Application-Oriented Chemical Optimization of a Metakaolin Based Geopolymer
Abstract
:1. Introduction
2. Experimental Section
2.1. Source Materials
| Properties | Value |
|---|---|
| Cord coating | Zinc |
| Number of filaments per cord | 38 |
| Cord area (mm2) | 1.78 |
| Cords per m | 210 |
| Tensile strength (MPa) | 2845 |
| Elongation at breakage (mm/mm) | 0.0135 |
| Tensile Modulus of Elasticity Ef (GPa) | 210 |
2.2. Optimization of the Geopolymer Matrix
2.2.1. Specimens Preparation
| sample | 1.07 | 1.40 | 1.75 | 1.90 |
| r | 0 | 0.67 | 1.34 | 1.67 |
| Chemical composition | Na2O·(SiO2)2.15·Al2O3·(H2O)10.5 | Na2O·(SiO2)2.80·Al2O3·(H2O)10.5 | Na2O·(SiO2)3.50·Al2O3·(H2O)10.5 | Na2O·(SiO2)3.80·Al2O3·(H2O)10.5 |
| Mix Design (g) | – | – | – | – |
| Sodium Silicate | – | 60 (No. 1) | 120 (No. 1) | 139 (No. 2) |
| Solid NaOH | – | – | 20 | 6.3 |
| NaOH (aq) | 110 (10 M) | 64 (15 M) | – | – |
| Metakaolin | 100 | 100 | 100 | 100 |
2.2.2. Specimens Characterization
