1. Introduction
Reflective liquid crystal displays (LCDs) are suitable for portable information systems because of their low power consumption. The reflective guest-host mode liquid crystal displays (GH-LCDs), wherein dichroic dyes are dissolved in liquid crystals were developed and attracted much attention [
1]. However, the absorption of a polarizing plate is very high (~50%) and brightness is diminished drastically. In order to improve display performances, it is necessary to select a LCD mode operating without a polarizing plate. Some LCD modes without a polarizing plate were reported [
2,
3,
4,
5,
6,
7,
8,
9,
10]. Among them, thin film transistor (TFT) driven reflective phase change GH-LCDs have a relatively wide viewing angle, high reflectance and are very promising display modes for future applications [
9,
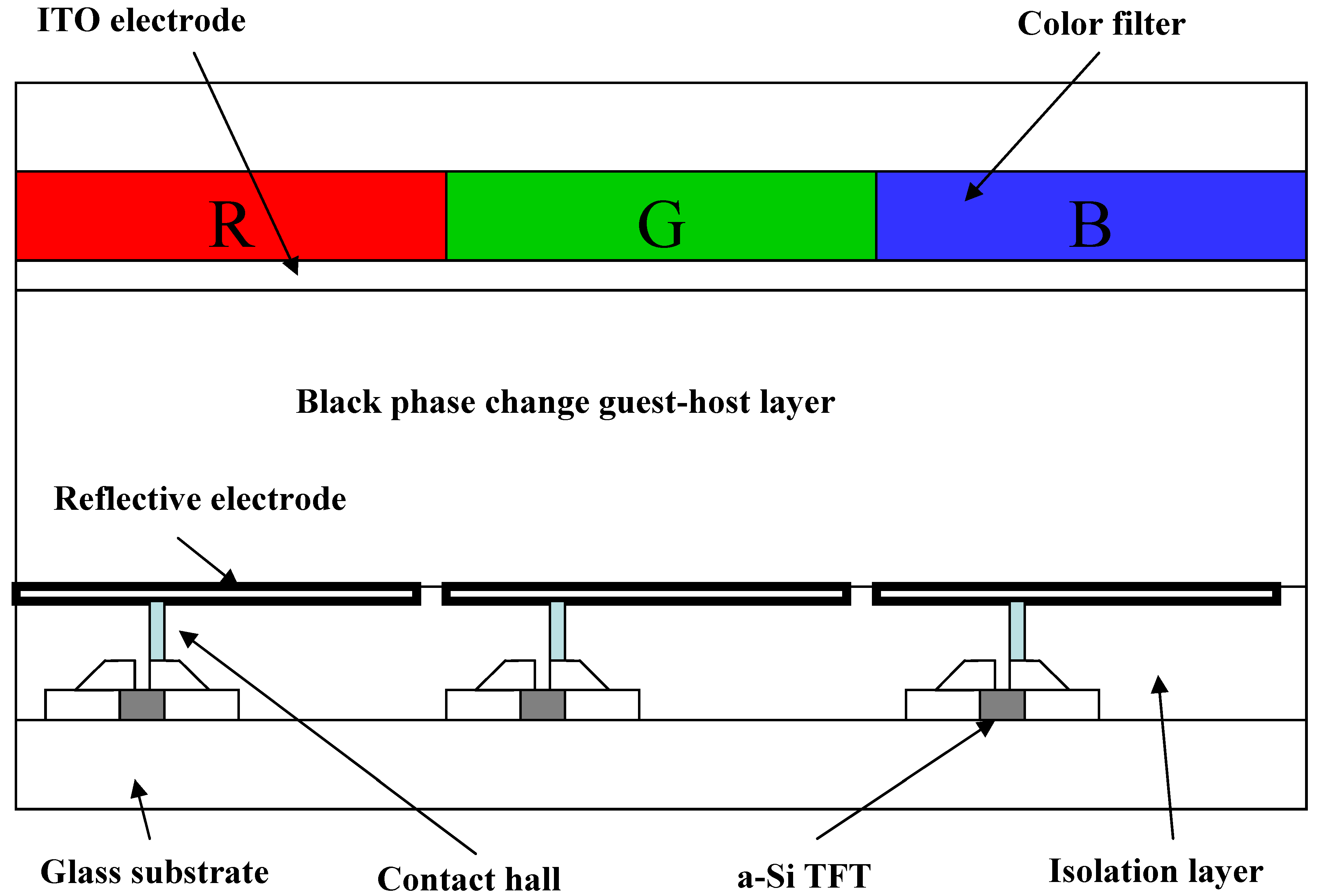
10]. The schematic structure of reflective phase change GH-LCD with color filter consisting of red, green and blue is shown in
Figure 1.
Figure 1.
The structure of the reflective phase change GH-LCD.
Figure 1.
The structure of the reflective phase change GH-LCD.
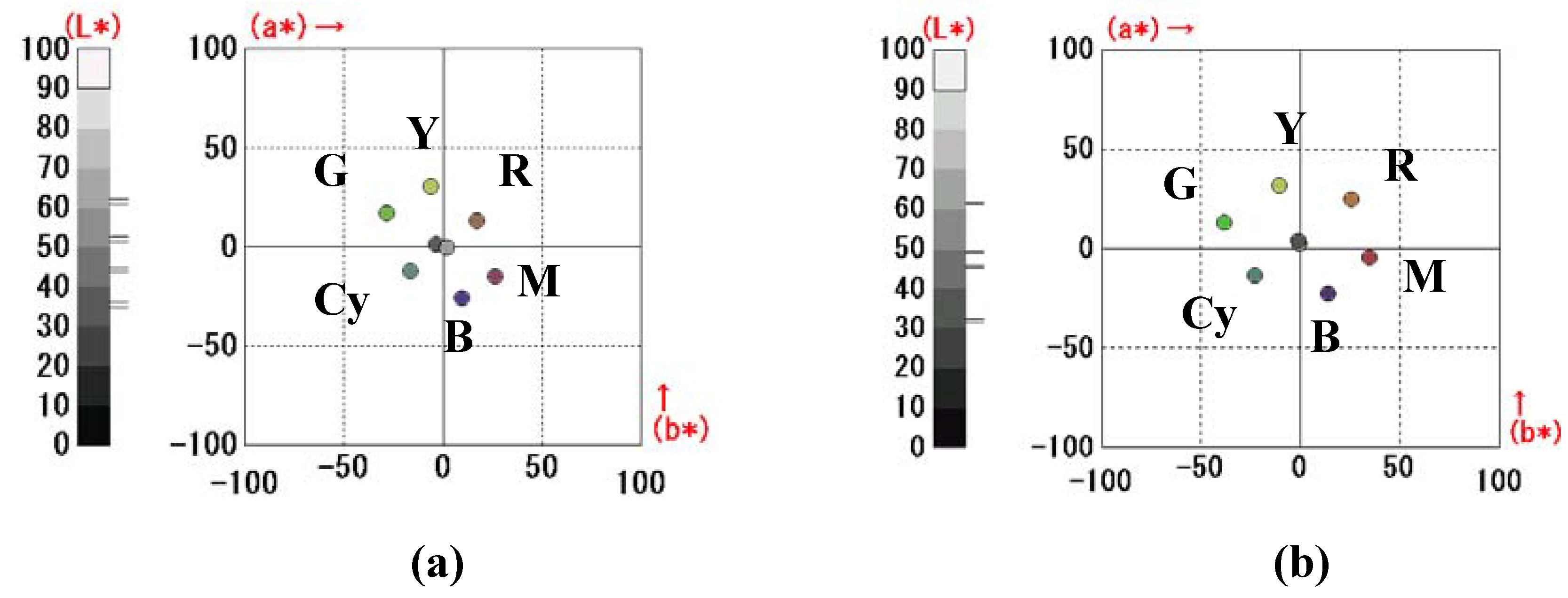
In the case of the RGB color filter GH-LCDs using black GH liquid crystals, color images are created by color filters and the black GH liquid crystal layer has a light-bulb function. The contrast is high and color reproduction areas are large. However, since the color filters always absorb the light, light utilization efficiency is limited. This means that images obtained from displays of this type are insufficient in some cases, with the insufficiency being particularly marked when the displays are viewed in dark places. On the other hand, bright images were obtained by using color filters consisting of yellow, magenta and cyan [
9]. However, the color reproduction areas are considered to be drastically decreased compared with RGB color filter GH-LCDs.
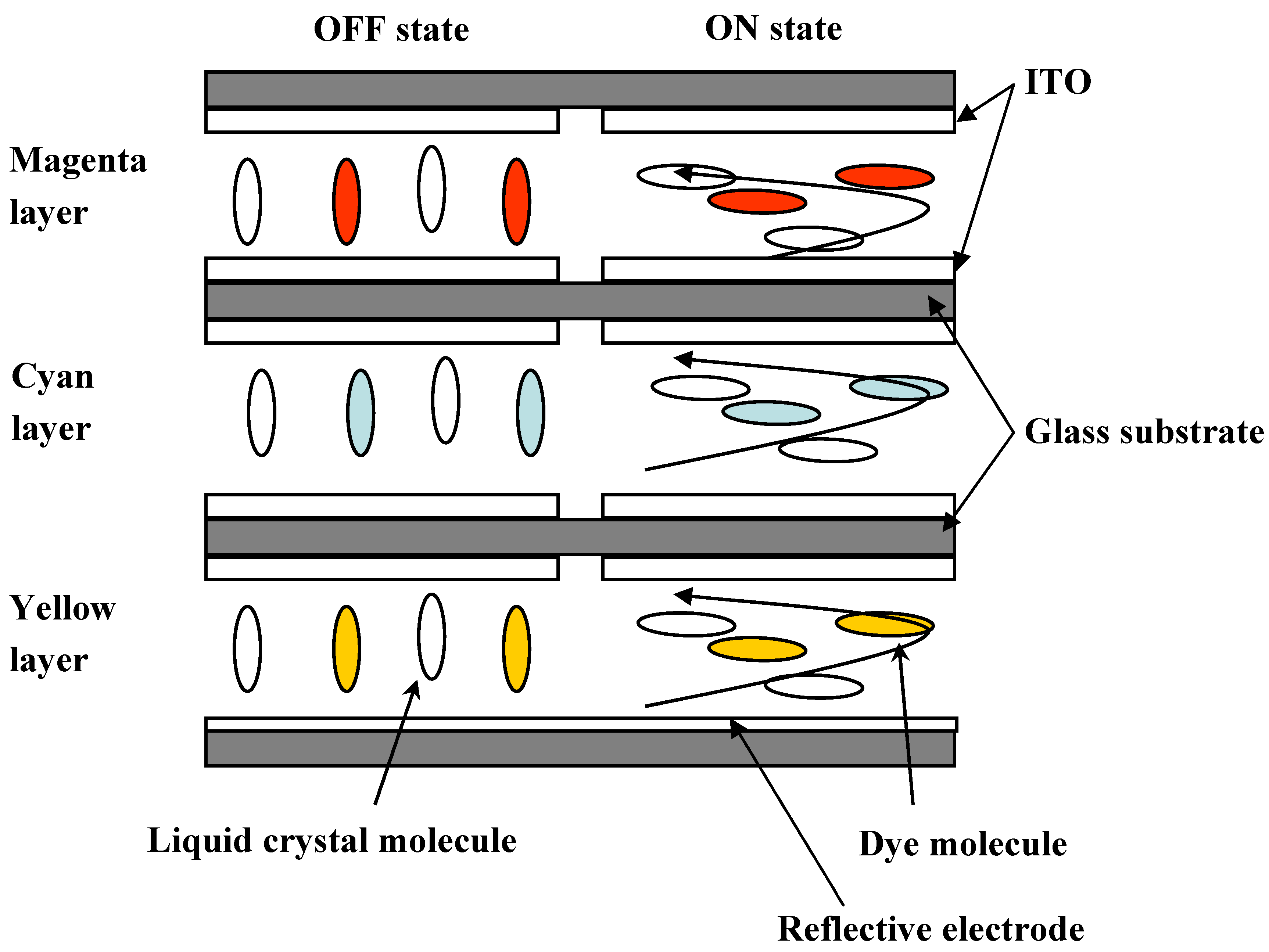
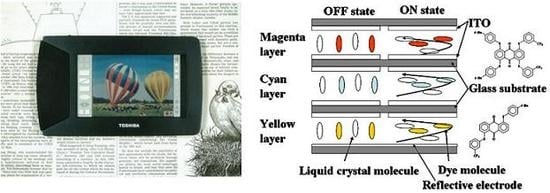
The concept of the three-layer GH-LCDs, which require no color filters and a polarizing plate, has been reported [
11]. The reflective three-layer GH-LCDs, consisting of yellow, magenta and cyan guest-host layers, are most desirable because a bright full-color image is expected to be realized [
12,
13,
14,
15,
16]. The schematic structure is shown in
Figure 2.
