1. Introduction
Microcrystalline cellulose (MCC) is a porous, white, odorless, crystalline powder, which has been derived by partial depolymerization of cellulose [
1]. It is obtained by the hydrolysis of wood or cotton linters using dilute mineral acid. In this context, the pulp is initially immersed in hot dilute acid, which dissolves the amorphous segments of the cellulosic chain, leaving the micro fibrils exhibiting a microcrystalline texture [
2]. After achieving a certain degree of polymerization, the samples are withdrawn from the acid hydrolysis bath, washed, dried and ground to yield microcrystalline cellulose with a specific particle size and moisture content. It is chemically inactive and hydroscopic, having a particle size of usually 20–80 µm, with a degree of polymerization lower than 350 [
3,
4]. Due to its insolubilities in common reagents, like water, organic solvent and dilute acids, its lubricating properties, as well as its hygroscopic tendencies, it has been used in the cosmetics and food industries as a fat replacement [
5,
6]. It is used extensively by pharmaceutical industries as diluents or binders for oral tablets [
7]. Furthermore, it is used for the dry and wet formulation of capsules. It is also used for pelletization during the direct compression process [
7].
The major sources of MCC are wood pulp and cotton fiber. Recently, some other non-woody biomass, such as soybean, corn stalk, oath and rice hulls, as well as sugar beet pulp [
8,
9], bagasse and maize cob [
10,
11], wheat, barley and oath straw [
12], groundnut shell and rice husks [
13], reed stalks [
14] and cereal straw [
15], have been used. Indian bamboo [
16] and
Luffa cylindrica [
17] have been identified as prospective sources of MCC. During the acid hydrolysis of cellulosic materials, amorphous regions are disintegrated, resulting in a highly crystalline substrate with different degrees of the crystallinity index [
18]. The amorphous regions are selectively hydrolyzed by strong mineral acids, such as hydrochloric, nitric and sulfuric acids [
19,
20]. However, to ensure the degradation of amorphous regions while keeping the crystalline region intact can enhance the yield and crystallinity of the MCC. Previous researchers used different types of transition metal salt catalyst, such as FeCl
3, CuCl
2 and AlCl
3, during acid hydrolysis of cellulose [
21]. Another finding showed that a minute amount of FeSO
4 can enhance the reducing sugar content during the hydrolysis of coniferous sawdust [
22]. It has been reported that hydrolysis efficiency can be improved significantly by the presence of Fe
3+ and H
+ ions simultaneously in the reaction medium [
23]. Several studies have been conducted for the extraction of cellulose from different types of biomass. However, limited studies were performed to pretreat microcrystalline cellulose to improve its crystallinity by the transition metal ion catalyzed acid hydrolysis process by optimizing the process parameters by statistical regression analysis.
RSM is a combination of mathematical and statistical analyses of experimental results that can establish an empirical relationship between process variables with desired responses or product characteristics. It provides a complete experimental design for data exploration, model fitting, as well as process optimization [
24,
25]. The main goal of this study is to pretreat the MCC in the presence of FeCl
3 catalyst during the acid hydrolysis process. Three individual factors of temperature, time and concentration of metal ion catalyst were selected to investigate the hydrolysis mechanism of MCC in hydrochloric acid medium. The responses measured were the crystallinity of the hydrocellulose and the yield, to develop corresponding mathematical models. The effect of the process variables on both of the responses was analyzed in terms of ANOVA analysis. The upcoming perspective of this research is to apply the pretreated MCC to lab-scale, as well as large-scale pilot plant experiments to produce nanocellulose with the desired properties.
2.3. Process Variables Optimization
A numerical optimization is also carried out for both responses. Overall optimal conditions are evaluated. The criteria applied for graphical optimization are to maximize the yield and crystallinity index of the product by keeping the reaction variables within the range studied. Under the optimum condition, the corresponding predicted response values of the percentage of crystallinity and yield are 82.37 and 88.41, respectively. The sample has been prepared under optimum conditions, and the experimental results are compared with the predicted values. The percentage of deviation between the predicted and experimental conditions is evaluated and presented in
Table 4.
Table 4.
Process parameter optimization for the acid hydrolysis of MCC in the presence of FeCl3.
Table 4.