3. Results and Discussion
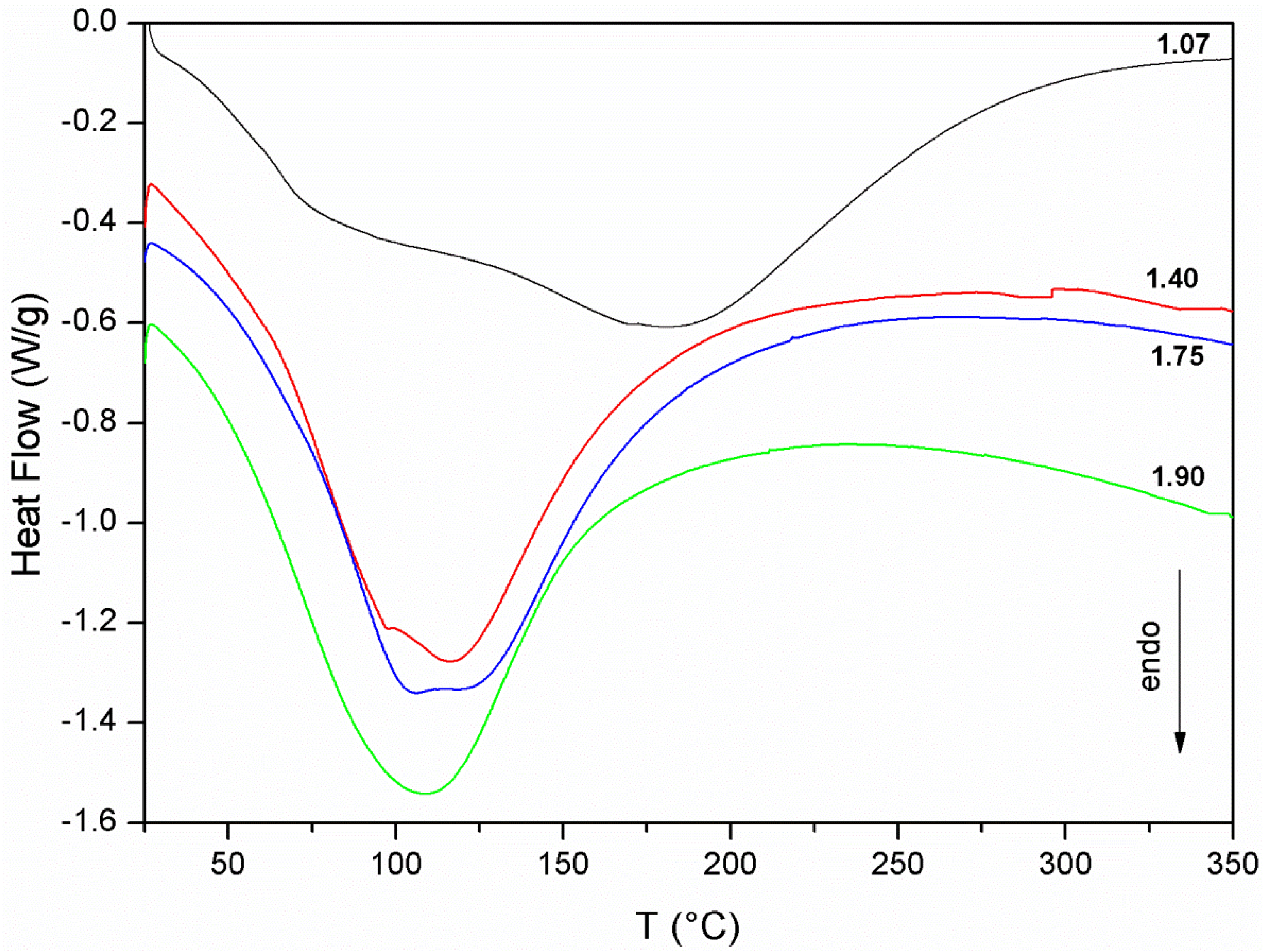
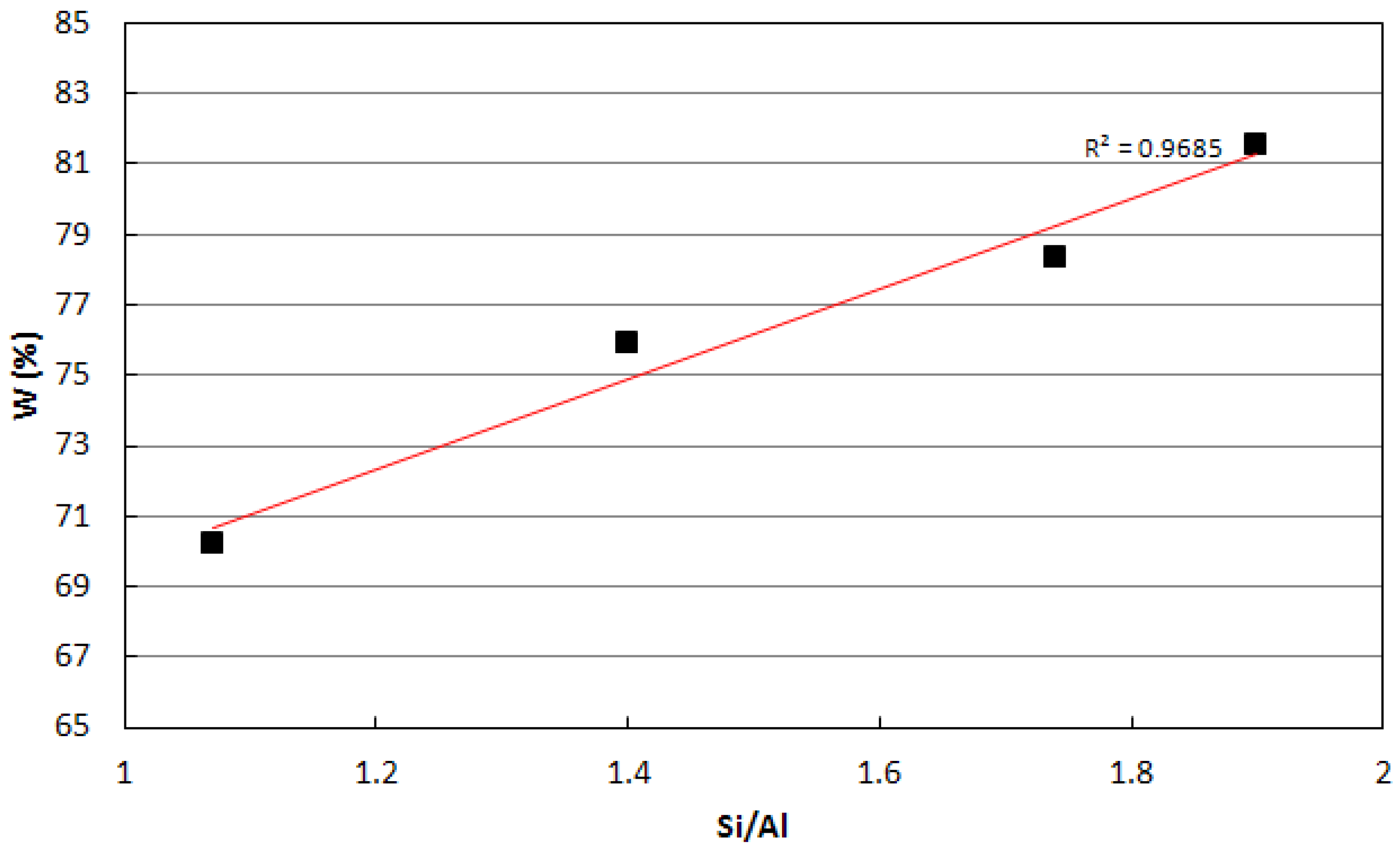
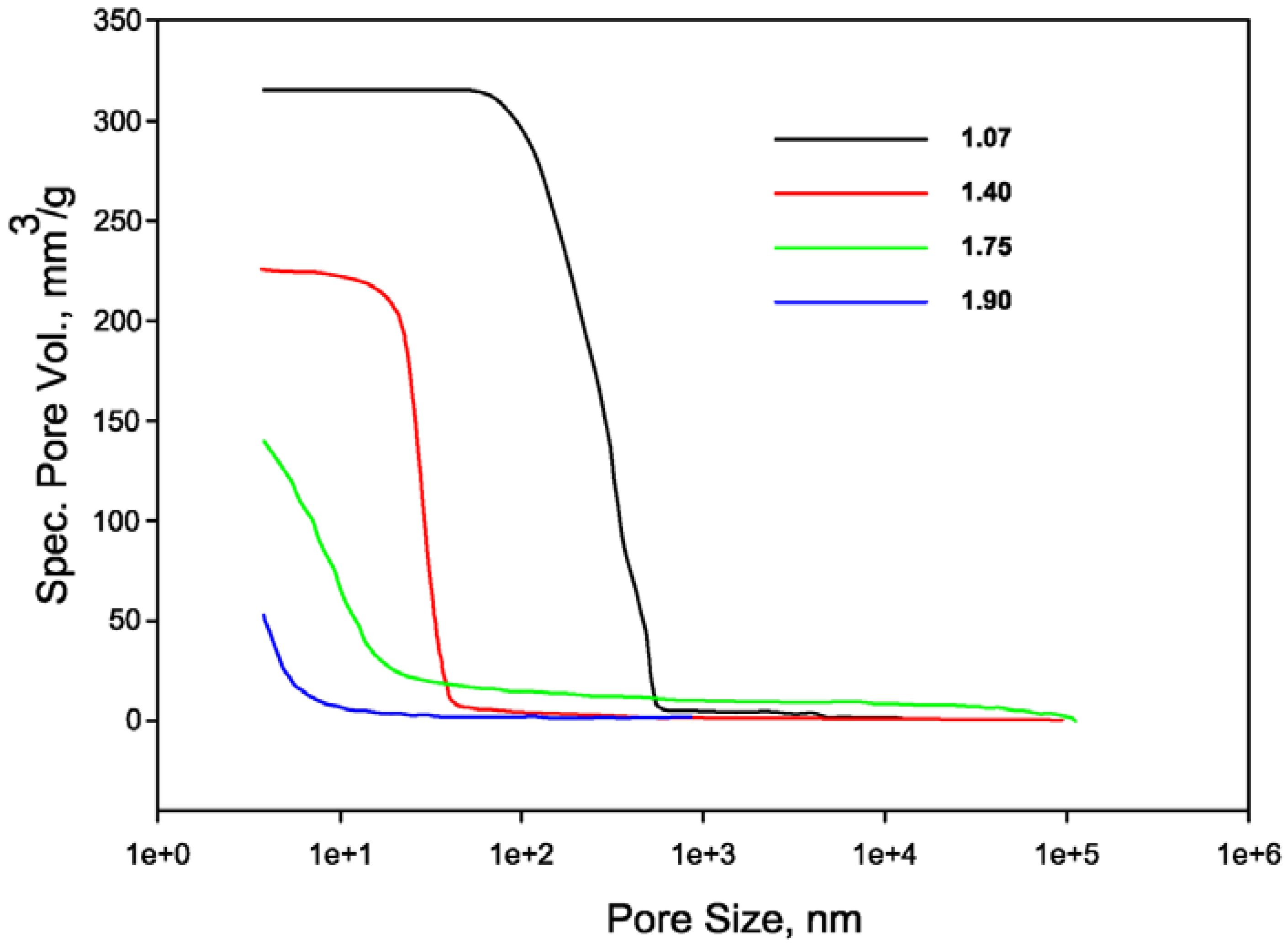
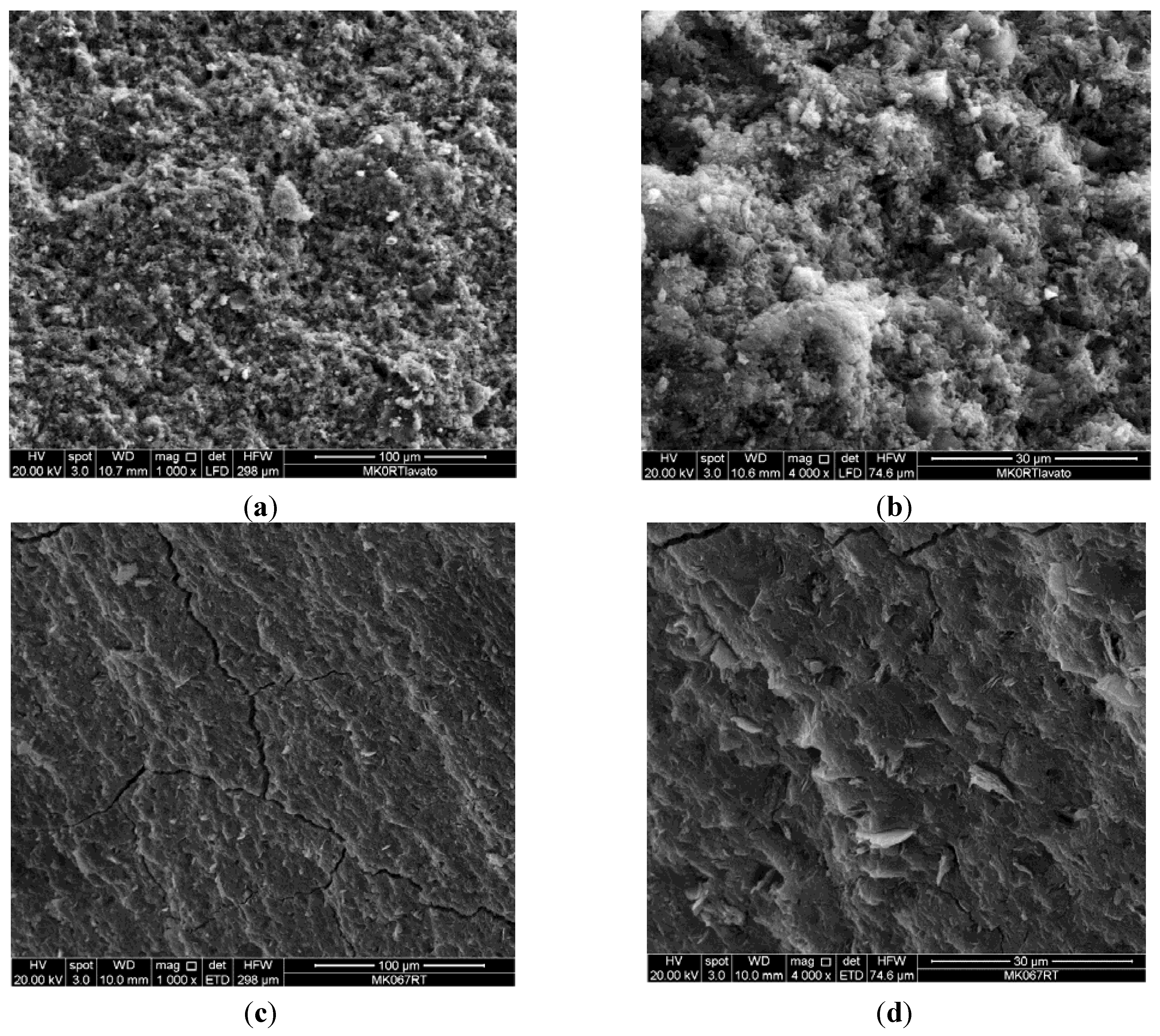
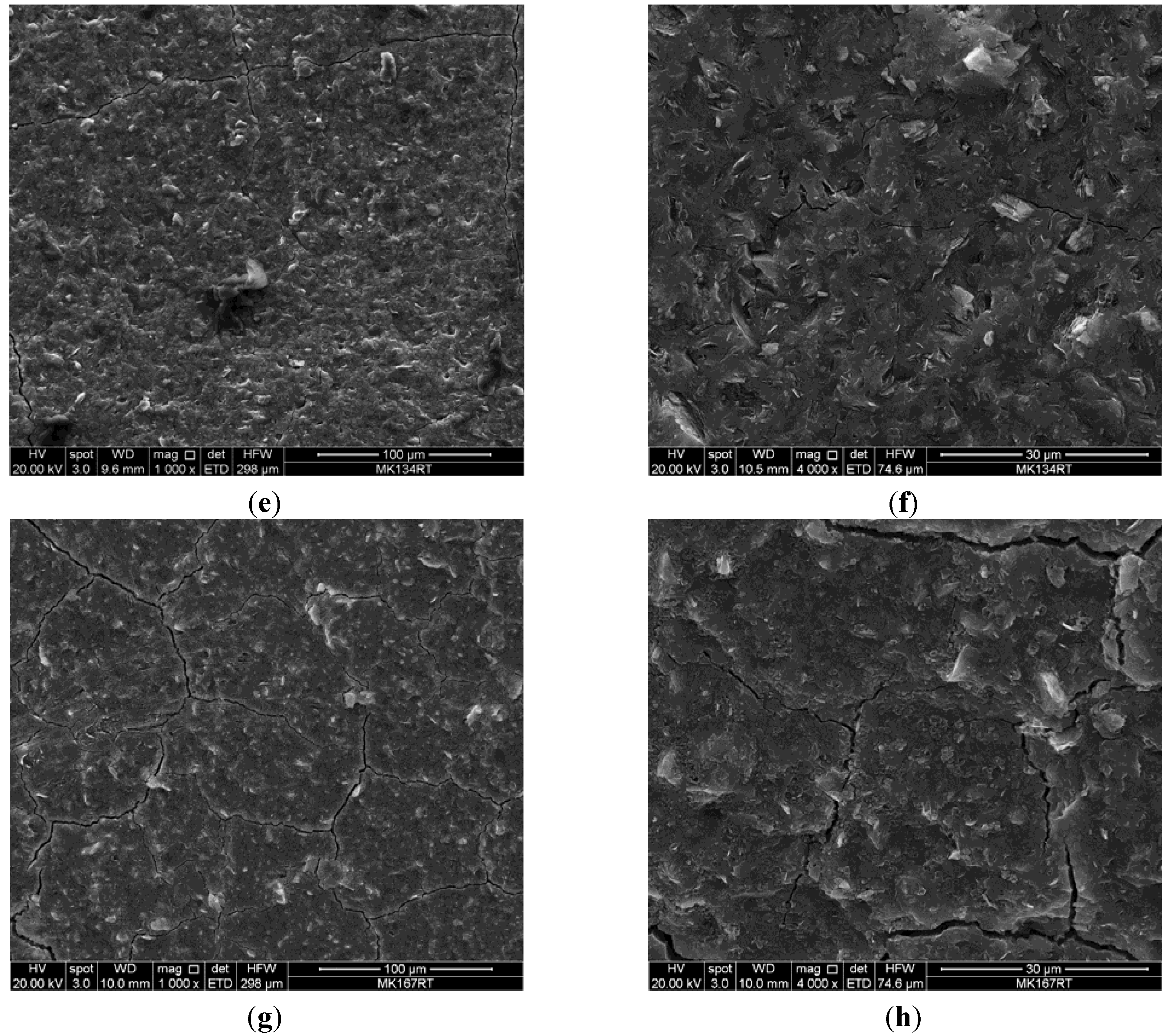

4. Application
4.1. Optimization of the Geopolymeric Adhesive
4.2. Application of the Geopolymer Based Reinforcing System
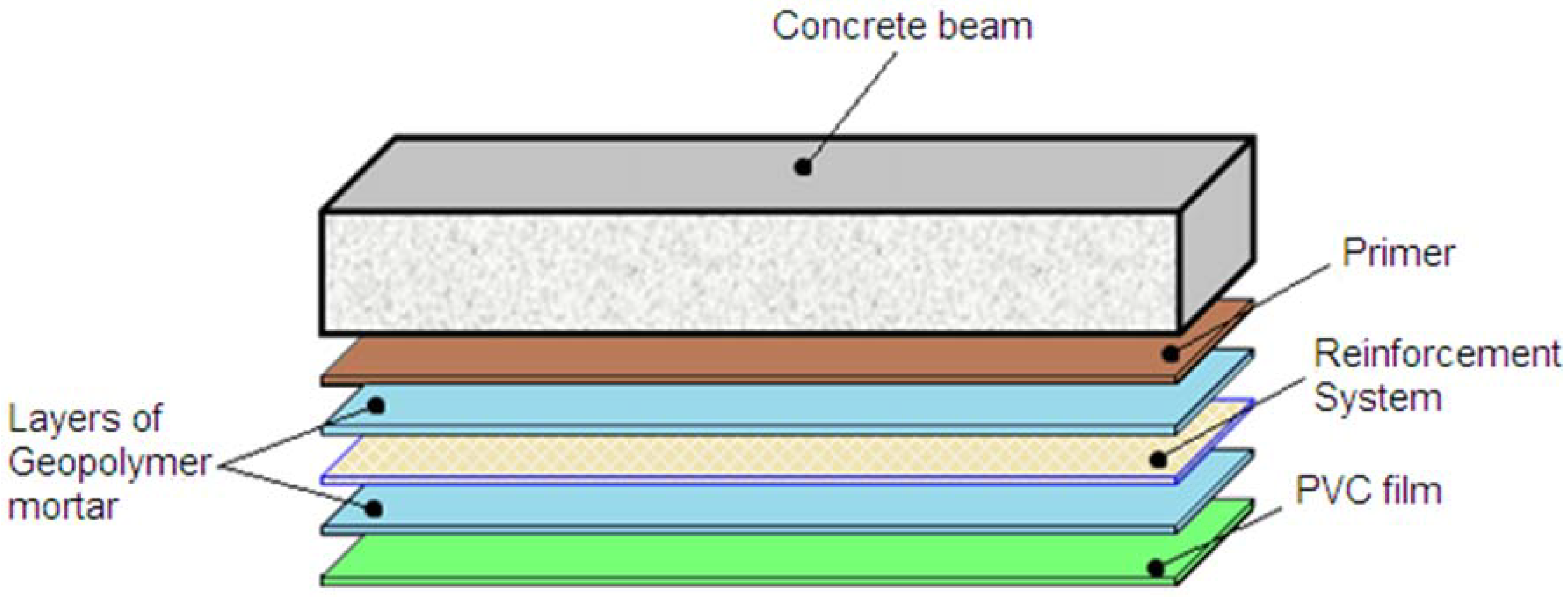
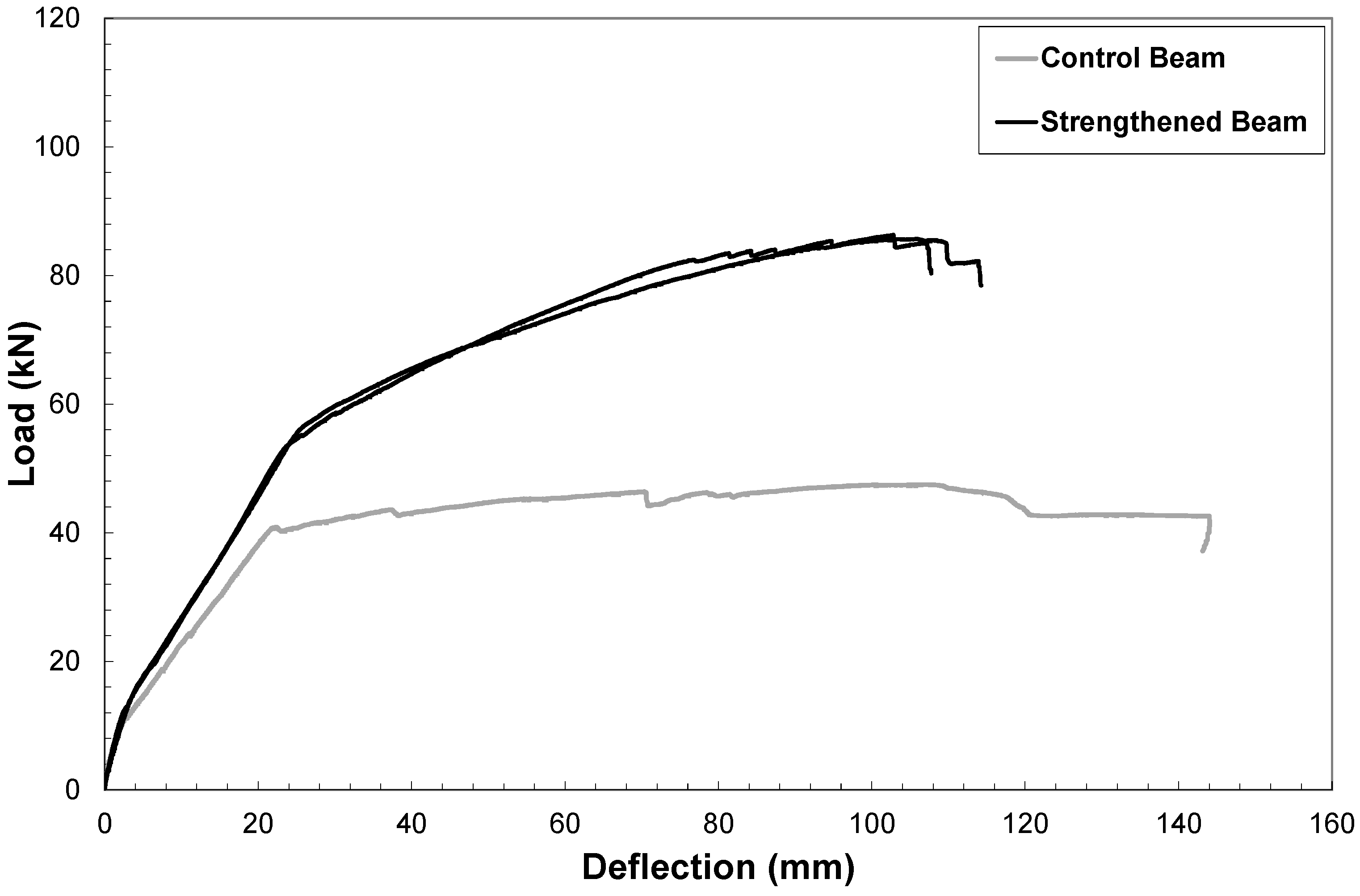

4. Conclusions
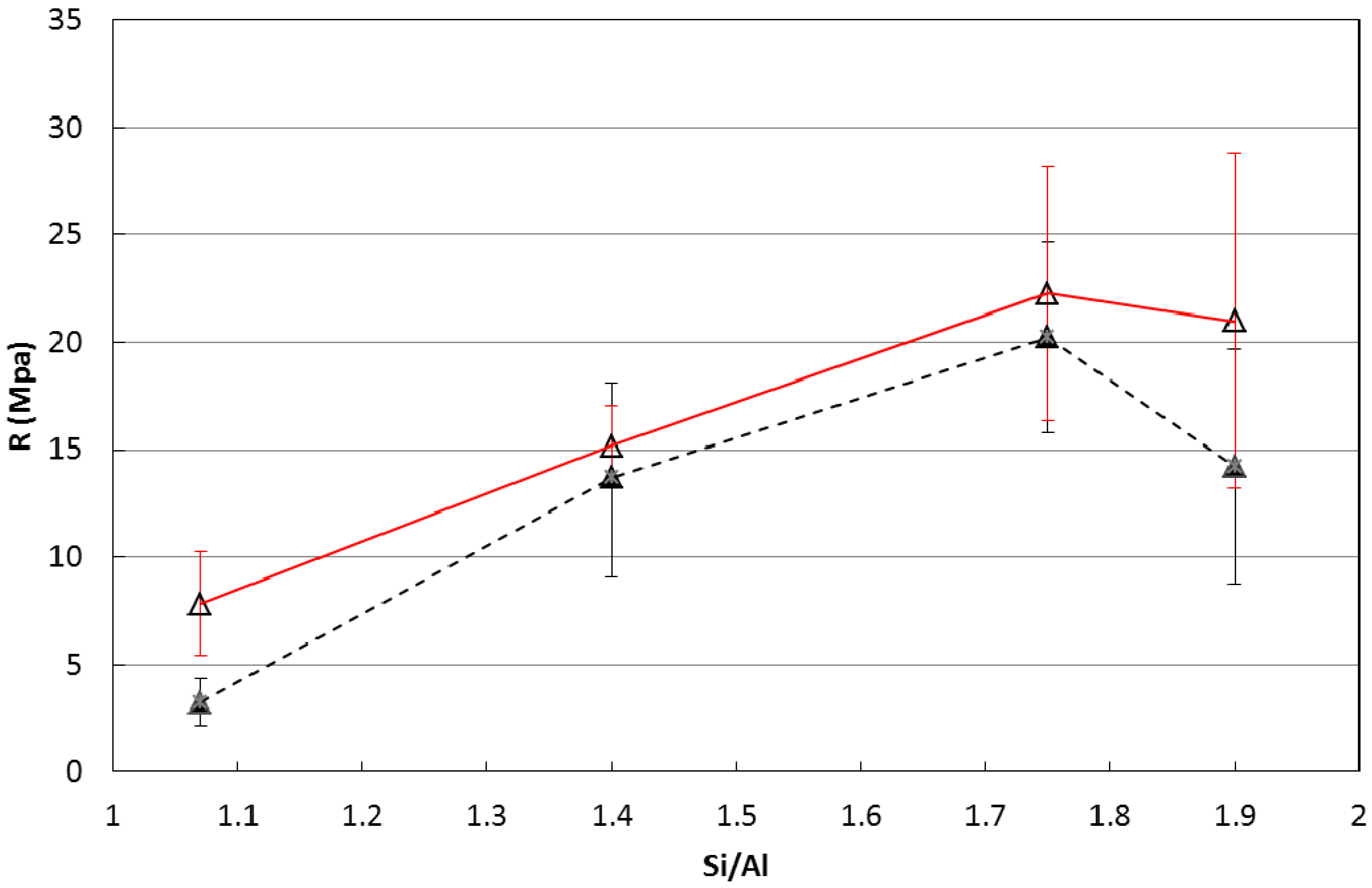
- Curing of metakaolin based geopolymers at ambient conditions is critical because a substantial material shrinkage can occur. This issue is related to the amount of evaporable water of the sample, which was found to increase with the Si/Al ratio of the geopolymer mixture.
- A medium-high value of Si/Al ratio = 1.75 was found to be the best compromise between mechanical performances and shrinkage issues. Nonetheless, the addition of a fine quartz powder as filler and the control of the early water evaporation were found to be crucial in obtaining the best performances.
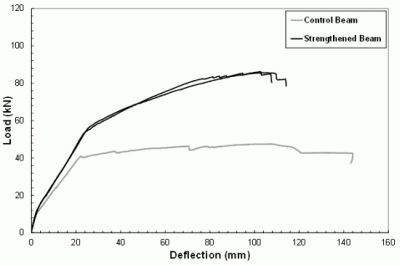
- The results of preliminary tests conducted by employing the mortar as matrix of a composite with long steel fibers to strengthen reinforced concrete beams were greatly encouraging as the ultimate load capacity of the beams increased more than 100% with respect to the plain beam and no debonding between geopolymer matrix and concrete substrate was observed.
