Synthesis and Properties of Octithiophene Dication Sterically Segregated by Annelation with Bicyclo[2.2.2]octene Units
Abstract
:1. Introduction
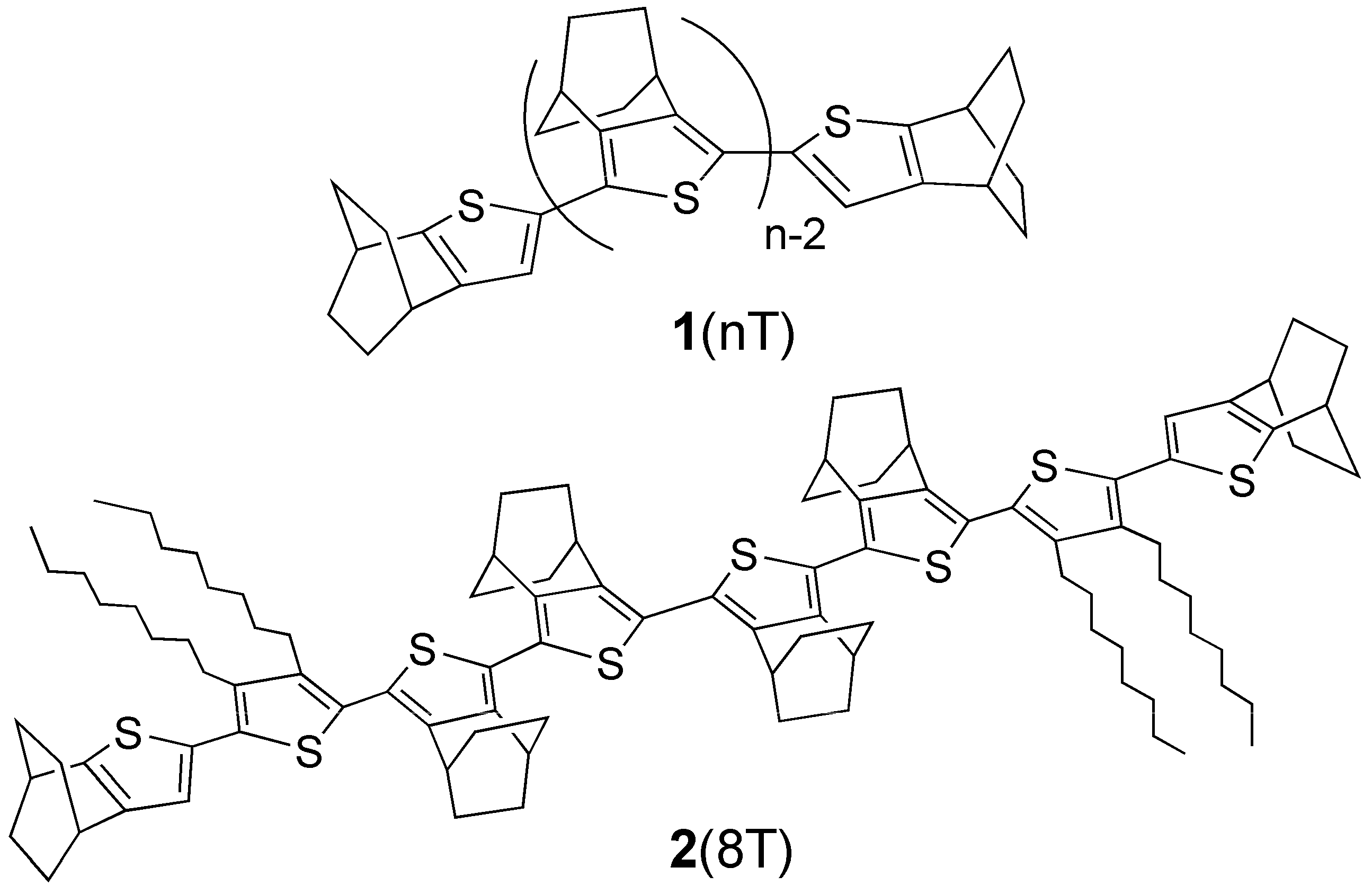
2. Results and Discussion
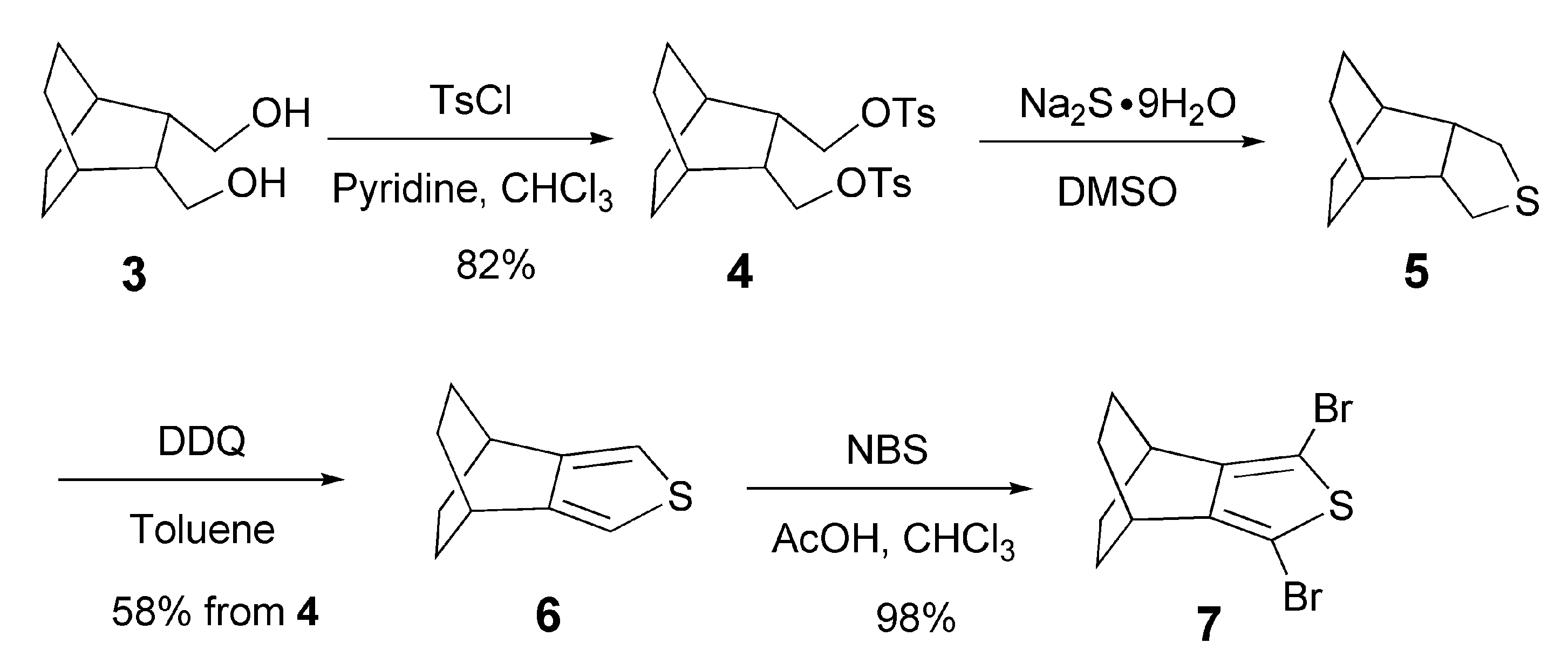
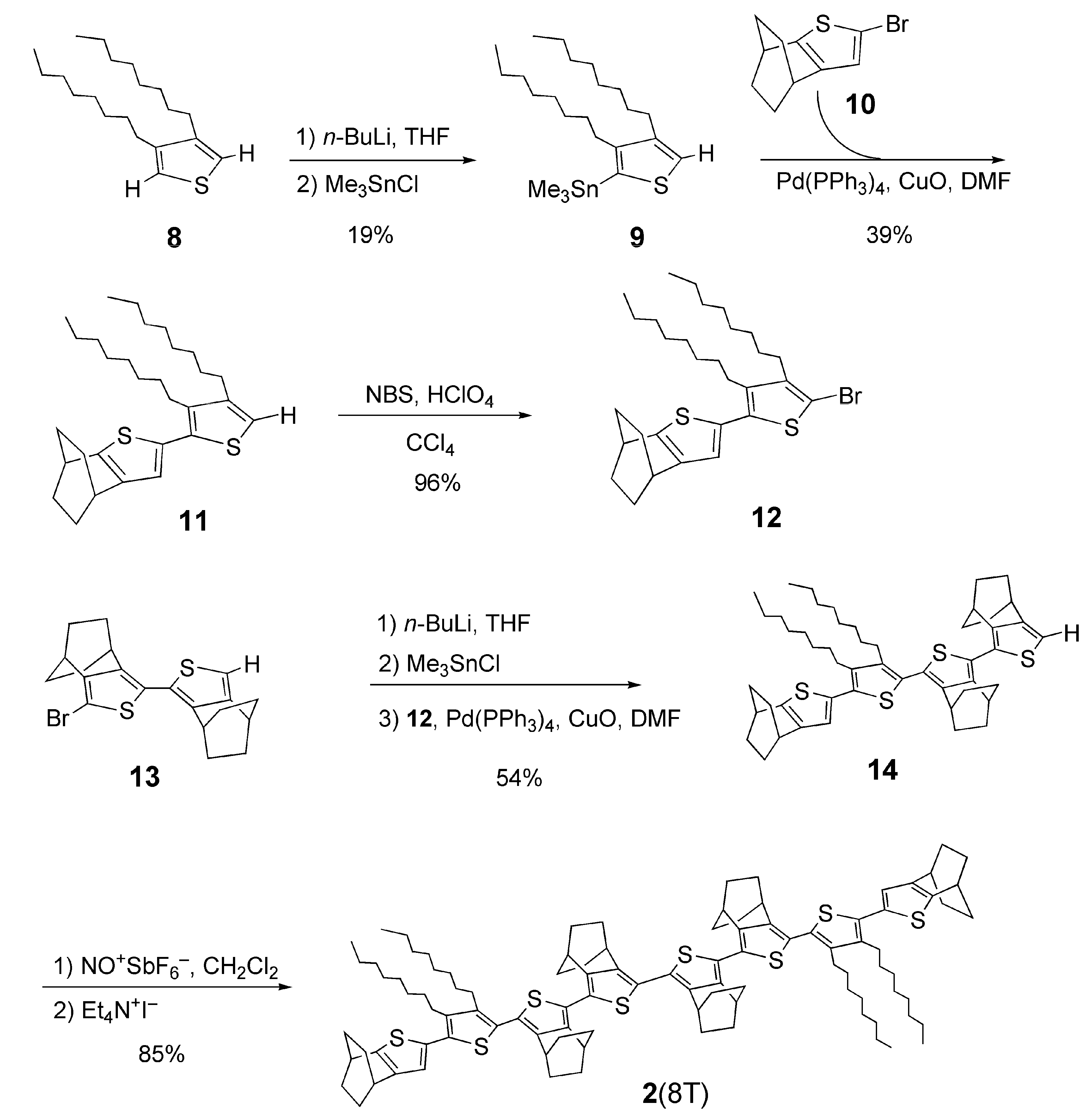
2.1. Synthesis
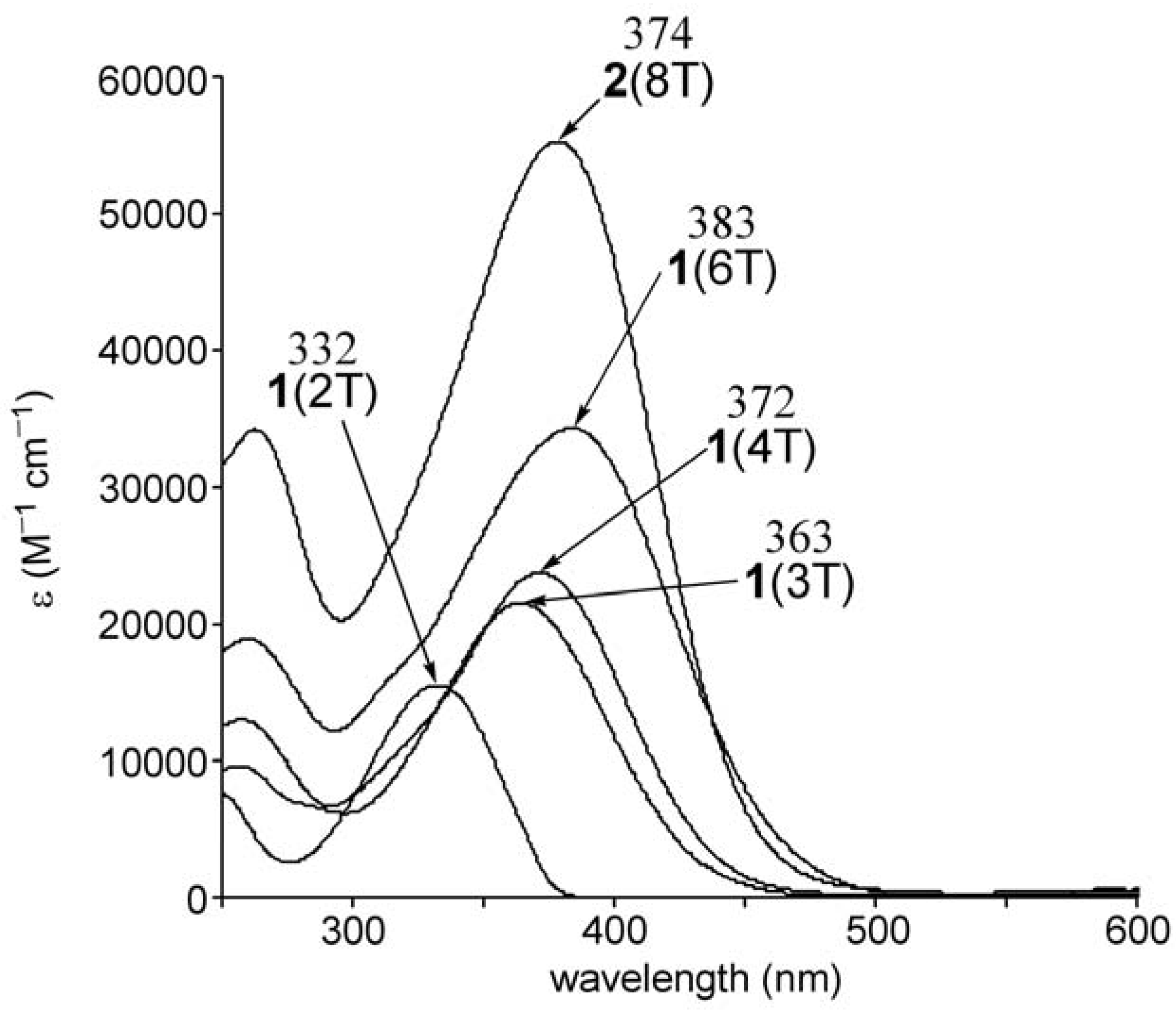
