Use of Slag/Sugar Cane Bagasse Ash (SCBA) Blends in the Production of Alkali-Activated Materials
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
- i.
- uncontrolled burning of sugarcane bagasse to obtain heat;
- ii.
- collection of ash generated by a scrubber;
- iii.
- obtained ashes were mixed with water generated from sugar cane washing and then, deposited in the lagoon;
- iv.
- settled solids from lagoon were collected and then dried at 105 °C;
- v.
- collected ashes were ground in a laboratory ball mill (steel balls) for 20 min, obtaining a mean particle diameter of about 26.8 μm.
2.2. Physico-Chemical and Mechanical Tests
2.3. Preliminary Study Using BFS
2.4. Study on Binders Containing SCBA
- 100% BFS + 0% SCBA (mixture 100/0);
- 85% BFS + 15% SCBA (mixture 85/15);
- 75% BFS + 25% SCBA (mixture 75/25);
- 60% BFS + 40% SCBA (mixture 60/40).
3. Results and Discussion
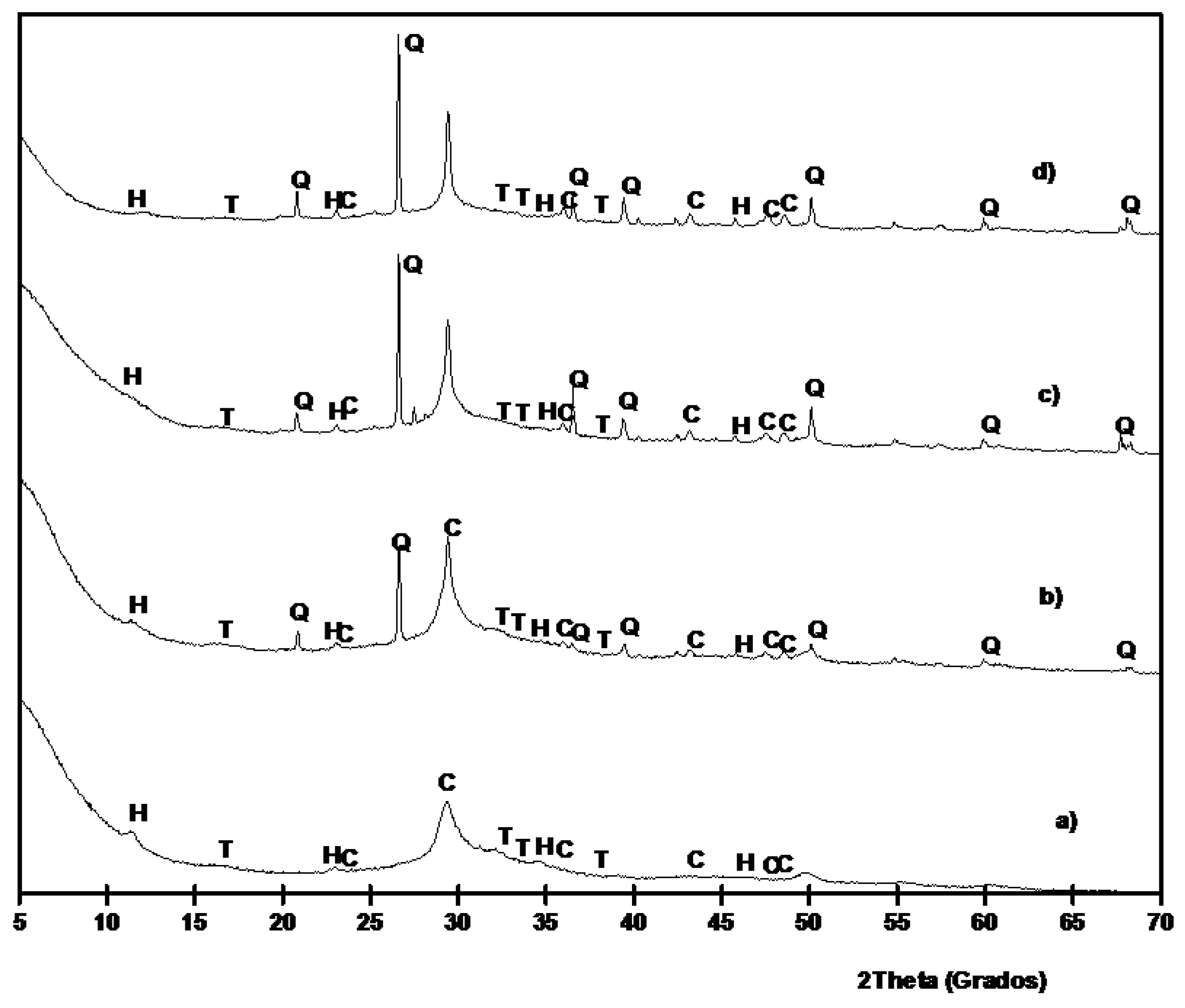
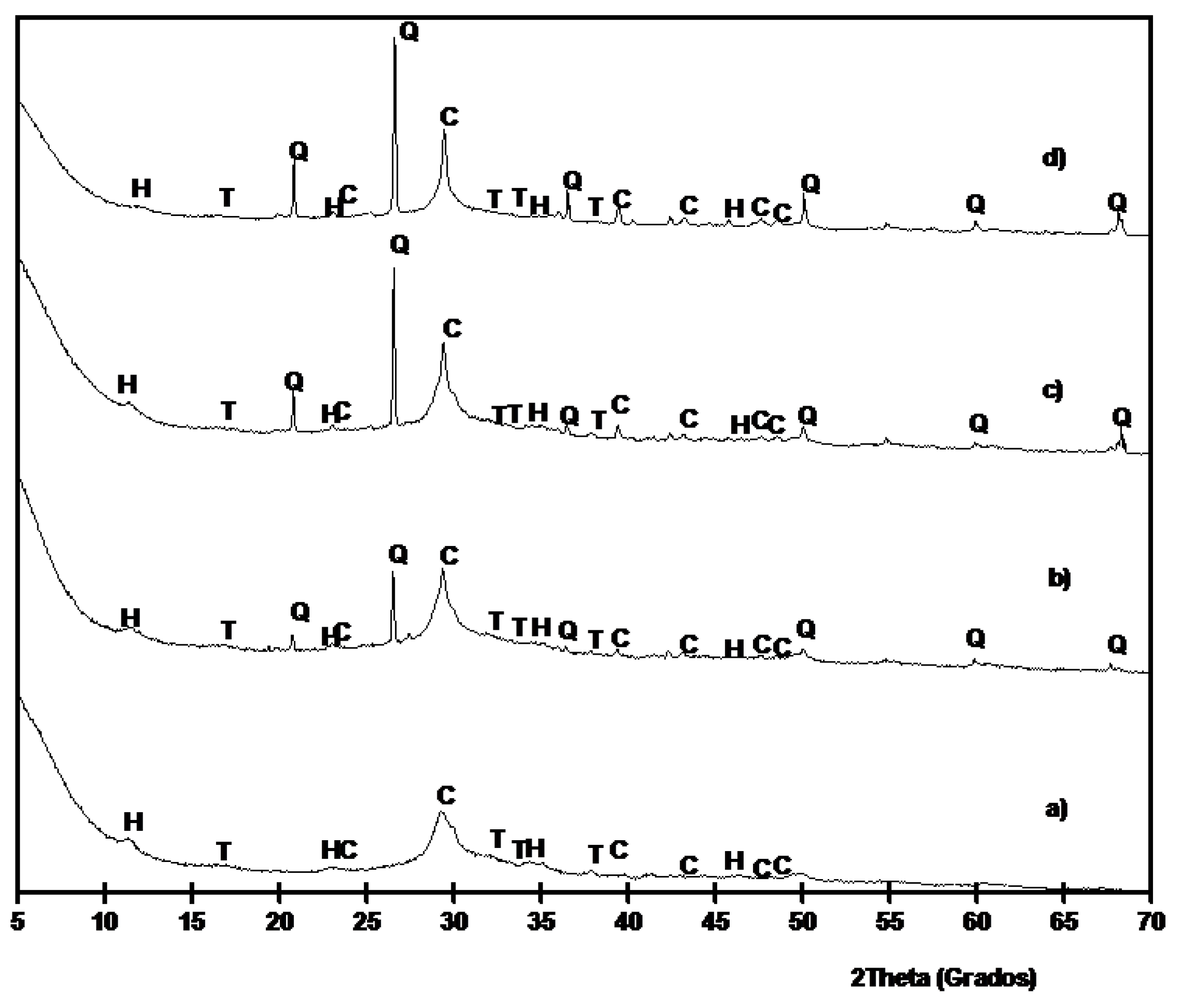
3.1. Chemical and Mineralogical Characterization of BFS and SCBA
| Oxide | BFS | SCBA |
|---|---|---|
| SiO2 | 30.19 | 31.41 |
| Al2O3 | 10.66 | 7.57 |
| Fe2O3 | 1.31 | 6.02 |
| CaO | 39.53 | 16.06 |
| MgO | 7.50 | 1.07 |
| Na2O | 0.87 | 0.14 |
| K2O | 0.58 | 1.58 |
| SO3 | 1.95 | 0.78 |
| TiO2 | 0.51 | 2.09 |
| MnO | 0.40 | 0.10 |
| Chloride | 0.44 | 0.14 |
| LOI | 5.62 | 32.20 |
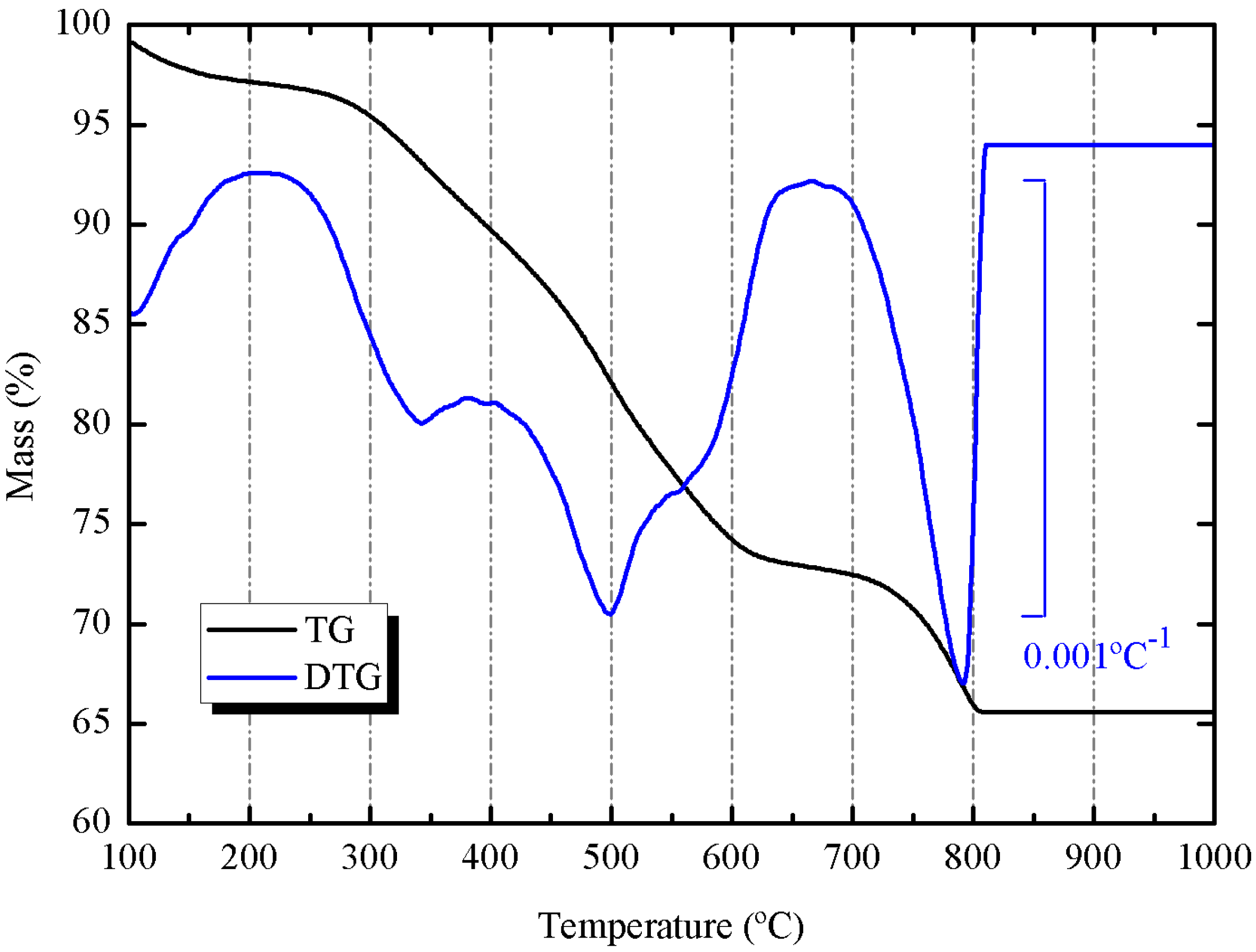
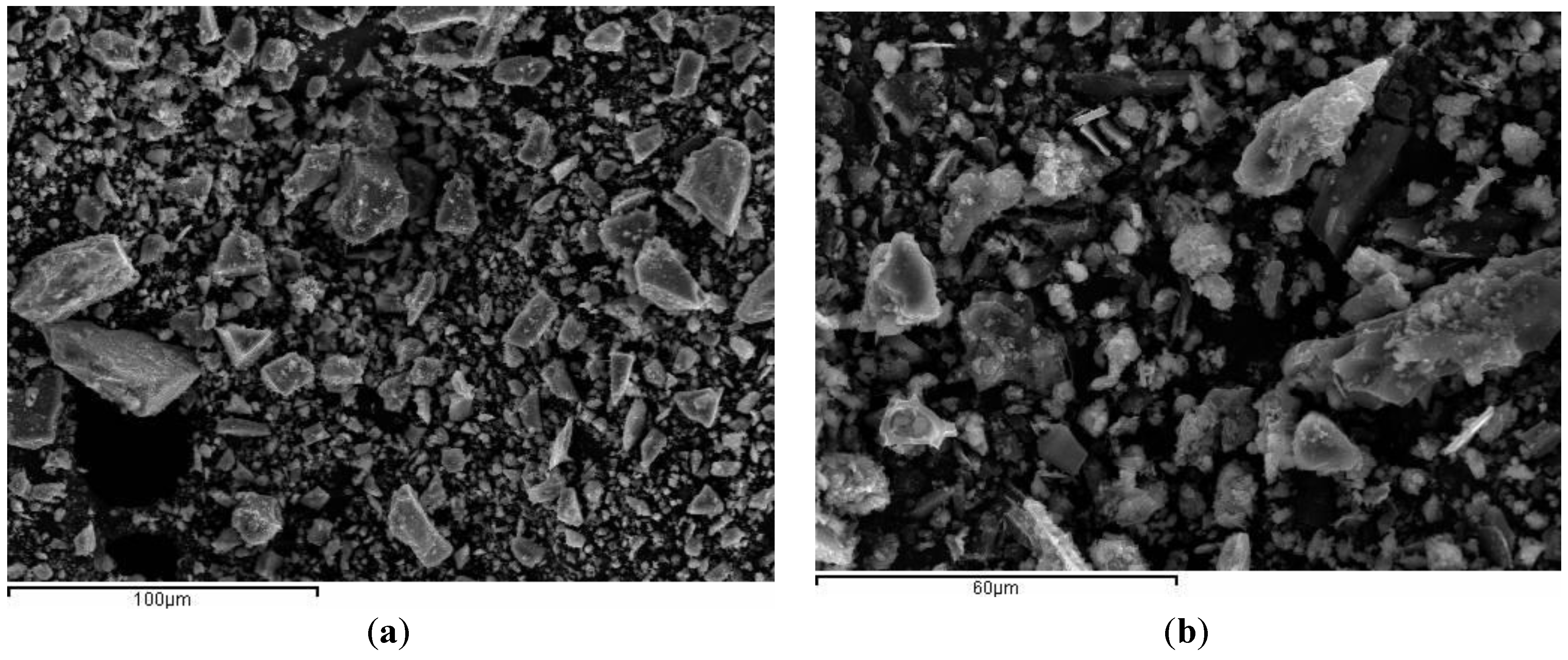
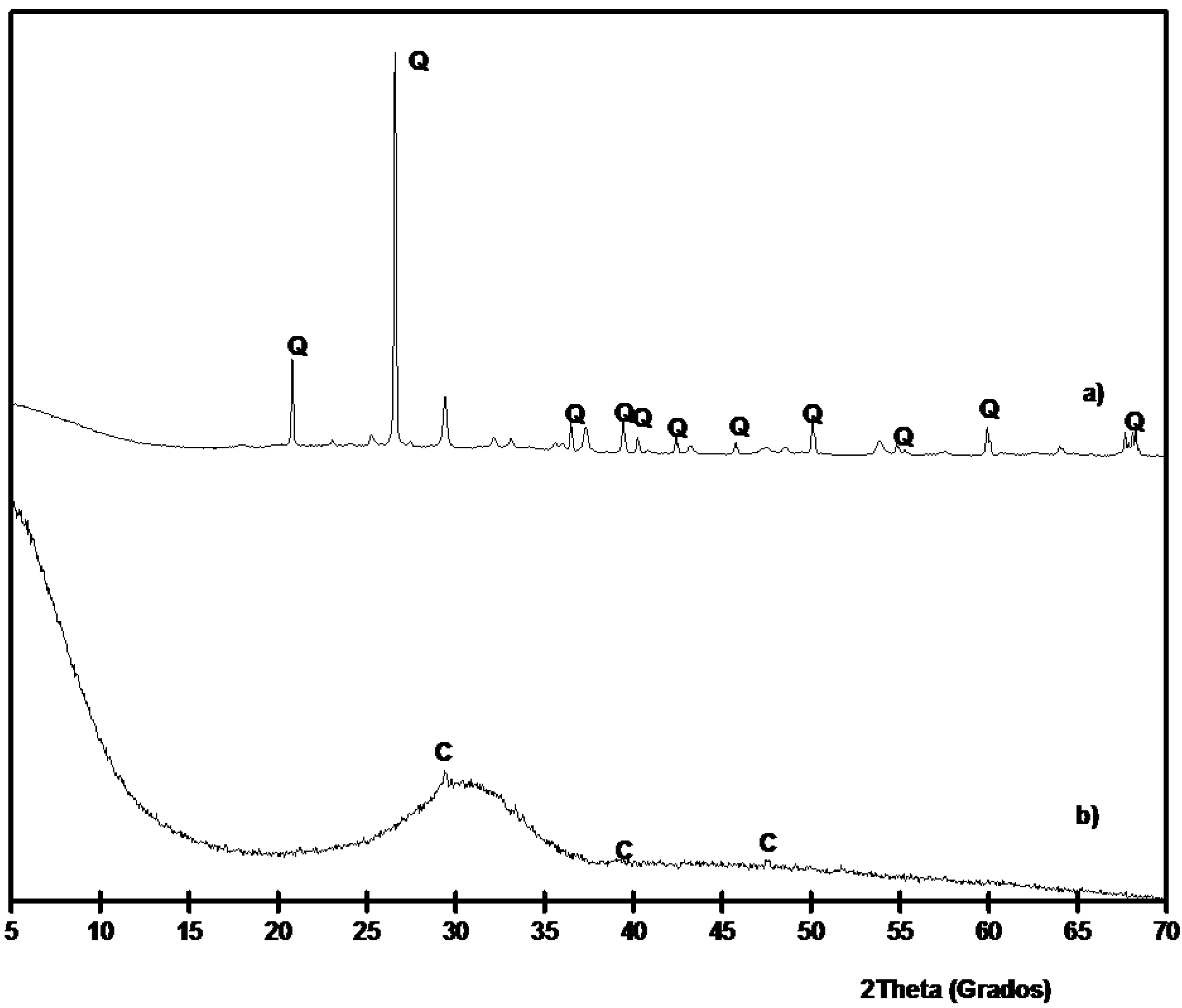
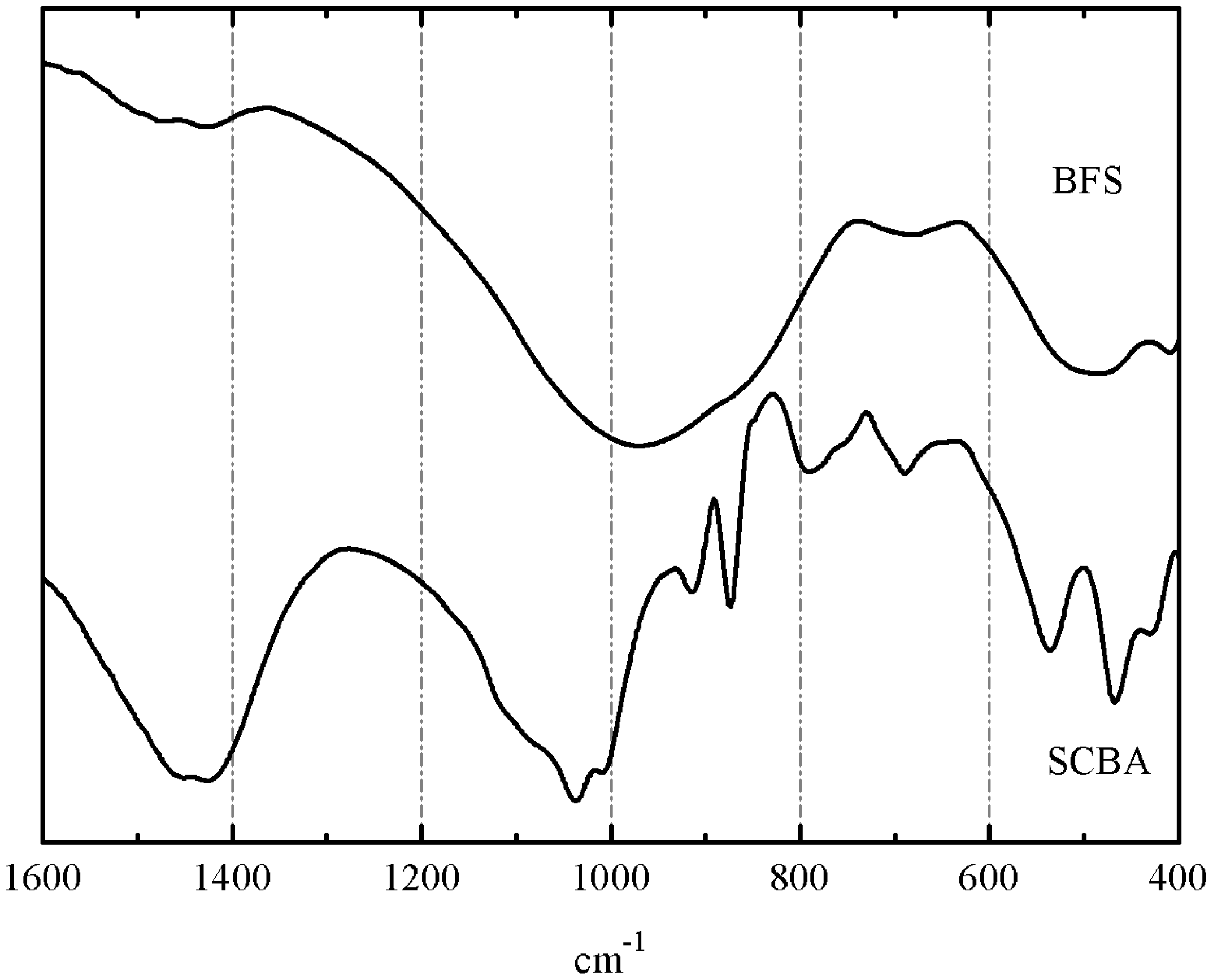
3.2. Preliminary Results