References
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Xu, H.; van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Cioffi, R.; Maffucci, L.; Santoro, L. Optimization of geopolymer synthesis by calcination and polycondensation of a kaolinitic residue. Resour. Conserv. Recycl. 2003, 40, 27–38. [Google Scholar] [CrossRef]
- Steveson, M.; Sagoe-Crentsil, K. Relationships between composition, structure and strength of inorganic polymers. Part I. Metakaolin-derived inorganic polymers. J. Mater. Sci. 2005, 40, 2023–2036. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Brew, D.R.M.; Fletcher, R.A.; Vagana, R. Formation of aluminosilicate geopolymers from 1:1 layer-lattice minerals pre-treated by various methods: A comparative study. J. Mater. Sci. 2007, 42, 4667–4674. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Geopolymerisation of low calcium ferronickel slags. J. Mater. Sci. 2007, 42, 3073–3082. [Google Scholar] [CrossRef]
- Andini, S.; Cioffi, R.; Colangelo, F.; Montagnaro, F.; Santoro, L. Coal fly ash as raw material for the manufacture of geopolymer-based products. Waste Manag. 2008, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Ferone, C.; Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Use of reservoir clay sediments as raw materials for geopolymer binders. Adv. Appl. Ceram. 2012. Available online: http://www.ingentaconnect.com/content/maney/aac/pre-prints (accessed on 22 February 2013). [CrossRef]
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J. Effect of the alkali metal activator on the properties of fly-ash-based geopolymers. Ind. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Rahier, H.; Simons, W.; van Mele, B.; Biesemans, M. Low-temperature synthesized aluminosilicate glasses. Part III. Influence of the composition of the silicate solution on production, structure and properties. J. Mater Sci. 1997, 32, 2237–2247. [Google Scholar] [CrossRef]
- Palomo, A.; Macias, A.; Blanco, M.T.; Puertas, F. Physical, Chemical and Mechanical Characterization of Geopolymers. In Proceedings of the 9th International Congress on the Chemistry of Cement, New Delhi, India, 23–28 November 1992; National Council for Cement and Building Materials (NCCBM): New Delhi, India, 1992; pp. 505–511. [Google Scholar]
- Schmücker, M.; MacKenzie, K.J.D. Microstructure of sodium polysialate siloxo geopolymer. Ceram. Int. 2005, 31, 433–437. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer, Chemistry and Applications, 3rd ed.; Institute Geopolymere: Saint-Quentin, France, 2011; pp. 10–11. [Google Scholar]
- Ferone, C.; Roviello, G.; Colangelo, F.; Cioffi, R.; Tarallo, O. Novel hybrid organic-geopolymer materials. Appl. Clay Sci. 2013, 73, 42–50. [Google Scholar] [CrossRef]
- Palomo, A.; Blanco-Varela, M.T.; Granizo, M.L.; Puertas, F.; Vasquez, T.; Grutzeck, M.W. Chemical stability of cementitious materials based on metakaolin. Cem. Concr. Res. 1999, 29, 997–1004. [Google Scholar] [CrossRef]
- Habert, G.; d’Espinose de Lacaillerie, J.B.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag. 2012, 32, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, F.; Cioffi, R.; Lavorgna, M.; Verdolotti, L.; de Stefano, L. Treatment and recycling of asbestos-cement containing waste. J. Hazard. Mater. 2011, 195, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, R.; Colangelo, F.; Montagnaro, F.; Santoro, L. Manufacture of artificial aggregate using MSWI bottom ash. Waste Manag. 2011, 31, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lorenzen, L. The potential use of geopolymeric materials to immobilise toxic metals: Part I. Theory and applications. Miner. Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Kriven, W.M.; Bell, J.L.; Gordon, M. Geopolymer refractories for the glass manufacturing industry. Ceram. Eng. Sci. Proc. 2004, 25, 57–79. [Google Scholar]
- Lancellotti, I.; Kamseu, E.; Michelazzi, M.; Barbieri, L.; Corradi, A.; Leonelli, C. Chemical stability of geopolymers containing municipal solid waste incinerator fly ash. Waste Manag. 2010, 30, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Andini, S.; Cioffi, R.; Colangelo, F.; Ferone, C.; Montagnaro, F.; Santoro, L. Characterization of geopolymer materials containing MSWI fly ash and coal fly ash. Adv. Sci. Technol. 2010, 69, 123–128. [Google Scholar] [CrossRef]
- Giancaspro, J.W.; Papakonstantinou, C.G.; Balaguru, P. Flexural response of inorganic hybrid composites with E-glass and carbon fibers. J. Eng. Mater. Technol. 2010, 132, 1–8. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Mechanical performances of weathered coal fly ash based geopolymer bricks. Procedia Eng. 2011, 21, 745–752. [Google Scholar] [CrossRef]
- American Concrete Institute (ACI) Committee 440. Guide for the Design and Construction of Externally Bonded FRP Systems for Strengthening Concrete Structures–ACI 440.2r-08 2008; ACI: Farmington Hills, MI, USA, 2008. [Google Scholar]
- Canadian Standards Association (CSA). Canadian Highway Bridge Design Code–CAN/CSA-S6-06 2006; CSA: Mississauga, ON, Canada, 2006. [Google Scholar]
- Katz, A.; Berman, N.; Bank, L.C. Effect high temperature on the bond strength of FRP rebars. J. Compos. Constr. 1999, 3, 73–81. [Google Scholar] [CrossRef]
- Saafi, M. Effect of fire on FRP reinforced concrete members. Compos. Struct. 2002, 58, 11–20. [Google Scholar] [CrossRef]
- Bisby, L.A.; Green, M.F.; Kodur, V.K.R. Modeling the behavior of fiber reinforced polymer-confined concrete columns exposed to fire. J. Compos. Constr. 2005, 9, 15–24. [Google Scholar] [CrossRef]
- Roviello, A.; Buono, A.; Carella, A.; Roviello, G.; Cassinese, A.; Barra, M.; Biasucci, M. Regioregular poly[3-(4-alkoxyphenyl)thiophene]s. J. Polym. Sci. Polym. Chem. 2007, 45, 1758–1770. [Google Scholar] [CrossRef]
- Hashemi, S.; Al-Mahaidi, R. Flexural performance of CFRP textile-retrofitted RC beams using cement-based adhesives at high temperature. Constr. Build. Mater. 2012, 28, 791–797. [Google Scholar] [CrossRef]
- Gamage, J.C.P.H.; Al-Mahaidi, R.; Wong, M.B. Bond characteristics of CFRP plated concrete members under elevated temperatures. Compos. Struct. 2006, 75, 199–205. [Google Scholar] [CrossRef]
- Williams, B.; Bisby, L.; Kodur, V.; Green, M.; Chowdhury, E. Fire insulation schemes for FRP-strengthened concrete slabs. Compos. Part A Appl. S. 2006, 37, 1151–1160. [Google Scholar] [CrossRef]
- Sumida, A.; Mutsuyoshi, H. Mechanical properties of newly developed heat-resistant FRP bars. J. Adv. Concr. Technol. 2008, 6, 157–170. [Google Scholar] [CrossRef]
- Nigro, E.; Manfredi, G.; Cosenza, E.; Zappoli, M. Effects of High Temperature on the Performances of RC Bridge Decks Strengthened with Externally Bonded FRP Reinforcement. In Proceedings of 2nd International fib Conference, Naples, Italy, 5–8 June 2006. paper No. 12-2.
- Balaguru, P.; Kurtz, S.; Rudolph, J. Geopolymer for repair and rehabilitation of reinforced concrete beams. 1997. Available online: http://www.geopolymer.org (accessed on 7 May 2013).
