In Vivo Osteogenic Differentiation of Human Embryoid Bodies in an Injectable in Situ-Forming Hydrogel
Abstract
:1. Introduction
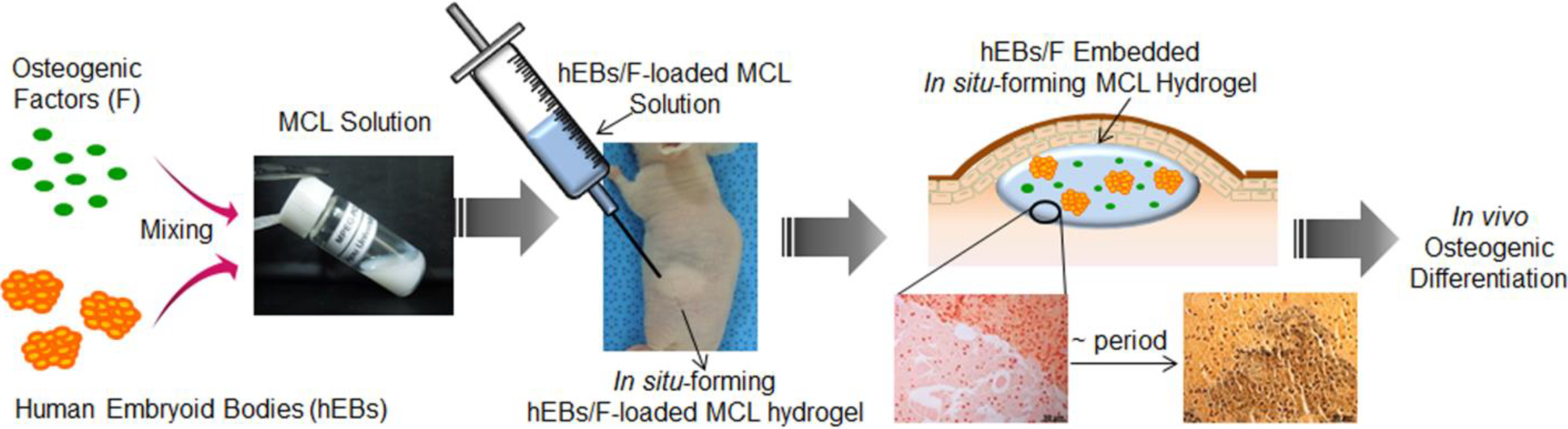
2. Results and Discussion
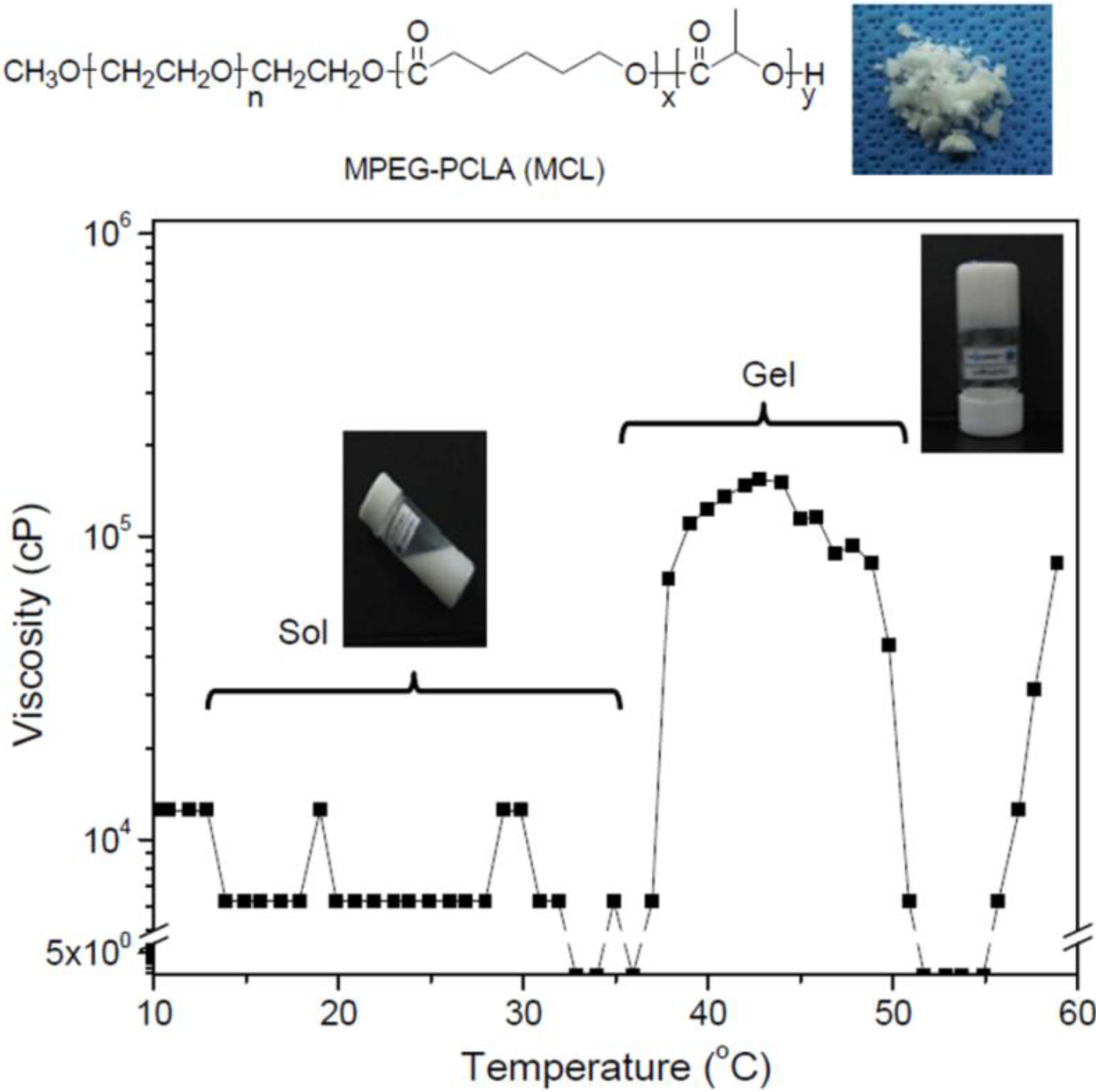
2.1. Preparation of an Injectable in Situ-Forming Hydrogel
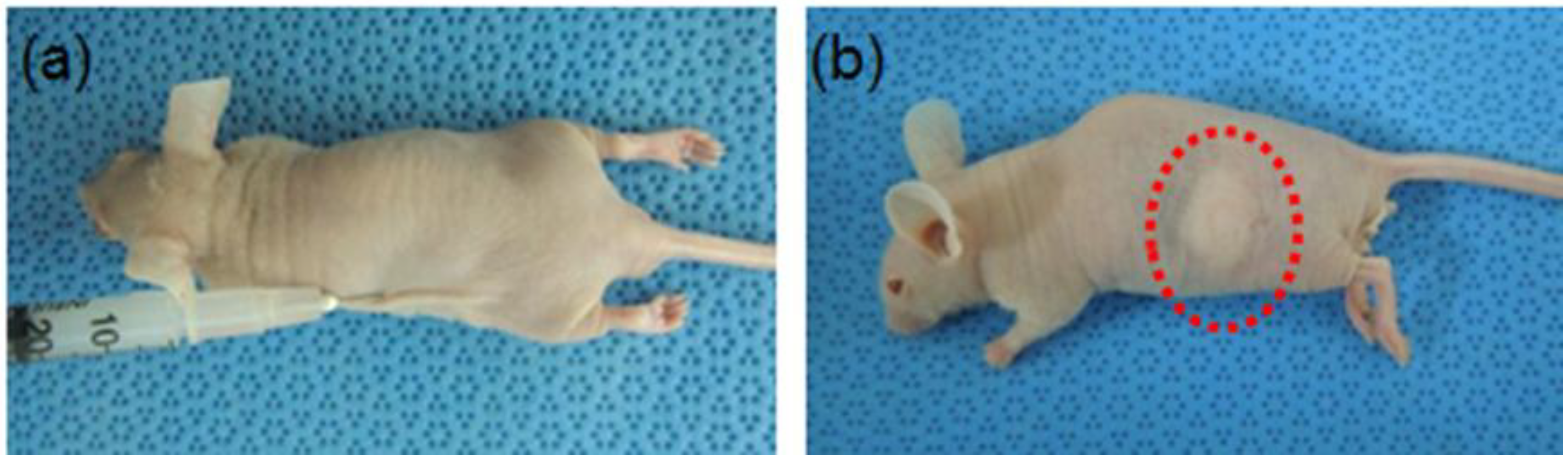
2.2. In Vivo Gelation

2.3. In Vivo Staining
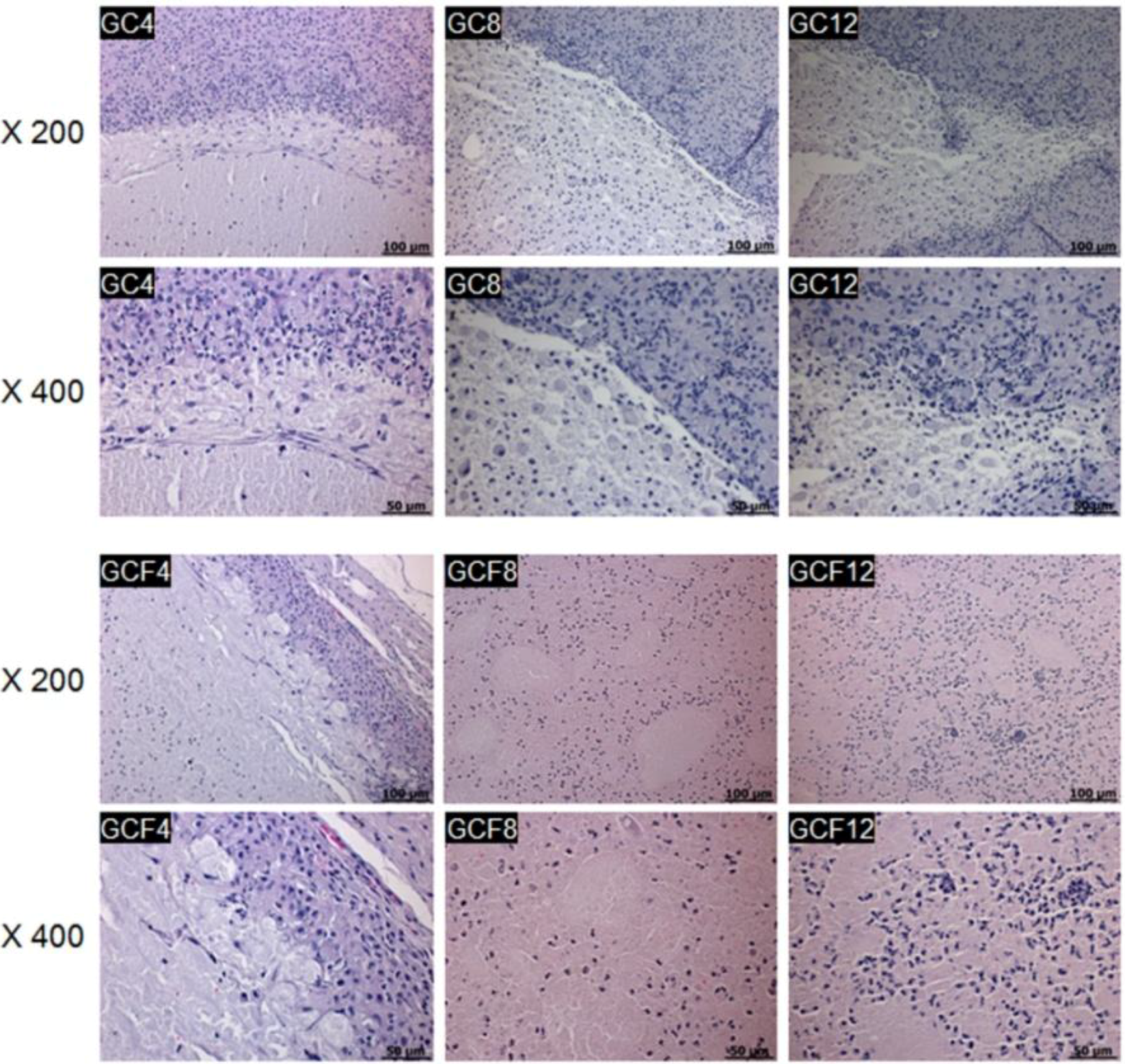
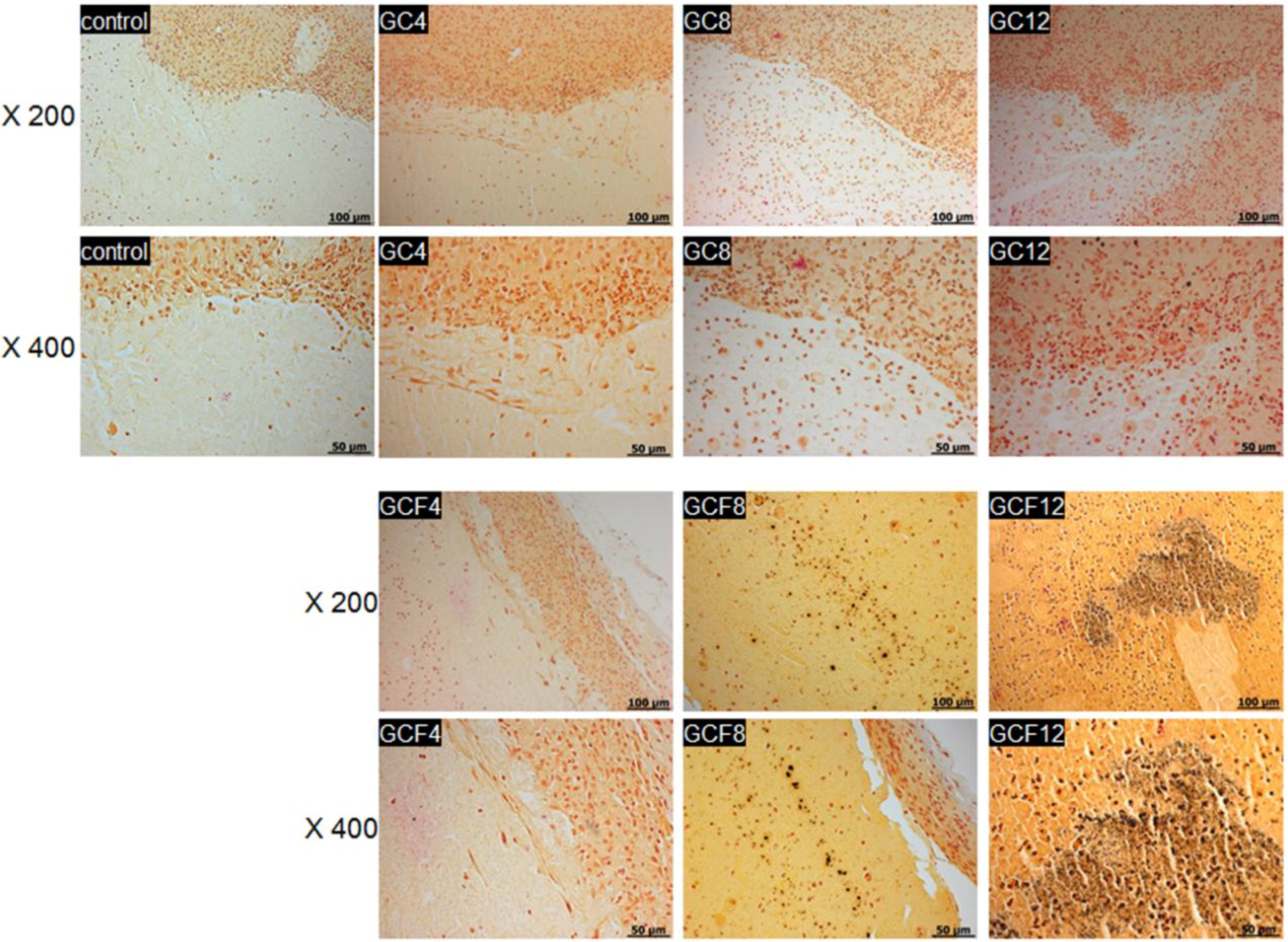
3. Experimental Section
3.1. Synthesis of MCL Diblock Copolymers
3.2. Viscosity Measurements
3.3. In Vivo Degradation of Hydrogels
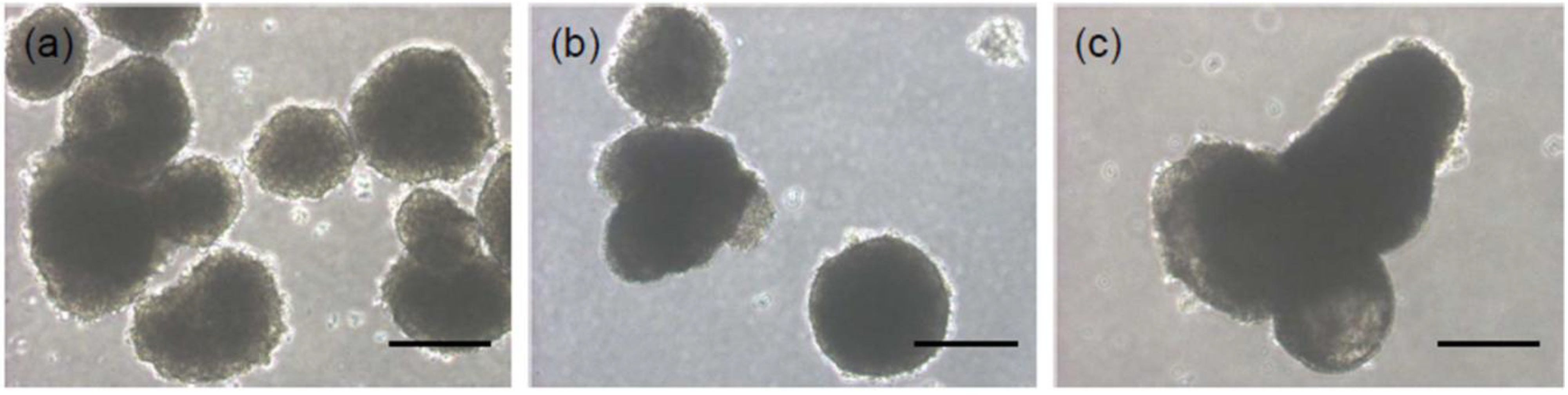
3.4. EB Formation
3.5. In Vivo Experiment
3.6. Histological Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Muschler, G.F.; Nakamoto, C.; Griffith, L.G. Engineering principles of clinical cell-based tissue engineering. J. Bone Joint Surg. Am. 2004, 86-A, 1541–1558. [Google Scholar]
- Hollister, S.J.; Murphy, W.L. Scaffold translation: Barriers between concept and clinic. Tissue Eng. Part B Rev. 2011, 17, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.H.; Min, B.H.; Chun, H.J.; Han, D.K.; Lee, H.B. Polymeric scaffolds for regenerative medicine. Polym. Rev. 2011, 51, 23–52. [Google Scholar] [CrossRef]
- Kim, M.S.; Khang, G.; Lee, H.B. Gradient polymer surfaces for biomedical applications. Prog. Polym. Sci. 2008, 33, 138–164. [Google Scholar] [CrossRef]