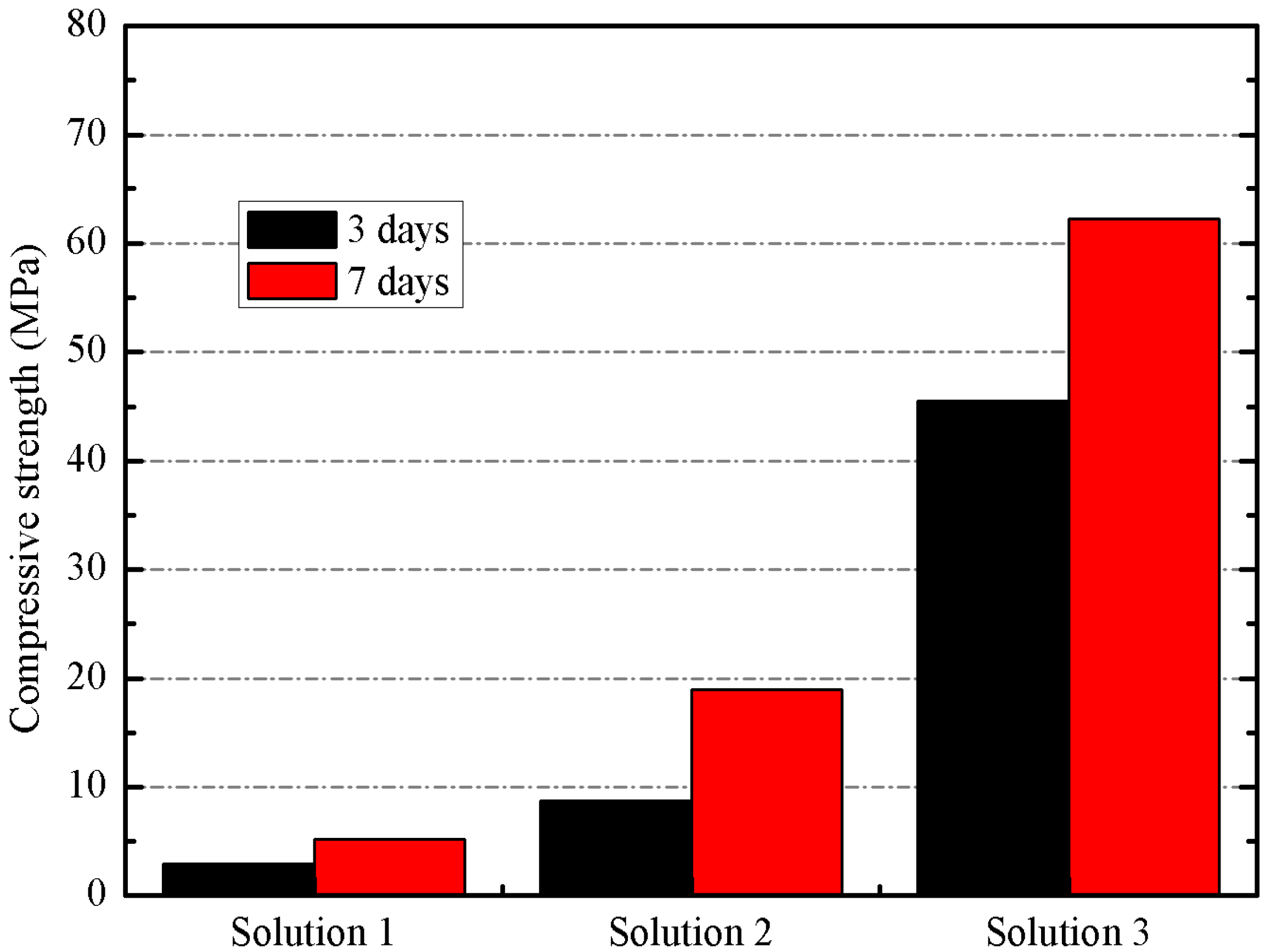
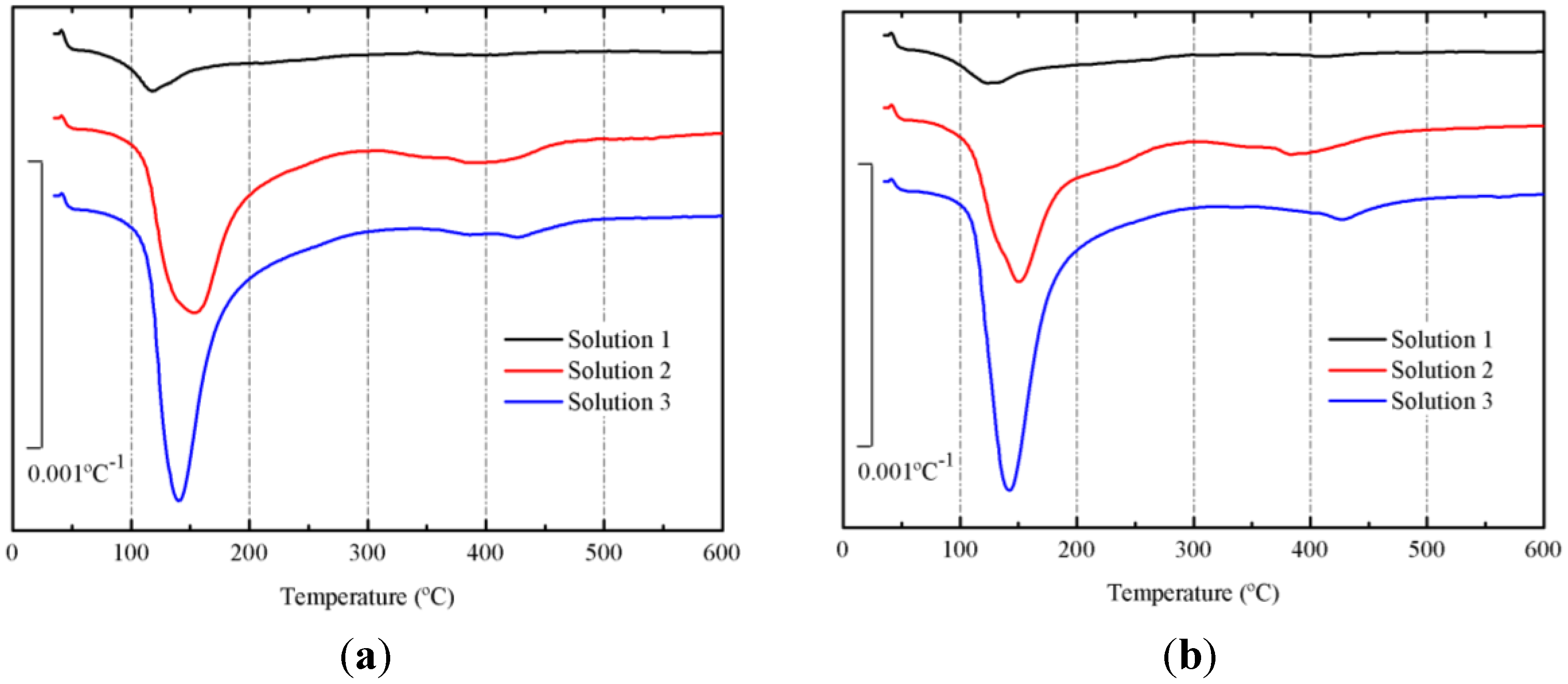
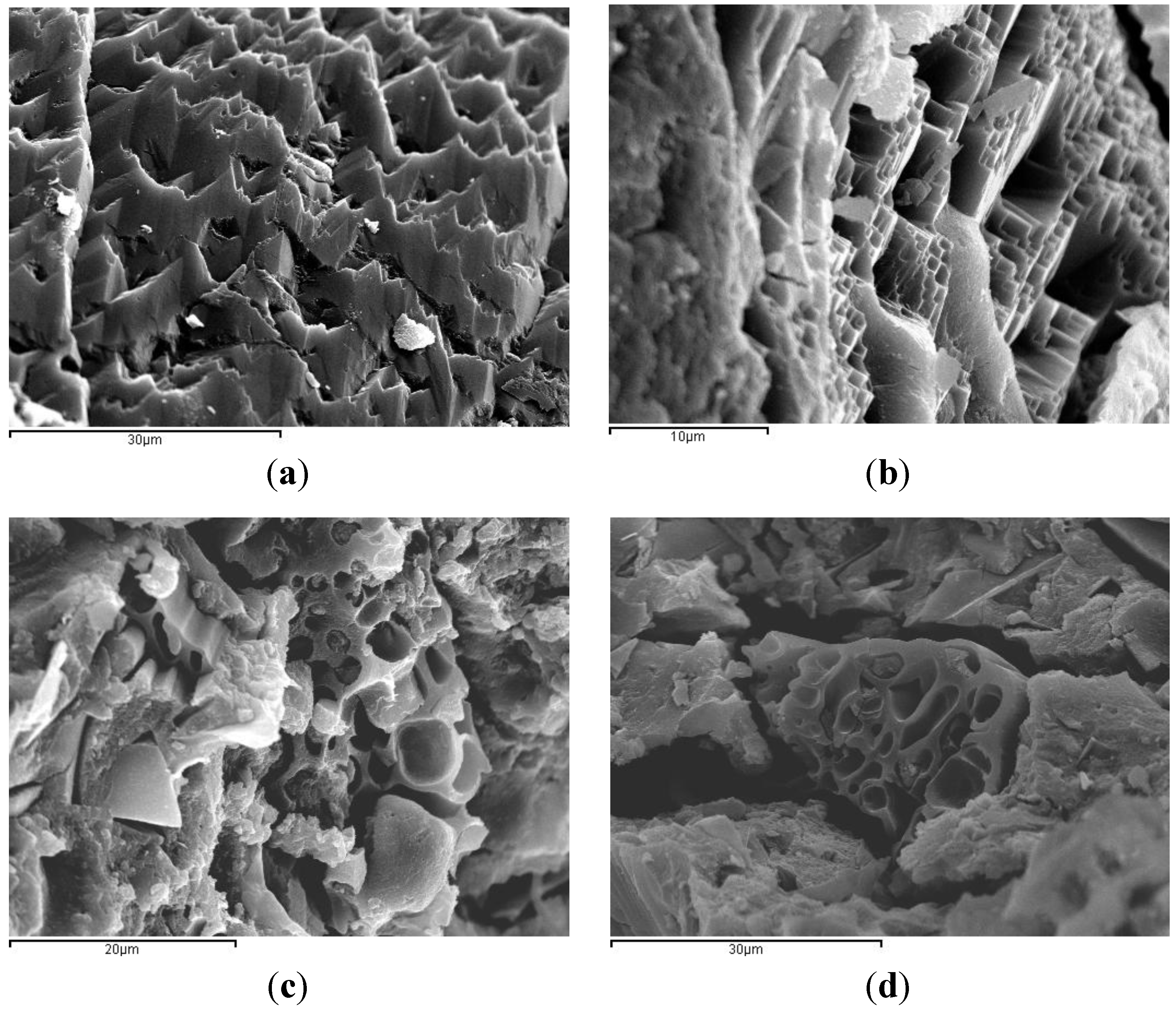
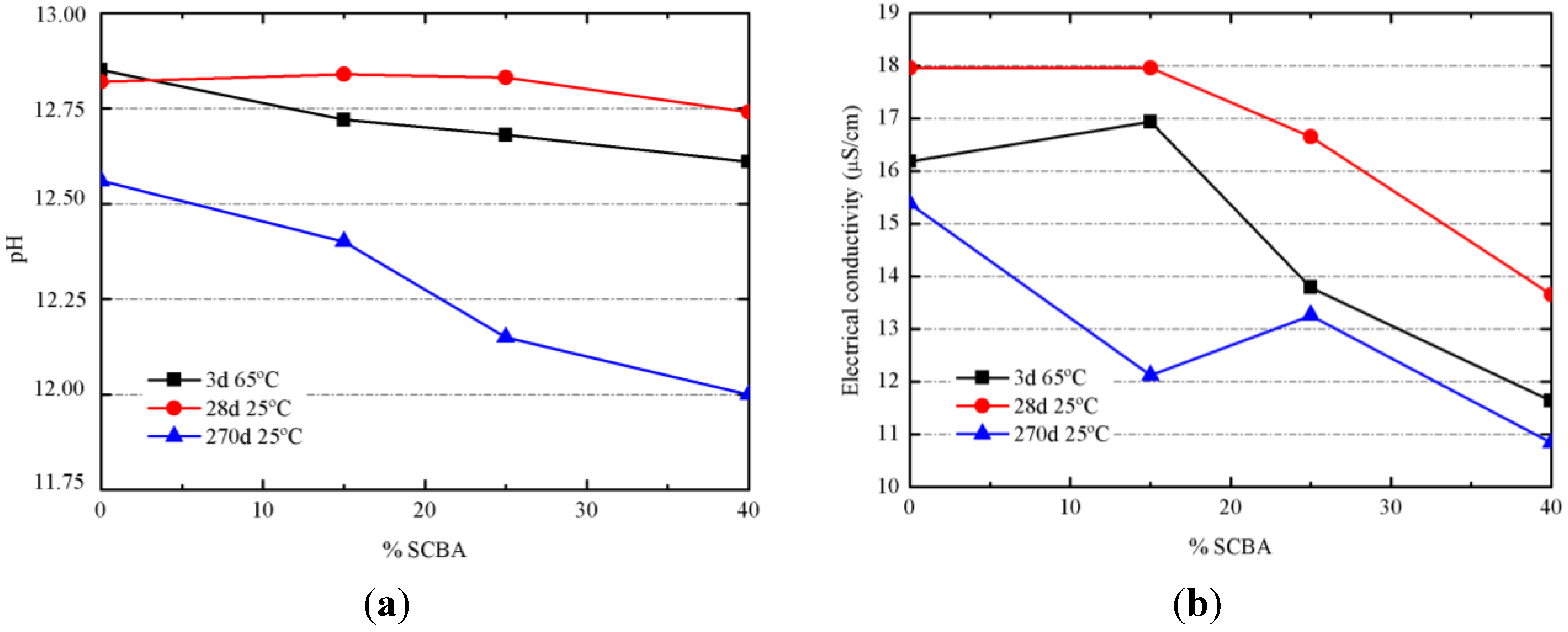
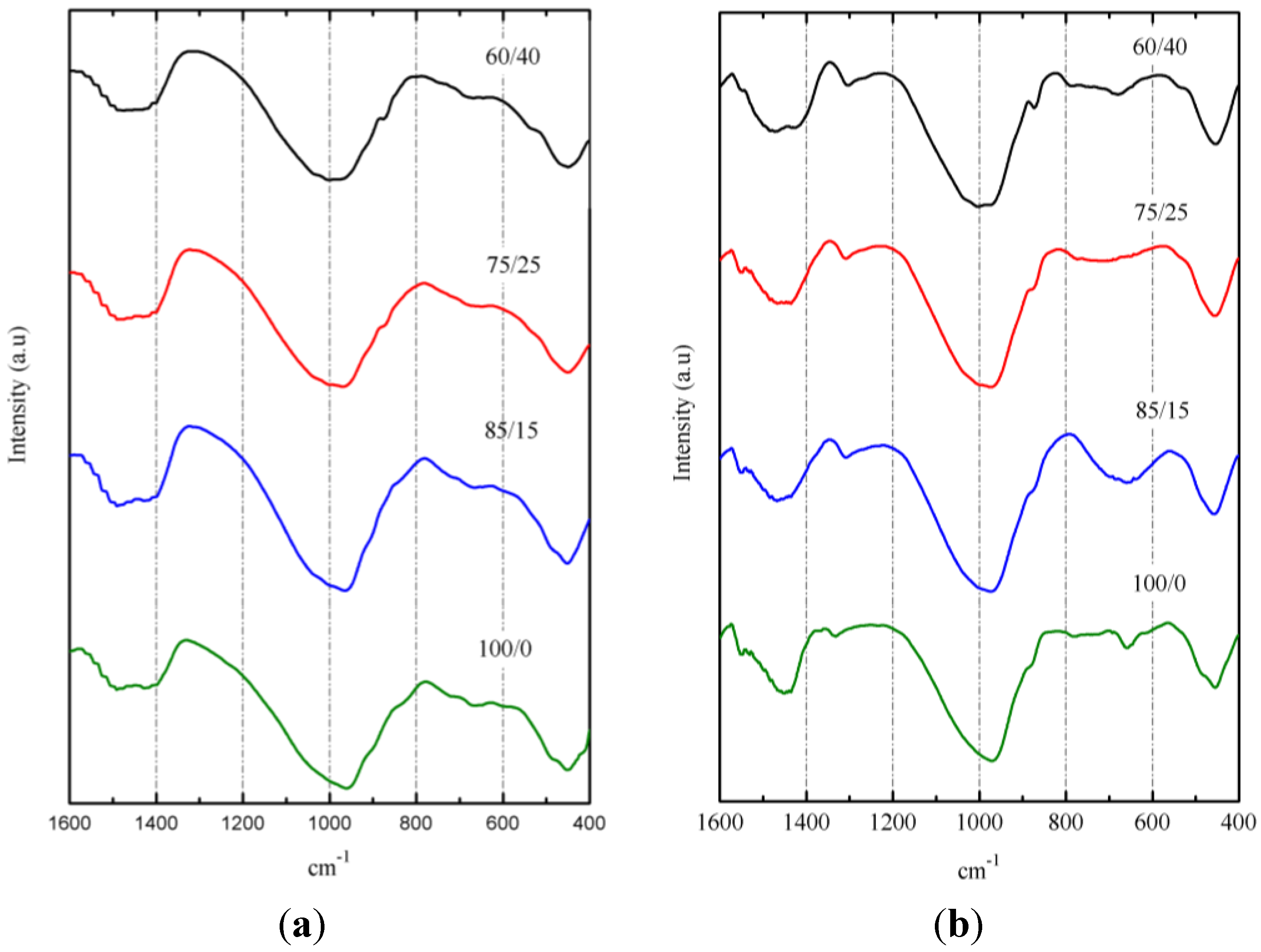
3.3. Results on Binders Containing SCBA
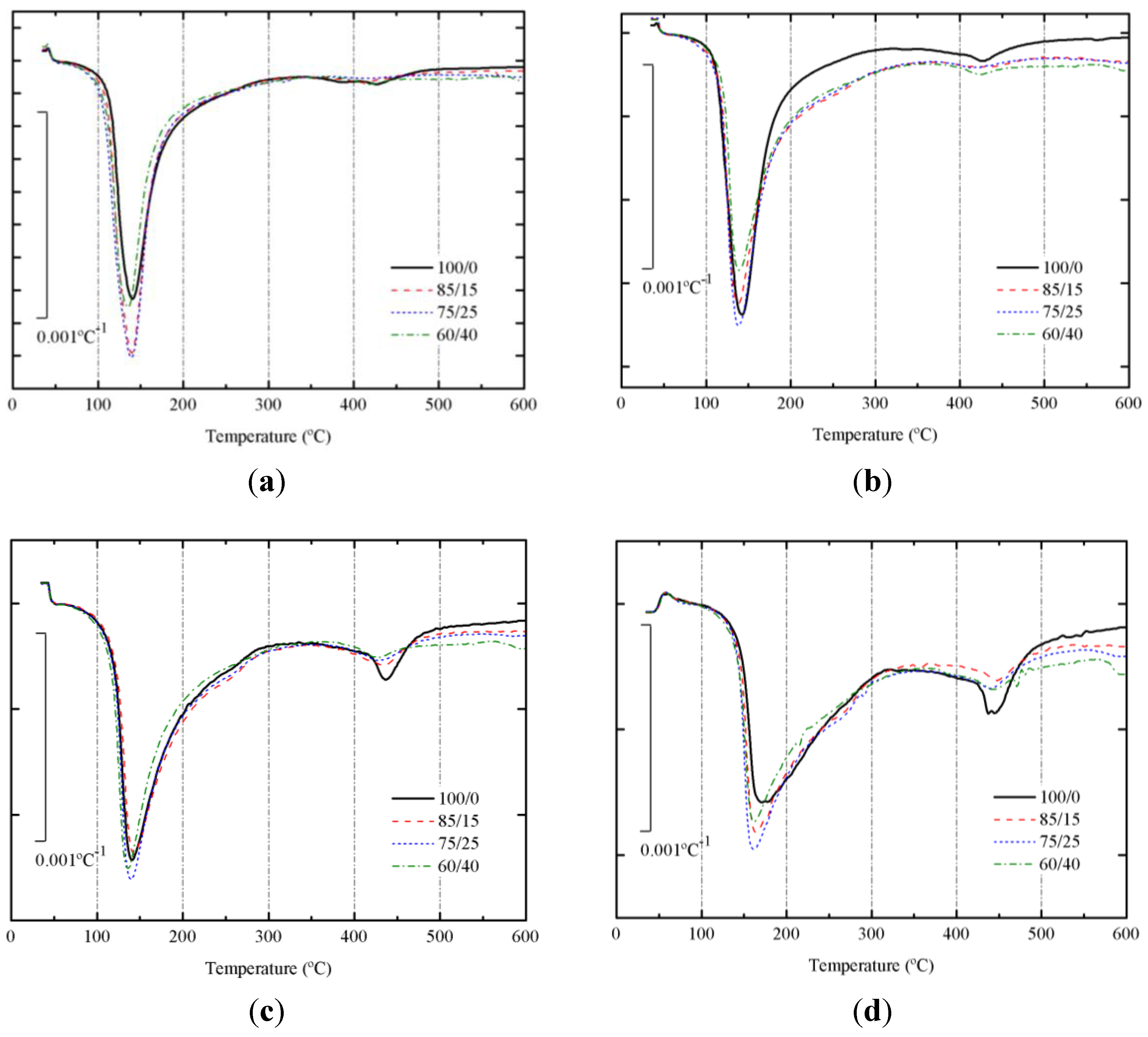
| Mix BFS/SCBA | Mass loss in pastes in different curing conditions (days–temperature) and temperature at the highest mass loss rate (°C, in parentheses) | |||
|---|---|---|---|---|
| 3 d–65 °C | 7 d–65 °C | 28 d–20 °C | 270 d–20 °C | |
| 100/0 | 18.15 (140) | 19.28 (143) | 15.69 (141) | 15.58 (171) |
| 85/15 | 20.00 (139) | 17.93 (139) | 16.36 (143) | 15.87 (164) |
| 75/25 | 21.42 (139) | 18.34 (138) | 16.77 (140) | 17.25 (162) |
| 60/40 | 19.15 (135) | 17.53 (139) | 16.33 (136) | 16.81 (161) |
