Pulmonary Inflammation of Well-Dispersed Multi-Wall Carbon Nanotubes Following Intratracheal Instillation: Toxicity by Fiber of 1–5 µm in Length
Abstract
:1. Introduction
2. Results
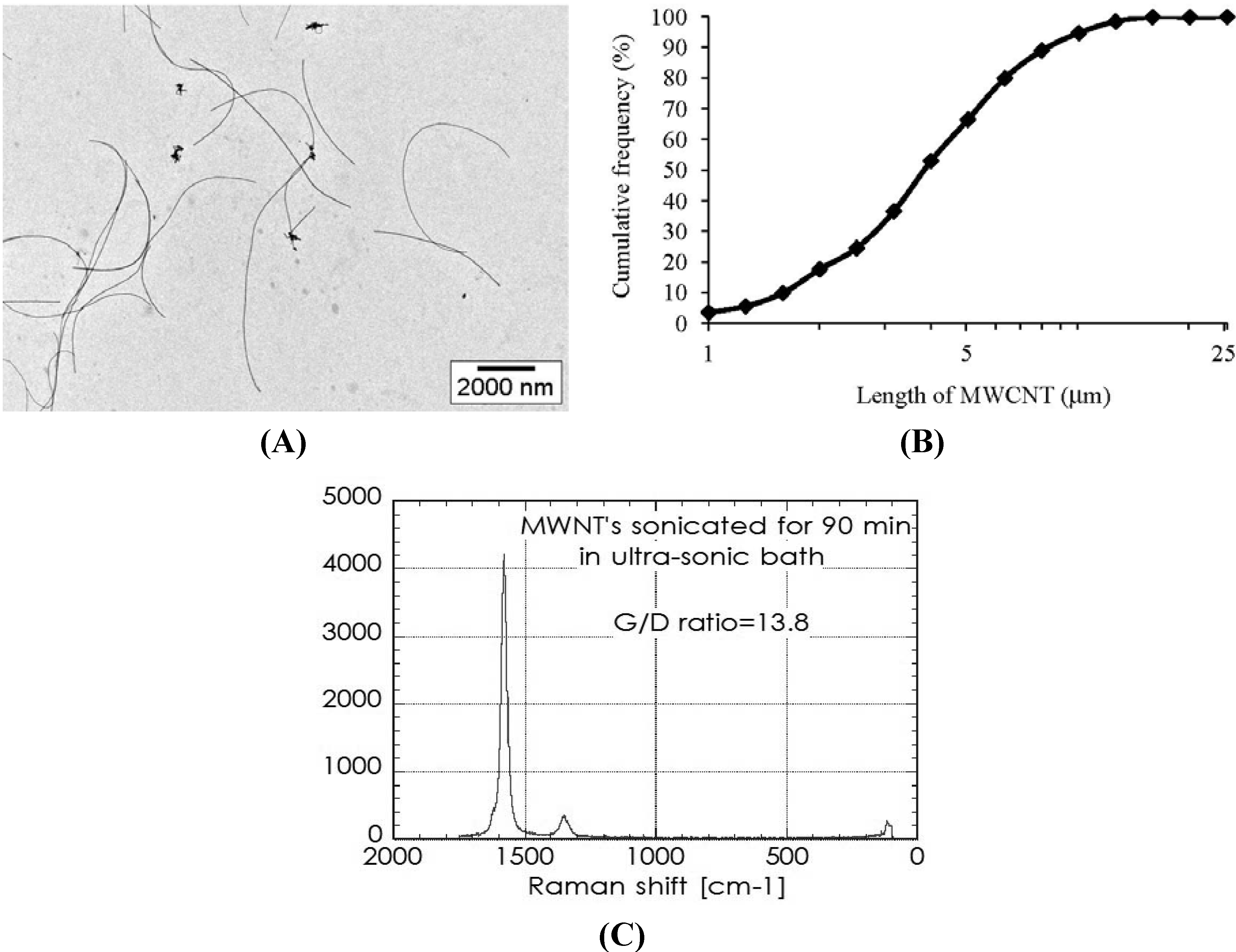
2.1. Characterization of MWCNT in the Dispersion
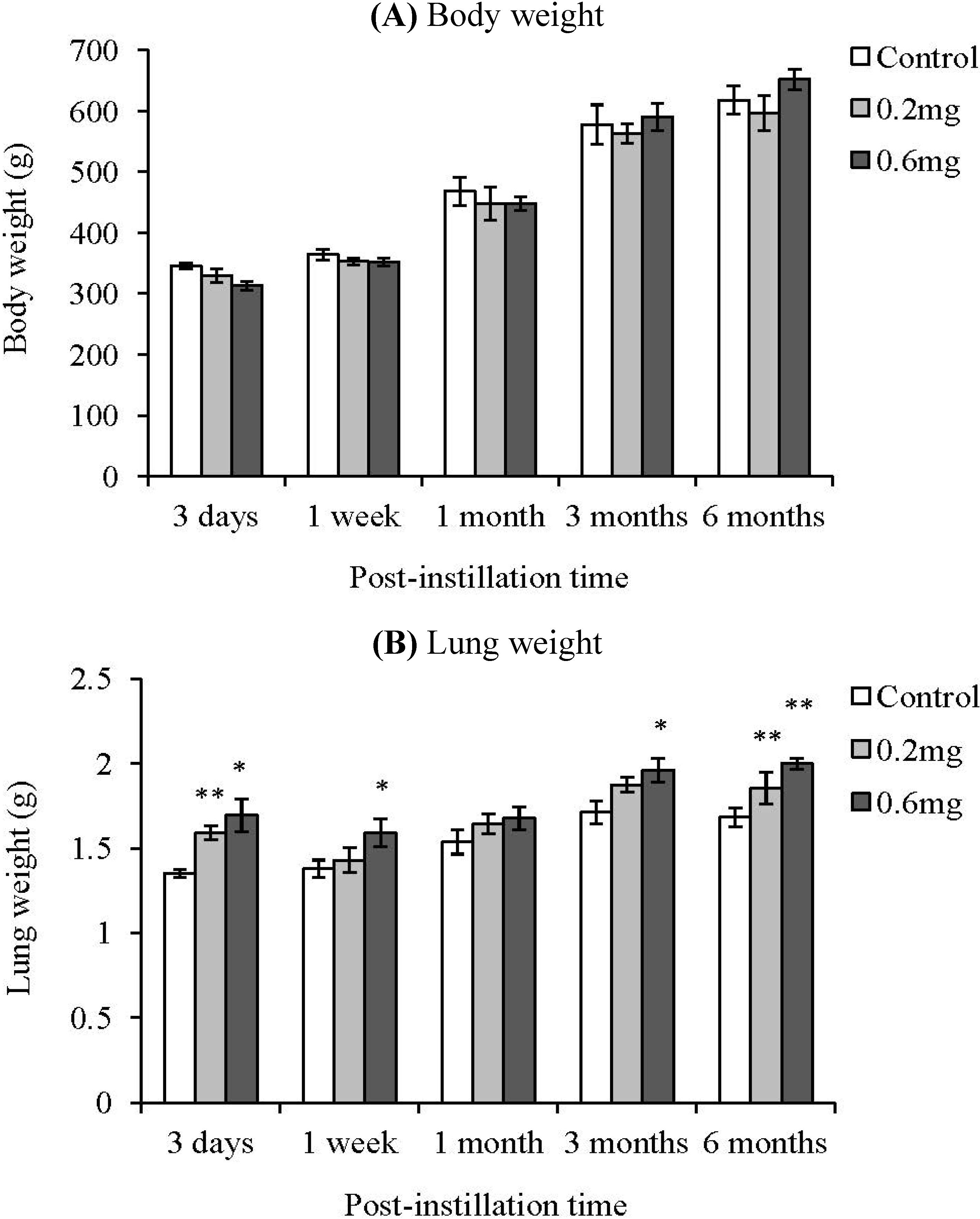
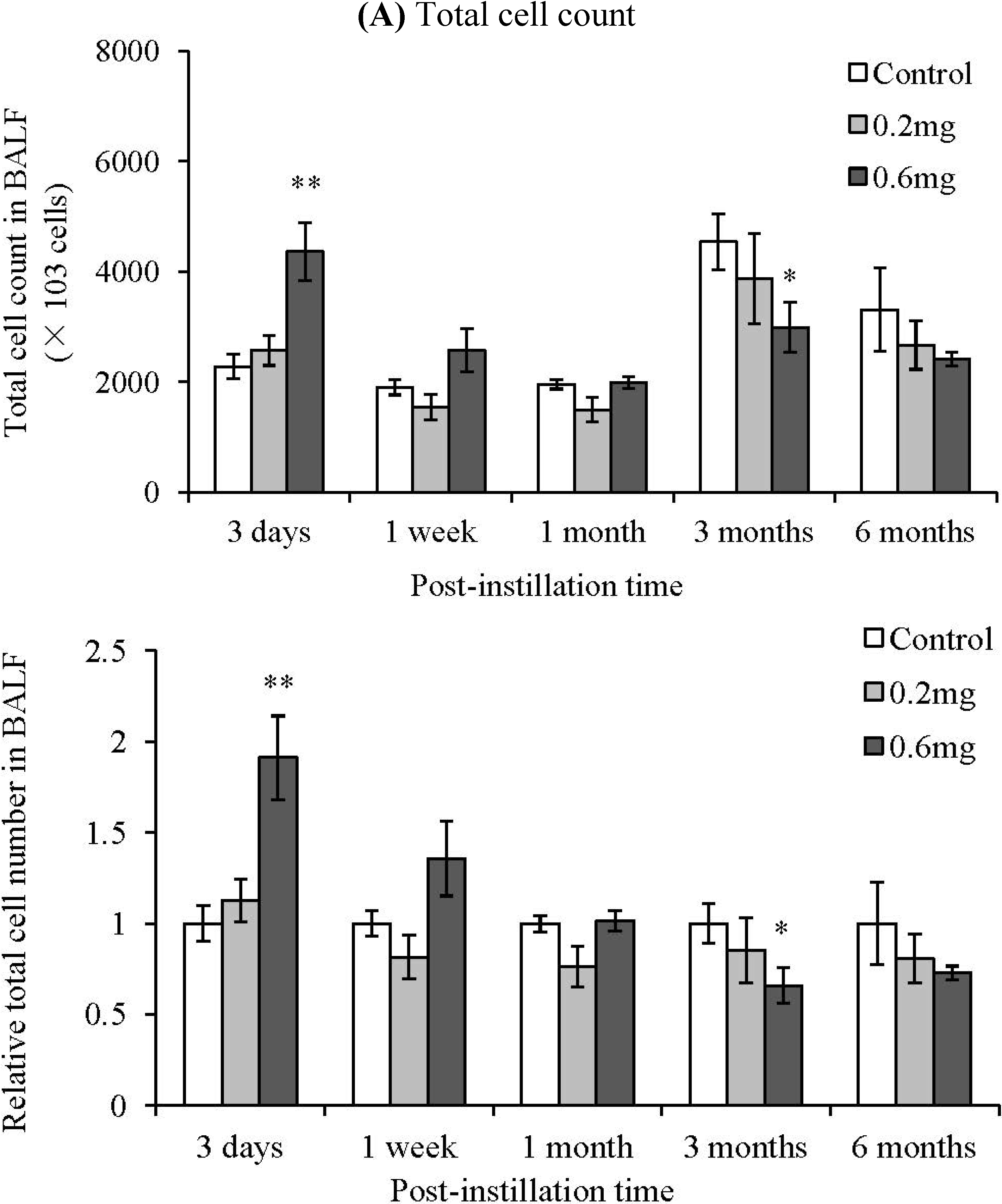
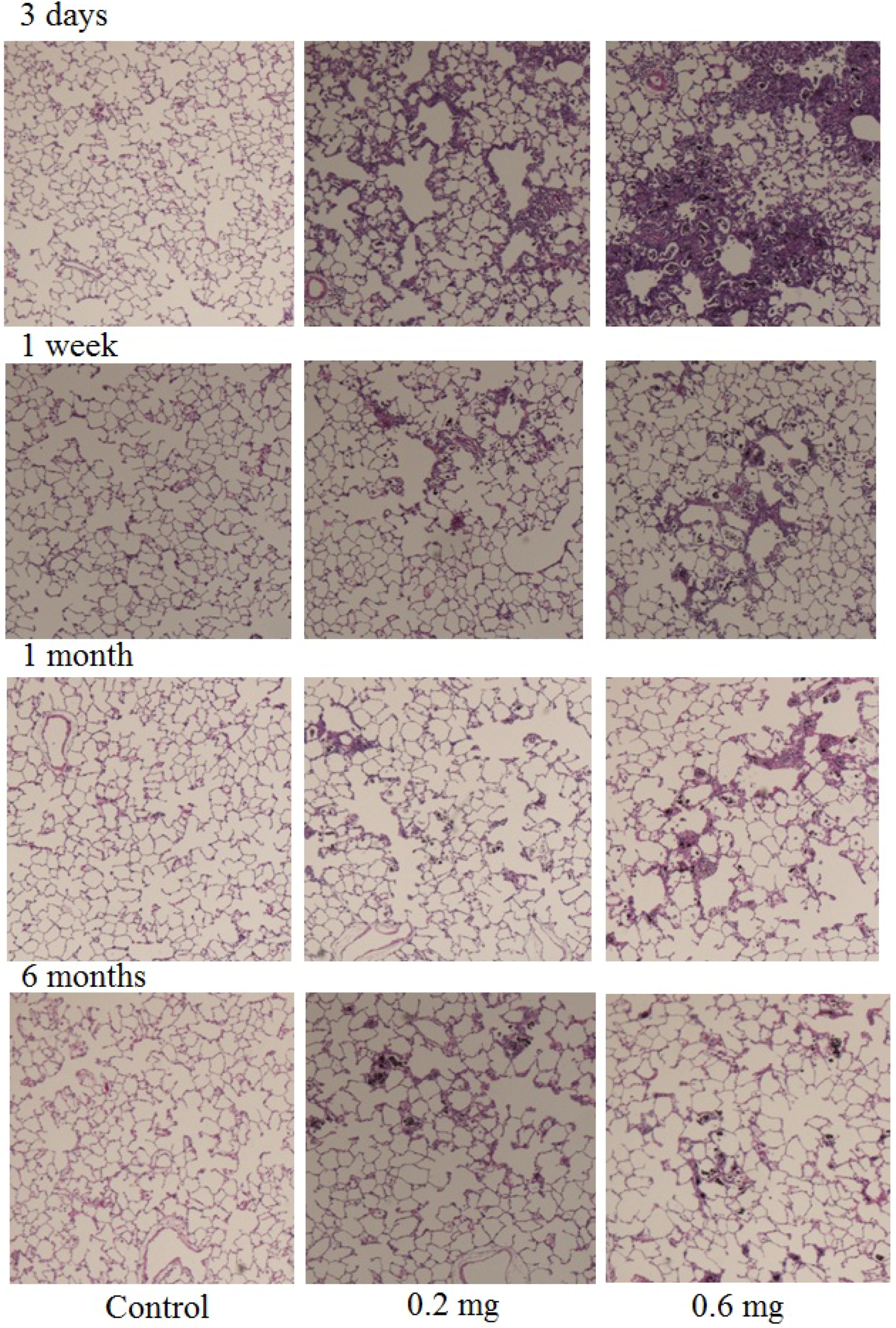
2.2. Analysis of Cells in BALF after Intratracheal Instillation

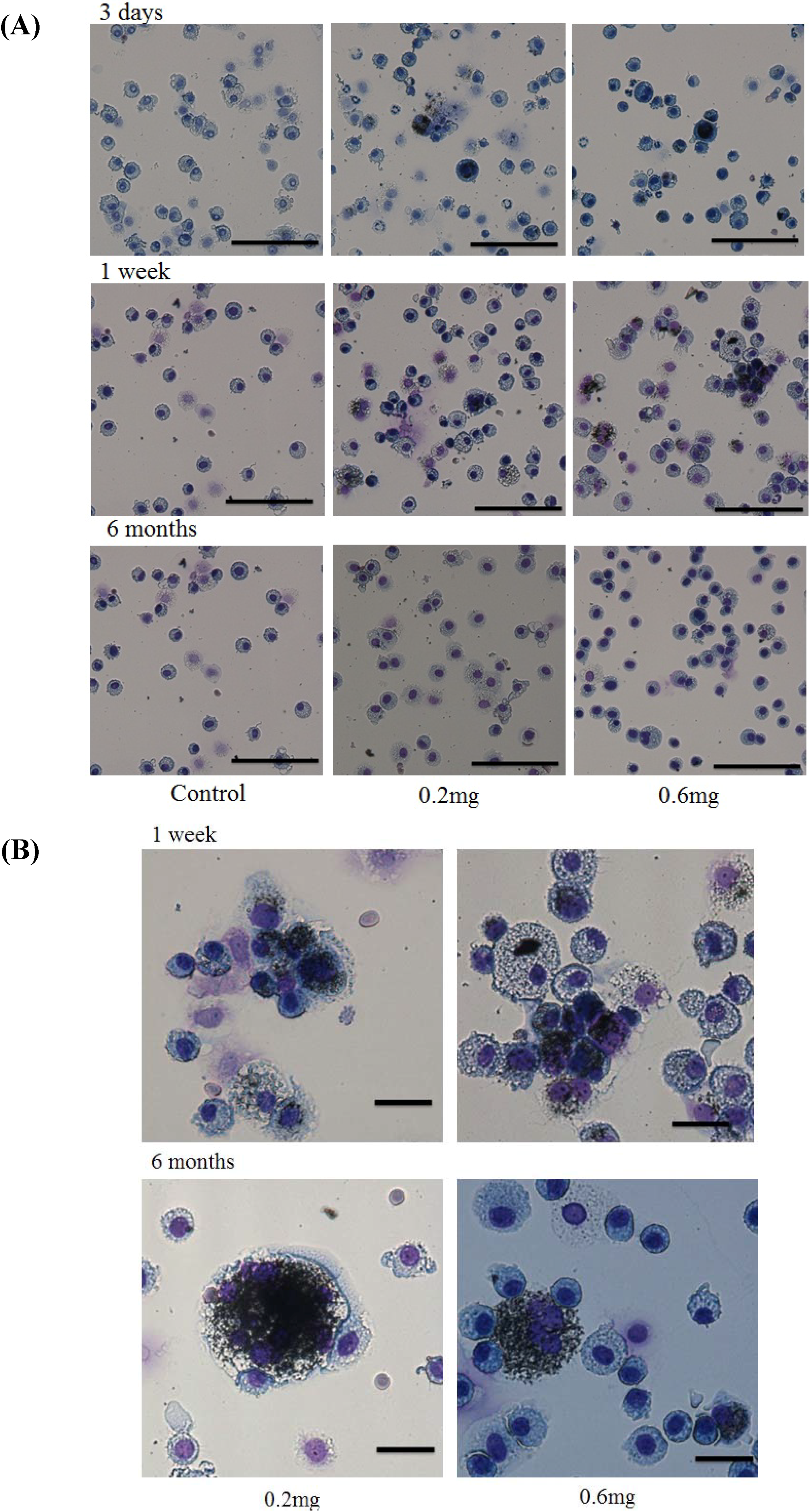
2.3. Uptake of CNT by Macrophages
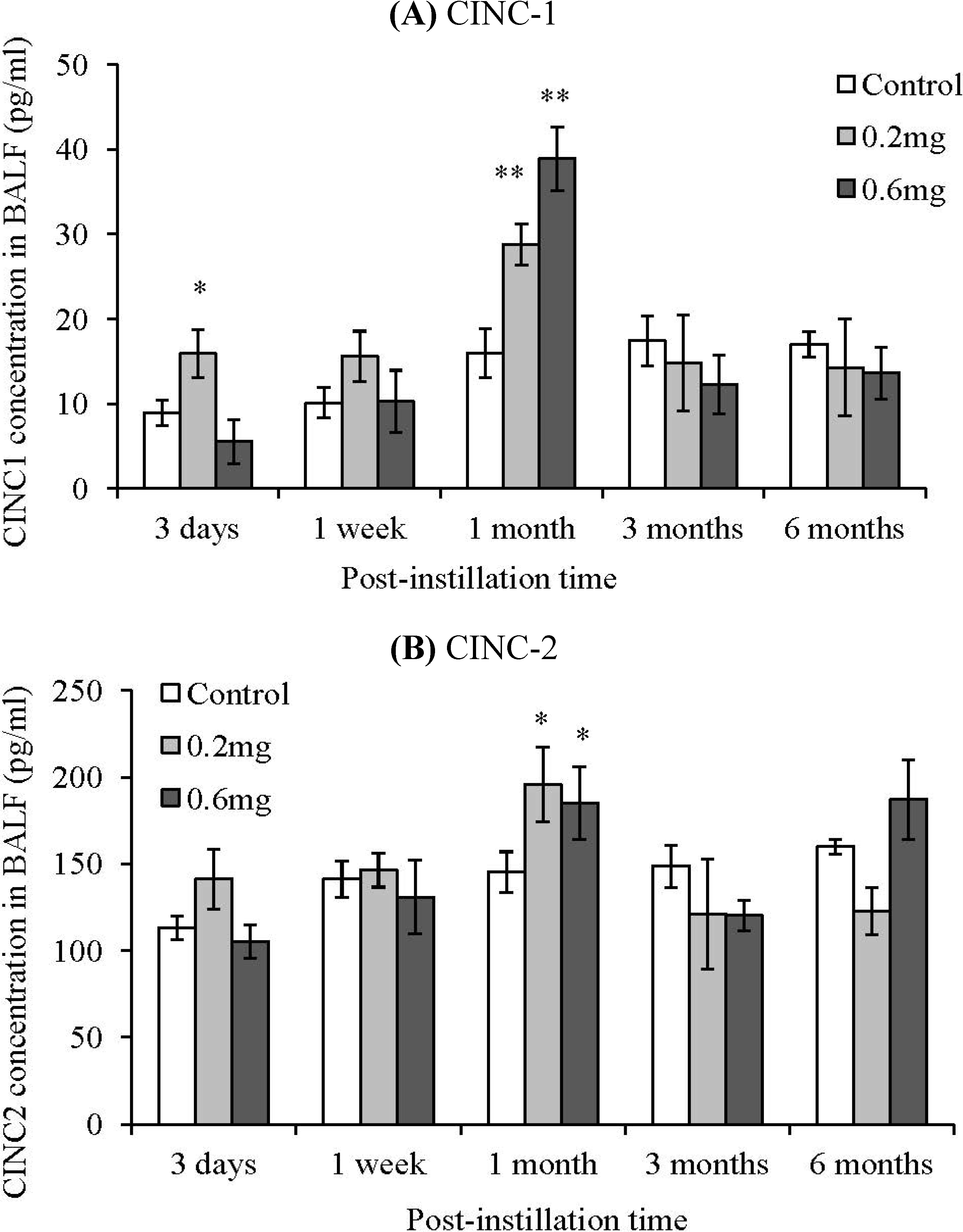
2.4. CINC Concentration in BALF
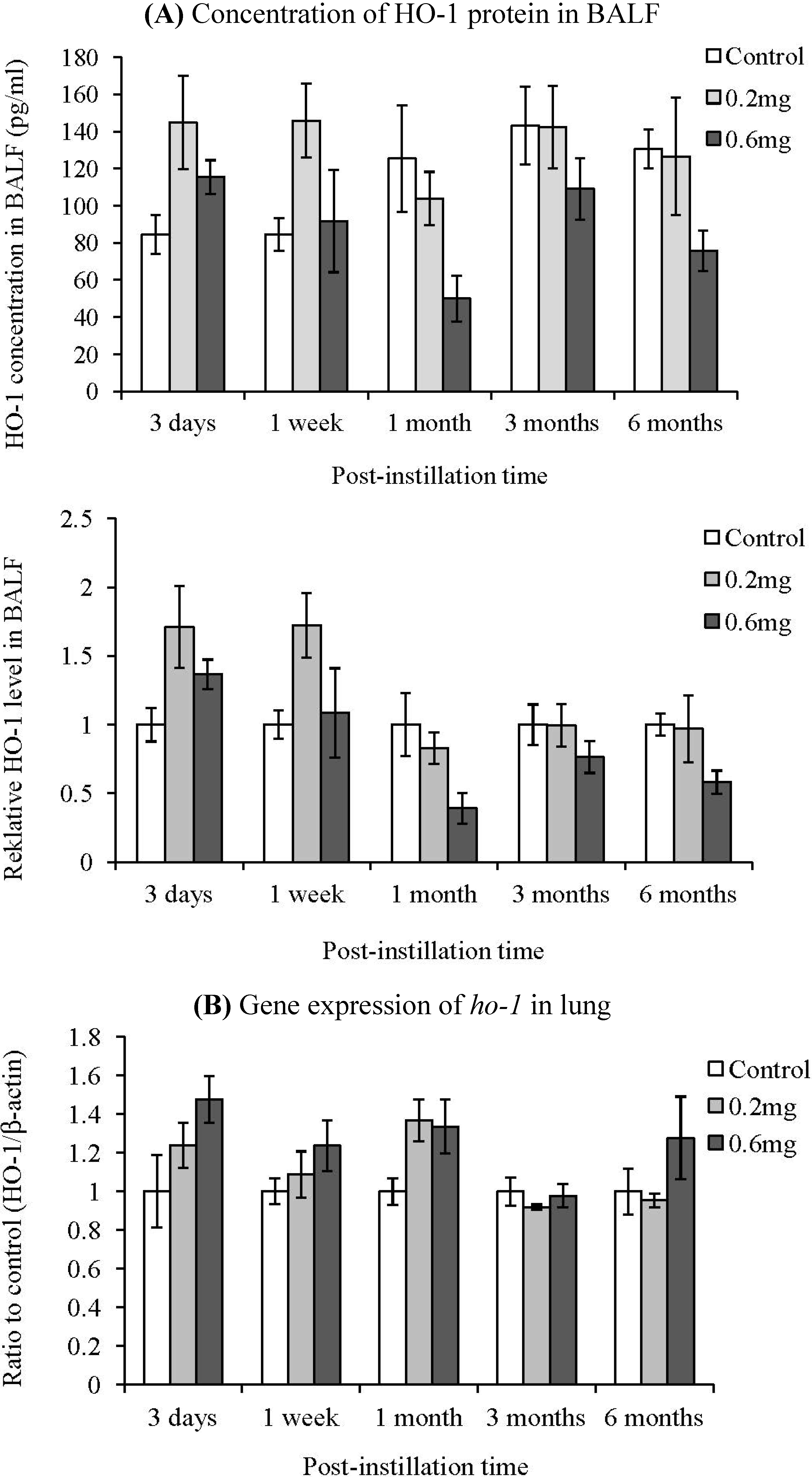
2.5. HO-1 Concentration in BALF
3. Experimental Section
3.1. MWCNTs
3.2. Animals
3.3. Preparation of MWCNT Dispersions
3.4. Characterization of MWCNT
3.5. Intratracheal Instillation of MWCNT
3.6. Observation of Cells in the BALF
3.7. Measurement of Chemokines and HO-1 in the BALF
3.8. Detection of Gene Expression of ho-1 in Lung
3.9. Tissue Preparation for Hematoxylin-Eosin Staining
3.10. Statistical Analysis
4. Discussion
Acknowledgment
References
