Polyamide 6 Nanocomposites with Inorganic Particles Modified with Three Quaternary Ammonium Salts
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methodology
2.2.1. Preparation of Organoclay
2.2.2. Nanocomposites Preparation
2.2.3. Characterization
X-Ray Fluorescence (XRF)
X-Ray Diffraction (XRD)
Thermogravimetry (TG)
Transmission Electron Microscope (TEM)
Mechanical Properties
3. Results
3.1. X-Ray Fluorescence (XFR)
| Oxides | MMT (%) | OMMT-Cet (%) | OMMT-Gen (%) | OMMT-Dod (%) |
|---|---|---|---|---|
| SiO2 | 62.85 | 61.72 | 63.55 | 62.69 |
| Al2O3 | 18.02 | 21.19 | 18.66 | 26.77 |
| Fe2O3 | 11.25 | 9.88 | 11.91 | 7.30 |
| MgO | 2.06 | 2.91 | 1.60 | - |
| CaO | 1.78 | - | 0.96 | 0.90 |
| TiO2 | 1.22 | 1.00 | 1.28 | 0.77 |
| Na2O | 1.12 | - | - | - |
| K2O | 0.85 | 0.58 | 0.84 | 0.68 |
| Cl | - | - | 0.76 | 0.33 |
| Br | - | 1.07 | - | - |
| others | 0,46 | 0.63 | 0.44 | 0.56 |
3.2. X-Ray Diffraction (XRD)
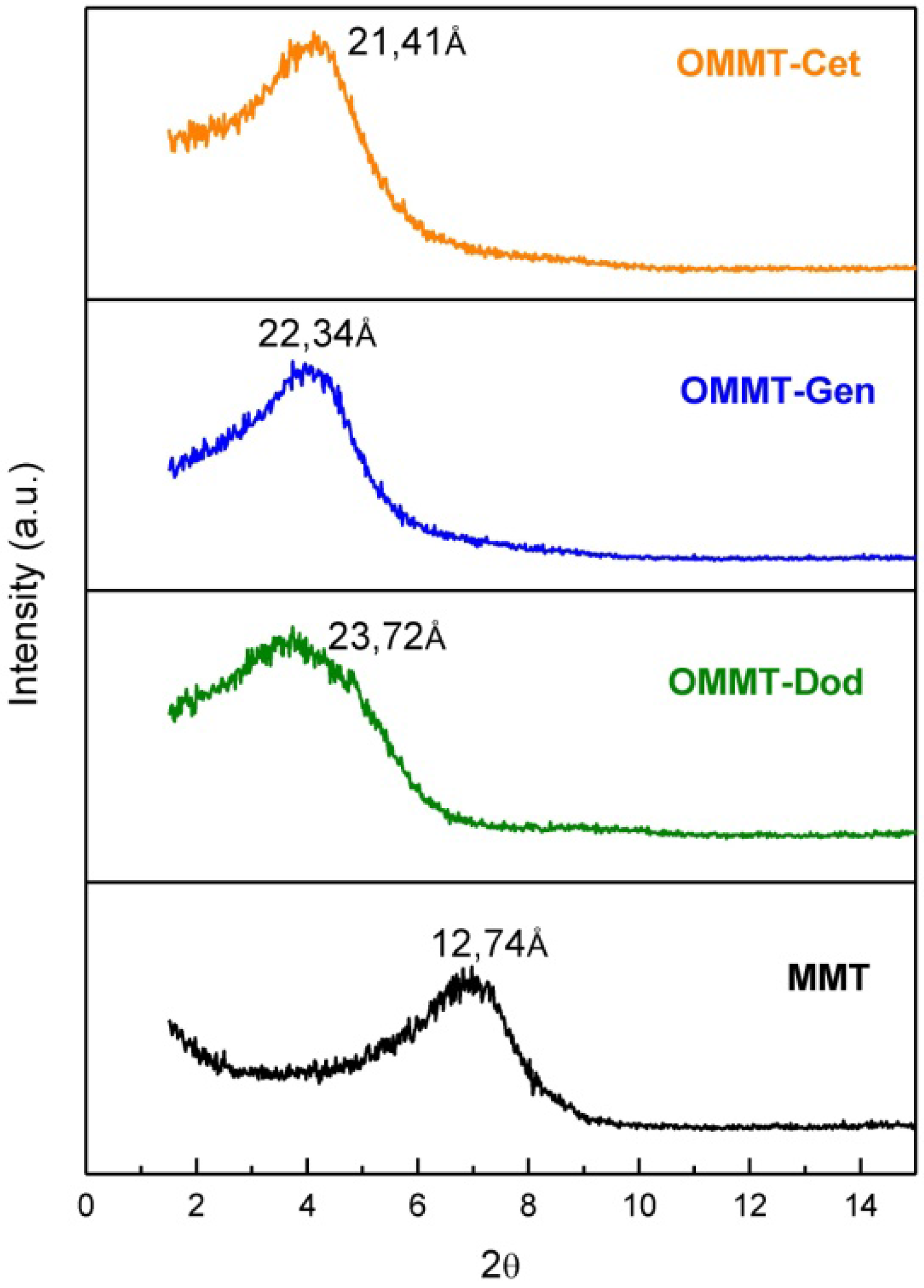
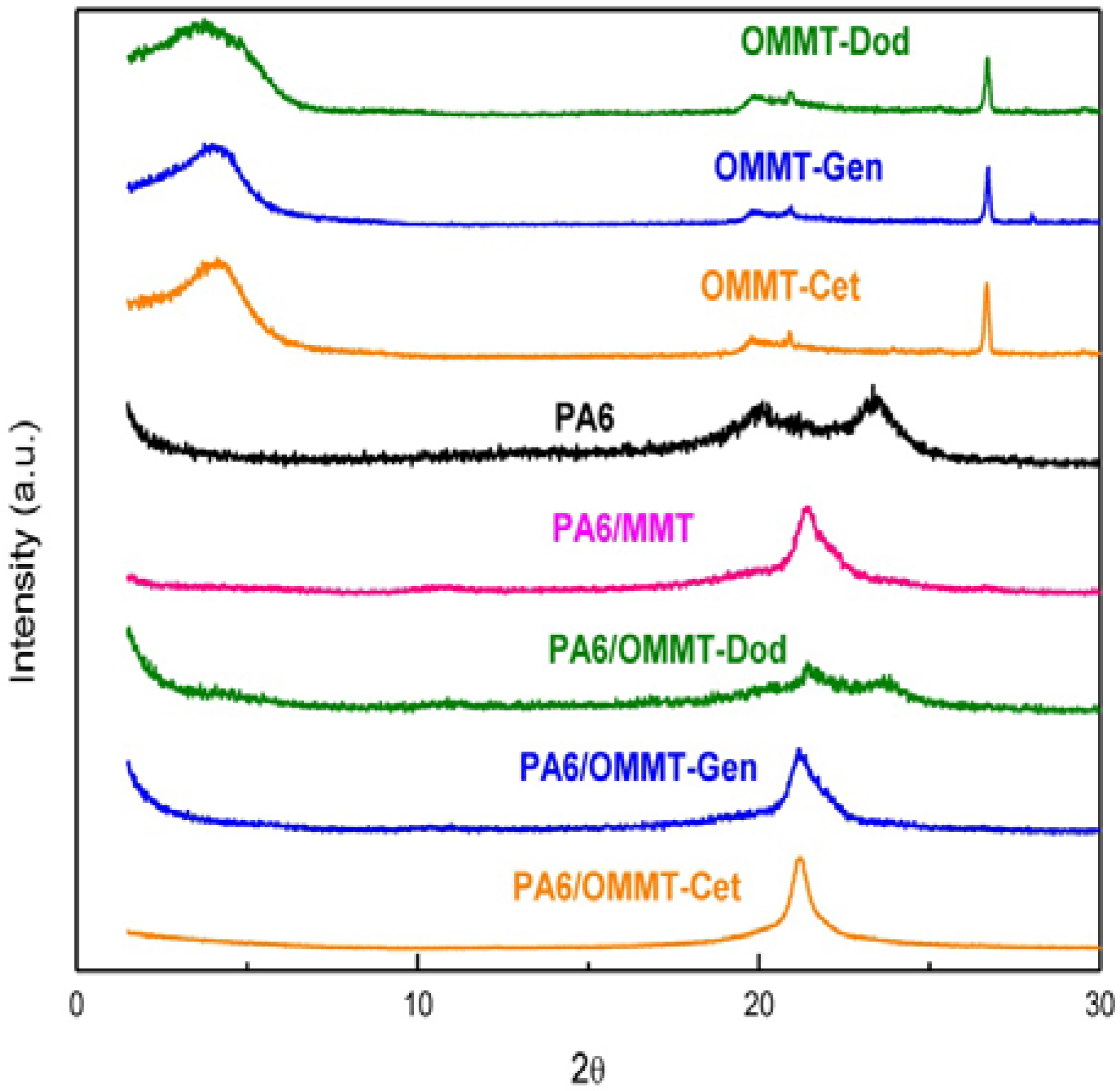
3.3. Transmission Electron Microscope (TEM)