| Mixtures | Rc (MPa) | Rf (MPa) | ||
|---|---|---|---|---|
| 3 days | 7 days | 3 days | 7 days | |
| 100/0 | 45.5 ± 2.9 | 62.2 ± 2.6 | 5.80 ± 0.3 | 5.39 ± 1.1 |
| 85/15 | 53.5 ± 2.0 | 51.2 ± 0.4 | 5.31 ± 0.4 | 2.94 ± 0.6 |
| 75/25 | 49.0 ± 2.7 | 52.8 ± 1.9 | 5.31 ± 0.6 | 4.00 ± 0.4 |
| 60/40 | 42.8 ± 0.9 | 43.2 ± 0.3 | 3.84 ± 0.5 | 3.19 ± 0.4 |
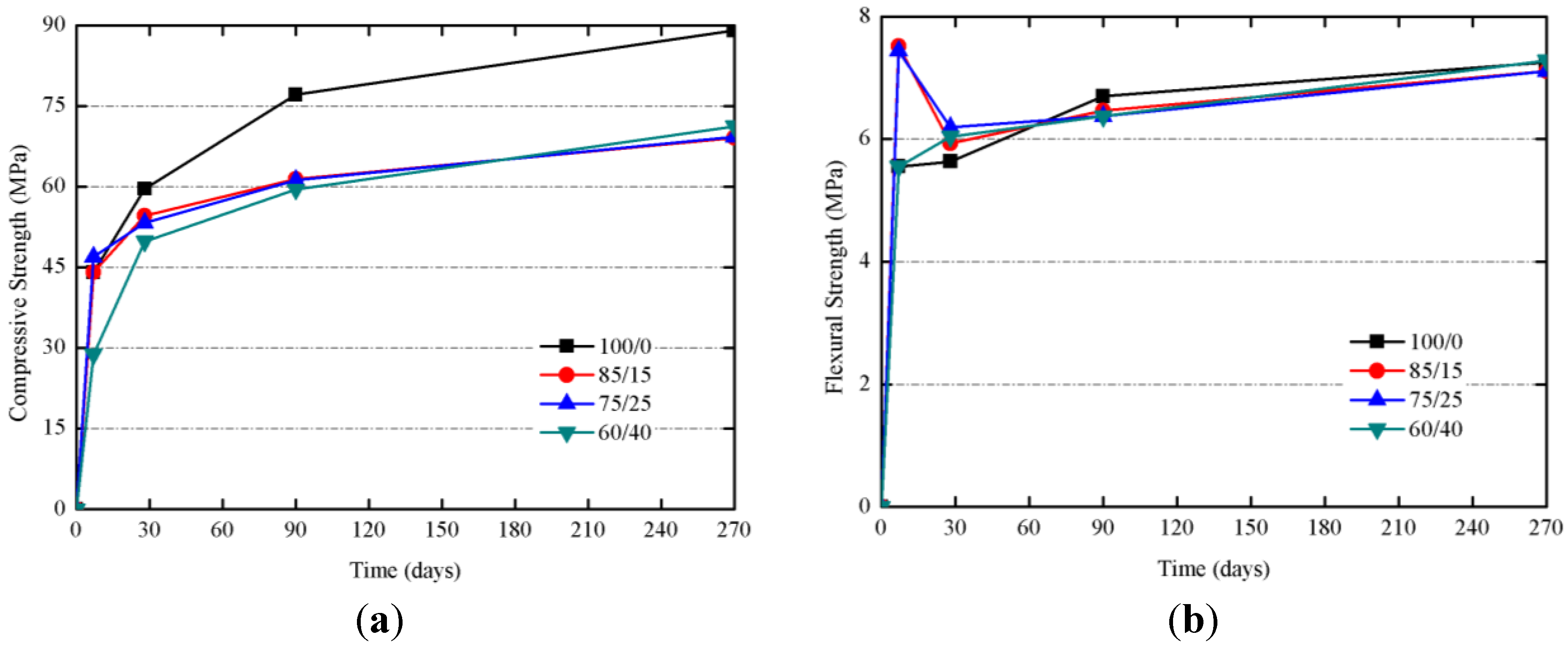
| Mixtures | Total porosity (%) | Total pore area (m2/g) | Median pore diameter | Volume (mL of Hg/g of mortar) | Hg retained (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Volume (nm) | Area (nm) | >1 μm | 1 μm–50 nm | 50–10 nm | <10 nm | ||||
| 100/0 | 9.43 | 0.251 | 15,683.0 | 5.8 | 0.0381 | 0.0019 | 0.0001 | 0.0004 | 81.64 |