- Morimoto, Y.; Kobayashi, N.; Shinohara, N.; Myojo, T.; Tanaka, I.; Nakanishi, J. Hazard assessments of manufactured nanomaterials. J. Occup. Health 2010, 52, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Horie, M.; Kobayashi, N.; Shinohara, N.; Shimada, M. Inhalation toxicity assessment of carbon-based nanoparticles. Acc. Chem. Res. 2012. In Press. [Google Scholar]
- Schinwald, A.; Murphy, F.A.; Prina-Mello, A.; Poland, C.A.; Byrne, F.; Movia, D.; Glass, J.R.; Dickerson, J.C.; Schultz, D.A.; Jeffree, C.E.; Macnee, W.; Donaldson, K. The threshold length for fiber-induced acute pleural inflammation: Shedding light on the early events in asbestos-induced mesothelioma. Toxicol. Sci. 2012, 128, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Donaldson, K. Length-dependent pleural inflammation and parietal pleural responses after deposition of carbon nanotubes in the pulmonary airspaces of mice. Nanotoxicology 2012. In Press. [Google Scholar]
- Liu, D.; Wang, L.; Wang, Z.; Cuschieri, A. Different cellular response mechanisms contribute to the length-dependent cytotoxicity of multi-walled carbon nanotubes. Nanoscale Res. Lett. 2012, 7, 361:1–361:10. [Google Scholar]