- Gronau, G.; Krishnaji, S.T.; Kinahan, M.E.; Giesa, T.; Wong, J.Y.; Kaplan, D.L.; Buehler, M.J. A review of combined experimental and computational procedures for assessing biopolymer structure-process-property relationships. Biomaterials 2012, 3, 8240–8255. [Google Scholar] [CrossRef]
- Peramo, A. Medical implants as delivery platforms for tissue engineering and regenerative medicine applications. Curr. Tissue Eng. 2012, 1, 36–40. [Google Scholar] [CrossRef]
- Walther, A.; Hoyer, B.; Springer, A.; Mrozik, B.; Hanke, T.; Cherif, C.; Pompe, W.; Gelinsky, M. Novel textile scaffolds generated by flock technology for tissue engineering of bone and cartilage. Materials 2012, 5, 540–557. [Google Scholar]
- Cho, S.Y.; Park, H.H.; Jin, H.J. Controlling pore size of electrospunsilk fibroin scaffold for tissue engineering. Polym.-Korea 2012, 36, 651–655. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.J.; Chun, H.J.; Kim, C.H. Thermosensitive hydrogels for tissue engineering. Tissue Eng. Regen. Med. 2011, 8, 117–123. [Google Scholar]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, J.H.; Ahn, H.H.; Lee, J.H.; Lee, J.Y.; Lee, B.; Lee, H.B.; Kim, M.S. The osteogenic differentiation of rat muscle-derived stem cells in vivo within in situ-forming chitosan scaffolds. Biomaterials 2008, 29, 4420–4428. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Lee, J.S.; Choi, J.W.; Min, B.H.; Chang, J.W.; Lim, J.Y.; Kim, C.H. Transplantation of autologous chondrocytes seeded on a fibrin/hyaluronic acid composite gel into vocal fold in rabbits: Preliminary results. Tissue Eng. Regen. Med. 2012, 9, 203–208. [Google Scholar] [CrossRef]