Process parameter optimization for the acid hydrolysis of MCC in the presence of FeCl3.
| Hydrolysis temperature (°C) | Hydrolysis time (h) | Catalyst concentration (M) | Percentage of crystallinity (y1) | Percentage yield (y2) |
|---|
| Predicted | Experimental | Error | Predicted | Experimental | Error |
|---|
| 90.99 | 6 | 1 | 82.37 | 83.46 | 1.32 | 88.41 | 86.98 | 1.62 |
Under the same experimental conditions of temperature and time (
Table 4), the experiment was conducted without FeCl
3 catalyst in the presence of HCl acid (2.5 M) only. The percentage of crystallinity obtained was 79.25%. However, in the presence of both catalyst and acid together, the percentage of crystallinity increased significantly up to 83.46%. A similar experiment was also conducted using optimum conditions of temperature and time (
Table 4), in the presence of H
2SO
4 (2.5 M) and Fe
2(SO
4)
3 (1 M). However, the percentage of crystallinity obtained was 51.84%. The lower crystallinity obtained from sulfuric acid can be explained due to it being a weaker acid with lower K
a value, thus dissociating in water sparingly. Sulfate is a stronger conjugate base than chloride in water.
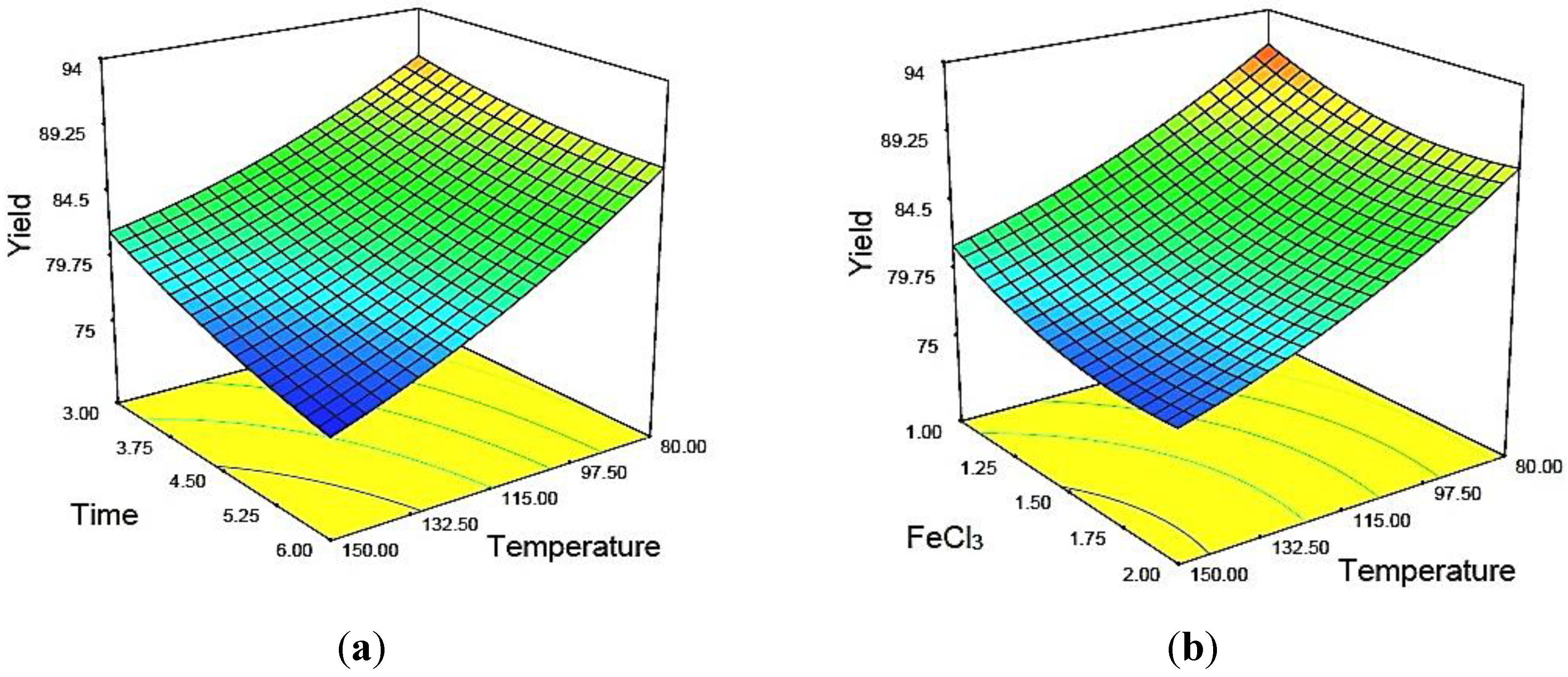
Figure 2a was constructed to show the effects of temperature and time on the percentage of crystallinity of MCC, where catalyst concentration was kept constant at 1.5.