- Wang, J.; Sun, P.; Bao, Y.; Liu, J.; An, L. Cytotoxicity of single-walled carbon nanotubes on PC12 cells. Toxicol. In Vitro 2011, 25, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.; Seaton, A.; Stone, V.; Brown, S.; Macnee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Mühlfeld, C.; Poland, C.A.; Duffin, R.; Brandenberger, C.; Murphy, F.A.; Rothen-Rutishauser, B.; Gehr, P.; Donaldson, K. Differential effects of long and short carbon nanotubes on the gas-exchange region of the mouse lung. Nanotoxicology 2012, 6, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Manshian, B.B.; Jenkins, G.J.; Williams, P.M.; Wright, C.; Barron, A.R.; Brown, A.P.; Hondow, N.; Dunstan, P.R.; Rickman, R.; Brady, K.; Doak, S.H. Single-walled carbon nanotubes: Differential genotoxic potential associated with physico-chemical properties. Nanotoxicology 2012, in press. [Google Scholar]
- Fenoglio, I.; Aldieri, E.; Gazzano, E.; Cesano, F.; Colonna, M.; Scarano, D.; Mazzucco, G.; Attanasio, A.; Yakoub, Y.; Lison, D.; Fubini, B. Thickness of multiwalled carbon nanotubes affects their lung toxicity. Chem. Res. Toxicol. 2012, 25, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Hirohashi, M.; Ogami, A.; Oyabu, T.; Myojo, T.; Todoroki, M.; Yamamoto, M.; Hashiba, M.; Mizuguchi, Y.; Lee, B.W.; Kuroda, E.; Shimada, M.; Wang, W.N.; Yamamoto, K.; Fujita, K.; Endoh, S.; Uchida, K.; Kobayashi, N.; Mizuno, K.; Inada, M.; Tao, H.; Nakazato, T.; Nakanishi, J.; Tanaka, I. Pulmonary