- Baek, E.J.; Shin, B.K.; Nho, Y.C.; Lim, Y.M.; Park, J.S.; Park, J.S.; Huh, K.M. Preparation of poloxamer-based hydrogels using electron beam and their evaluation for buccalmucoadhesive drug delivery. Polym.-Korea 2012, 36, 182–189. [Google Scholar] [CrossRef]
- Cho, M.H.; Kim, K.S.; Ahn, H.H.; Shin, Y.N.; Kim, M.S.; Khang, G.; Lee, B.; Lee, H.B. Chitosan gel as an in situ-forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng. Part A 2008, 14, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, J.Y.; Kang, Y.M.; Kim, E.S.; Lee, B.; Chun, H.J.; Kim, J.H.; Min, B.H.; Lee, H.B.; Kim, M.S. Electrostatic crosslinked in situ-forming in vivo scaffold for rat bone marrow mesenchymal stem cells. Tissue Eng. Part A 2009, 15, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kang, Y.M.; Kim, E.S.; Kang, M.L.; Lee, B.; Kim, J.H.; Min, B.H.; Park, K.; Kim, M.S. In vitro and in vivo release of albumin from an electrostatically crosslinked in situ-forming gel. J. Mater. Chem. 2010, 20, 3265–3271. [Google Scholar] [CrossRef]
- Khor, E.; Wu, H.; Lim, L.Y.; Guo, C.M. Chitin-methacrylate: Preparation, characterization and hydrogel formation. Materials 2011, 4, 1728–1746. [Google Scholar]