Figure 2b depicts the three-dimensional response surfaces with a contour plot that shows the combined effects of two significant variables, hydrolysis temperature and time on the percentage of crystallinity, where the hydrolyzing time was fixed at the zero level, which was 4.5 h. In this work, all three variables studied were found to have synergistic effects on the percentage of crystallinity of MCC. The percentage of crystallinity was increased significantly when the temperature and time were maximal (
Figure 2a). This was expected, as the progressive increase of temperature and hydrolyzing time would increase the diffusion of acids into the amorphous region of cellulose. Enhanced contact time would cause physical swelling of MCC. Consequently, the surface area of the sample would increase, resulting in higher hydrolysis efficiency. Increasing the concentration of the metal ion catalyst increased the percentage of crystallinity. Basically, Fe
3+ can form a coordination complex with water to yield H
+ ions, according to Equation (3):
Figure 2.
Response surface and contour plots of the combined effects of: (a) temperature and time; (b) temperature and catalyst concentration on the percentage of crystallinity (y1) of acid hydrolyzed MCC when the other two variables were at center points.
Figure 2.
Response surface and contour plots of the combined effects of: (a) temperature and time; (b) temperature and catalyst concentration on the percentage of crystallinity (y1) of acid hydrolyzed MCC when the other two variables were at center points.
H
+ ions will increase the acidity of the solution, which would disintegrate the glycosidic linkage between glucose units of cellulose. The oxygen atoms of the glucose unit in cellulose can readily form an intermediate complex by absorbing Fe
3+ ions. This would increase the pyranose bond length and bond angle. Thus, the activation energy would be lowered, resulting in greater hydrolysis efficiency [
27,
28].
Figure 3a,b shows the three-dimensional response surfaces that were constructed to reveal the effects of catalytic acid hydrolysis reaction variables on MCC yield.
Figure 3a represents the combined effect of temperature and time on the response, where the catalyst concentration was fixed at the zero level (1.5).
Figure 3b illustrates the effect of temperature and catalyst concentration on the same response, where time was fixed at the zero leve1 (4.5 h). In general, yield was found to decrease with increasing temperature, time and catalyst concentration. As can be seen from both plots (
Figure 3a,b), temperature was more dominant relative to the yield as compared to the other two variables. The lowest yield was obtained when the temperature was at the maximum point (173.86 °C, Sample 10) within the studied range, as depicted by the design matrix of
Table 5. This was due to the chemical degradation of cellulose in acid hydrolyzing medium [
27,
28].
2.4. Physiochemical Characterization of the Hydrocellulose
The changes in the structures of the treated MCC particles due to acid hydrolysis in the presence of FeCl
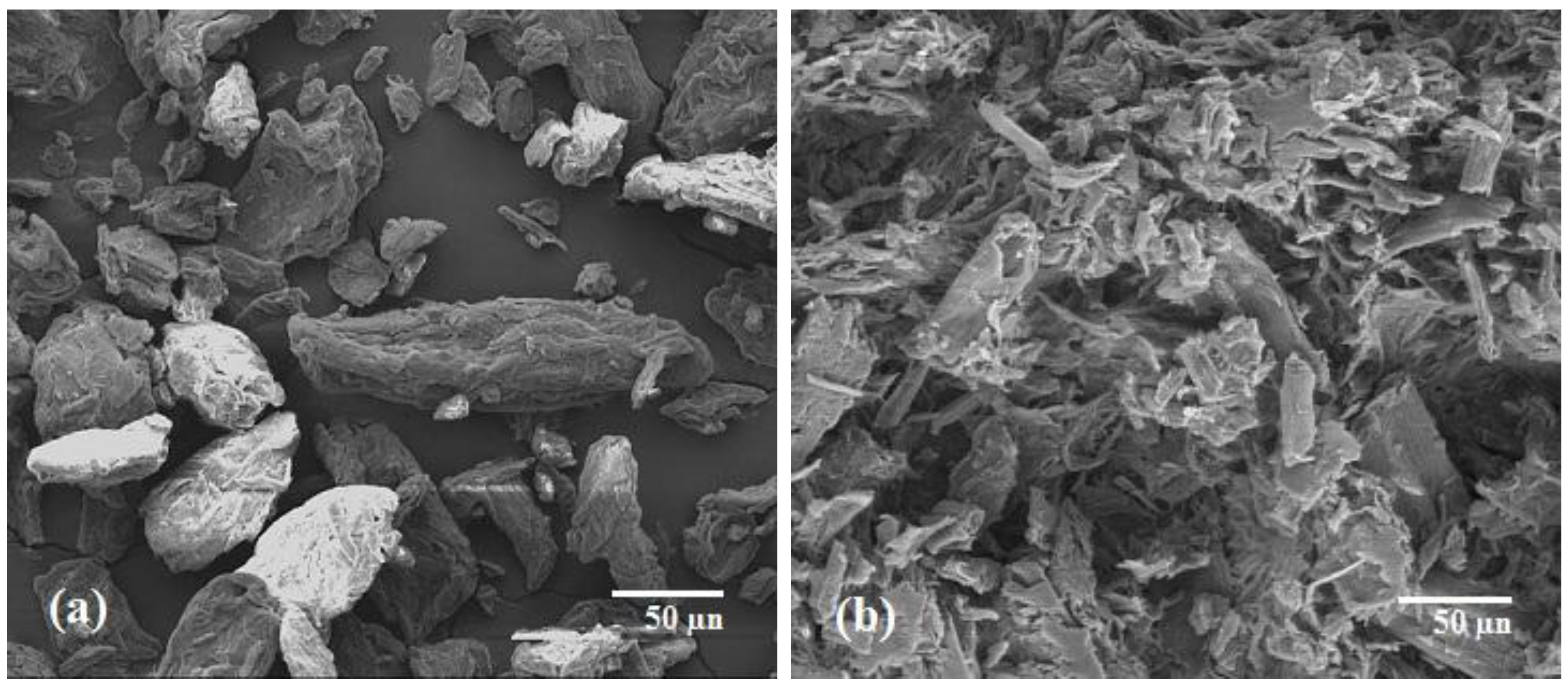
3 catalyst were confirmed by the images obtained from scanning electron microscopy (
Figure 4).