2.2. UV-vis spectra and cyclic voltammetry of neutral oligothiophenes
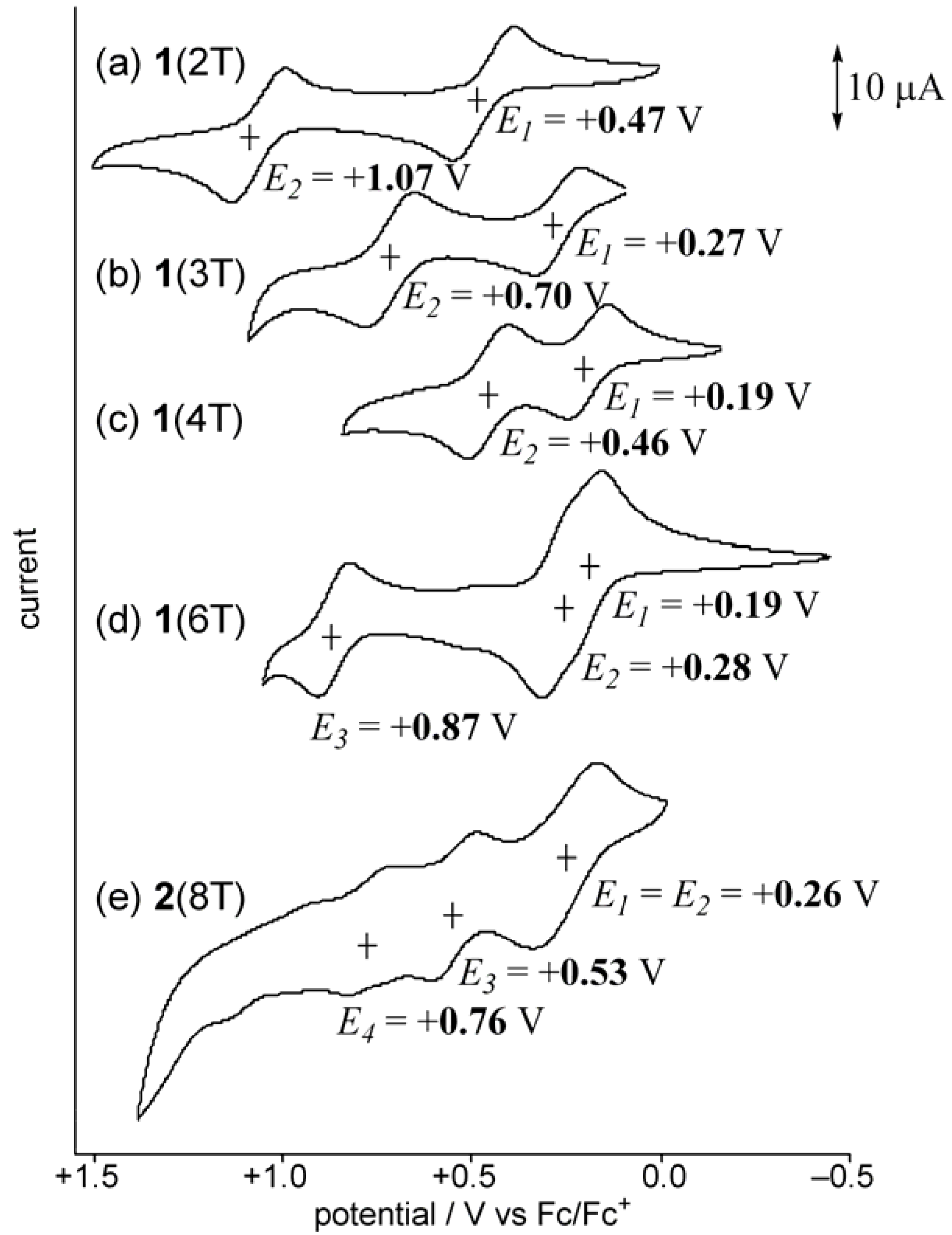
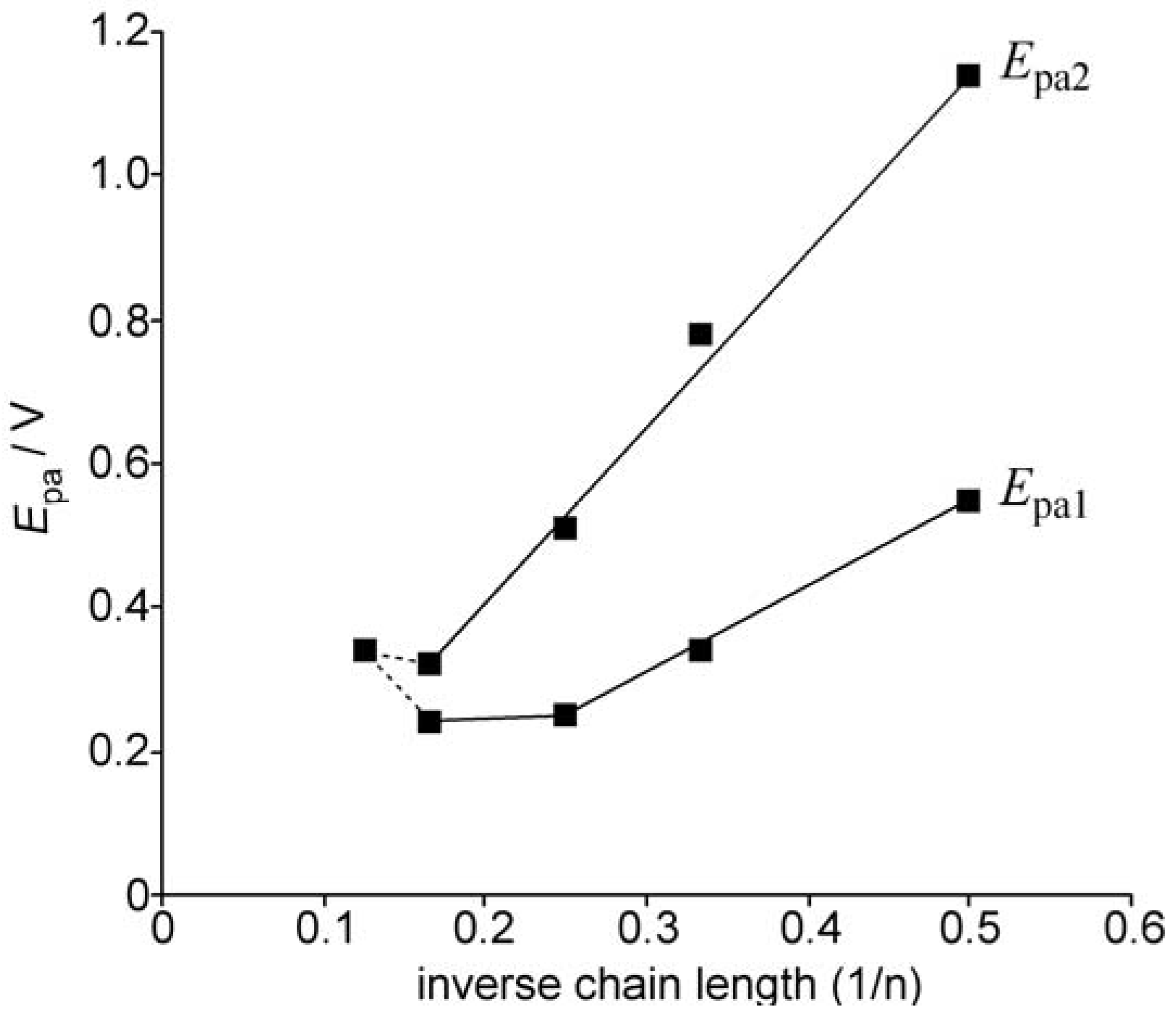
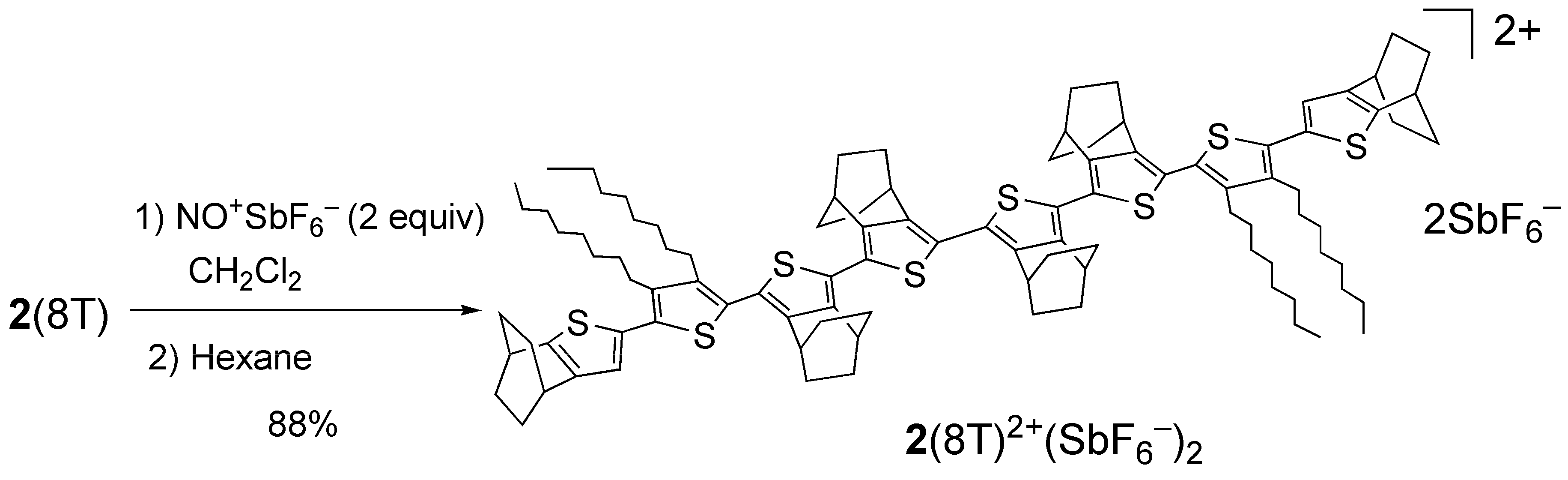
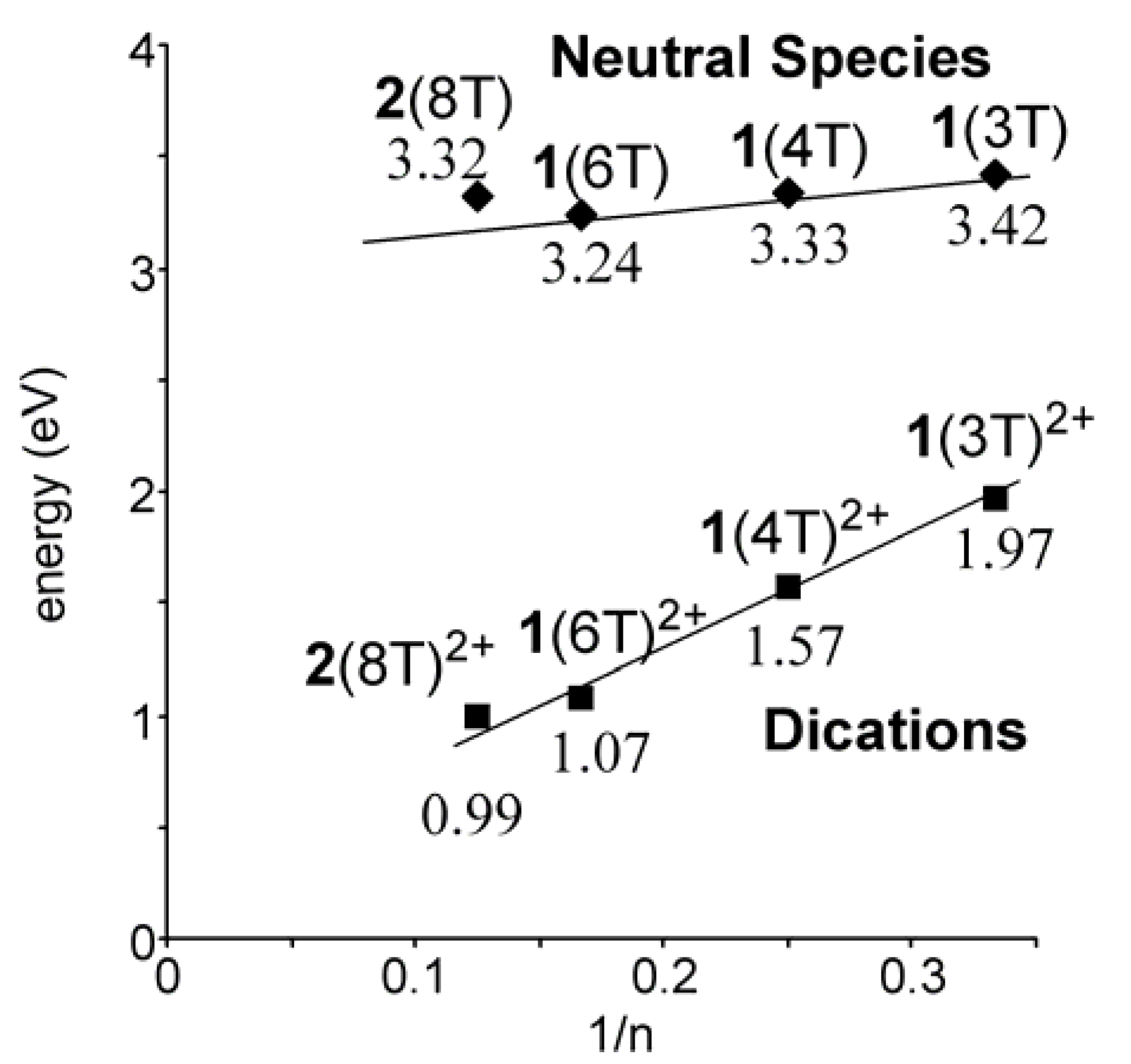
2.3. Preparation of a dication salt of 2(8T) and its properties
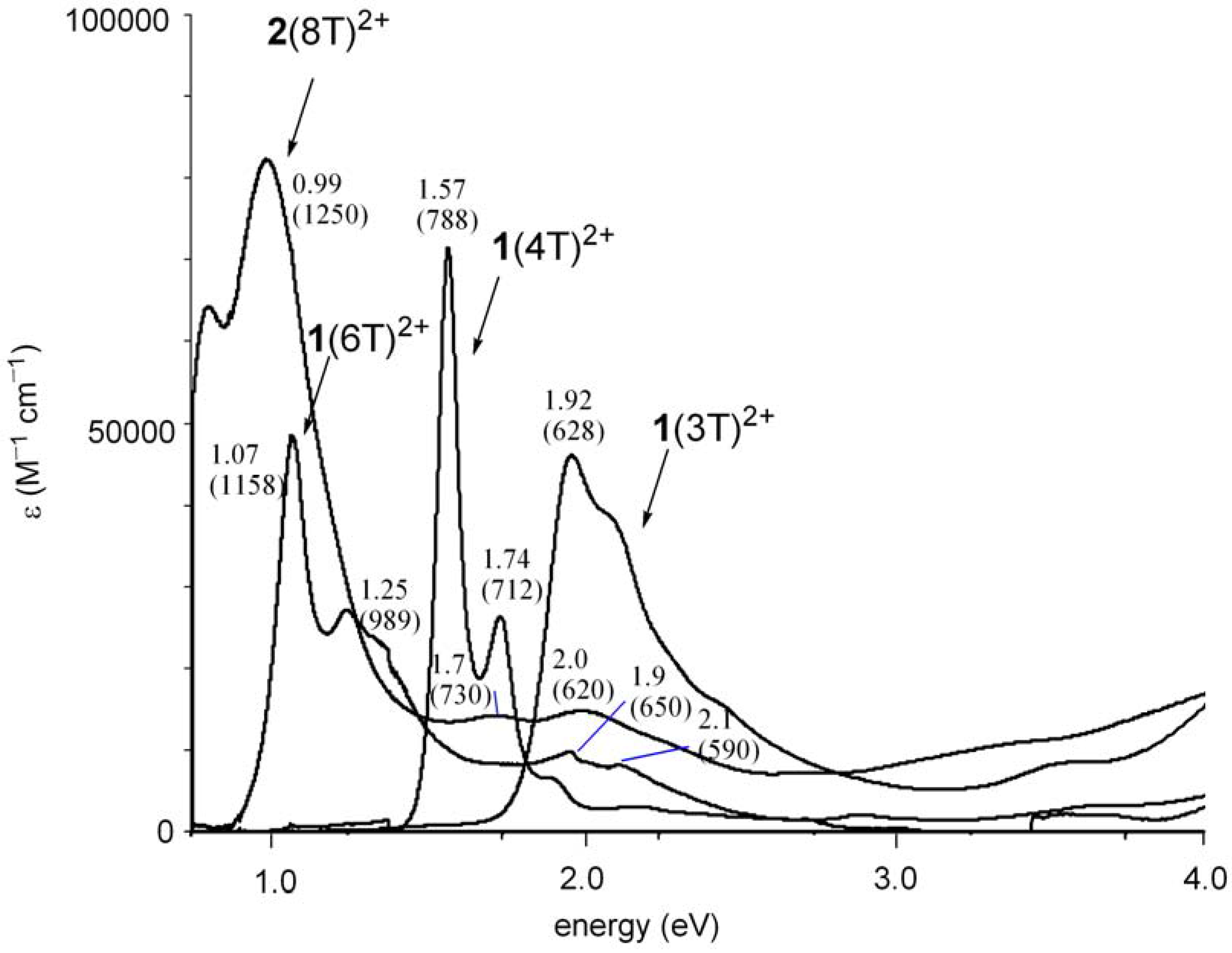
| Molecule | State | E1 | E2 | E3 | E4 |