| 85/15 | 12.58 | 1.918 | 17,706.1 | 7.2 | 0.0517 | 0.0033 | 0.0006 | 0.0032 | 86.53 |
| 75/25 | 9.82 | 2.897 | 7154.3 | 6.9 | 0.0351 | 0.0047 | 0.0008 | 0.0047 | 74.35 |
| 60/40 | 11.30 | 5.321 | 4823.1 | 6.6 | 0.0375 | 0.0070 | 0.0018 | 0.0083 | 70.59 |
| Mixtures | Total porosity (%) | Total pore area (m2/g) | Median pore diameter | Volume (mL of Hg/g of mortar) | Hg retained (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Volume (nm) | Area (nm) | >1 μm | 1 μm–50 nm | 50–10 nm | <10 nm | ||||
| 100/0 | 6.80 | 2.070 | 10,813.8 | 8.2 | 0.0229 | 0.0024 | 0.0021 | 0.0021 | 71.29 |
| 85/15 | 7.48 | 1.154 | 8835.9 | 8.2 | 0.0278 | 0.0037 | 0.0008 | 0.0017 | 77.57 |
| 75/25 | 7.62 | 1.989 | 6903.3 | 7.3 | 0.0256 | 0.0051 | 0.0010 | 0.0032 | 75.64 |