Figure 4a shows the structure of untreated MCC, which was comparatively irregular, flat and rod-like aggregates. The untreated MCC contained a rough surface with numerous individual cellulose whiskers connected by strong hydrogen bonding [
29,
30,
31].
Figure 4b shows images of acid-treated MCC, which were swollen, resulting in the MCC being partially digested by the acid. The aggregated MCC particles were fragmented due to chemical swelling and acid hydrolysis. Minute amounts of holes were observed over the surface of the treated MCC, resulting in a higher hydrolysis rate.
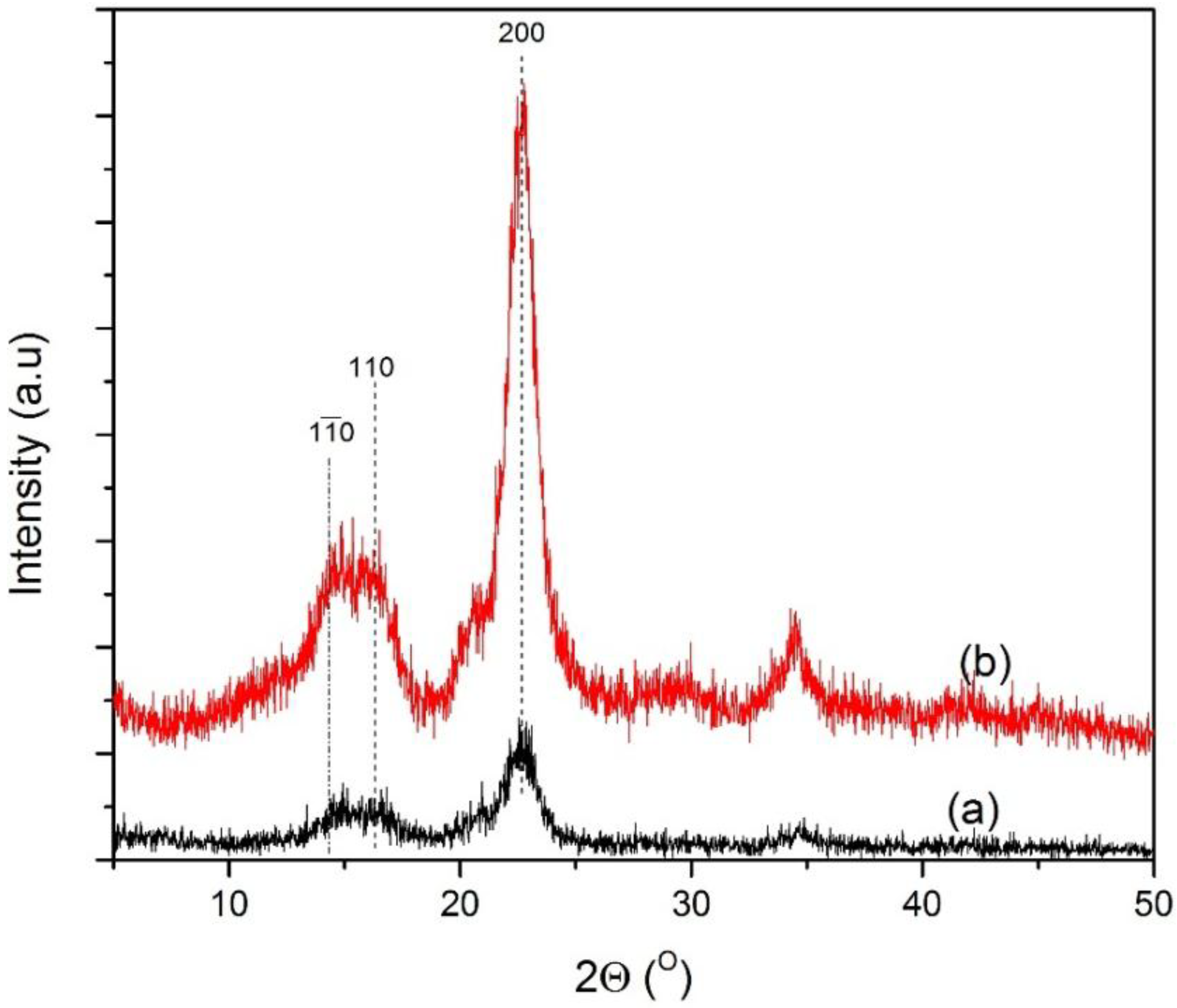
The XRD patterns of the untreated MCC and pretreated MCC are illustrated in
Figure 5. It was reported earlier that the major diffraction peak for cellulose can be observed for 2θ ranging between 22° and 23° as the primary peak, whereas a secondary peak is in the range of 16° to 18° [
32,
33]. From the XRD distribution pattern, noticeable peaks can be observed within the mentioned range for both the untreated and pretreated MCC. This reflects the presence of the crystalline and amorphous structure of the cellulose constituent. Similar types of curves and peaks were observed for both samples. It seems that catalytic acid hydrolysis did not disrupt the whole structure of the cellulosic matrix. However, the pretreated sample showed higher peak intensity, which might be due to the partial breaking up of glycoside linkages inside the amorphous region, whereas the crystalline region was almost unaltered. The percentage of crystallinity observed for untreated and treated samples under optimum conditions were 56.05% and 83.46%, respectively.
Figure 3.
Response surface and contour plots of the combined effects of: (a) temperature and time; (b) temperature and catalyst concentration on the percentage yield (y2) of acid hydrolyzed MCC when the other two variables were at center points.
Figure 3.
Response surface and contour plots of the combined effects of: (a) temperature and time; (b) temperature and catalyst concentration on the percentage yield (y2) of acid hydrolyzed MCC when the other two variables were at center points.