toxicity of well-dispersed multi-wall carbon nanotubes following inhalation and intratracheal instillation. Nanotoxicology 2012, 6, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7, 5:1–5:17. [Google Scholar] [CrossRef]
- Mercer, R.R.; Hubbs, A.F.; Scabilloni, J.F.; Wang, L.; Battelli, L.A.; Schwegler-Berry, D.; Castranova, V.; Porter, D.W. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part. Fibre Toxicol. 2010, 7, 28:1–28:11. [Google Scholar] [CrossRef]
- Bermudez, E.; Mangum, J.B.; Wong, B.A.; Asgharian, B.; Hext, P.M.; Warheit, D.B.; Everitt, J.I. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol. Sci. 2004, 77, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.R.; Scabilloni, J.; Wang, L.; Kisin, E.; Murray, A.R.; Schwegler-Berry, D.; Shvedova, A.A.; Castranova, V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L87–L97. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Sundaram, S.K.; Teeguarden, J.G.; Riley, B.J.; Fifield, L.S.; Jacobs, J.M.; Addleman, S.R.; Kaysen, G.A.; Moudgil, B.M.; Weber, T.J. Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxicol. Sci. 2007, 100, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Seki, T.; Furuya, M.; Ohara, N. Acute functional enhancement of circulatory neutrophils after intratracheal instillation with diesel exhaust particles in rats. Inhal. Toxicol. 2005, 17, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Yasuhara, T.; Ishiyama, S.; Kawashima, H.; Miyasaka, M.; Miyazaki, T. The role of leukocytes during acute phase inflammation in crystalline silica-induced lung injury. Exp. Lung Res. 2001, 27, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Kyono, H.; Kohyama, N.; Otaki, N.; Serita, F.; Toya, T.; Kagawa, J. Acute biological effects of intratracheally instilled titanium dioxide whiskers compared with nonfibrous titanium dioxide and amosite in rats. Inhal. Toxicol. 1999, 11, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Huvenne, W.; Pérez-Novo, C.A.; Derycke, L.; De Ruyck, N.; Krysko, O.; Maes, T.; Pauwels, N.; Robays, L.; Bracke, K.R.; Joos, G.; Brusselle, G.; Bachert, C. Different regulation of cigarette smoke induced inflammation in upper versus lower airways. Respir. Res. 2010, 11, 100:1–100:9. [Google Scholar] [CrossRef]
