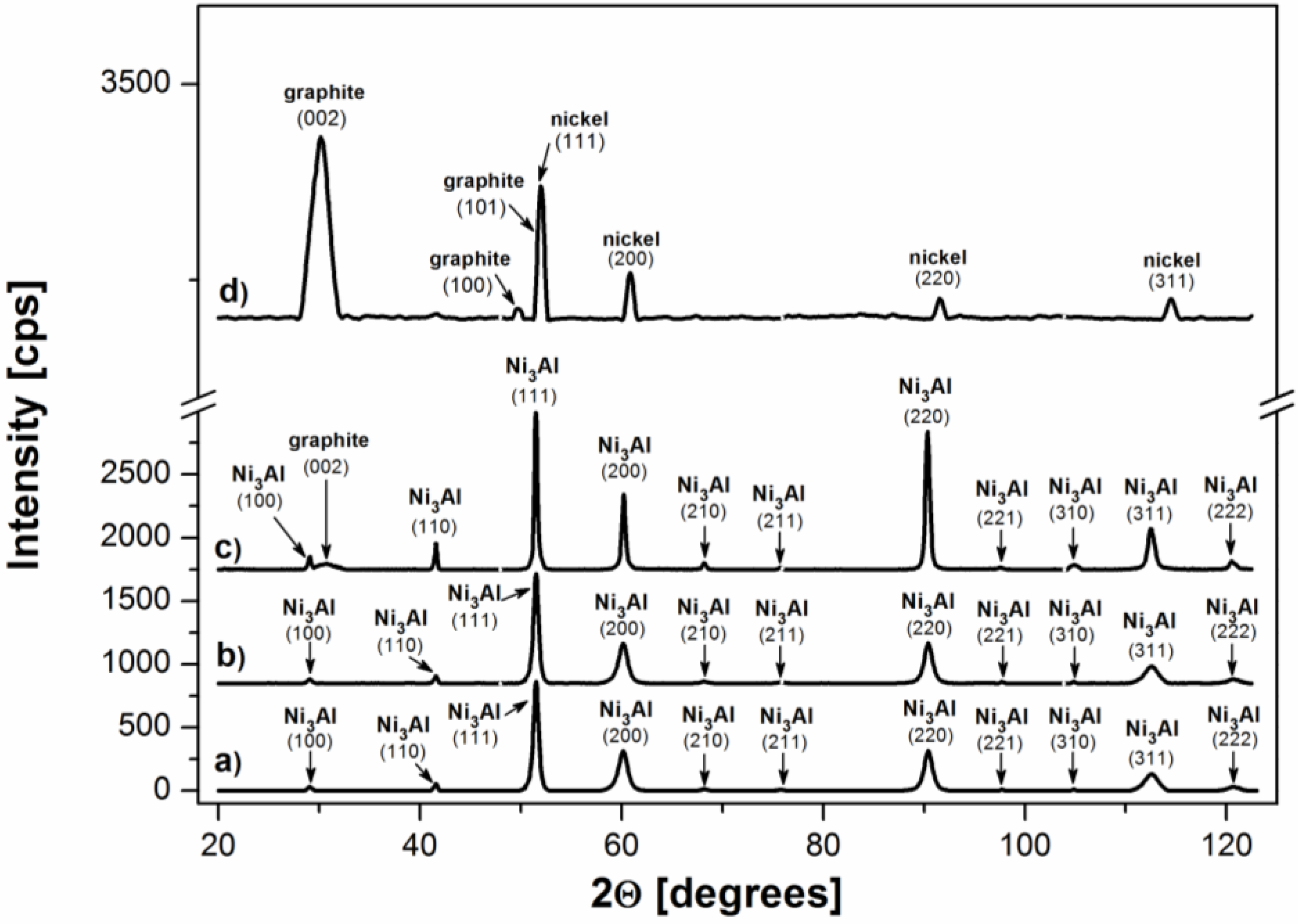
3. Materials and Methods
4. Conclusions
- (1)
- The conversion rate of cyclohexane is approximately 99% ± 1% at 600 °C and 87% ± 4% at 500 °C, what is a promising result.
- (2)
- The SEM analyses of the deposit formed on the Ni3Al foil after decomposition revealed existence of CNFs, in both platelet and tubular morphologies.