Figure 2.
The schematic structure of the three-layer reflective GH-LCD using negative liquid crystals.
Figure 2.
The schematic structure of the three-layer reflective GH-LCD using negative liquid crystals.
Each GH layer is driven by TFT individually, and black, white, yellow, cyan, magenta, red, green, blue and their intermediate colors are created by all pixels followed by constructing full-color images. The properties of dichroic dyes are important for the realization of high performances of the three-layer GH-LCDs. In particular, absorption spectra (color), solubilities and dichroic ratios in liquid crystals are the dominant properties.
Dichroic dyes have been developed mainly to make black dyes for monochrome displays by mixing [
17]. The spectrum requirements of dyes for the three-layer GH-LCDs are much higher than those for monochrome displays, and are even higher than those for photography from the viewpoint of color contrast [
14]. Azo dyes have been actively investigated [
6], but they are unsuitable for three-layer GH-LCDs because of their excessively wide absorption spectra that reduce their color reproduction area. On the other hand, anthraquinone dyes not only satisfy the spectrum requirement, but also have wide color selectivity and excellent photostability [
18,
19,
20,
21,
22,
23].
In particular, the anthraquinone dichroic dyes with the phenylthio groups or the anilino groups at α positions are intramolecular charge transfer complexes and reported to be dissolved in cyanobiphenyl liquid crystals to some extent [
22,
23]. However, the cyanobiphenyl liquid crystals can’t be applied to the thin film transistor liquid crystal displays (TFT-LCDs) because they require high resistance values.
Fluorinated liquid crystals with high resistance were developed for TFT-LCDs [
24,
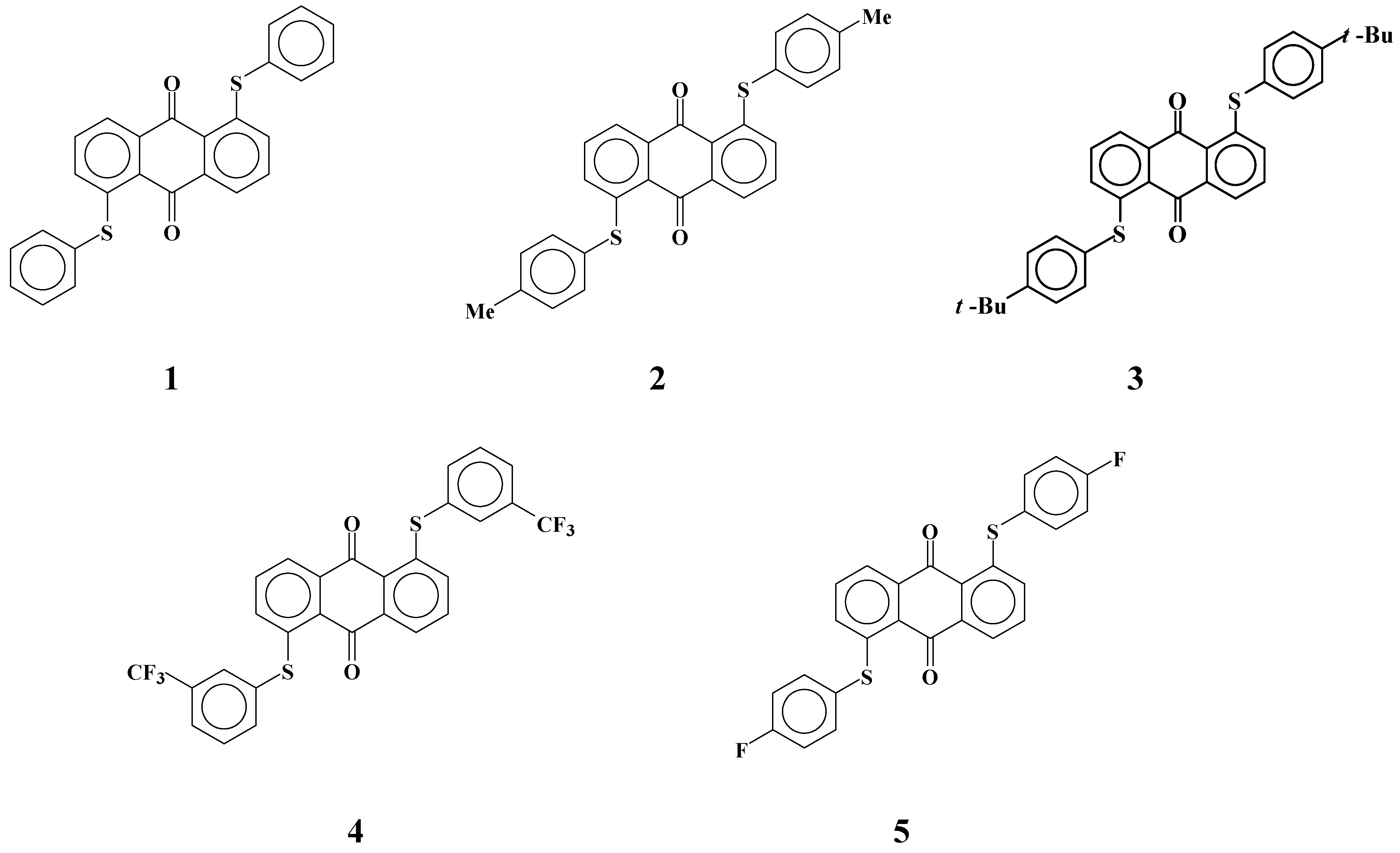
25]. However, the solubilities of yellow anthraquinone dyes
1-
5 with phenylthio groups (
Figure 3) in fluorinated liquid crystals were much smaller than those in cyanobiphenyl liquid crystals [
26]. This problem is most serious for yellow anthraquinone dyes having a small absorption coefficient, and therefore larger concentrations of dyes are required. Moreover, GH-LCDs require dichroic dyes with large solubility even at low temperatures to protect dyes from recrystallization in the liquid crystal cell.
The purpose of this study is to enlarge the performances of the three-layer GH-LCDs by realizing highly soluble anthraquinone dichroic dyes in fluorinated liquid crystals even at low temperatures. The ultimate aim is to develop portable information systems with full-color images.
Figure 3.
The molecular structures of yellow anthraquinone dichroic dyes with phenylthio groups used in this study.
Figure 3.
The molecular structures of yellow anthraquinone dichroic dyes with phenylthio groups used in this study.
2. Molecular Structures and Properties of Anthraquinone Dichroic Dyes
2.1. The fundamental molecular structures of dichroic dyes for the three-layer GH-LCDs
The preferable wavelength ranges of yellow, magenta and cyan colors of the three-layer GH-LCDs are as follows from the analogy of photographic dyes: yellow 425-470 nm, magenta 525-560 nm, and cyan 630-670 nm. The Pariser-Parr-Pople configuration interaction (PPP-CI) quantum chemical calculation method [
27] was used to select the molecular structures of dyes [
14]. The PPP calculation deals only with π-electrons. Nevertheless, the results are sufficiently reliable because π-electrons determine most of the optical and electrical properties. Various properties of large molecules can be calculated without exact knowledge of the coordinates of atoms in dye molecules.
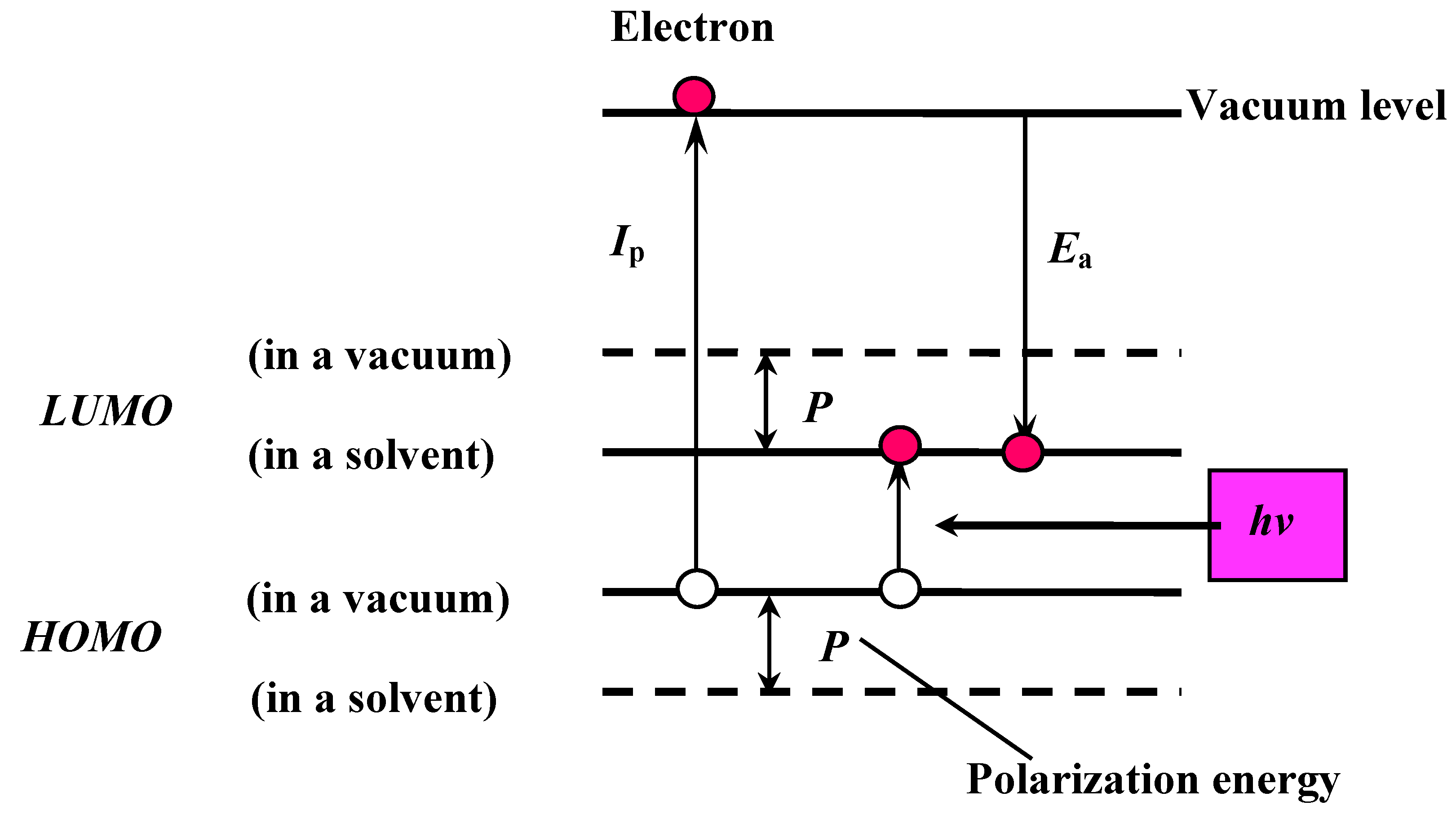
The PPP method is semi-empirical. The determination of the calculation parameters is the most important step to obtain reliable and quantitative results. The correlation of molecular energy level between in a vacuum and in a solvent is shown in
Figure 4.
Figure 4.
The correlation of molecular energy levels between in a vacuum and in a solvent.
Figure 4.
The correlation of molecular energy levels between in a vacuum and in a solvent.
The optical properties of the studied dyes are those in liquid crystals, not those in a vacuum. However, quantum calculations deal with molecules in a vacuum. In liquid crystal solvent, ionic species are stabilized by the polarization energy (P). The polarization energy is generated by reorientation of atoms or molecules and by localization of electric charges. Therefore, in liquid crystals, obtained ionization potential values (Ip) decrease, whereas obtained electron affinity values (Ea) increase. In the calculation, this corresponds to the fact that HOMO and LUMO energy levels become substantially shallower and deeper, respectively. Excited states obtained by light absorption have polar structures and are generally stabilized by the polarization energy. Therefore, estimation of P is important in the PPP method.
In order to estimate the polarization energy (P), Ip parameters in the PPP program were modified so that these parameters for donor heteroatoms were decreased and those for acceptor heteroatoms were increased. In the calculation, an Ip increment corresponds to an Ea increment.
Calculated suitable fundamental molecular structures of yellow, magenta and cyan anthraquinone dyes for the three-layer GH-LCDs are shown in
Table 1 [
14].
Yellow and magenta anthraquinone dyes
A~
C were synthesized according to reference 22. Cyan dyes
D and
E were newly synthesized [
28]. The absorption coefficient (
ε) of yellow anthraquinone dye
A is small, and is consistent with the calculated small oscillator strength (
f = 0.37).