Figure 4.
SEM images: (a) untreated MCC; and (b) acid hydrolyzed MCC in the presence of FeCl3 catalyst.
Figure 4.
SEM images: (a) untreated MCC; and (b) acid hydrolyzed MCC in the presence of FeCl3 catalyst.
Table 5.
Experimental design matrix for acid hydrolysis of microcrystalline cellulose (MCC) in the presence of FeCl3 catalyst.
Table 5.
Experimental design matrix for acid hydrolysis of microcrystalline cellulose (MCC) in the presence of FeCl3 catalyst.
| Sample ID | Run | Type of point | Level (coded factors) | Reaction variables (actual factors) | Percentage of crystallinity | Percentage yield |
|---|
| Temperature x1, (°C) | Time, x2, (h) | Catalyst concentration, x3 (M) | y1 | y2 |
|---|
| S-1 | 1 | Fact | −1 | −1 | −1 | 150 | 6 | 2 | 92.44 | 76.89 |
| S-2 | 2 | Fact | +1 | −1 | −1 | 150 | 6 | 1 | 88.99 | 78.77 |
| S-3 | 3 | Fact | −1 | +1 | −1 | 80 | 6 | 2 | 80.99 | 88.99 |
| S-4 | 4 | Fact | +1 | +1 | −1 | 80 | 3 | 2 | 78.99 | 90.99 |
| S-5 | 5 | Fact | −1 | −1 | +1 | 80 | 3 | 1 | 77.09 | 92.33 |
| S-6 | 6 | Fact | +1 | −1 | +1 | 150 | 3 | 2 | 86.77 | 80.99 |
| S-7 | 7 | Fact | −1 | +1 | +1 | 80 | 6 | 1 | 79.99 | 91.45 |
| S-8 | 8 | Fact | +1 | +1 | +1 | 150 | 3 | 1 | 84.99 | 84.89 |
| S-9 | 9 | Axial | −1.412 | 0 | 0 | 56.14 | 4.5 | 1.5 | 70.99 | 93.78 |
| S-10 | 10 | Axial | +1.412 | 0 | 0 | 173.86 | 4.5 | 1.5 | 85.67 | 75.89 |
| S-11 | 11 | Axial | 0 | −1.412 | 0 | 115 | 1.98 | 4.5 | 79.89 | 87.99 |
| S-12 | 12 | Axial | 0 | +1.412 | 0 | 115 | 7.02 | 4.5 | 90.34 | 78.98 |
| S-13 | 13 | Axial | 0 | 0 | −1.412 | 115 | 4.5 | 0.66 | 83.89 | 83.89 |
| S-14 | 14 | Axial | 0 | 0 | +1.412 | 115 | 4.5 | 2.34 | 84.09 | 84.09 |
| S-15 | 15 | Center | 0 | 0 | 0 | 115 | 4.5 | 1.5 | 83.99 | 81.88 |
| S-16 | 16 | Center | 0 | 0 | 0 | 115 | 4.5 | 1.5 | 83.09 | 82.77 |
| S-17 | 17 | Center | 0 | 0 | 0 | 115 | 4.5 | 1.5 | 82.78 | 81.67 |
| S-18 | 18 | Center | 0 | 0 | 0 | 115 | 4.5 | 1.5 | 82.99 | 82.99 |
| S-19 | 19 | Center | 0 | 0 | 0 | 115 | 4.5 | 1.5 | 82.58 | 81.99 |
| S-20 | 20 | Center | 0 | 0 | 0 | 115 | 4.5 | 1.5 | 83.55 | 82.99 |
Figure 5.