- Kim, M.S.; Hyun, H.; Khang, G.; Lee, H.B. Preparation of thermosensitived diblock copolymers consisting of MPEG and polyesters. Macromolecules 2006, 39, 3099–3102. [Google Scholar] [CrossRef]
- Kim, M.S.; Hyun, H.; Seo, K.S.; Cho, Y.H.; Khang, G.; Lee, H.B. Preparation and characterization of MPEG-PCL diblock copolymers with thermo-responsive sol-gel-sol phase transition. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5413–5423. [Google Scholar] [CrossRef]
- Kang, Y.M.; Lee, S.H.; Lee, J.Y.; Son, J.S.; Kim, B.S.; Lee, B.; Chun, H.J.; Min, B.H.; Kim, J.H.; Kim, M.S. A biodegradable, injectable, gel system based on MPEG-b-(PCL-ran-PLLA) diblock copolymers with an adjustable therapeutic window. Biomaterials 2010, 31, 2453–2834. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Kim, D.Y.; Kwon, D.Y.; Kang, H.J.; Kim, J.H.; Min, B.H.; Kim, M.S. An injectable biodegradable temperature-responsive gel with an adjustable persistence window. Biomaterials 2012, 33, 2823–2834. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Kim, M.S.; Jeong, S.C.; Kim, Y.H.; Lee, S.Y.; Khang, G.; Lee, H.B. Preparation of diblock copolymers consisting of methoxy poly(ethyleneglycol) and poly(ε-caprolactone)/poly(L-lactide) and their degradation property. Polym. Eng. Sci. 2006, 46, 1242–1249. [Google Scholar] [CrossRef]
- Kim, J.I.; Lee, S.H.; Kang, H.J.; Kwon, D.Y.; Kim, D.Y.; Kang, W.S.; Kim, J.H.; Kim, M.S. Examination of phase transition behavior of ion group functionalized MPEG-b-PCL diblock copolymers. Soft Matter 2011, 7, 8650–8656. [Google Scholar] [CrossRef]