|---|---|---|---|---|---|
| 3T2+ | closed-shell | 2.62 (0.68) | 3.02 (0.56) | ||
| 4T2+ | closed-shell | 2.19 (1.62) | 2.97 (0.21) | ||
| open-shell | 1.88 (0.37) | 2.24 (1.11) | 3.04 (0.15) | ||
| 6T2+ | closed-shell | 1.59 (2.78) | 2.98 (0.16) | ||
| open-shell | 1.40 (1.44) | 1.80 (0.87) | 2.09 (0.10) | 2.23 (0.26) | |
| 8T2+ | closed-shell | 1.22 (3.69) | 2.67 (0.17) | ||
| open-shell | 1.10 (2.01) | 1.57 (0.64) | 1.87 (0.93) |
3. Conclusions
4. Experimental Section
4.1. General
4.2. cis-2,3-Bis(tosyloxymethyl)bicyclo[2.2.2]octane (4)
4.3. 3,4-bicyclo[2.2.2]octanothiophene (6)
4.4. 2,5-dibromo-3,4-bicyclo[2.2.2]octanothiophene (7)
4.5. 2-(Trimethylstannyl)-3,4-dioctylthiophene (9)
4.6. 4,5-Bicyclo[2.2.2]octeno-3’,4’-dioctyl-2,2’-bithiophene (11)
4.7. 5’-Bromo-4,5-bicyclo[2.2.2]octeno-3’,4’-dioctyl-2,2’-bithiophene (12)
4.8. 4,5-Bicyclo[2.2.2]octeno-3’’,4’’:3’’’,4’’’-bis(bicyclo[2.2.2]octeno)-3’,4’-dioctyl-2,2’:5’,2’’:5’’,2’’’-quarterthiophene (14)
4.9. Octithiophene 2(8T)
4.10. Dication salt 2(8T)2+(SbF6–)2
Acknowledgements
References and Notes
- Hotta, S. Handbook of Organic Conductive Molecules and Polymers; Nalwa, H.S., Ed.; Wiley: Chichester, UK, 1997; Volume 2, Chapter 8. [Google Scholar]
- Baüerle, P. Electronic Materials: The Oligomeric Approach; Müllen, K., Wegner, G., Eds.; Wiley-VCH: Weinheim, Germany, 1998; Chapter 2. [Google Scholar]
- Fichou, D. (Ed.) Handbook of Oligo- and Polythiophenes; Wiley-VCH: Weinheim, Germany, 1999.