Microcrystalline cellulose: (a) untreated MCC; and (b) acid hydrolyzed in the presence of FeCl3 catalyst. The percentage of crystallinity of the untreated MCC was determined as 56.05%, and this value increased to 83.46% after treatment with FeCl3.
Figure 5.
Microcrystalline cellulose: (a) untreated MCC; and (b) acid hydrolyzed in the presence of FeCl3 catalyst. The percentage of crystallinity of the untreated MCC was determined as 56.05%, and this value increased to 83.46% after treatment with FeCl3.
The FTIR spectra of the MCC samples (
Figure 6a) and pretreated MCC sample (
Figure 6b) are shown in
Figure 6. The FTIR spectra obtained before and after acid swelling in the presence of catalyst revealed the absence of strong chemical reactions. This showed that the treatment could not change the chemical structure of the cellulosic fragments [
32]. The peaks around 3400, 2900, 1400 and 900 cm
−1 in the untreated and treated samples exhibited native cellulose I [
32]. The peaks around 672 cm
−1 changed their frequency level and were observed around 669 cm
−1 in the pretreated sample. The peaks around 901 and 900 cm
−1 showed the rocking vibration of the –C–H band in cellulose. The band at 1163 and 1164 cm
−1 ascribed to the –C–O–C– stretch of the β-1, 4-glycosidic linkage is prominent for both untreated and pretreated MCC samples. The bands at 1436 and 1432 cm
−1 endorsed the asymmetric bending and wagging of the –CH
2 group. This showed the intermolecular hydrogen attraction at the C
6 group [
34]. Due to the absorption of water, a small sharp peak around 1648 and 1646 cm
−1 was identified in both samples [
35].
The peak at 2900 cm−1 represented the C–H stretching in both samples. The broad absorption peak in the range of 3000 to 3600 cm−1 in both samples represented the stretching of the H-bonded –OH groups. Two additional minor peaks around 3736 cm−1 and 3842 cm−1 appeared for the –OH groups in pretreated sample.