- Menna, C.; Asprone, D.; Ferone, C.; Colangelo, F.; Balsamo, A.; Prota, A.; Cioffi, R.; Manfredi, G. Use of geopolymers for composite external reinforcement of RC members. Compos. Part B Eng. 2013, 45, 1667–1676. [Google Scholar] [CrossRef]
- Rowles, M.; O’Connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The effect of composition and temperature on the properties of fly ash- and kaolinite-based geopolymers. Chem. Eng. J. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Perera, D.S.; Uchida, O.; Vance, E.R.; Finnie, K.S. Influence of curing schedule on the integrity of geopolymers. J. Mater. Sci. 2007, 4, 3099–3106. [Google Scholar] [CrossRef]
- Kuenzel, C.; Vandeperre, L.J.; Donatello, S.; Boccaccini, A.R.; Cheeseman, C. Ambient temperature drying shrinkage and cracking in metakaolin-based geopolymers. J. Am. Ceram. Soc. 2012, 95, 3270–3277. [Google Scholar] [CrossRef]
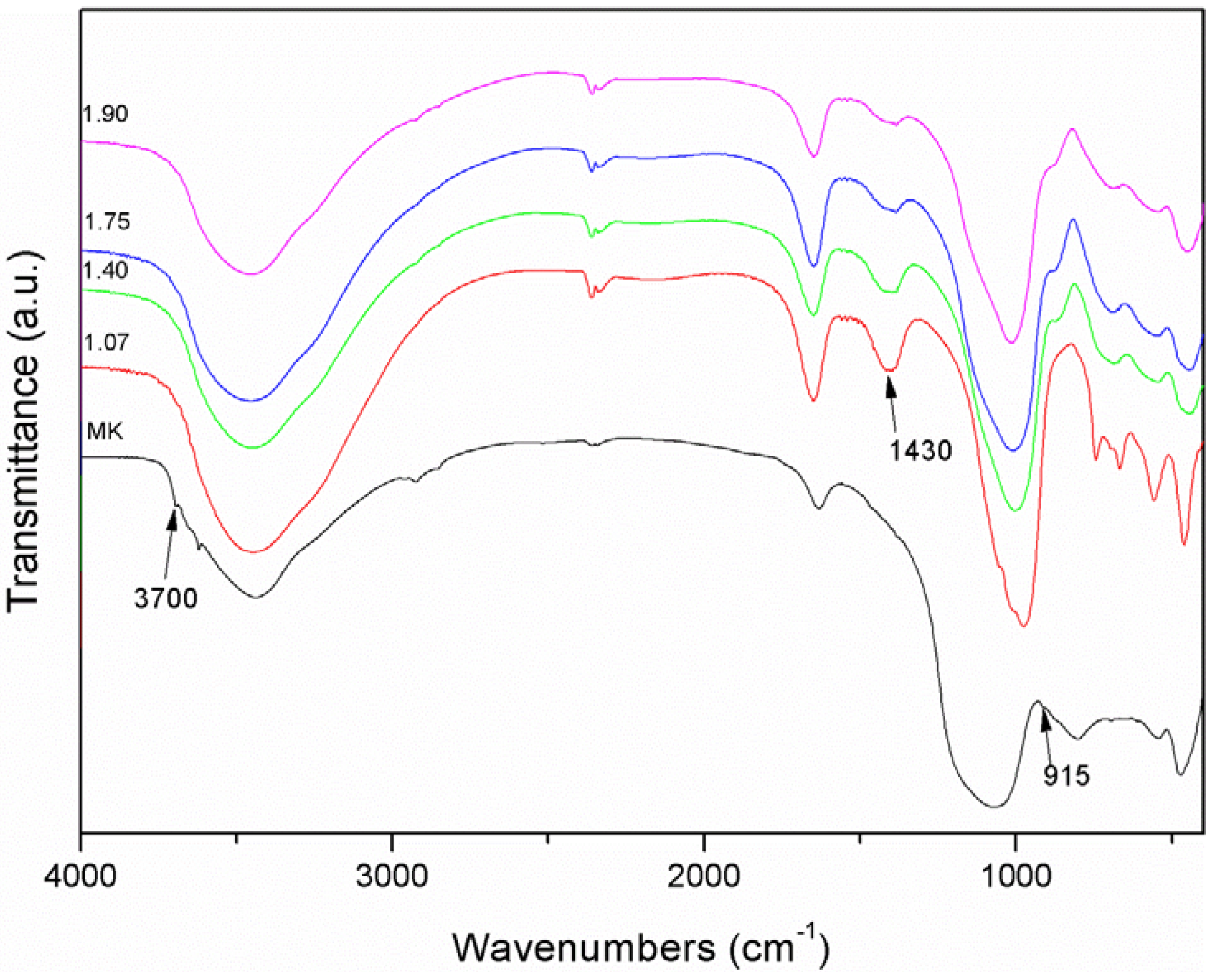
- Russell, J.D.; Fraser, A.R.; Wilson, M.J. Clay Mineralogy: Spectroscopic and Chemical Determinative Methods; Chapman & Hall: London, UK, 1996; pp. 11–67. [Google Scholar]
- Zhang, Z.; Yao, X.; Zhu, H.; Hua, S.; Chen, Y. Activating process of geopolymer source material: Kaolinite. J. Wuhan Univ. Technol. Mater. Sci. 2009, 24, 132–136. [Google Scholar] [CrossRef]
- Bosch Reig, F.; Gimeno Adelantado, J.V.; Moya Moreno, M.C.M. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Duxson, P.; Lukey, G.C.; Separovic, F.; van Deventer, J.S.J. Effect of alkali cations on aluminium incorporation in geopolymeric gels. Ind. Eng. Chem. Res. 2005, 44, 832–839. [Google Scholar] [CrossRef]
- Provis, J.L.; Rees, C.A. Geopolymer Synthesis Kinetics. In Geopolymers: Structure, Processing, Properties and Industrial Applications; Provis, J.L., van Deventer, J.S.J., Eds.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2009; pp. 118–136. [Google Scholar]
- Aronne, A.; Esposito, S.; Ferone, C.; Pansini, M.; Pernice, P. FTIR study of the thermal transformation of barium exchanged zeolite A to celsian. J. Mater. Chem. 2002, 12, 3039–3045. [Google Scholar] [CrossRef]
- Provis, J.L.; Yong, S.L.; Duxson, P. Nanostructure/Microstructure of Metakaolin Geopolymers. In Geopolymers: Structure, Processing, Properties and Industrial Applications; Provis, J.L., van Deventer, J.S.J., Eds.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2009; pp. 72–88. [Google Scholar]
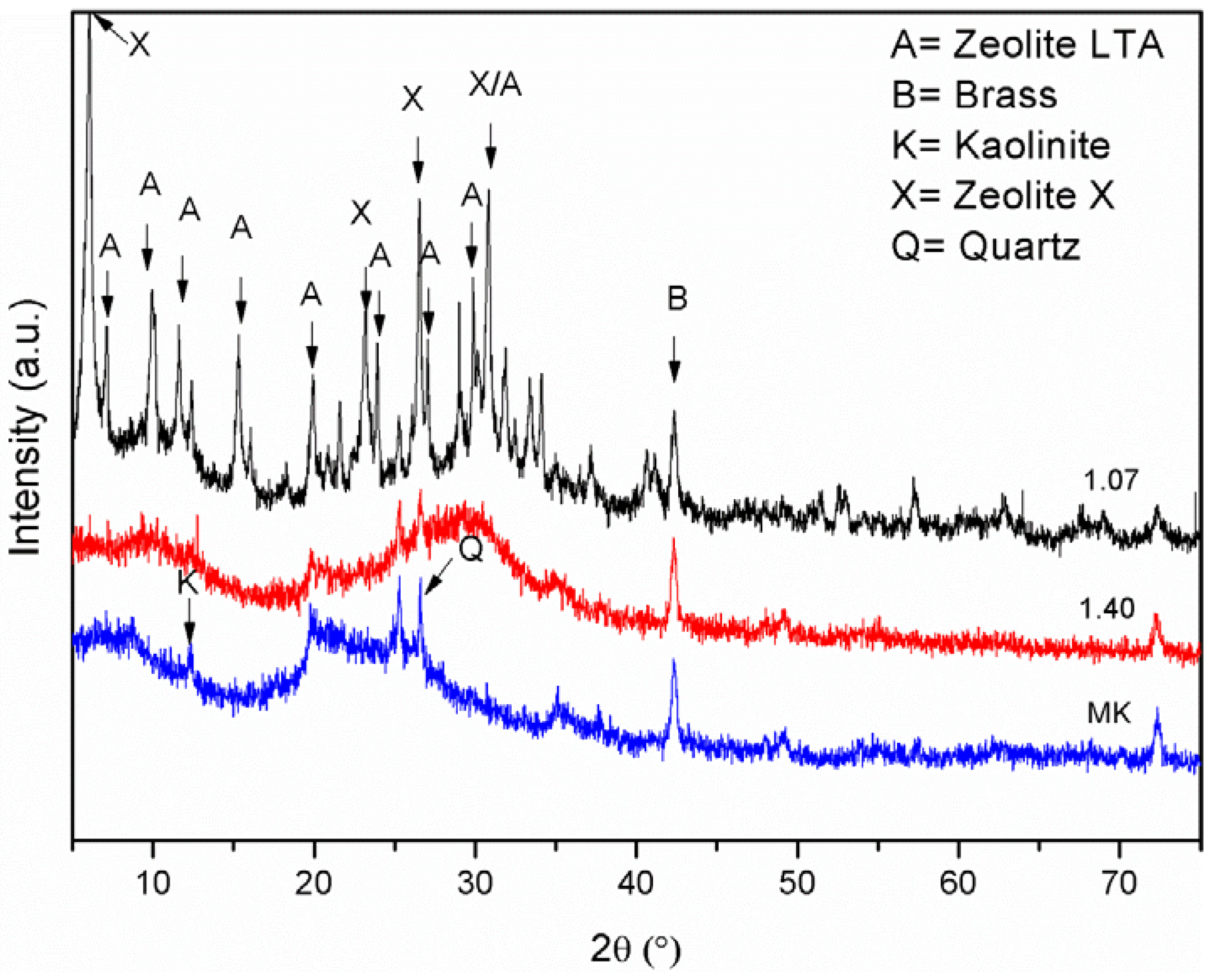
- Chandrasekhar, S.; Pramada, P.N. Microwave assisted synthesis of zeolite: A from metakaolin. Microporous Mesoporous Mater. 2008, 108, 152–161. [Google Scholar] [CrossRef]
- Demortier, A.; Gobelz, N.; Lelieur, J.P.; Duhayon, C. Infrared evidence for the formation of an intermediate compound during the synthesis of zeolite Na-A from metakaolin. Int. J. Inorg. Mater. 1999, 1, 129–134. [Google Scholar] [CrossRef]