- Otsubo, T.; Aso, Y.; Takimiya, K. Synthesis, optical, and conductive properties of long oligothiophenes and their utilization as molecular wires. Bull. Chem. Soc. Jpn. 2001, 74, 1789–1801. [Google Scholar] [CrossRef]
- Salzner, U. Investigation of charge carriers in doped thiophene oligomers through theoretical modeling of their UV/Vis spectra. J. Phys. Chem. A 2008, 112, 5458–5466, and references cited therein. [Google Scholar] [CrossRef] [PubMed]
- Brédas, J.L.; Street, G.B. Polarons, bipolarons, and solitons in conducting polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Van Haare, J.A.E.H.; Havinga, E.E.; van Dongen, J.L.J.; Janssen, R.A.J.; Cornil, J.; Brédas, J.-L. Redox states of long oligothiophenes: Two polarons on a single chain. Chem. Eur. J. 1998, 4, 1509–1522. [Google Scholar] [CrossRef]
- Miller, L.L.; Mann, K.R. π-Dimers and π-stacks in solution and in conducting polymers. Acc. Chem. Res. 1996, 29, 417–423. [Google Scholar] [CrossRef]
- Graf, D.D.; Campbell, J.P.; Miller, L.L.; Mann, K.R. Single-crystal X-ray structure of the cation radical of 3‘,4‘-dibutyl-2,5‘‘-diphenyl-2,2‘:5‘,2‘‘-terthiophene: definitive evidence for π-stacked oxidized oligothiophenes. J. Am. Chem. Soc. 1996, 118, 5480–5481. [Google Scholar] [CrossRef]
- Graf, D.D.; Duan, R.G.; Campbell, J.P.; Miller, L.L.; Mann, K.R. From Monomers to π-stacks. A comprehensive study of the structure and properties of monomeric, π-dimerized, and π-stacked forms of the cation radical of 3‘,4‘-dibutyl-2,5‘‘-diphenyl-2,2‘:5‘,2‘‘-terthiophene. J. Am. Chem. Soc. 1997, 119, 5888–5899. [Google Scholar]
- Yamazaki, D.; Nishinaga, T.; Tanino, N.; Komatsu, K. Terthiophene radical cations end-capped by bicyclo[2.2.2]octene units: formation of bent π-dimers mutually attracted at the central position. J. Am. Chem. Soc. 2006, 128, 14470–14471. [Google Scholar]
- Gao, Y.; Liu, C.-G.; Jiang, Y.-S. Electronic structure of thiophene oligomer dications: an alternative interpretation from the spin-unrestricted DFT study. J. Phys. Chem. A 2002, 106, 5380–5384. [Google Scholar] [CrossRef]
- Zade, S.S.; Bendikov, M. Theoretical study of long oligothiophene dications: bipolaron vs polaron pair vs triplet state. J. Phys. Chem. B 2006, 110, 15839–15846. [Google Scholar] [CrossRef] [PubMed]
- Kurata, T.; Mohri, T.; Takimiya, K.; Otsubo, T. Conductive, magnetic, and optical properties of sterically hindered dodecithiophenes. Evidence for the coexistence of bipolaron and π-dimer. Bull. Chem. Soc. Jpn. 2007, 80, 1799–1807. [Google Scholar] [CrossRef]
- Ie, Y.; Han, A.; Otsubo, T.; Aso, Y. Completely encapsulated oligothiophenes up to 12-mer. Chem. Commun. 2009, 3020–3022. [Google Scholar] [CrossRef]
- Wakamiya, A.; Yamazaki, D.; Nishinaga, T.; Kitagawa, T.; Komatsu, K. Synthesis and properties of novel oligothiophenes surrounded by bicyclo[2.2.2]octene frameworks. J. Org. Chem. 2003, 68, 8305–8314. [Google Scholar] [CrossRef]
- Nishinaga, T.; Wakamiya, A.; Yamazaki, D.; Komatsu, K. Crystal structures and spectroscopic characterization of radical cations and dications of oligothiophenes stabilized by annelation with bicyclo[2.2.2]octene units: sterically segregated cationic oligothiophenes. J. Am. Chem. Soc. 2004, 126, 3163–3174. [Google Scholar]
- Nishinaga, T.; Komatsu, K. Persistent π radical cations: self-association and its steric control in the condensed phase. Org. Biomol. Chem. 2005, 3, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K.; Nishinaga, T. Synthesis and properties of cationic π-conjugated systems stabilized by bicyclo[2.2.2]octene units. Synlett 2005, 187–202. [Google Scholar] [CrossRef]
- Reddy, V.P.; Olah, G.A.; Prakash, G.K.S. Stable carbocations. 292. 2-Methylbicyclo[3.2.2]non-3-en-2-yl cation involving rearrangement of 1,3'-spirocyclopropylbicyclo[2.2.2]oct-2'-yl cation. J. Org. Chem. 1993, 58, 7622–7623. [Google Scholar] [CrossRef]
- Lok, K.P.; Jakovac, I.J.; Jones, J.B. Enzymes in organic synthesis. 34. Preparations of enantiomerically pure exo- and endo-bridged bicyclic [2.2.1] and [2.2.2] chiral lactones via stereospecific horse liver alcohol dehydrogenase catalyzed oxidations of meso diols. J. Am. Chem. Soc. 1985, 107, 2521–2526. [Google Scholar]