Figure 6.
Microcrystalline cellulose: (a) untreated MCC; and (b) acid hydrolyzed in the presence of FeCl3 catalyst.
Figure 6.
Microcrystalline cellulose: (a) untreated MCC; and (b) acid hydrolyzed in the presence of FeCl3 catalyst.
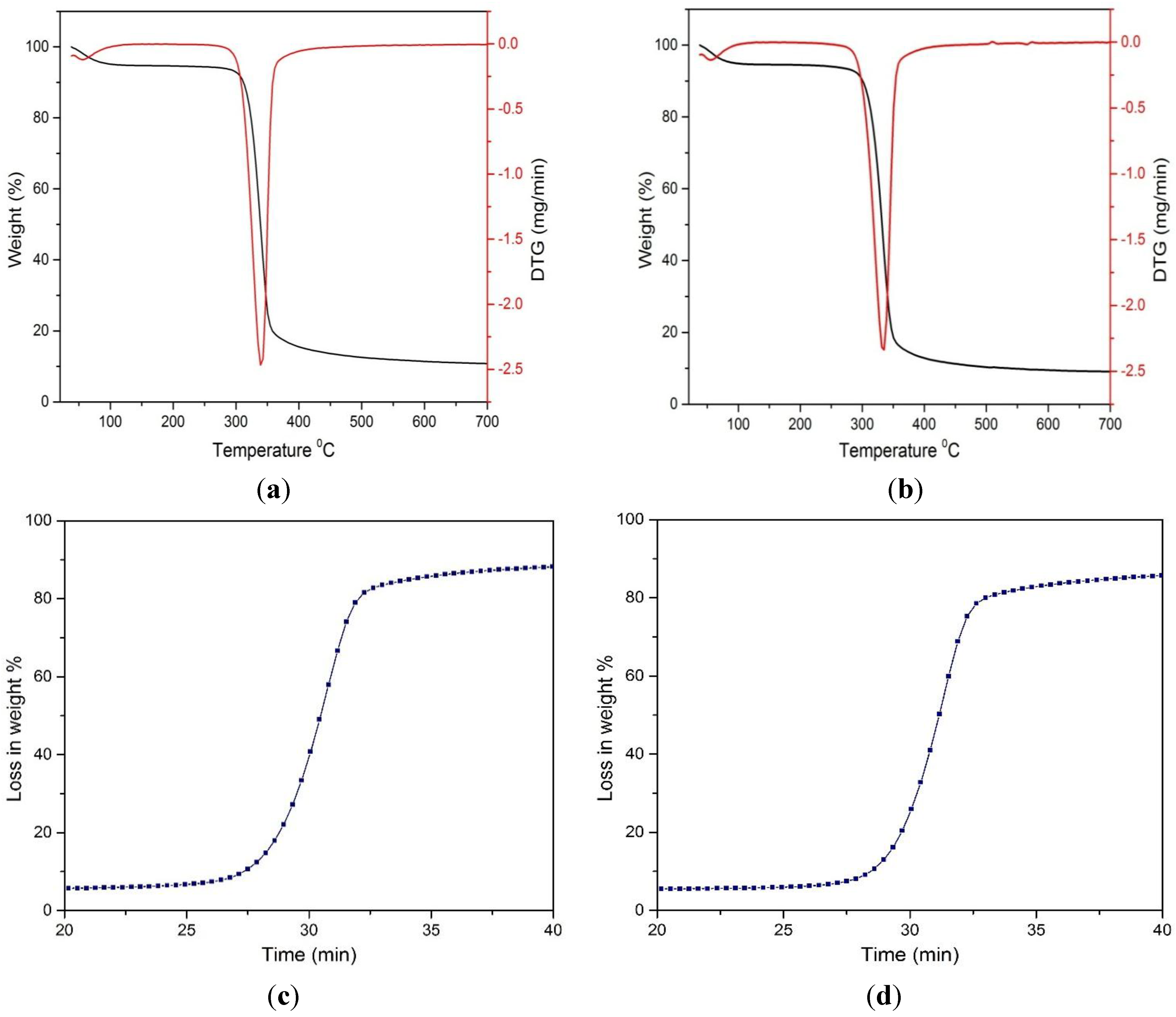
Thermogravimetric (TGA) and derivative thermogram (DTA) analyses were performed for untreated MCC and the FeCl
3-catalyzed acid hydrolyzed MCC sample. The TGA and DTA curves obtained are illustrated in
Figure 7.
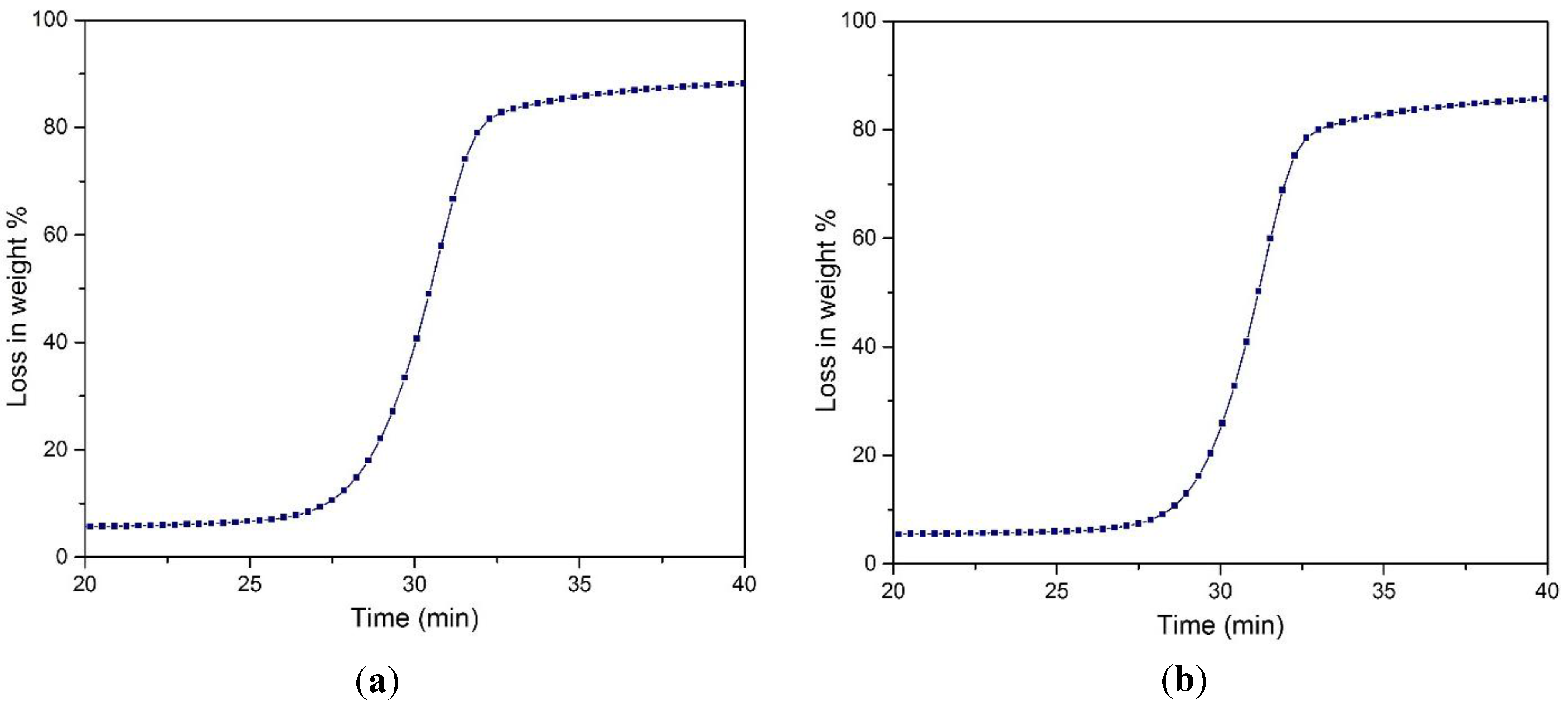
Figure 7.
Thermogravimetric analysis of: (a) untreated MCC; (b) hydrolyzed MCC; and relation between weight loss % and time of (c) untreated MCC; (d) treated MCC in the presence of FeCl3 catalyst.
Figure 7.
Thermogravimetric analysis of: (a) untreated MCC; (b) hydrolyzed MCC; and relation between weight loss % and time of (c) untreated MCC; (d) treated MCC in the presence of FeCl3 catalyst.
The thermal degradation data (onset temperature, T
on, 10% weight loss temperature, T
10%, and 50% weight loss temperature, T
50%), along with the residual weight loss around 700 °C, including the peak degradation temperature, are provided in
Table 6.
Table 6.
Thermogravimetric analysis of untreated and treated MCC.
Table 6.
Thermogravimetric analysis of untreated and treated MCC.
| Sample | Degradation temperature °C | DTG peak temperature | Residual weight loss at 700 °C |
|---|
| Ton | T10% | T50% | Tmax |
|---|
| MCC | 258 | 310 | 333 | 332 | 9.00 |
| Treated MCC | 280 | 320 | 341 | 339 | 10.58 |
It is evident from the curves that initially, the loss of weight occurred in the range of 100–120 °C, which is associated with the moisture content of the sample. After that, the loss in weight of the cellulosic materials increased to a greater extent. However, the loss in weight percentages was greater