| 60/40 | 9.61 | 1.535 | 8554.4 | 7.6 | 0.0348 | 0.0064 | 0.0011 | 0.0021 | 84.35 |
| Mixtures | Total porosity (%) | Total pore area (m2/g) | Median pore diameter | Volume (mL of Hg/g of mortar) | Hg retained (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Volume (nm) | Area (nm) | >1 μm | 1 μm–50 nm | 50–10 nm | <10 nm | ||||
| 100/0 | 8.78 | 1.287 | 7738.9 | 6.4 | 0.0405 | 0.0033 | 0.0001 | 0.0021 | 69.53 |
| 85/15 | 9.69 | 5.713 | 1423.5 | 7.4 | 0.0319 | 0.0127 | 0.0032 | 0.0092 | 74.13 |
| 75/25 | 8.60 | 3.173 | 1933.6 | 6.7 | 0.0339 | 0.0109 | 0.0043 | 0.0048 | 83.97 |
| 60/40 | 12.53 | 3.881 | 1125.6 | 7.7 | 0.0427 | 0.0306 | 0.0030 | 0.0053 | 83.15 |
4. Conclusions
Acknowledgments
References
- Aïtcin, P.C. Cements of yesterday and today: Concrete of tomorrow. Cem. Concr. Res. 2000, 30, 1349–1359. [Google Scholar] [CrossRef]
- Flatt, R.; Roussel, R.; Cheeseman, C.R. Concrete: An eco-material that needs to be improved. J. Eur. Ceram. Soc. 2012, 32, 2787–2798. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnerfeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Societé Generale. Chinese Construction Bubble–Preparing for a Potential Burst. 2011. Available online: http://pt.scribd.com/doc/58599536/SocGenChinaConstruction (accessed on 20 May 2013).
- Damtoft, J.S.; Glavind, M.; Munch-Petersen, C. Danish Centre for Green Concrete. In Proceedings of Third CANMET/ACI International Symposium, Sustainable Development of Cement and Concrete, Supplementary Papers, San Francisco, CA, USA, 16–19 September 2001; pp. 401–418.