- Kang, Y.M.; Kim, G.H.; Kim, J.I.; Kim, D.Y.; Lee, B.N.; Yoon, S.M.; Kim, J.H.; Kim, M.S. In vivo efficacy of an intratumorally injected in situ-forming doxorubicin/poly(ethylene glycol)-b-polycaprolactoned iblock copolymer. Biomaterials 2011, 32, 4556–4564. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, K.S.; Kang, Y.M.; Kim, E.S.; Hwang, S.J.; Lee, H.B.; Min, B.H.; Kim, J.H.; Kim, M.S. In vivo efficacy of paclitaxel-loaded injectable in situ-forming gel against subcutaneous tumor growth. Int. J. Pharm. 2010, 392, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Kim, Y.H.; Song, I.B.; Lee, J.W.; Kim, M.S.; Khang, G.; Park, K.; Lee, H.B. In vitro and in vivo release of albumin using a biodegradable MPEG-PCL diblock copolymer as an in situ gel-forming carrier. Biomacromolecules 2007, 8, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.H.; Kim, K.S.; Lee, J.H.; Lee, J.Y.; Lee, I.W.; Chun, H.J.; Kim, J.H.; Lee, H.B.; Kim, M.S. In vivo osteogenic differentiation of human adipose-derived stem cells in an injectable in situ-forming gel scaffold. Tissue Eng. Part A 2009, 15, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, S.K.; Kim, S.H.; Hyun, H.; Khang, G.; Lee, H.B. In vivo osteogenic differentiation of rat bone marrow stromal cells in thermosensitive MPEG-PCL diblock copolymer gel. Tissue Eng. 2006, 12, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Kim, Y.Y.; Oh, S.K.; Chung, S.G.; Ku, S.Y.; Kim, S.H.; Choi, Y.M.; Moon, S.Y. Alterations of proliferative and differentiation potentials of human embryonic stem cells during long-term culture. Exp. Mol. Med. 2008, 40, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.Y.; Ku, S.Y.; Kim, S.H.; Choi, Y.M.; Moon, S.Y. The effect of estrogen compounds on human embryoid bodies. Reprod. Sci. 2012, 20, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Ku, S.Y.; Jang, J.; Oh, S.K.; Kim, H.S.; Kim, S.H.; Choi, Y.M.; Moon, S.Y. Use of long-term cultured embryoid bodies may enhance cardiomyocyte differentiation by BMP2. Yonsei Med. J. 2008, 49, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Parsons, X.H. An engraftable human embryonic stem cell neuronal lineage-specific derivative retains embryonic chromatin plasticity for scale-up CNS regeneration. J. Regen. Med. Tissue Eng. 2012, 1, 1–12. [Google Scholar] [CrossRef]
- Kim, J.; Moon, S.H.; Lee, S.H.; Lee, D.R.; Chung, H.M. Isolation and in vivo study of human embryonic stem cells derived endothelial cells to investigate feasibility for vascular tissue regeneration. Tissue Eng. Regen. Med. 2006, 3, 451–456. [Google Scholar]