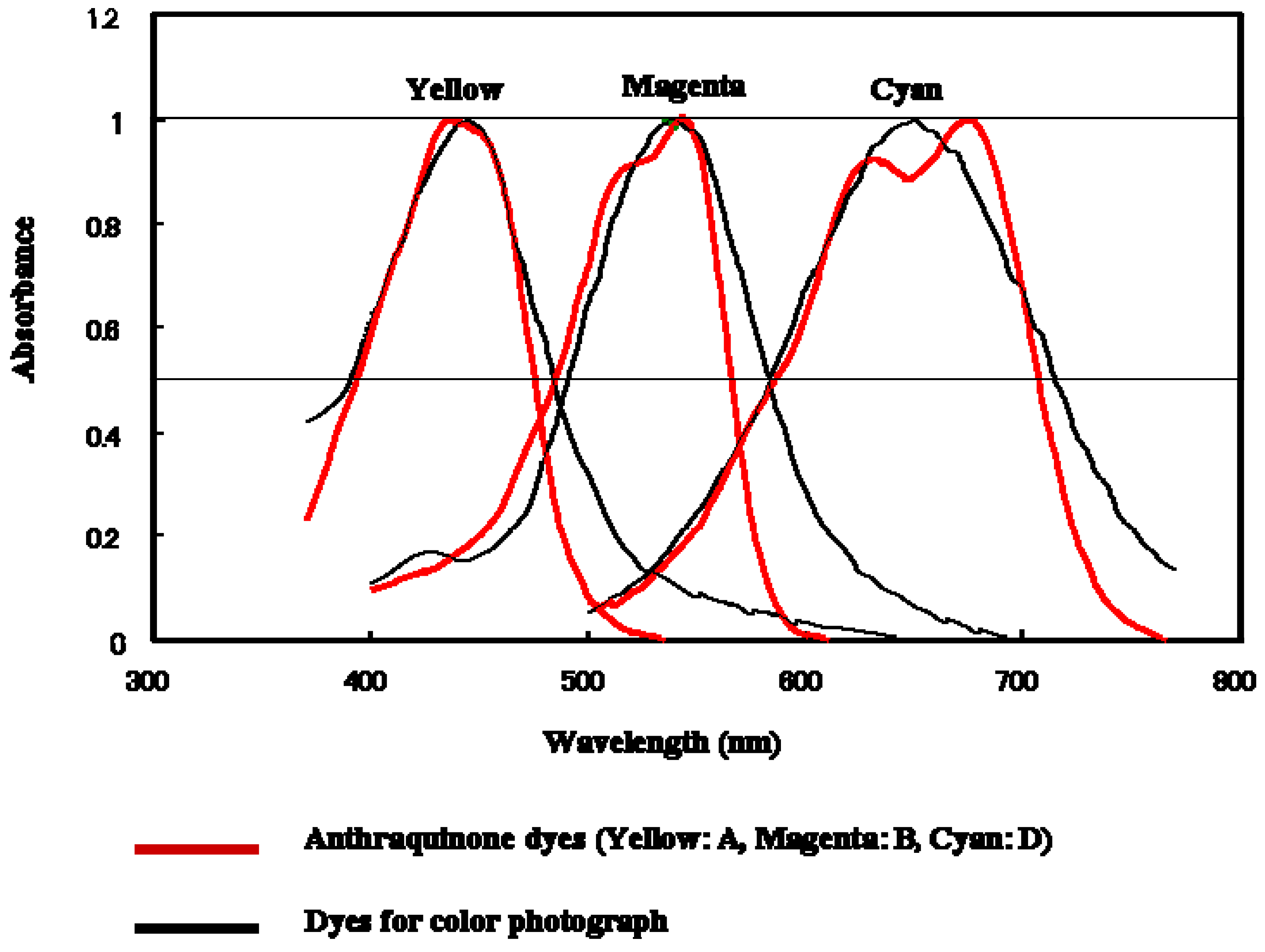
Figure 5 indicates absorption spectra in dilute ethyl acetate solutions. The spectrum features were suitable for the three-layer GH-LCDs. In concentrated liquid crystal solutions, λ
obs increased by 10~15 nm. The half-width values of anthraquinone dyes were narrower than those of photographic dyes resulting in purer color image.
The simple PPP-CI method was found to be extremely useful in investigation related to guest dyes. Yellow, magenta and cyan dyes could be optimized from the viewpoint of absorption properties.
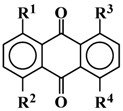
Table 1.
Extracted molecular structures of yellow, magenta and cyan dyes for the three-layer GH-LCDs from the viewpoint of absorption spectra.
![]()
Table 1.
Extracted molecular structures of yellow, magenta and cyan dyes for the three-layer GH-LCDs from the viewpoint of absorption spectra. ![]()
| | Color | R1 | R2 | R3 | R4 | λobs(nm)1) | λcal(nm)2) | f 3) |
|---|
| A | Yellow | SPh | H | H | SPh | 435 | 433 | 0.37 |
| B | Magenta | SPh | SPh | SPh | SPh | 524 | 513 | 0.52 |
| C | Magenta | SPh | SPh | OH | OH | 537 | 541 | 0.63 |
| D | Cyan | SPh | SPh | NH(p-n-Bu-Ph) | NH(p-n-Bu-Ph) | 646 | 664 | 0.78 |
| E | Cyan | SPh | SPh | NH(n-Bu) | NH(n-Bu) | 638 | 598 | 0.71 |
| 1) Maximum wavelength of absorption spectra (measured in ethyl acetate at room temperature). |
| 2) Calculated maximum wavelength. |
| 3) Oscillator strength. |
Figure 5.
The comparison of absorption spectra of anthraquinone dyes for the three-layer GH-LCDs and photographic dyes.
Figure 5.
The comparison of absorption spectra of anthraquinone dyes for the three-layer GH-LCDs and photographic dyes.
2.2. Development of yellow anthraquinone dyes with two different phenylthio groups at 1 and 5 positions for the three-layer GH-LCDs
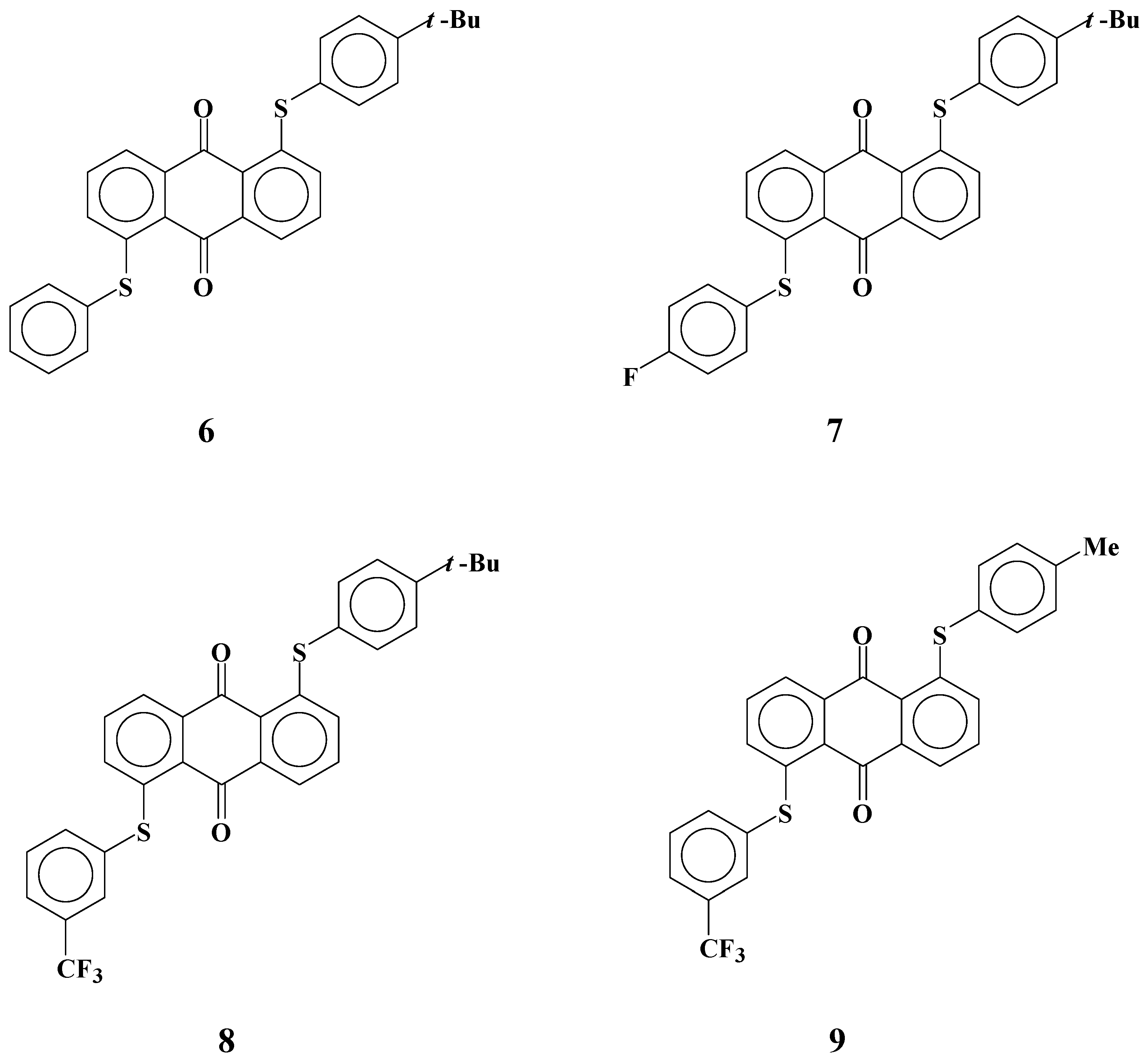
Several yellow anthraquinone dyes
6-
9 with phenylthio groups at the 1 and 5 positions were synthesized (
Figure 6) [
26,
29]. Two different phenylthio groups were introduced to form asymmetric molecular structures to be dissolved in fluorinated liquid crystals. Dye
6 has the simplest asymmetric molecular structure. The molecular structure of dye
7 includes a fluorine atom at the
para position of a non-substituted phenylthio group to clarify the effect of introduction of fluorine atom on solubility.
Figure 6.
The molecular structures of yellow anthraquinone dyes with two different phenylthio groups at 1 and 5 positions.
Figure 6.
The molecular structures of yellow anthraquinone dyes with two different phenylthio groups at 1 and 5 positions.
The molecular structure of dye
8 is the same as that of dye
6, except that a trifluoromethyl group is added at the
meta position of a non-substituted phenylthio group, and dye
9 is the same as dye
8 except that it has a methyl group at the
para position instead of a
t-butyl group. The solubilities of these dyes in fluorinated liquid crystals at room temperature and low temperatures and their dichroic ratios are shown in
Table 2. The fluorinated liquid crystal material used in this study was LIXON 5052 XX produced by Chisso Corp. Solubilities of some dichroic dyes in several fluorinated liquid crystal materials were measured and LIXON 5052 XX was selected as the standard in view of its preferable results. The properties of LIXON 5052XX are as follows: dielectric constant (horizontal) is 7.0; dielectric constant (vertical) is 3.3; and nematic and liquid phase change temperature is 376.8 K.
Table 2.
The relationships between molecular structures of yellow anthraquinone dyes and their solubilities and dichroic ratios.
Table 2.
The relationships between molecular structures of yellow anthraquinone dyes and their solubilities and dichroic ratios.
| Dye | Solubility (wt%)1) | Dichroic ratio |
|---|
| | r. t. | low temp. 2) | |
|---|
| 6 | 4.0 | 1.7 | 1.7 |
| 7 | 3.1 | 2.1 | 2.1 |
| 8 | 5.8 | 3.5 | 3.5 |
| 9 | 0.66 | 0.38 | 0.38 |
| 1) Measured in fluorinated liquid crystals (LIXON 5052XX). |
| 2) 267-268 K. |
As shown in
Table 2, unlike symmetric dyes, asymmetric anthraquinone dyes
6-
8 are soluble to some extent in fluorinated liquid crystals. In particular, the solubility of dye
8 at room temperature reached 5.8 wt%, and remained at a high level (3.5 wt%), even at low temperatures. To realize bright and clear colors, solubility above 3 wt% is required for yellow anthraquinone dyes with relatively small absorption coefficients. No previous yellow anthraquinone dichroic dyes were reported to satisfy this level of solubility. The solubility of dye
6 was large at room temperature and decreases little even at low temperatures. However, dye
8 with a trifluoromethyl group at the
meta position was more soluble than dye
6 without a trifluoromethyl group. In general, it is expected that trifluoromethyl group will induce a large dipole moment in dye structures. Therefore, the interaction between dye molecules will increase, resulting in low solubility. However, to compare dyes
6 and
8, introduction of a trifluoromethyl group enlarges the solubilities. Another important feature of these asymmetric dyes is that the solubilities at low temperatures were very large, whereas solubilities usually drastically decrease at low temperatures compared with those at room temperature. Two possibilities are considered. One is that the introduction of a trifluoromethyl group at a
meta position induces greater molecular asymmetry and interactions between dye molecules decreased. The other is that the introduction of a local polar structure that causes an electrostatic interaction with liquid crystal molecules is effective for enlarging the solubility. Dichroic ratios of dyes
6~
9 are large enough (10~11) for application to GH-LCDs with efficient contrast.