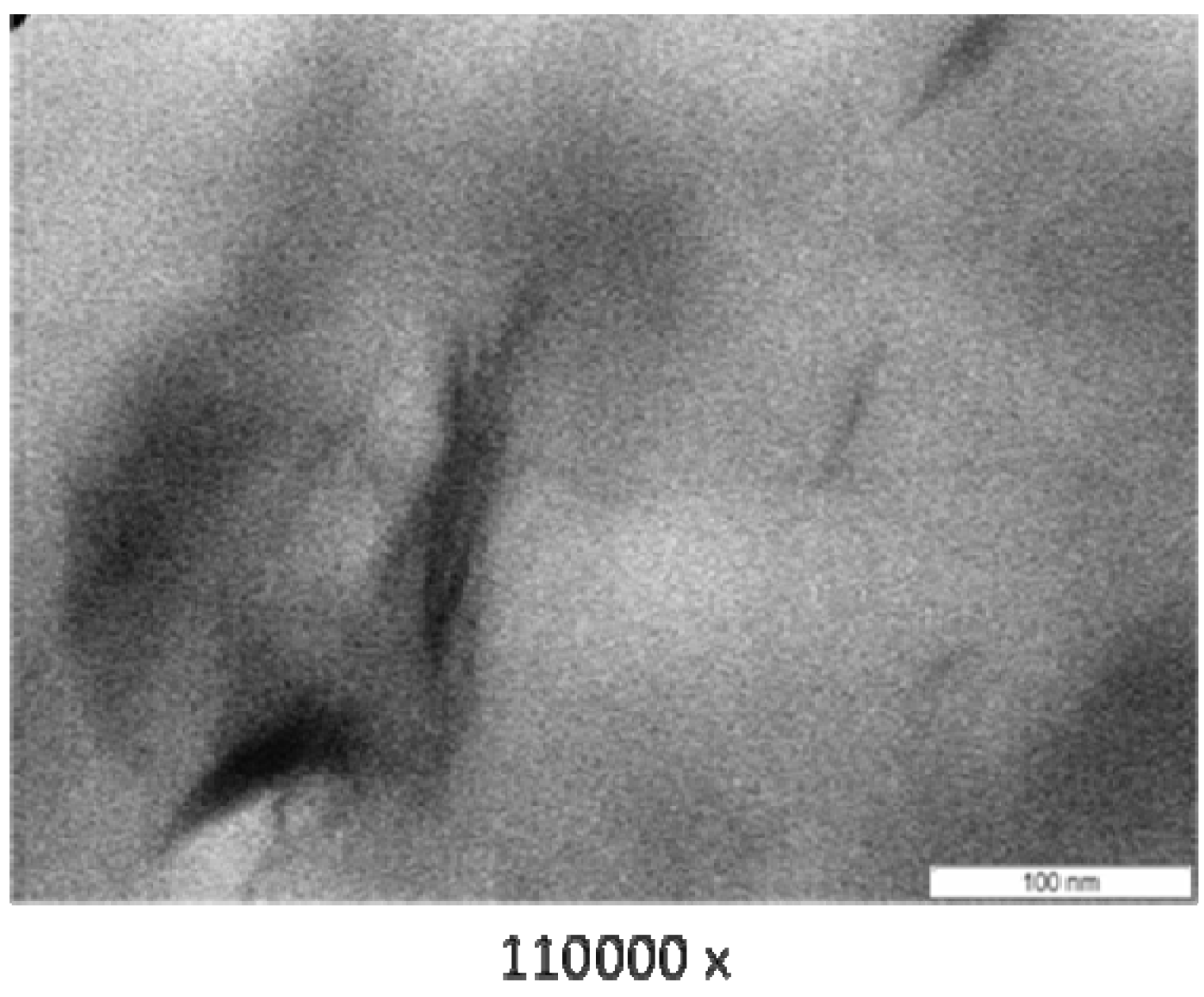


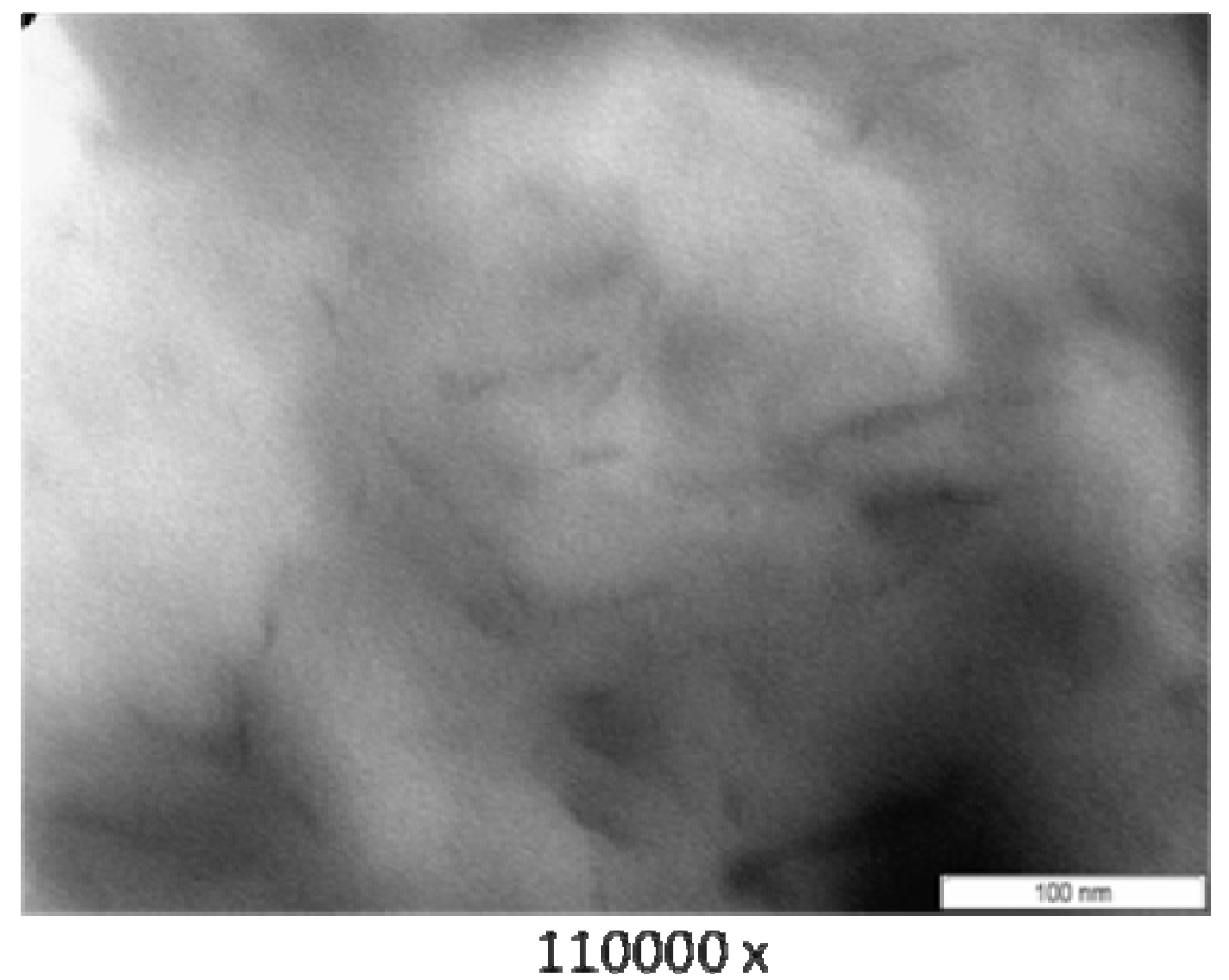
3.4. Thermogravimetry (TG)
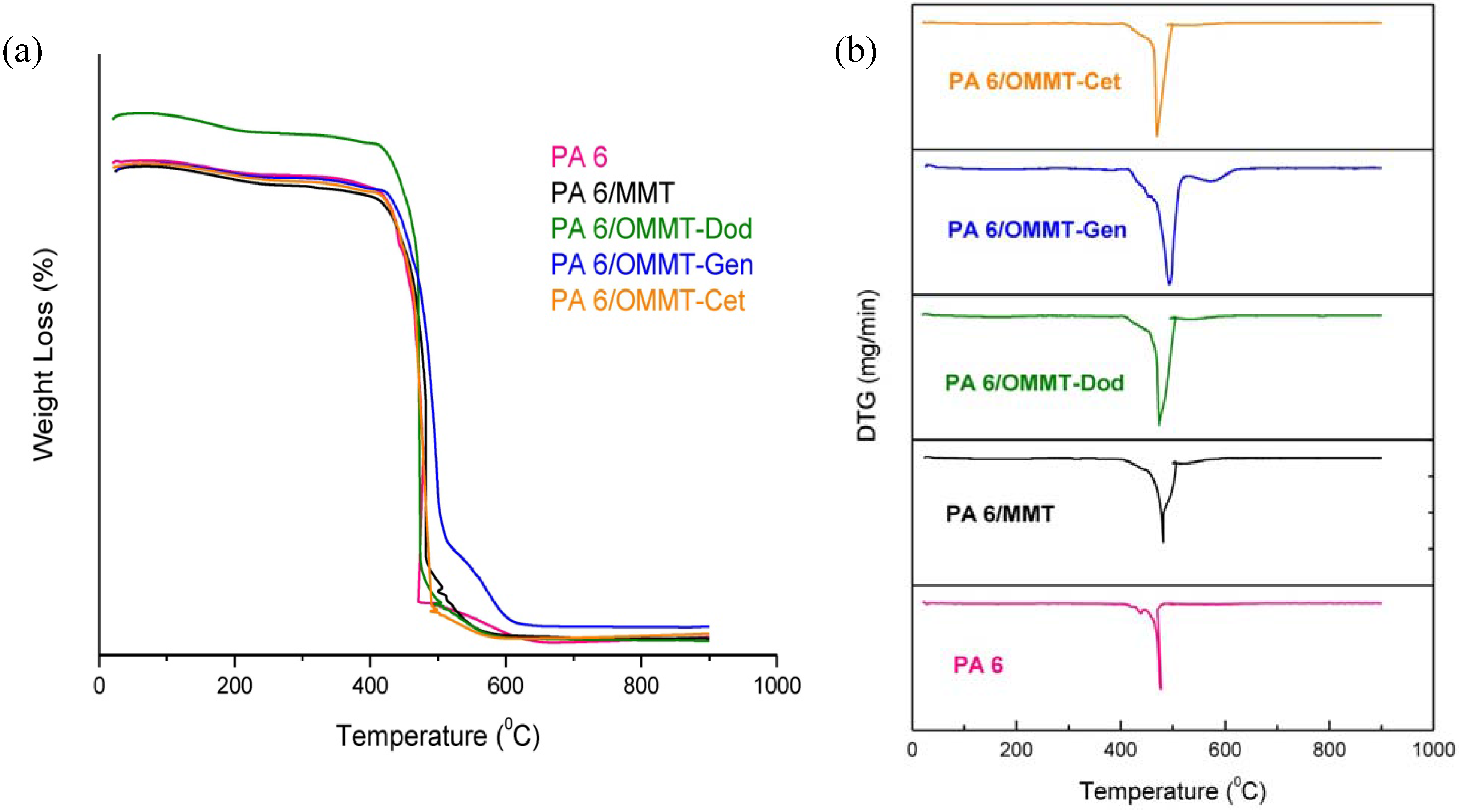
3.5. Mechanical Properties
| Specimens | Tensile Modulus (GPa) | Yield Strength (MPa) | Elongation at Yield (%) | Izod Impact Strength (J/m) |
|---|---|---|---|---|
| Pure PA6 | 2.15 ± 0.09 | 64.97 ± 0.56 | 16.06 ± 0.65 | 45.78 ± 1.6 |
| PA6/MMT | 2.40 ± 0.04 | 68.52 ± 0.90 | 11.54 ± 0.41 | 44.33 ± 1.0 |
| PA6/OMMT-Dod | 2.71 ± 0.13 | 71.07 ± 1.1 | 12.66 ± 0.07 | 28.17 ± 1.41 |
| PA6/OMMT-Gen | 2.48 ± 0.24 | 71.18 ± 0.28 | 11.03 ± 0.71 | 34.65 ± 1.91 |
| PA6/OMMT-Cet | 2.42 ± 0.13 | 69.70 ± 0.49 | 12.98 ± 0.44 | 48.98 ± 4.27 |
4. Conclusions
Acknowledgments
References
- Varlot, K.; Reynaud, E.; Kloppfer, M.H.; Vigier, G.; Varlet, J. Clay-reinforced polyamide: Preferential orientation of the montmorillonite sheets and the polyamide crystalline lamellae. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1360–1370. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer-layered silicate nanocomposite: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Cho, J.W.; Paul, D.R. Nylon 6 nanocomposites by melt compounding. Polymer 2001, 42, 1083–1094. [Google Scholar] [CrossRef]
- Liu, T.; Tjiu, W.C.; He, C.; Na, S.S.; Chung, T.S. A processing-induced clay dispersion and its effect on the structure and properties of polyamide 6. Polym. Int. 2004, 53, 392–399. [Google Scholar] [CrossRef]
- Bilotti, E.; Zhang, R.; Deng, H.; Quero, F.; Fischer, H.R.; Peijs, T. Sepiolite needle-like clay for PA6 nanocomposites: An alternative to layered silicates? Compos. Sci. Technol. 2009, 69, 2587–2595. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon-6-clay hybrid. J. Mater. Res. 1993, 6, 1185–1189. [Google Scholar] [CrossRef]
- Dubois, A.L. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Gloaguen, J.M.; Lefebvre, J.M. Plastic deformation behaviour of thermoplastic/clay nanocomposites. Polymer. 2001, 42, 5841–5847. [Google Scholar] [CrossRef]
- Fornes, T.D.; Paul, D.R. Modelling properties of nylon6/clay nanocomposites using composite theories. Polymer 2003, 44, 4993–5013. [Google Scholar] [CrossRef]
- Wang, N.; Qinghong, F.; Yawei, S.; Zhang, J. Microstructure and properties of polypropylene composites filled with coincorporation of MCM-41(with template) and OMMT nanoparticles prepared by melt-compounding. Mater. Sci. Eng. A 2009, 512, 32–38. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G. Non-isothermal crystallization kinetics of polyamide-6/graphite oxeide nanocompósitos. Thermochim. Acta 2009, 500, 13–20. [Google Scholar] [CrossRef]
- Pinnavaia, T.J.; Beall, G.W. Polymer-Clay Nanocomposites; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Fornes, T.D.; Hunter, D.L.; Paul, D.R. Effect of sodium montmorillonite source on nylon 6/clay nanocompositos. Polymer 2004, 45, 2321–2331. [Google Scholar] [CrossRef]
- Liu, L.M.; Qi, Z.N.; Zhu, X.G. Studies on nylon 6 clay nanocompósitos by melt-intercalation process. J. Appl. Polym Sci. 1999, 71, 1133–1138. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D.; Pfaender, R. Influence of accelerated aging on clay-reinforced polyamide 6. J. Polym. Degrad. Stab. 2009, 94, 389–396. [Google Scholar] [CrossRef]