- Stenberg, J.; Elovsson, M.; Strehl, R.; Kilmare, E.; Hyllner, J.; Lindahl, A. Sustained embryoid body formation and culture in a non-laborious three dimensional culture system for human embryonic stem cells. Cytotechnology 2011, 63, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Kim, H.S.; Kim, H.S. Derivation and characterization of new human embryonic stem cell lines: SNUhES1, SNUhES2, and SNUhES3. Stem Cells 2005, 23, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Kim, H.S.; Kim, H.S. Methods for expansion of human embryonic stem cells. Stem Cells 2005, 23, 605–609. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, D.Y.; Kim, Y.Y.; Lee, H.B.; Moon, S.Y.; Ku, S.-Y.; Kim, M.S. In Vivo Osteogenic Differentiation of Human Embryoid Bodies in an Injectable in Situ-Forming Hydrogel. Materials 2013, 6, 2978-2988. https://doi.org/10.3390/ma6072978
Kim DY, Kim YY, Lee HB, Moon SY, Ku S-Y, Kim MS. In Vivo Osteogenic Differentiation of Human Embryoid Bodies in an Injectable in Situ-Forming Hydrogel. Materials. 2013; 6(7):2978-2988. https://doi.org/10.3390/ma6072978
Chicago/Turabian StyleKim, Da Yeon, Yoon Young Kim, Hai Bang Lee, Shin Yong Moon, Seung-Yup Ku, and Moon Suk Kim. 2013. "In Vivo Osteogenic Differentiation of Human Embryoid Bodies in an Injectable in Situ-Forming Hydrogel" Materials 6, no. 7: 2978-2988. https://doi.org/10.3390/ma6072978
APA StyleKim, D. Y., Kim, Y. Y., Lee, H. B., Moon, S. Y., Ku, S.-Y., & Kim, M. S. (2013). In Vivo Osteogenic Differentiation of Human Embryoid Bodies in an Injectable in Situ-Forming Hydrogel. Materials, 6(7), 2978-2988. https://doi.org/10.3390/ma6072978