2.3. Yellow anthraquinone dyes with a different phenylthio group restricted in their flexibility
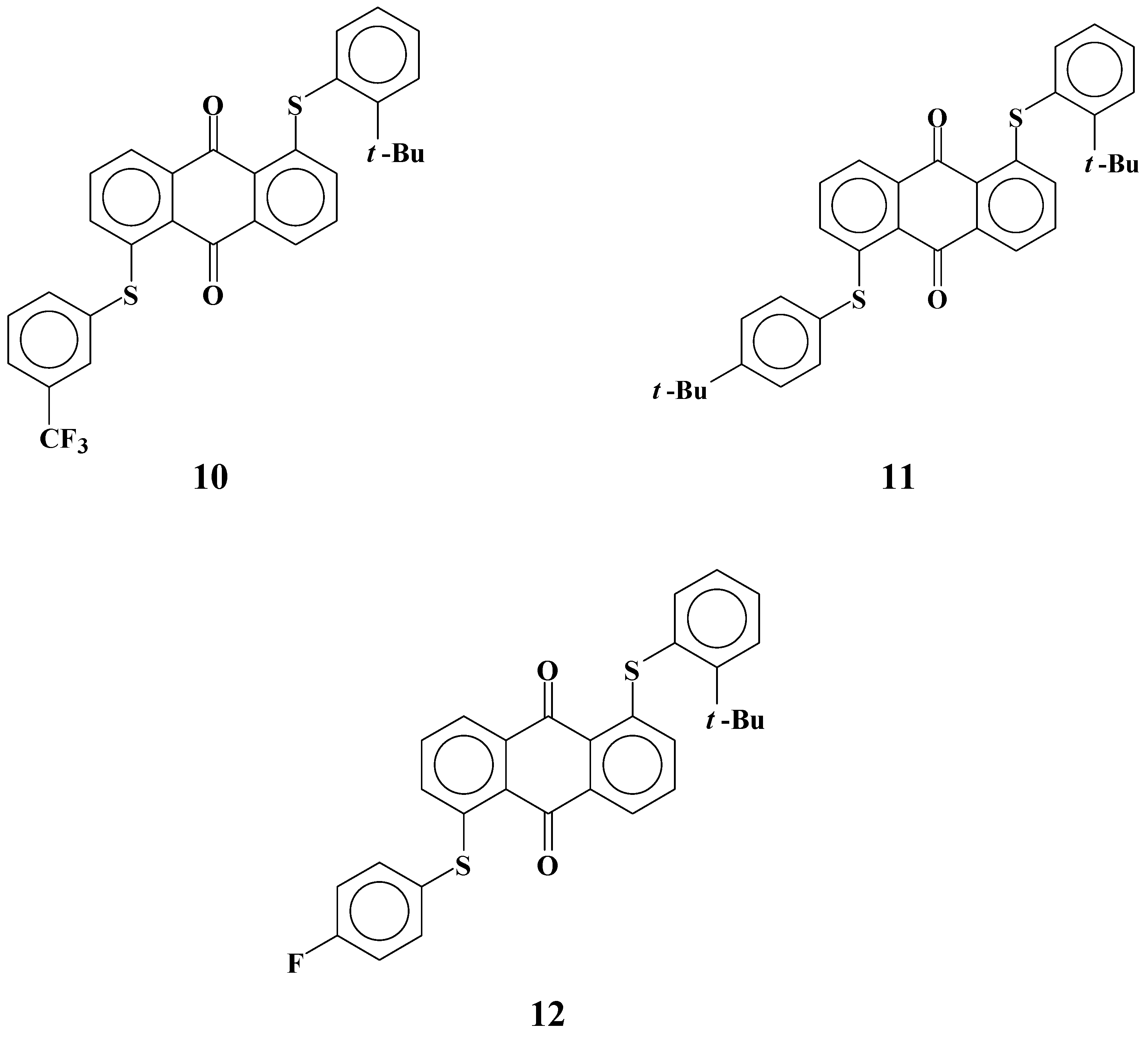
When electrostatic interactions between dye
8 and liquid crystal molecules increase the solubility, the flexibility of phenylthio groups has a predominant role in forming a suitable conformation enabling strong interactions of dyes and liquid crystals. Yellow anthraquinone dyes
10-
12 with phenylthio groups at the
ortho positions that restrict the flexibility of substituents were synthesized to clarify this thesis [
29] (
Figure 7). The solubilities of these dyes in fluorinated liquid crystals at room temperature and low temperatures and their dichroic ratios are shown in
Table 3.
Figure 7.
The molecular structures of yellow anthraquinone dyes with an ortho-substituted phenylthio group.
Figure 7.
The molecular structures of yellow anthraquinone dyes with an ortho-substituted phenylthio group.
Table 3.
The relationships between molecular structures of yellow anthraquinone dyes and their solubilities and dichroic ratios.
Table 3.
The relationships between molecular structures of yellow anthraquinone dyes and their solubilities and dichroic ratios.
| Dye | Solubility (wt%)1) | Dichroic ratio |
|---|
| | r. t. | Low temp.2) | |
|---|
| 10 | 1.0 | 0.5 | 7.2 |
| 11 | 1.8 | 1.6 | 7.9 |
| 12 | 1.4 | 1.3 | 7.8 |
| 1) Measured in fluorinated liquid crystals (LIXON 5052 XX). |
| 2) 267 - 268 K. |
Solubilities of ortho-substituted dye 10 are much smaller than those of para-substituted dye 8, despite their having the same molecular structure except for the position of the t-butyl group. Comparison of dyes 7 and 12 leads to the same conclusion. These results indicate that flexibility of phenylthio substituents has a significant role in increasing the solubilities. Dichroic ratios of dyes 10 and 12 are much lower than those of dyes 8 and 7, respectively. This drastic decrease of dichroic ratios cannot be fully explained by the smaller molecular length of dyes 10-12. Non-fluorinated dye 11 was investigated to distinguish the effects of ortho-substitution, and the same levels of solubilities and dichroic ratios as dyes 10 and 12 were obtained.
From these results, the hypothesis of a dichroic dye in liquid crystals was established. A dichroic dye in liquid crystals forms a suitable conformation to adjust to the liquid crystal phase, which is different from the conformation in isotropic solvents. In this conformation, the solubilities and dichroic ratios increase because the interactions between the dye and liquid crystal molecules are strengthened. The dyes with limited flexibility make it hard to form a suitable conformation and have inferior properties.
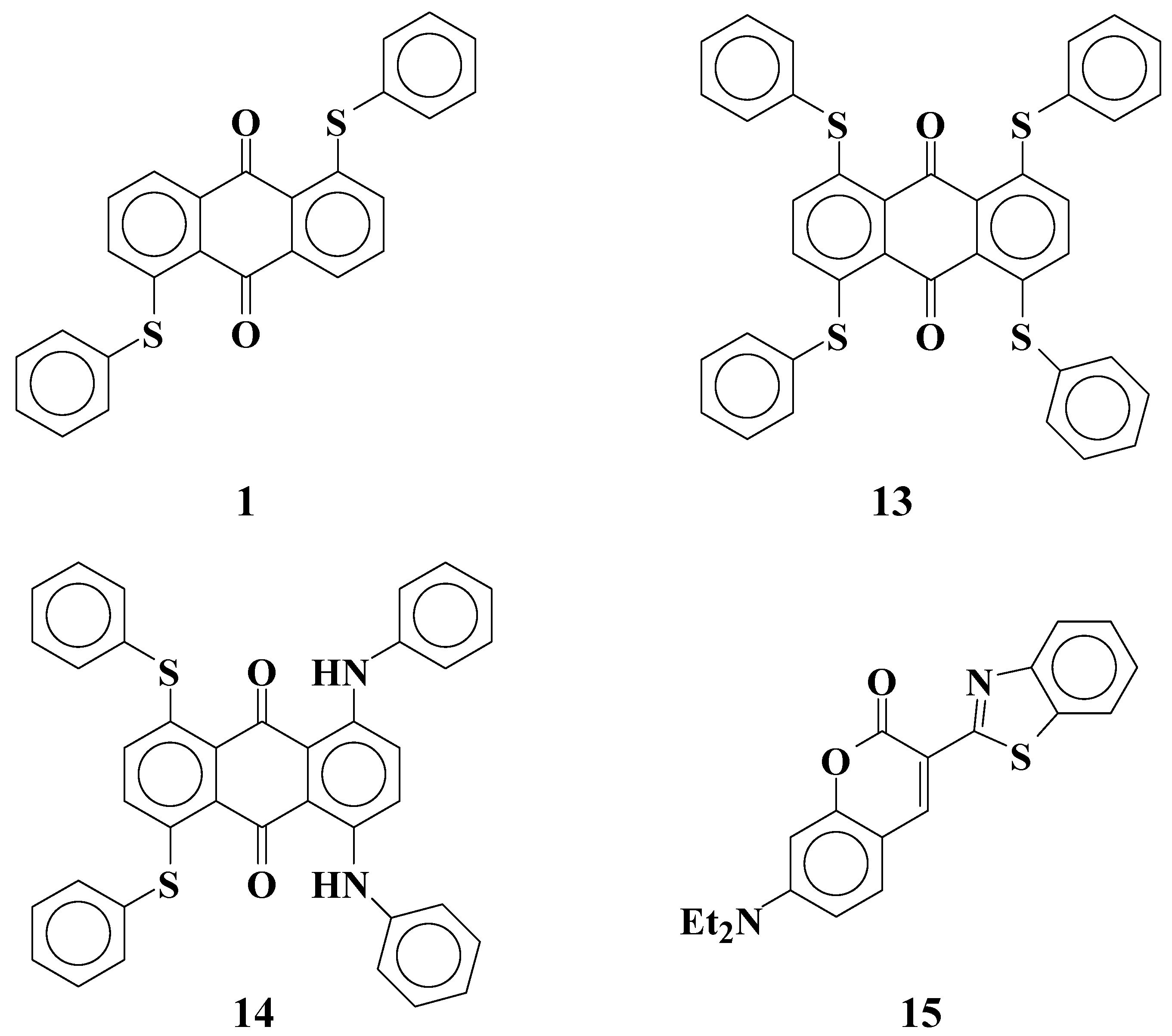
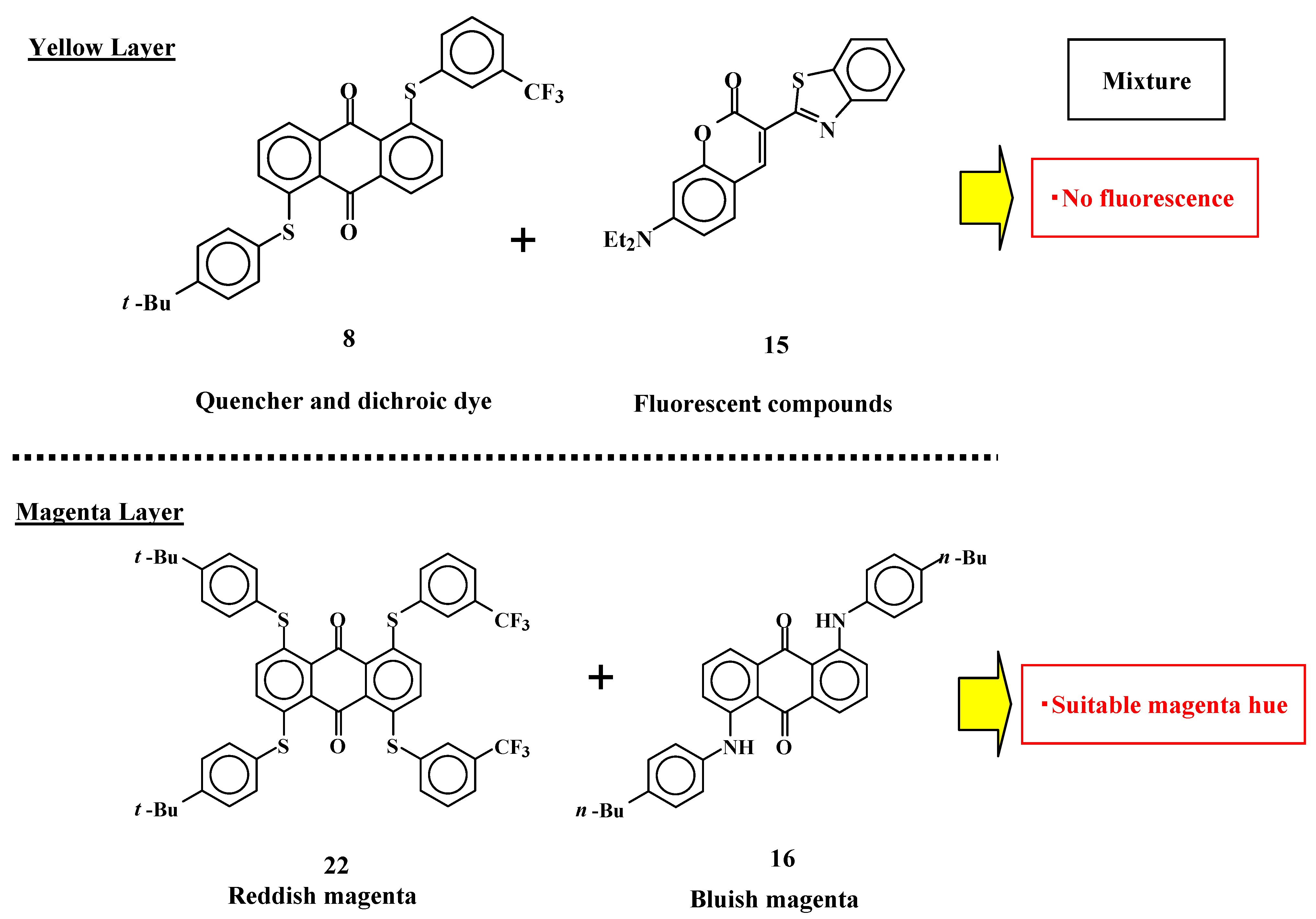
2.4. The mixture of anthraquinone dyes with two phenylthio groups and the coumarin dye as a yellow layer of three-layered GH-LCDs [30]
Table 4 lists the observed and calculated maximum absorption wavelength (λ
obsd and λ
calcd, respectively), the observed half bandwidth of absorption spectra (
HW: the width of the absorbance range when the absorbance is half of the maximum), and calculated oscillator strengths (
f) of anthraquinone dyes and coumarin dye (
Figure 8).