- (3)
- Nickel nanoparticles were found in the CNFs formed during the reaction. The Ni-ended CNFs were obtained during the catalytic decomposition of cyclohexane on the Ni3Al intermetallic foil.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sikka, V.K.; Deevi, S.C.; Viswanathan, S.; Swindeman, R.W.; Santella, M.L. Advances in processing of Ni3Al-based intermetallics and applications. Intermetallics 2000, 8, 1329–1137. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K. Nickel and iron aluminides: An overview on properties, processing, and applications. Intermetallics 1996, 4, 357–375. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K.; Liu, C.T. Processing, properties, and applications of nickel and iron aluminides. Prog. Mater. Sci. 1997, 42, 177–192. [Google Scholar] [CrossRef]
- Kim, S.H.; Oh, M.H.; Kishida, K.; Hirano, T.; Wee, D.M. Cyclic oxidation behavior and recrystallization of cold-rolled Ni3Al foils. Mater. Lett. 2004, 58, 2867–2871. [Google Scholar] [CrossRef]
- Chun, D.H.; Xu, Y.; Demura, M.; Kishida, K.; Wee, D.M.; Hirano, T. Spontaneous catalytic activation of Ni3Al thin foils in methanol decomposition. J. Catal. 2006, 243, 99–107. [Google Scholar] [CrossRef]
- Chun, D.H.; Xu, Y.; Demura, M.; Kishida, K.; Oh, M.H.; Hirano, T.; Wee, D.M. Catalytic Properties of Ni3Al Foils for Methanol Decomposition. Catal. Lett. 2006, 106, 71–75. [Google Scholar] [CrossRef]
- Arkatova, L.A.; Pakhnutov, O.V.; Shmakov, A.N.; Naiborodenko, Y.S.; Kasatsky, N.G. Pt-implanted intermetallides as the catalysts for CH4–CO2 reforming. Catal. Today 2011, 171, 156–167. [Google Scholar] [CrossRef]
- Bojar, Z.; Jóźwik, P.; Bystrzycki, J. Tensile properties and fracture behavior of nanocrystalline Ni3Al intermetallic foil. Scr. Mater. 2006, 55, 399–402. [Google Scholar] [CrossRef]
- Borodians’ka, H.; Demura, M.; Kishida, K.; Hirano, T. Fabrication of thin foils of binary Ni-Al γ/γ′ two-phase alloys by cold rolling. Intermetallics 2002, 10, 255–262. [Google Scholar] [CrossRef]
- Demura, M.; Kishida, K.; Suga, Y.; Takanashi, M.; Hirano, T. Fabrication of thin Ni3Al foils by cold rolling. Scr. Mater. 2002, 47, 267–272. [Google Scholar] [CrossRef]
- Li, Y.F.; Guo, J.T.; Zhou, L.Z.; Ye, H.Q. Effect of recrystallization on room-temperature mechanical properties of Zr-doped Ni3Al alloy. Mater. Lett. 2004, 58, 1853–1856. [Google Scholar] [CrossRef]
- Jóźwik, P.; Bojar, Z.; Winiarek, P. Catalytic activity of Ni3Al foils in decomposition of selected chemical compounds. Mater. Eng. 2010, 3, 654–657. [Google Scholar]
- Liu, J.; Chai, Y.; Liu, B.; Wu, Y.; Li, X.; Tang, Z.; Liu, Y.; Liu, C. The catalytic performance of Ni2P/Al2O3 catalyst in comparison with Ni/Al2O3 catalyst in dehydrogenation of cyclohexane. Appl. Catal. A General. 2014, 469, 434–441. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, X.; Wang, Y.; Chen, W. An efficient oxidation of cyclohexane over Au@TiO2/MCM-41 catalyst prepared by photocatalytic reduction method using molecular oxygen as oxidant. Catal. Commun. 2014, 46, 228–233. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, X.; Yu, H.; Peng, F.; Wang, H.; Ning, G. sp2- and sp3-hybridized carbon materials as catalysts for aerobic oxidation of cyclohexane. Catal. Sci. Technol. 2013, 3, 2654–2660. [Google Scholar] [CrossRef]
- Tanaka, A.; Seong-Ho, Y.; Mochida, I. Preparation of highly crystalline nanofibers on Fe and Fe–Ni catalysts with a variety of graphene plane alignments. Carbon 2004, 42, 591–597. [Google Scholar] [CrossRef]
- Rodriguez, N.M.; Chambers, A.; Baker, R.T.K. Catalytic engineering of carbon nanostructures. Langmuir 1995, 11, 3862–3866. [Google Scholar] [CrossRef]
- Martin-Gullon, I.; Vera, J.; Conesa, J.A.; Gonzalez, J.L.; Merino, C. Differences between carbon nanofibers produced using Fe and Ni catalysts in a floating catalyst reactor. Carbon 2006, 44, 1572–1580. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Norek, M.; Jóźwik, P.; Jankiewicz, B.; Stępniowski, W.J.; Bojar, Z. Catalytic stability and surface analysis of microcrystalline Ni3Al thin foils in methanol decomposition. Appl. Surf. Sci. 2014, 293, 169–176. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwik, P.; Salerno, M.; Stępniowski, W.J.; Bojar, Z.; Krawczyk, K. Decomposition of Cyclohexane on Ni3Al Thin Foil Intermetallic Catalyst. Materials 2014, 7, 7039-7047. https://doi.org/10.3390/ma7107039
Jóźwik P, Salerno M, Stępniowski WJ, Bojar Z, Krawczyk K. Decomposition of Cyclohexane on Ni3Al Thin Foil Intermetallic Catalyst. Materials. 2014; 7(10):7039-7047. https://doi.org/10.3390/ma7107039
Chicago/Turabian StyleJóźwik, Paweł, Marco Salerno, Wojciech J. Stępniowski, Zbigniew Bojar, and Krzysztof Krawczyk. 2014. "Decomposition of Cyclohexane on Ni3Al Thin Foil Intermetallic Catalyst" Materials 7, no. 10: 7039-7047. https://doi.org/10.3390/ma7107039
APA StyleJóźwik, P., Salerno, M., Stępniowski, W. J., Bojar, Z., & Krawczyk, K. (2014). Decomposition of Cyclohexane on Ni3Al Thin Foil Intermetallic Catalyst. Materials, 7(10), 7039-7047. https://doi.org/10.3390/ma7107039