- Wu, M.L.; Ho, Y.C.; Lin, C.Y.; Yet, S.F. Heme oxygenase-1 in inflammation and cardiovascular disease. Am. J. Cardiovasc. Dis. 2011, 1, 150–158. [Google Scholar] [PubMed]
- Wu, M.L.; Layne, M.D.; Yet, S.F. Heme oxygenase-1 in environmental toxin-induced lung disease. Toxicol. Mech. Methods 2012, 22, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Fukui, H.; Endoh, S.; Maru, J.; Miyauchi, A.; Shichiri, M.; Fujita, K.; Niki, E.; Hagihara, Y.; Yoshida, Y.; Morimoto, Y.; Iwahashi, H. Comparison of acute oxidative stress on rat lung induced by nano and fine-scale, soluble and insoluble metal oxide particles: NiO and TiO2. Inhal. Toxicol. 2012, 24, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Sellamuthu, R.; Umbright, C.; Roberts, J.R.; Cumpston, A.; McKinney, W.; Chen, B.T.; Frazer, D.; Li, S.; Kashon, M.; Joseph, P. Molecular insights into the progression of crystalline silica-induced pulmonary toxicity in rats. J. Appl. Toxicol. 2012. [Google Scholar] [CrossRef]
- He, X.; Young, S.H.; Schwegler-Berry, D.; Chisholm, W.P.; Fernback, J.E.; Ma, Q. Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-κB signaling, and promoting fibroblast-to-myofibroblast transformation. Chem. Res. Toxicol. 2011, 12, 2237–2248. [Google Scholar] [CrossRef]