Table 4.
The observed and calculated properties of anthraquinone and coumarin dyes.
Table 4.
The observed and calculated properties of anthraquinone and coumarin dyes.
| Dye | λobsd1) | λcalcd | HW (nm) | f |
|---|
| 1 | 435 | 433 | 82 | 0.37 |
| 13 | 524 | 513 | 84 | 0.52 |
| 14 | 646 | 664 | 124 | 0.78 |
| 15 | 441 | 437 | 64 | 1.3 |
| 1) Measured in ethyl acetate. |
Figure 8.
The molecular structures of calculated anthraquinone and coumarin dyes.
Figure 8.
The molecular structures of calculated anthraquinone and coumarin dyes.
From the results listed in
Table 4, the
ε value of yellow anthraquinone dye
1 is predicted to be small, because its calculated oscillator strength (
f = 0.37) is small. On the other hand, coumarin dye
15 is expected to have a large
ε value (
f = 1.30) and act as a yellow dichroic dye. However, there are problems with coumarin dye
15, namely, its strong fluorescence adversely affects the hues it exhibit. To diminish the fluorescence of coumarin dye
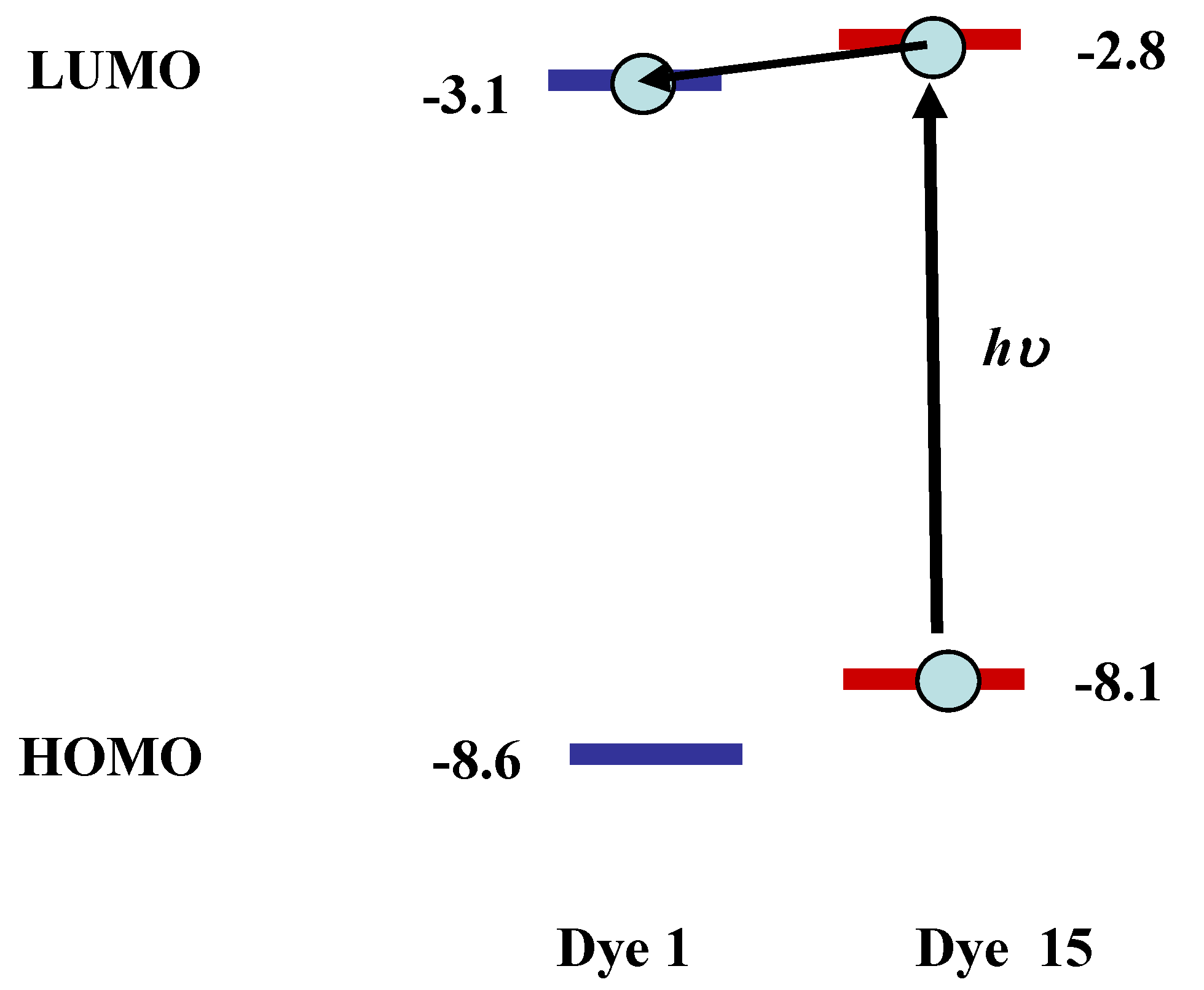
15, selection of an efficient quencher is considered to be effective. Yellow dichroic dye quencher that diminishes fluorescence of coumarin dye efficiently is the best one. The calculated HOMO and LUMO energy levels of anthraquinone dye
1 and coumarin dye
15 are shown in
Figure 9.
Figure 9.
The calculated energy levels (eV) of anthraquinone dye 1 and coumarin dye 15.
Figure 9.
The calculated energy levels (eV) of anthraquinone dye 1 and coumarin dye 15.
The lower HOMO and LUMO energy levels of anthraquinone dye 1 relative to coumarin dye 15 indicate that electron transfer will occur from coumarin dye in the excited state to anthraquinone dye. Strong fluorescence of coumarin dye is expected to be quenched through the mechanism.
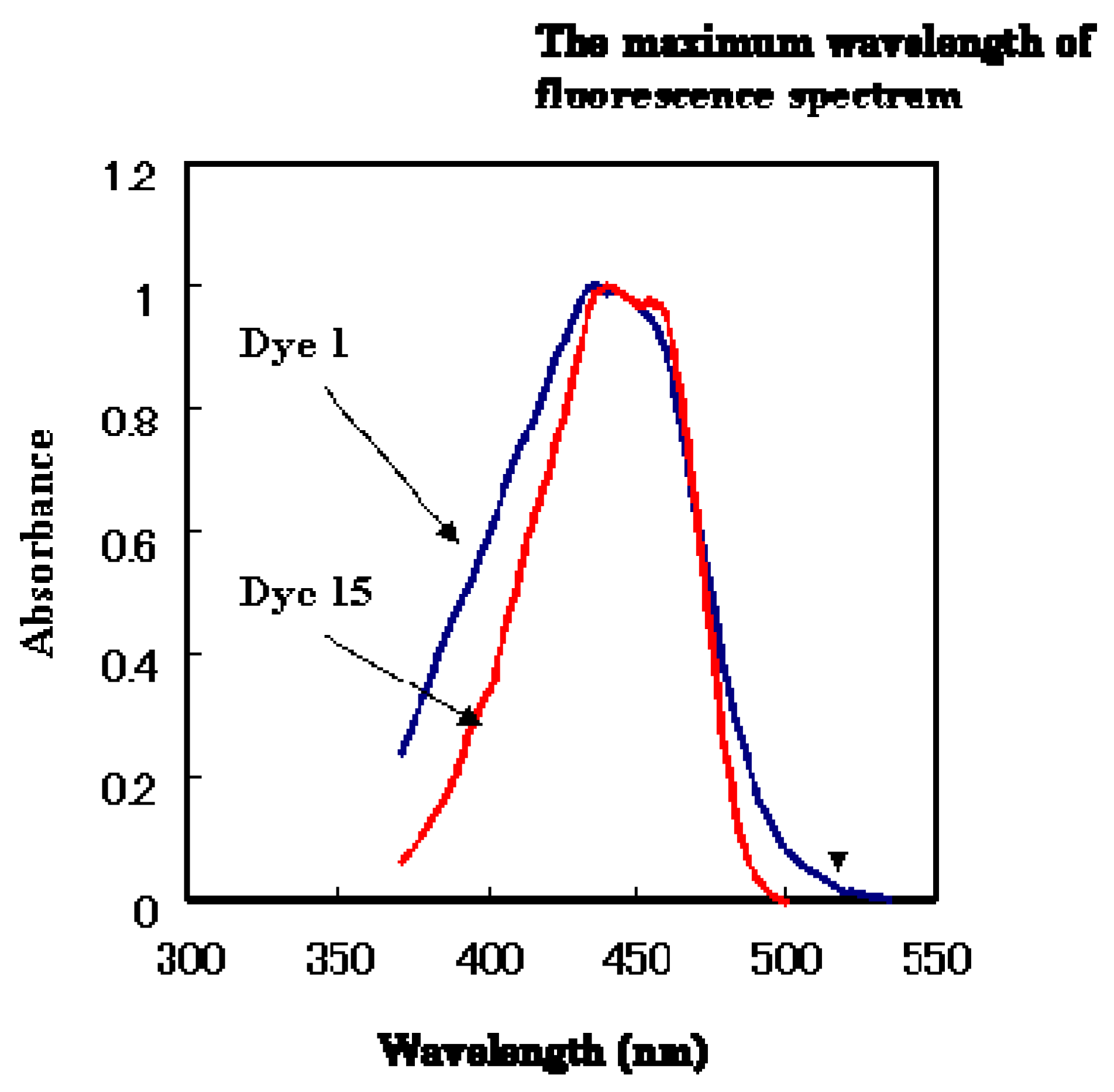
Figure 10.
The absorption spectra of anthraquinone dye 1 and coumarin dye 15 (in ethyl acetate).
Figure 10.
The absorption spectra of anthraquinone dye 1 and coumarin dye 15 (in ethyl acetate).
There is another possible fluorescence quenching mechanism. The absorption spectra of dyes
1 and
15 are shown in
Figure 10. The wider absorption tail (>500 nm) of dye
1 relative to dye
15 implies that fluorescence is quenched by energy transfer. For these two reasons, anthraquinone dye
1 is thought to behave as an efficient quencher of coumarin dye
15. The fluorescence spectra of the mixtures of anthraquinone dye
1 and coumarin dye
15 in ethyl acetate at 297 K are shown in
Figure 11.