- Calderon, J.U.; Lennox, B.; Kamal, M.R. Thermally stable phosphonium-montmorillonite organoclays. Appl. Clay Sci. 2008, 40, 90–98. [Google Scholar] [CrossRef]
- He, H.; Ding, Z.; Zhu, J.; Yuan, P.; Xi, Y.; Yang, D.; Frost, R.L. Thermal characterization of surfactant-modified montmorillonites. Clays Clay Miner. 2005, 53, 287–293. [Google Scholar] [CrossRef]
- Hedley, C.B.; Yuan, G.; Teng, B.K.G. Thermal analysis of montmorillonites modified with quaternary phosphonium and ammonium surfactants. Appl. Clay Sci. 2007, 35, 180–188. [Google Scholar] [CrossRef]
- Leite, A.M.D.; Maia, L.F.; Paz, R.A.; Araujo, E.M.; Lira, H.L. Thermal properties from membrane of polyamide 6/montmorillonite clay nanocomposites obtained by immersion precipitation method. J. Therm. Anal. Calorim. 2009, 97, 577–580. [Google Scholar] [CrossRef]
- Araujo, E.M.; Barbosa, R.; Oliveira, A.D.; Morais, C.R.S.; Melo, T.J.A.; Souza, G.A. Thermal and mechanical properties of PE/organoclay nanocompósitos. J. Therm. Anal. Calorim. 2007, 87, 811–814. [Google Scholar] [CrossRef]
- Araújo, E.M.; Barbosa, R.; Rodrigues, A.W.B.; Melo, T.J.A.; Ito, E.N. Processing and characterization of polyethylene/Brazilian clay nanocomposites. Mater. Sci. Eng. A 2007, 445–446, 141–147. [Google Scholar] [CrossRef]
- Kozaka, M.; Domka, L. Adsorption of the quaternary ammonium salts on montmorillonite. J. Phys. Chem. Solids. 2004, 65, 441–445. [Google Scholar] [CrossRef]
- Kwolek, T.; Hodorowicz, M.; Stadnicka, K.; Czapkiewicz, J.; Kwolek, T. Adsorption isotherms of homologous alkyldimethylbenzylammonium bromides on sodium montmorillonite. J. Colloid Interface Sci. 2003, 264, 14–19. [Google Scholar] [CrossRef]
- Khanna, Y.P.; Khun, W.P. Measurement of crystalline index in nylons by DSC: Complexities and recommendations. J. Polym. Sci. 1997, 35, 2219–2231. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Araujo, E.M.; Leite, A.M.D.; Paz, R.A.d.; Medeiros, V.d.N.; Melo, T.J.A.d.; Lira, H.d.L. Polyamide 6 Nanocomposites with Inorganic Particles Modified with Three Quaternary Ammonium Salts. Materials 2011, 4, 1956-1966. https://doi.org/10.3390/ma4111956
Araujo EM, Leite AMD, Paz RAd, Medeiros VdN, Melo TJAd, Lira HdL. Polyamide 6 Nanocomposites with Inorganic Particles Modified with Three Quaternary Ammonium Salts. Materials. 2011; 4(11):1956-1966. https://doi.org/10.3390/ma4111956
Chicago/Turabian StyleAraujo, Edcleide Maria, Amanda Melissa Damião Leite, Rene Anisio da Paz, Vanessa da Nóbrega Medeiros, Tomas Jeferson Alves de Melo, and Hélio de Lucena Lira. 2011. "Polyamide 6 Nanocomposites with Inorganic Particles Modified with Three Quaternary Ammonium Salts" Materials 4, no. 11: 1956-1966. https://doi.org/10.3390/ma4111956
APA StyleAraujo, E. M., Leite, A. M. D., Paz, R. A. d., Medeiros, V. d. N., Melo, T. J. A. d., & Lira, H. d. L. (2011). Polyamide 6 Nanocomposites with Inorganic Particles Modified with Three Quaternary Ammonium Salts. Materials, 4(11), 1956-1966. https://doi.org/10.3390/ma4111956