- Roy, D.M. Alkali-activated cements opportunities and challenges. Cem. Concr. Res. 2000, 29, 249–254. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers, Structure, Processing, Properties and Industrial Applications; Woodhead Publishing Limited: Cambridge, UK, 2009. [Google Scholar]
- McLellan, B.; Williams, R.; Lay, J.; van Riessen, A.; Corder, G. Costs and carbon emissions for geopolymer pastes in comparison to ordinary Portland cement. J. Clean. Product. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review: Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Alkali activation of australian slag cements. Cem. Concr. Res. 1999, 29, 113–120. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.J.; Puertas, F. Alkali-activated slag mortars: Mechanical strength behaviour. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. The Thermal evolution of metakaolin geopolymers: Part 2—Phase stability and structural development. J. Non-Cryst. Solids 2007, 353, 2186–2200. [Google Scholar] [CrossRef]
- Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Cheeseman, C.R.; Payá, J. Alkali activation of vitreous calcium aluminosilicate derived from glass fiber waste. J. Sustain. Cement-Based Mater. 2012, 1, 83–93. [Google Scholar] [CrossRef]
- Puertas, F.; García-Díaz, I.; Barba, A.; Gazulla, M.F.; Palacios, M.; Gómez, M.P.; Martínez-Ramírez, S. Ceramic wastes as alternative raw materials for Portland cement clínker production. Cem. Concr. Compos. 2008, 30, 798–805. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Cheeseman, C.R.; Payá, J. Properties and microstructure of alkali-activated red clay brick waste. Constr. Build. Mater. 2013, 43, 98–106. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Tungsten mine waste geopolymeric binder: Preliminary hydration products investigations. Constr. Build. Mater. 2009, 23, 200–209. [Google Scholar] [CrossRef]
- Payá, J.; Borrachero, M.V.; Monzó, J.; Soriano, L.; Tashima, M.M. A new geopolymeric binder from hydrated-carbonated cement. Mater. Lett. 2012, 74, 223–225. [Google Scholar] [CrossRef]
- Tashima, M.M.; Akasaki, J.L.; Melges, J.L.P.; Soriano, L.; Monzó, J.; Payá, J.; Borrachero, M.V. Alkali activated materials based on fluid catalytic cracking catalyst residue (FCC): Influence of SiO2/Na2O and H2O/Na2O ratio on mechanical strength and microstructure. Fuel 2013, 108, 833–839. [Google Scholar] [CrossRef]
- Kourti, I.; Rani, D.A.; Boccaccini, A.R.; Cheeseman, C.R. Production of geopolymers using glass produced from DC plasma treatment of air pollution control (APC) residues. J. Hazard. Mater. 2010, 176, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Onisei, S.; Pontikes, Y.; van Gerven, T.; Angelopoulos, V.N.; Velea, T.; Predica, V.; Moldovan, P. Synthesis of inorganic polymers using fly ash and primary lead slag. J. Hazard. Mater. 2012, 205–206, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Allahverdi, A.; Kani, E.N.; Yazdanipour, M. Effects of blast-furnace slag on natural pozzolan-based geopolymer cement. Ceram. Silik. 2011, 55, 68–78. [Google Scholar]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.; Mejía de Gutiérrez, R.; Gordillo, M.; Provis, J.L. Mechanical and termal characterisation of geopolymers base don silicate-activated metakaolin/slag blends. J. Mater. Sci. 2011, 46, 5477–5486. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía de Gutiérrez, R. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Puertas, F.; Martinez-Ramirez, S.; Alonso, S.; Vazquez, T. Alkali-activated fly ash/slag cement—Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Frías, M.; Villar-Cociña, E.; Morales, E.V.; Savastano, H. Study of the pozzolanic reaction kinetics in sugar cane bagasse–clay ash/calcium hydroxide system: Kinetic parameters and pozzolanic activity. Adv. Cem. Res. 2009, 21, 23–30. [Google Scholar] [CrossRef]
- Cordeiro, G.C.; Toledo Filho, R.D.; Fairbairn, E.M.R. Effect of calcination temperature on the pozzolanic activity of sugar cane bagasse ash. Constr. Build. Mater. 2009, 23, 22–28. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Díaz-Piazón, L.; Ordoñez, L.M. Sugar-cane bagasse ash (SCBA): Studies on its properties for reusing in concrete production. J. Chem. Technol. Biotechnol. 2002, 77, 321–325. [Google Scholar] [CrossRef]
- Tippayasan, C.; Boonsalee, S.; Sajjavanich, S.; Ponzoni, C.; Kamseu, E.; Chaysuwan, D. Geopolymer development by powder of metakaolin and wastes in Thailand. Adv. Sci. Technol. 2010, 69, 63–68. [Google Scholar] [CrossRef]
- Castaldelli, V.N.; Tashima, M.M.; Melges, J.L.P.; Akasaki, J.L.; Monzó, J.; Borrachero, M.V.; Soriano, L.; Payá, J. Preliminary Studies on the Use of sugar Cane Bagasse Ash (SCBA) in the Manufacture of Alkali Activated Binders. In Proceedings of the 14th International Conference on Non-Conventional Materials and Technologies, João Pessoa, Brazil, 24–27 March 2013.
- Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- AENOR—Asociación Española de Normalización y Certificación. Methods of Testing Cement. Chemical Analysis. Determination of Reactive SiO2 Content in Cements, Pozzolans and Fly Ash; UNE 80–225 2003; AENOR: Madrid, Spain, 2012. [Google Scholar]
- Allahverdi, A.; Shaverdi, B.; Najafi Kani, E. Influence of sodium oxide on properties of fresh and hardened paste of alkali-activated blast-furnace slag. Int. J. Civ. Eng. 2010, 8, 304–314. [Google Scholar]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds,, 4th ed.; John Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Rocha-Rangel, E.; Alvarado, M.; Díaz-Cruz, M. Effect of temperature on the hydration of activated granulated blast furnace slag. Process. Prop. Adv. Ceram. Compos. IV 2012, 234, 29–35. [Google Scholar]
- Buchwald, A.; Hilbig, H.; Kaps, C. Alkali-activated metakaolin-slag blends—Performance and structure in dependence of their composition. J. Mater. Sci. 2007, 42, 3024–3032. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E. Effect of calcium additions on N–A–S–H cementitious gels. J. Am. Ceram. Soc. 2010, 93, 1934–1940. [Google Scholar]
- Clayden, N.J.; Esposito, S.; Aronne, A.; Pernice, P. Solid state 27Al NMR and FTIR study of lanthanum aluminosilicate glasses. J. Non-Cryst. Solids 1991, 11, 258–268. [Google Scholar]
- Ortego, J.D.; Barroeta, Y. Leaching effects on silicate polymerization, A FTIR and silicon-29 NMR study of lead and zinc in Portland cement. Environ. Sci. Technol. 1991, 25, 1171–1174. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Castaldelli, V.N.; Akasaki, J.L.; Melges, J.L.P.; Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Use of Slag/Sugar Cane Bagasse Ash (SCBA) Blends in the Production of Alkali-Activated Materials. Materials 2013, 6, 3108-3127. https://doi.org/10.3390/ma6083108
Castaldelli VN, Akasaki JL, Melges JLP, Tashima MM, Soriano L, Borrachero MV, Monzó J, Payá J. Use of Slag/Sugar Cane Bagasse Ash (SCBA) Blends in the Production of Alkali-Activated Materials. Materials. 2013; 6(8):3108-3127. https://doi.org/10.3390/ma6083108
Chicago/Turabian StyleCastaldelli, Vinícius N., Jorge L. Akasaki, José L.P. Melges, Mauro M. Tashima, Lourdes Soriano, María V. Borrachero, José Monzó, and Jordi Payá. 2013. "Use of Slag/Sugar Cane Bagasse Ash (SCBA) Blends in the Production of Alkali-Activated Materials" Materials 6, no. 8: 3108-3127. https://doi.org/10.3390/ma6083108
APA StyleCastaldelli, V. N., Akasaki, J. L., Melges, J. L. P., Tashima, M. M., Soriano, L., Borrachero, M. V., Monzó, J., & Payá, J. (2013). Use of Slag/Sugar Cane Bagasse Ash (SCBA) Blends in the Production of Alkali-Activated Materials. Materials, 6(8), 3108-3127. https://doi.org/10.3390/ma6083108