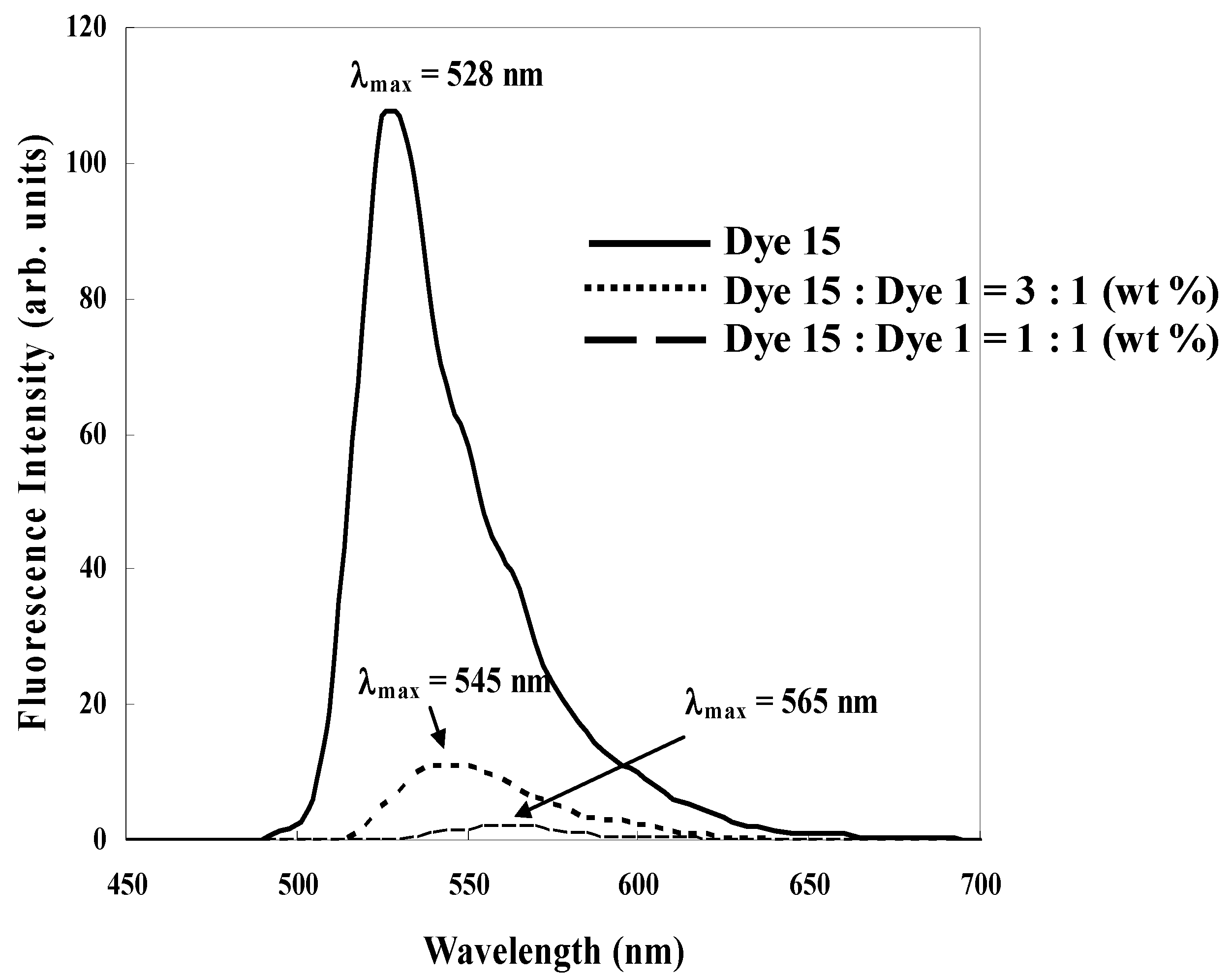
Figure 11.
The fluorescence spectra of the mixtures of anthraquinone dye 1 and coumarin dye 15.
Figure 11.
The fluorescence spectra of the mixtures of anthraquinone dye 1 and coumarin dye 15.
As the concentration of anthraquinone dye 1 increases to the constant concentration of coumarin dye 15, a rapid decrease of the fluorescence intensity was observed, as predicted. Only the ethyl acetate solution of anthraquinone dye 1 was found to have little fluorescence. Fluorescence intensities were measured in a very dilute solution. The fluorescence quenching was therefore considered to occur by energy transfer. Increasing the ratio of anthraquinone dye 1 to coumarin dye 15 causes the maximum wavelength of the fluorescence spectra to shift to a longer wavelength. This result indicates that an excitation complex of anthraquinone and coumarin dyes has been formed.
These studies show that the mixture of yellow anthraquinone dye with two phenylthio groups at the 1 and 5 positions and yellow coumarin dye gives a good yellow spectrum, weak fluorescence and large absorption coefficient, and effective for realizing GH-LCDs with high performances.
2.5. Magenta anthraquinone dyes with four phenylthio groups or two anilino groups
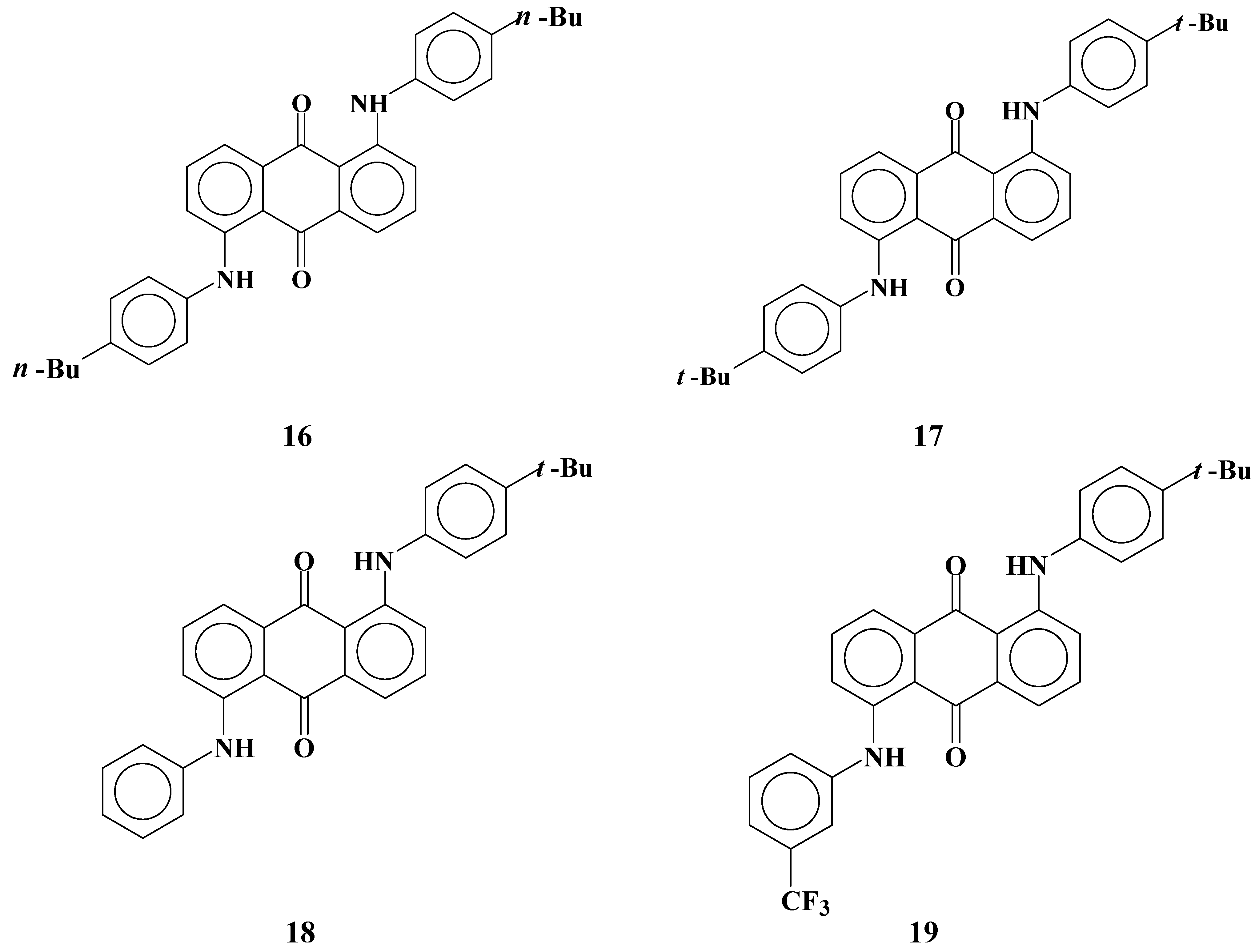
Anthraquinone dyes with two anilino groups at the 1 and 5 positions are widely known magenta dichroic dyes. Solubilities and dichroic ratios of dyes
16-
19 (
Figure 12) were investigated.
Figure 12.
The molecular structures of magenta anthraquinone dichroic dyes with two anilino groups at the 1 and 5 positions.
Figure 12.
The molecular structures of magenta anthraquinone dichroic dyes with two anilino groups at the 1 and 5 positions.
Table 5.
Solubilities and dichroic ratios of magenta anthraquinone dyes with two anilino groups at 1 and 5 positions.
Table 5.
Solubilities and dichroic ratios of magenta anthraquinone dyes with two anilino groups at 1 and 5 positions.
| Dye | Solubility (wt%)1) | Dichroic ratio |
|---|
| | r. t. | low temp.2) | |
|---|
| 16 | 2.3 | 0.11 | 6.7 |
| 17 | 0.23 | 0.04 | 6.7 |
| 18 | 1.6 | 0.6 | 6.7 |
| 19 | 0.35 | 0.03 | 5.5 |
| 1) Measured in fluorinated liquid crystals (LIXON 5052 XX). |
| 2) 267 - 268 K. |
Dye
16 with flexible substituents (
n-butyl groups) is highly soluble at room temperature. Dye
17 has the same molecular structures as dye
16 except that the substituents of the anilino groups were
t-butyl groups instead of
n-butyl groups, and solubilities are very low compared with those of dye
16. Introduction of flexible groups is effective for enlarging solubilities at room temperature. However, the solubility of dye
16 decreases severely at low temperatures [
26]. This result indicates that when the GH-LCDs are kept at low temperatures, dye crystallization may take place. Therefore, introduction of flexible substituents in dyes to increase solubility, which is the usual way of increasing solubility, is not suitable for molecular design of dyes used at low temperatures in large concentration.
The asymmetric dye
18 with two different anilino groups is highly soluble at room temperature, and the solubility decreased little even at low temperatures. The asymmetric dye
19, which has the same molecular structure as dye
18 but with trifluoromethyl groups introduced at
meta positions of an non-substituted anilino group was found to have a relatively lower solubility. Unlike in the case of dye
8 with phenylthio groups, introduction of trifluoromethyl groups has a negative effect on increasing solubility. Moreover, dichroic ratios of dyes shown in
Table 5 are much lower than those of dyes shown in
Table 2, even if the substituents of phenyl groups are the same. This is considered to be due to the difference in the connection part between an anthraquinone skeleton and phenylthio or anilino groups. Flexibility of anilino groups is considered to be inferior to that of phenylthio groups for the following reasons. The length of C-N bond is smaller than that of C-S bond. Bond orders of anthraquinone dyes with phenylthio and anilino groups were calculated by the PPP-CI method (
Table 6).
Table 6.
The bond orders of C-N and C-S bonds calculated by PPP-CI methods.
![]()
Table 6.
The bond orders of C-N and C-S bonds calculated by PPP-CI methods. ![]()
| X | Bond order |
|---|
| | Ph (C)-X | Anthraquinone (C)-X |
|---|
| S | 0.32 | 0.39 |
| NH | 0.44 | 0.54 |
The bond orders of C-N bonds were found to be larger than those of C-S bonds. Stronger double bonding properties of C-N bonds are considered to restrict the flexibility of anilino groups. Inner molecular hydrogen bond also restricts the flexibility of anilino groups. The anilino group signal in the NMR spectra of dye 16 is shifted to lower magnetic fields from the original chemical shift and is very broad [1H-NMR (400 MHz, CDCl3): δH 11.3 (2H, s, br, NH)]. These results means that intramolecular hydrogen bonds are formed in dye 16 and rotation of anilino groups are restricted.