- Birch, S.F.; Hunter, N.J.; Mcallan, D.T. Preparation and physical properties of sulfur compounds related to petroleum. VI. endo-4,7-Methano-cis-2-thiahydrindan and endo-4,7-ethano-cis-2-thiahydrindan. J. Org. Chem. 1956, 21, 970–974. [Google Scholar]
- Bäuerle, P.; Fischer, T.; Bidlingmeier, B.; Rabe, J.P.; Stabel, A. Oligothiophenes - Yet longer? Synthesis, characterization, and scanning tunneling microscopy images of homologous, isomerically pure oligo(alkylthiophene)s. Angew. Chem., Int. Ed. Engl. 1995, 34, 303–307. [Google Scholar] [CrossRef]
- Kubo, T.; Shimizu, A.; Uruichi, M.; Yakushi, K.; Nakano, M.; Shiomi, D.; Sato, K.; Takui, T.; Morita, Y.; Nakasuji, K. Singlet biradical character of phenalenyl-based Kekulé hydrocarbon with naphthoquinoid structure. Org. Lett. 2007, 9, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Sakamoto, M.; Nakasuji, K. Biradicaloid character of phenalenyl-based aromatic compounds with a small HOMO–LUMO gap. Polyhedron 2005, 24, 2522–2527. [Google Scholar] [CrossRef]
- Kubo, T.; Shimizu, A.; Sakamoto, M.; Uruichi, M.; Yakushi, K.; Nakano, M.; Shiomi, D.; Sato, K.; Takui, T.; Morita, Y.; Nakasuji, K. Angew. Chem. Int. Ed. 2005, 44, 6564–6568. [CrossRef]
- Takahashi, T.; Matsuoka, K.; Takimiya, K.; Otsubo, T.; Aso, Y. Extensive quinoidal oligothiophenes with dicyanomethylene groups at terminal positions as highly amphoteric redox molecules. J. Am. Chem. Soc. 2005, 127, 8928–8929. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.P.; Casado, J.; Hernández, V.; Navarrete, J.T.L.; Viruela, P.M.; Ortí, E.; Takimiya, K.; Otsubo, T. On the biradicaloid nature of long quinoidal oligothiophenes: experimental evidence guided by theoretical studies. Angew. Chem. Int. Ed. 2007, 46, 9057–9061. [Google Scholar] [CrossRef]
- Ortiz, R.P.; Casado, J.; González, S.R.; Hernández, V.; Navarrete, J.T.L.; Viruela, P.M.; Ortí, E.; Takimiya, K.; Otsubo, T. Quinoidal oligothiophenes: Towards biradical ground-state species. Chem. Eur. J. 2010, 16, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Embert, F.; Lère-Porte, J.-P.; Moreau, J.J.E.; Serein-Spirau, F.; Righib, A.; Sauvajol, J.-L. Synthesis and optical properties of (thienylene)–[1,6-dithienylhexa-1,3,5-trienylene] copolymers. J. Mater. Chem. 2001, 11, 718–722. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J. A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; Millam, J.M.; Iyengar, S.S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G.A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J.E.; Hratchian, H.P.; Cross, J.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A.J.; Cammi, R.; Pomelli, C.; Ochterski, J.W.; Ayala, P.Y.; Morokuma, K.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Zakrzewski, V.G.; Dapprich, S.; Daniels, A.D.; Strain, M.C.; Farkas, O.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Ortiz, J.V.; Cui, Q.; Baboul, A.G.; Clifford, S.; Cioslowski, J.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Gonzalez, C.; Pople, J.A. Gaussian 03, Revision D.01. Gaussian, Inc.: Wallingford CT, USA, 2004. [Google Scholar]
© 2010 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nishinaga, T.; Yamazaki, D.; Tateno, M.; Iyoda, M.; Komatsu, K. Synthesis and Properties of Octithiophene Dication Sterically Segregated by Annelation with Bicyclo[2.2.2]octene Units. Materials 2010, 3, 2037-2052. https://doi.org/10.3390/ma3032037
Nishinaga T, Yamazaki D, Tateno M, Iyoda M, Komatsu K. Synthesis and Properties of Octithiophene Dication Sterically Segregated by Annelation with Bicyclo[2.2.2]octene Units. Materials. 2010; 3(3):2037-2052. https://doi.org/10.3390/ma3032037
Chicago/Turabian StyleNishinaga, Tohru, Daisuke Yamazaki, Masaki Tateno, Masahiko Iyoda, and Koichi Komatsu. 2010. "Synthesis and Properties of Octithiophene Dication Sterically Segregated by Annelation with Bicyclo[2.2.2]octene Units" Materials 3, no. 3: 2037-2052. https://doi.org/10.3390/ma3032037
APA StyleNishinaga, T., Yamazaki, D., Tateno, M., Iyoda, M., & Komatsu, K. (2010). Synthesis and Properties of Octithiophene Dication Sterically Segregated by Annelation with Bicyclo[2.2.2]octene Units. Materials, 3(3), 2037-2052. https://doi.org/10.3390/ma3032037