- Fukui, H.; Horie, M.; Endoh, S.; Kato, H.; Fujita, K.; Nishio, K.; Komaba, L.K.; Maru, J.; Miyauhi, A.; Nakamura, A.; Kinugasa, S.; Yoshida, Y.; Hagihara, Y.; Iwahashi, H. Association of zinc ion release and oxidative stress induced by intratracheal instillation of ZnO nanoparticles to rat lung. Chem. Biol. Interact. 2012, 198, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, H.; Morimoto, Y.; Oyabu, T.; Hirohashi, M.; Ogami, A.; Yamato, H.; Kuroda, K.; Higashi, T.; Tanaka, I. Expression of heme oxygenase-1 in the lungs of rats exposed to crocidolite asbestos. Inhal. Toxicol. 2005, 17, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takeno, M.; Honma, K.; Yamauchi, H.; Saito, Y.; Sasaki, T.; Morikubo, H.; Nagashima, Y.; Takagi, S.; Yamanaka, K.; Kaneko, T.; Ishigatsubo, Y. Heme oxygenase-1, a potential biomarker of chronic silicosis, attenuates silica-induced lung injury. Am. J. Respir. Crit. Care Med. 2006, 174, 906–914. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Horie, M.; Stowe, M.; Kambara, T.; Lee, B.W.; Endoh, S.; Maru, J.; Oyabu, T.; Myojo, T.; Ogami, A.; Uchida, K.; et al. Pulmonary Inflammation of Well-Dispersed Multi-Wall Carbon Nanotubes Following Intratracheal Instillation: Toxicity by Fiber of 1–5 µm in Length. Materials 2012, 5, 2833-2849. https://doi.org/10.3390/ma5122833