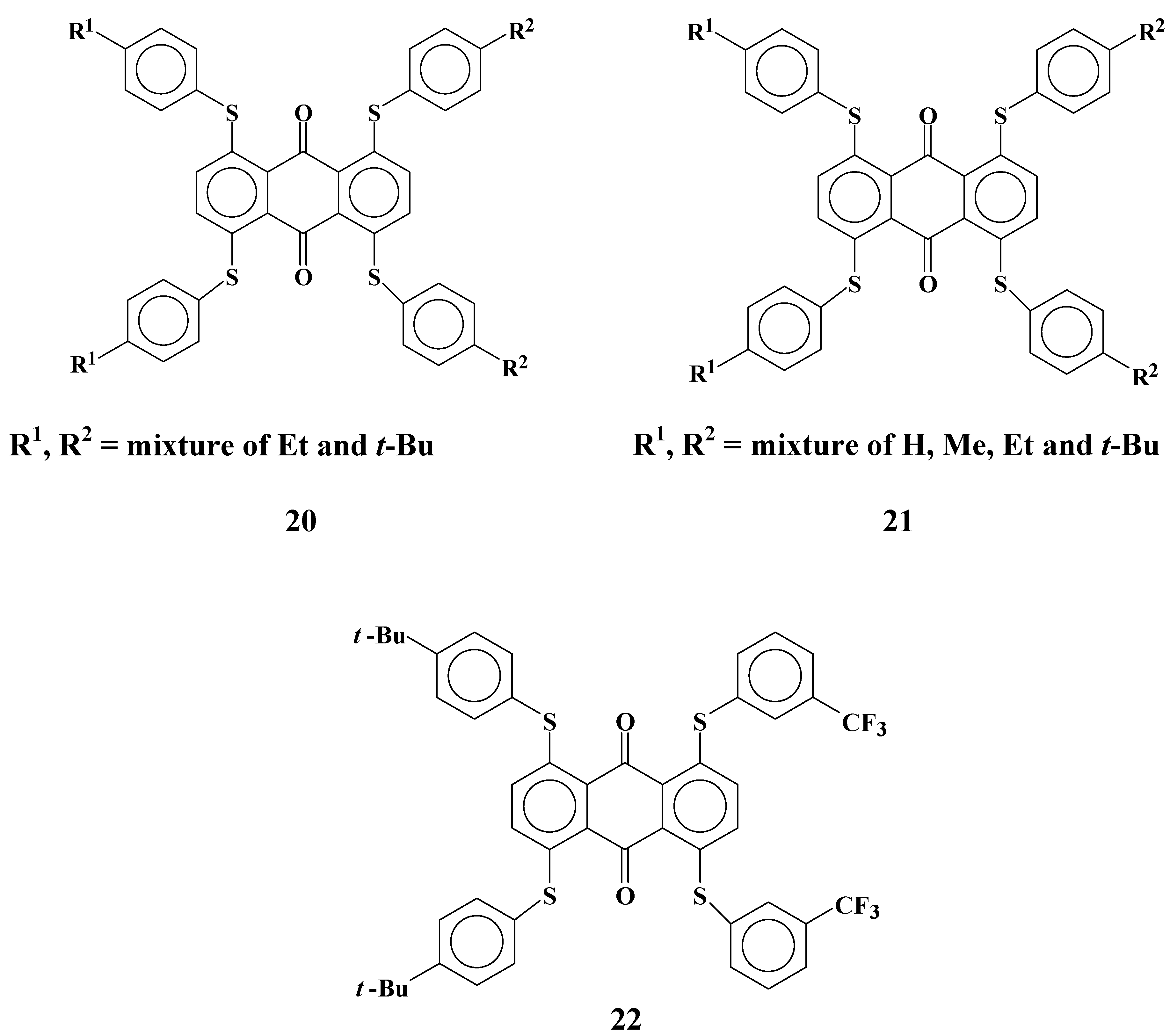
Anthraquinone dyes with phenylthio groups at the 1, 4, 5 and 8 positions are selected for magenta dichroic dyes for the three-layer GH-LCDs as described in
section 2.1. The synthesized molecular structures of magenta dyes
20-
22 are shown in
Figure 13. Solubilities and dichroic ratios of these dyes are listed in
Table 7.
Dyes 20 and 21 are mixtures of several dyes, but solubilities at low temperatures are not very large. On the other hand, as in the case of yellow anthraquinone dye 8, solubilities of dye 22 are very large even at low temperatures. These results mean that the combination of p-tert-butylphenylthio and m-trifluoromethylphenylthio groups drastically increases the solubility. Moreover, dichroic ratios of dyes 20-22 are preferable for the three-layer GH-LCDs with large contrast.
Figure 13.
The molecular structures of magenta dichroic dyes with phenylthio groups at the 1, 4, 5 and 8 positions.
Figure 13.
The molecular structures of magenta dichroic dyes with phenylthio groups at the 1, 4, 5 and 8 positions.
Table 7.
Solubilities and dichroic ratios of magenta anthraquinone dichroic dyes with phenylthio groups at the 1, 4, 5 and 8 positions.
Table 7.
Solubilities and dichroic ratios of magenta anthraquinone dichroic dyes with phenylthio groups at the 1, 4, 5 and 8 positions.
| Dye | Solubility (wt%)1) | Dichroic ratio |
|---|
| | r. t. | low temp.2) | |
|---|
| 20 | 0.31 | 0.031 | 10 |
| 21 | 1.35 | 0.18 | 10 |
| 22 | 1.70 | 1.5 | 10 |
| 1) measured in fluorinated liquid crystals (LIXON 5052 XX). |
| 2) 267 – 268 K. |
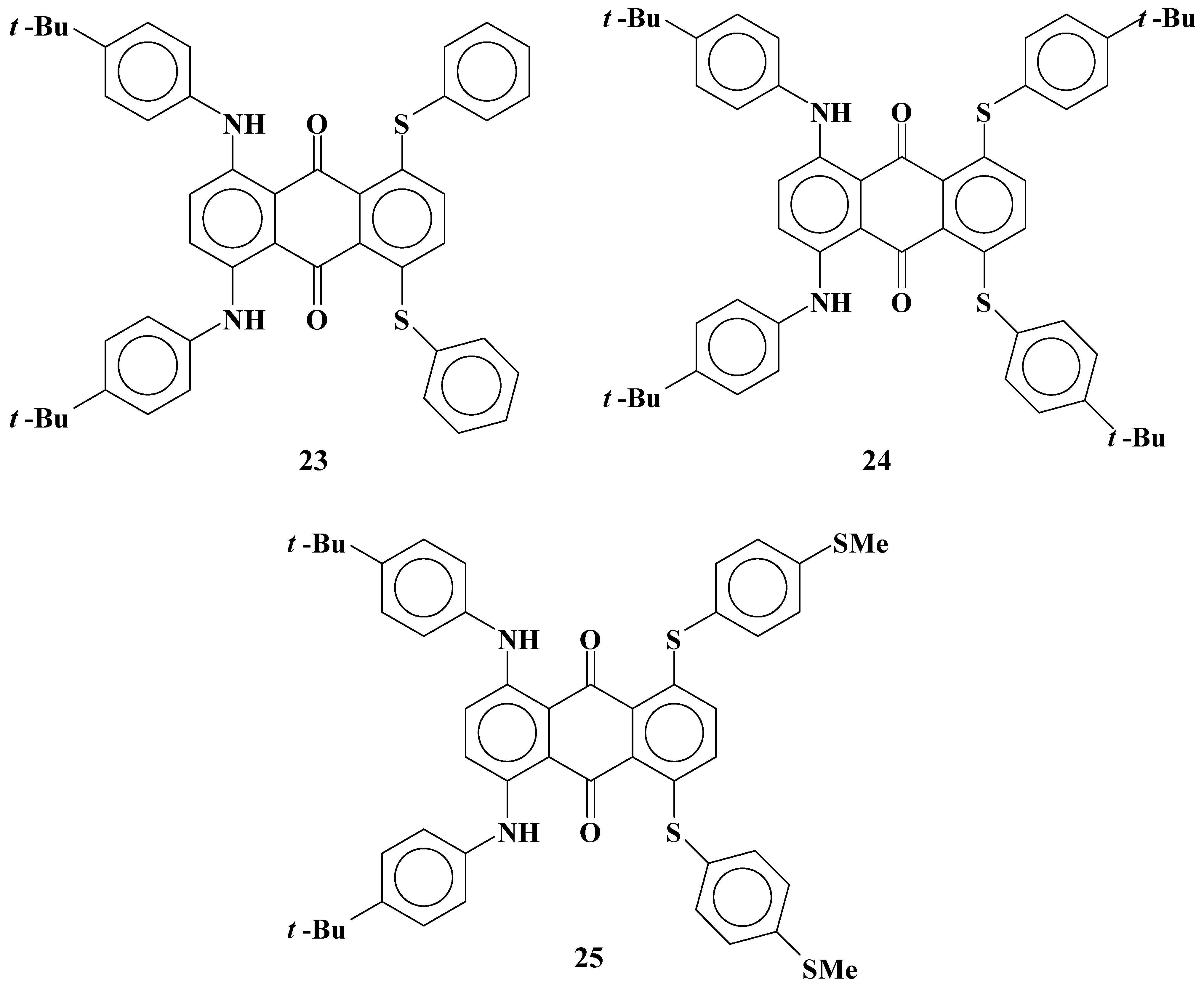
2.6. Cyan anthraquinone dyes with two phenylthio groups and two anilino groups
Cyan anthraquinone dyes
23~
25 with two phenylthio groups and two anilino groups were synthesized (
Figure 14).
Figure 14.
The molecular structures of cyan anthraquinone dyes with two phenylthio groups and two anilino groups.
Figure 14.
The molecular structures of cyan anthraquinone dyes with two phenylthio groups and two anilino groups.
Solubilites and dichroic ratios of dyes
23~
25 are shown in
Table 8.
Table 8.
Solubilities and dichroic ratios of cyan anthraquinone dichroic dyes with two phenylthio groups and two anilino groups.
Table 8.
Solubilities and dichroic ratios of cyan anthraquinone dichroic dyes with two phenylthio groups and two anilino groups.
| Dye | Solubility (wt%) 1) | Dichroic ratio |
|---|
| | r. t. | low temp. | |
|---|
| 23 | 0.06 | 0.04 | 5.1 |
| 24 | 0.09 | 0.06 | 5.7 |
| 25 | 0 | 0 | Cannot be measured |
| 1) Measured in fluorinated liquid crystals (LIXON 5052 XX). |
| 2) 267 – 268 K. |
Solubilities and dichroic ratios of anthraquinone dyes 23~25 are very low compared with those of dyes 20~22. This marked difference from the results of anthraquinone dyes with four phenylthio groups is considered to be due to the effects of the connection parts between an anthraquinone skeleton and substituents, namely, S or NH. This result is the same as that for dye 8 with two phenylthio groups and that for dye 19 with two anilino groups.
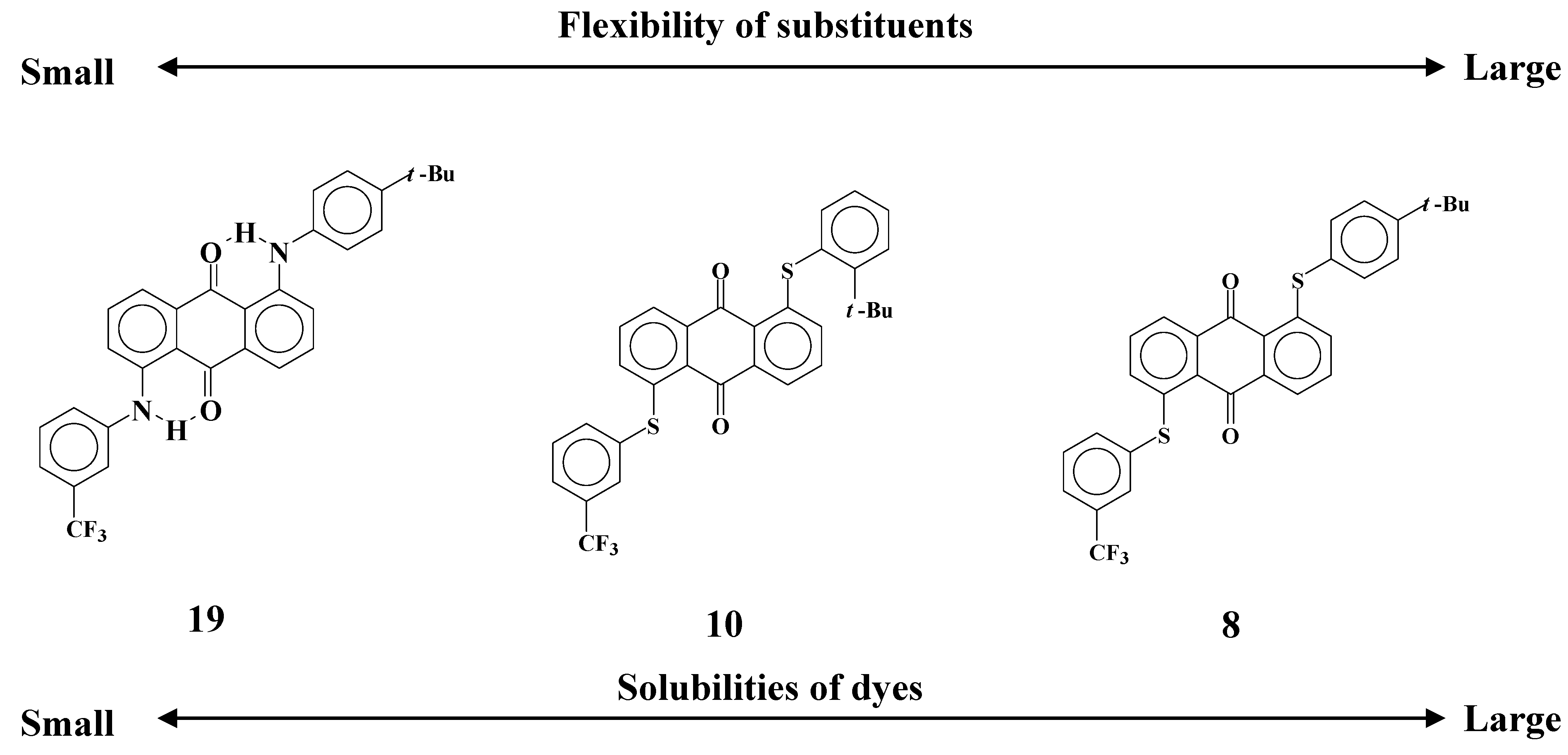
2.7. Difference of the structures of solvation in liquid crystals and isotropic phase
Comparison of dyes
8,
10 and
19 reveals that solubilities and dichroic ratios are closely related to the flexibility of the substituents as shown in
Figure 15.