for untreated sample than the treated one. This is obvious for the treated sample: the disordered amorphous region was decreased with the increase of the hydrogen bond crystalline region of the cellulosic matrix. Referring to the table, it was observed that chemical swelling and acid hydrolysis increased the onset temperature, Ton, 10% weight loss temperature, T10%, and 50% weight loss temperature, T50%. This was supported earlier by XRD data, where it was observed that for the treated MCC sample, the crystallinity index was increased, resulting in a higher onset degradation temperature.
The data obtained from TGA analysis were used to calculate the activation energy. The value ln(W
0 − W
inf./W
t − W
inf.) was plotted against time (t) and is shown in
Figure 8. Here:
Figure 8.
TGA analysis (a) the relation between ln(W0 − Winf.)/(Wt − Winf.) and time for the untreated MCC (b) the relation between ln(W0 − Winf.)/(Wt − Winf.) and time for the treated MCC.
Figure 8.
TGA analysis (a) the relation between ln(W0 − Winf.)/(Wt − Winf.) and time for the untreated MCC (b) the relation between ln(W0 − Winf.)/(Wt − Winf.) and time for the treated MCC.
From the figures, it was observed that there are mainly two parts; the first one is very quick, reflecting moisture loss within the initial 30 min, whereas the second one is due to thermal decomposition. The linearization of the plot gave a slope and intercepts. The slope rate constant was calculated to give the activation energy [
36,
37]. The activation energy calculated for untreated MCC was 185.66 kJ/mole, whereas for the treated one, it was around 211.38 kJ/mole. This can be ascribed to the degradation of the amorphous region of cellulose, resulting in a structure with a higher crystalline region after the treatment. Thus, the structure was a more compact and tight structure, which became difficult to attack [
2].