Horie M, Stowe M, Kambara T, Lee BW, Endoh S, Maru J, Oyabu T, Myojo T, Ogami A, Uchida K, et al. Pulmonary Inflammation of Well-Dispersed Multi-Wall Carbon Nanotubes Following Intratracheal Instillation: Toxicity by Fiber of 1–5 µm in Length. Materials. 2012; 5(12):2833-2849. https://doi.org/10.3390/ma5122833
Chicago/Turabian StyleHorie, Masanori, Mayumi Stowe, Tatsunori Kambara, Byeong Woo Lee, Shigehisa Endoh, Junko Maru, Takako Oyabu, Toshihiko Myojo, Akira Ogami, Kunio Uchida, and et al. 2012. "Pulmonary Inflammation of Well-Dispersed Multi-Wall Carbon Nanotubes Following Intratracheal Instillation: Toxicity by Fiber of 1–5 µm in Length" Materials 5, no. 12: 2833-2849. https://doi.org/10.3390/ma5122833
APA StyleHorie, M., Stowe, M., Kambara, T., Lee, B. W., Endoh, S., Maru, J., Oyabu, T., Myojo, T., Ogami, A., Uchida, K., Yamamoto, K., Kobayashi, N., Kuroda, E., Nakazato, T., & Morimoto, Y. (2012). Pulmonary Inflammation of Well-Dispersed Multi-Wall Carbon Nanotubes Following Intratracheal Instillation: Toxicity by Fiber of 1–5 µm in Length. Materials, 5(12), 2833-2849. https://doi.org/10.3390/ma5122833