Figure 15.
The relationships between flexibility of substituents and solubilities of dyes with analogous molecular structures.
Figure 15.
The relationships between flexibility of substituents and solubilities of dyes with analogous molecular structures.
In the liquid crystal phase, specific structures of solvation, which is different from the solvation in isotropic solutions, are formed and influence the solubility. And flexibility of substituents is very important for ensuring a suitable conformation matching the liquid crystal phase [
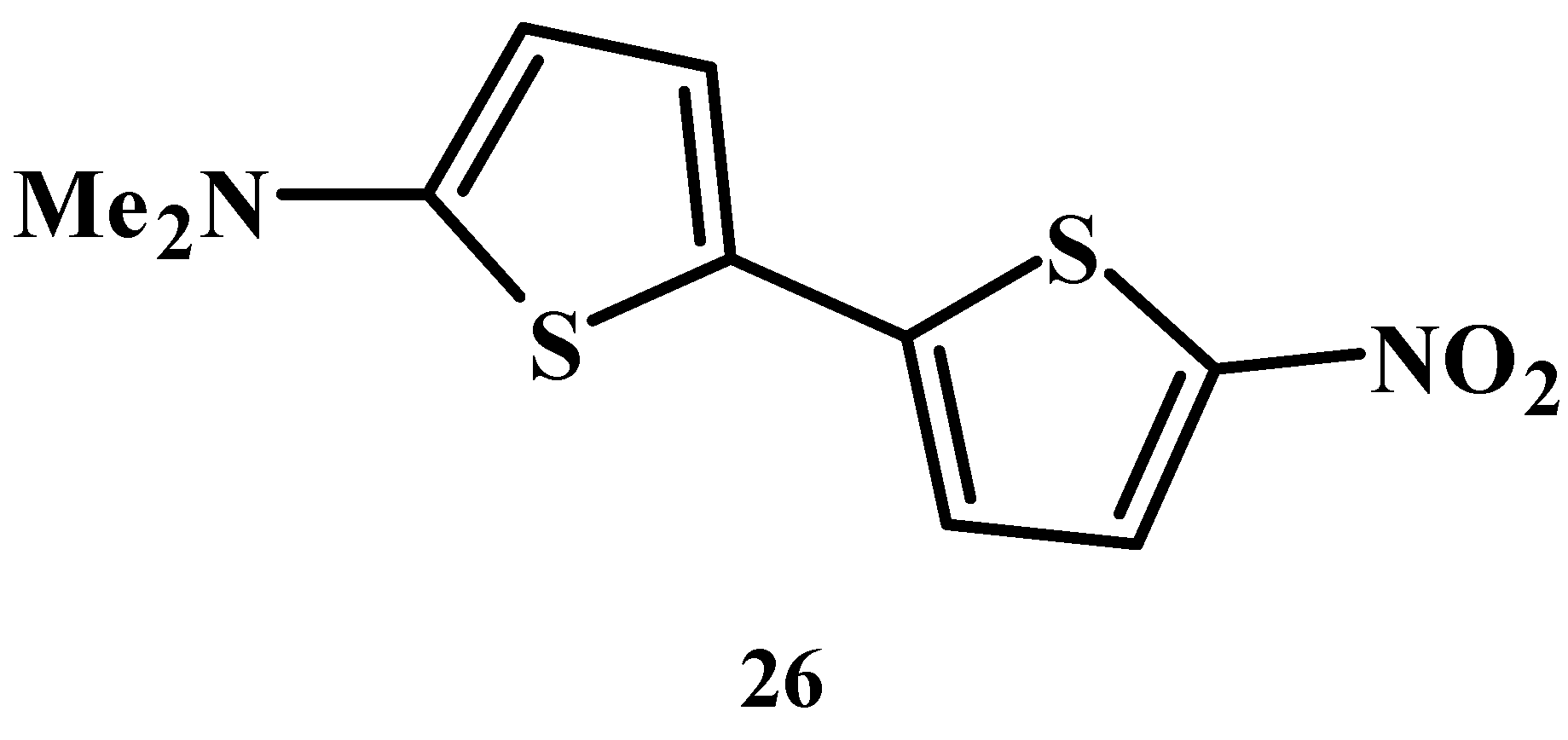
29]. Donor-acceptor-substituted oligothiophene compound
26 shown in
Figure 16 is known to show notable solvatochromic behaviors [
31,
32].
Figure 16.
The molecular structure of donor-acceptor-substituted oligothiophene compound 26.
Figure 16.
The molecular structure of donor-acceptor-substituted oligothiophene compound 26.
The solvatochromic behaviors, dielectric constants and solubilities of the dichroic dyes are thought to be correlated. Solvents with large dielectric constant behave as good ionic mediums. These solvents are considered effectively to stabilize the dyes in excited states, which have more polar structures than dyes in ground states. The stabilization of dyes in excited states induces longer wavelength shifts of the maximum absorption wavelengths of dyes; this is the origin of positive solvatochromic behaviors. On the other hand, solvents with large dielectric constants are considered to dissolve polar compounds such as dyes more effective than the solvents with small dielectric constant; thus solvatochromic behaviors can be correlated with the solubilities of the dyes.
The relationship between dipole moments and solubilities of the dyes is explained as follows. Since a dipole moment shows the value of the polarity of one molecule, a chemical compound such as a dye with a large polarity can be more easily dissolved as the dipole moment of a solvent becomes larger. Also, larger solvent dipole moments will stabilize dyes in their excited state more effectively. The stabilization of dyes in excited states induces the solvatochromic behaviors. Thus, a solvent with a larger dipole moment will dissolve a larger amount of dye, and it will increase the value of the solvatochromic behaviors.
In liquid crystals, maximum wavelengths of dyes shift to longer wavelength region compared with an isotropic solvent with close dipole moments and dielectric constant, indicating the formation of specific structures of solvation. To clarify this hypothesis, solvatochromic dye
26 was dissolved in fluorinated liquid crystals, and heated to the clearing point and maximum absorption wavelengths were observed continuously. Below the temperature of the clearing point, the maximum absorption wavelength hardly shifted. However, at temperatures above the clearing point, it shifted sharply to a shorter wavelength [
32]. This phenomenon was not due to the thermodynamic effects but indicated the change of structures of solvation because it was observed in various liquid crystals with different clearing points.
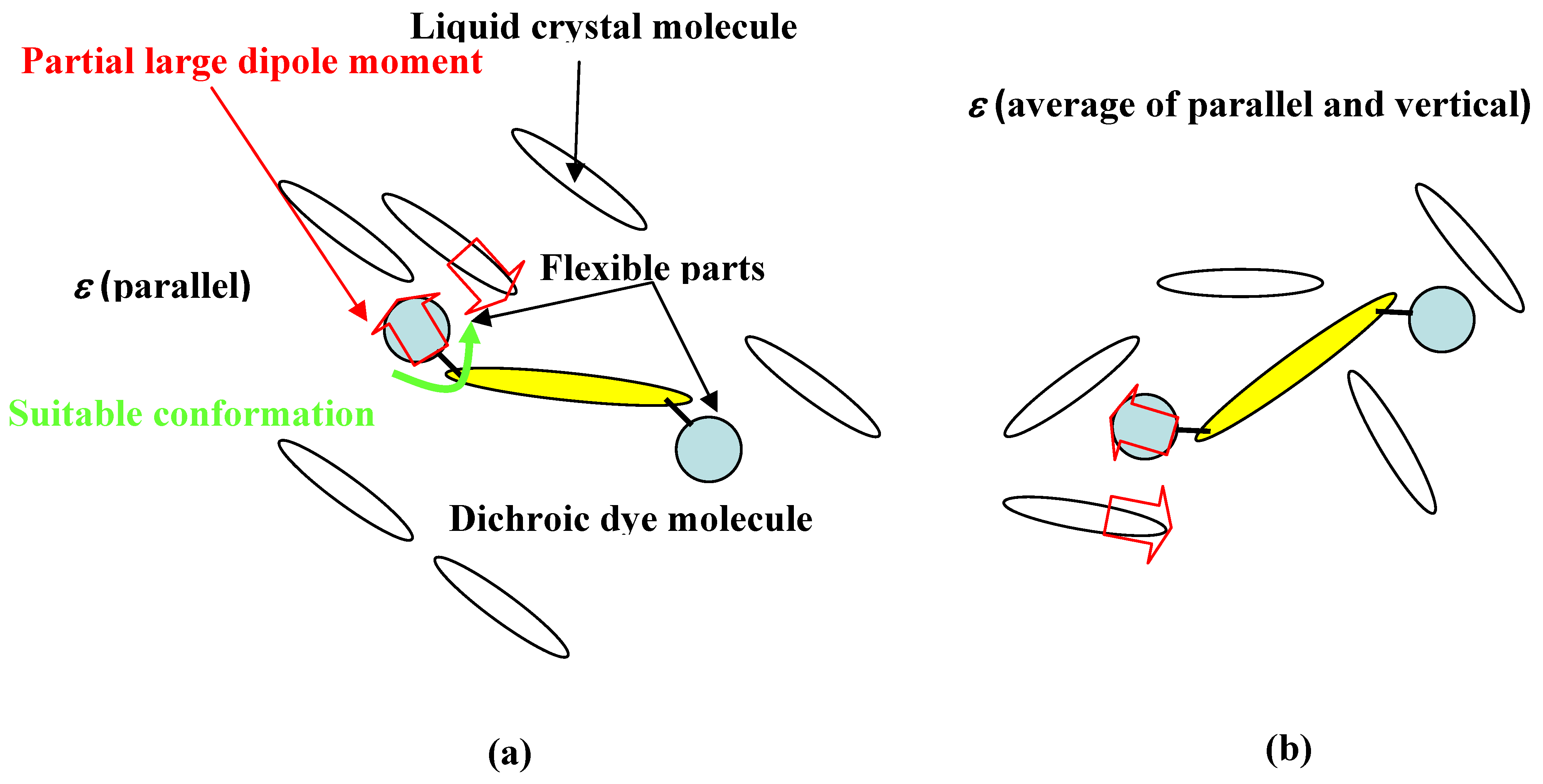
Figure 17.
The model of structures of solvation. (a) in liquid crystal phase. (b) in isotropic phase.
Figure 17.
The model of structures of solvation. (a) in liquid crystal phase. (b) in isotropic phase.
In the liquid crystal phase, the dye aligns to the direction of the long axis of liquid crystal molecules and packing is closer than in the isotropic phase. With liquid crystals and dye molecules parallel to each other, strong dipole-dipole interactions between a polar part of flexible substituent with suitable conformation and rigid polar part of liquid crystals are realized. Moreover, in the liquid crystal phase, dye is influenced by the parallel dielectric constant which is larger than the vertical dielectric constant in the case of positive liquid crystals, inducing longer wavelength shift.
On the other hand, in the isotropic phase (with liquid crystals heated above their clearing point), the structures of solvation are converted to be analogous to those of a normal solvent. Dipole-dipole interaction between liquid crystals and dye molecules is weaker than in the liquid crystal phase, and dye is influenced by the average of parallel and vertical dielectric constant. Above all, the intensity of solvation is stronger in the liquid crystal phase than in the isotropic phase, and dyes with polar flexible substituents are highly soluble, forming a suitable conformation to adjust to the liquid crystal phase [
29] (
Figure 17).