- Akolekar, D.; Chaffee, A.; Howe, R.F. The transformation of kaolin to low-silica X zeolite. Zeolites 1997, 19, 359–365. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Do geopolymers actually contain nanocrystalline zeolites? A reexamination of existing results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Lizcano, M.; Gonzalez, A.; Basu, S.; Lozano, K.; Radovic, M. Effects of water content and chemical composition on structural properties of alkaline activated metakaolin-based geopolymers. J. Am. Ceram. Soc. 2012, 95, 2169–2177. [Google Scholar] [CrossRef]
- Perera, D.S.; Vance, E.R.; Finnie, K.S.; Blackford, M.G.; Hanna, J.V.; Cassidy, D.J.; Nicholson, C.L. Disposition of water in metakaolinite-based geopolymers. Ceram. Trans. 2005, 185, 225–236. [Google Scholar]
- Colella, C. Use of Thermal Analysis in Zeolite Research and Applications. In Characterization Techniques of Glasses and Ceramics; Rincon, J.M.A., Romero, M., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 1999; pp. 112–137. [Google Scholar]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Yuan, X.; Fang, Y.; Gu., Y. Effect of quartz powder on the strength and shrinkage of alkali-activated slag cement. Adv. Mater. Res. 2013, 602–604, 972–975. [Google Scholar]
- Temuujin, J.; Minjigma, A.; Rickard, W.; Lee, M.; Williams, I.; van Riessen, A. Fly ash based geopolymer thin coatings on metal substrates and its thermal evaluation. J. Hazard. Mater. 2010, 180, 748–752. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferone, C.; Colangelo, F.; Roviello, G.; Asprone, D.; Menna, C.; Balsamo, A.; Prota, A.; Cioffi, R.; Manfredi, G. Application-Oriented Chemical Optimization of a Metakaolin Based Geopolymer. Materials 2013, 6, 1920-1939. https://doi.org/10.3390/ma6051920
Ferone C, Colangelo F, Roviello G, Asprone D, Menna C, Balsamo A, Prota A, Cioffi R, Manfredi G. Application-Oriented Chemical Optimization of a Metakaolin Based Geopolymer. Materials. 2013; 6(5):1920-1939. https://doi.org/10.3390/ma6051920
Chicago/Turabian StyleFerone, Claudio, Francesco Colangelo, Giuseppina Roviello, Domenico Asprone, Costantino Menna, Alberto Balsamo, Andrea Prota, Raffaele Cioffi, and Gaetano Manfredi. 2013. "Application-Oriented Chemical Optimization of a Metakaolin Based Geopolymer" Materials 6, no. 5: 1920-1939. https://doi.org/10.3390/ma6051920
APA StyleFerone, C., Colangelo, F., Roviello, G., Asprone, D., Menna, C., Balsamo, A., Prota, A., Cioffi, R., & Manfredi, G. (2013). Application-Oriented Chemical Optimization of a Metakaolin Based Geopolymer. Materials, 6(5), 1920-1939. https://doi.org/10.3390/ma6051920