Development of Thin Carbon-Ceramic Based Coatings in Roll-to-Roll Mode: Tribological and Corrosion Results on Stainless Steel
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Coatings
2.3. Characterization
2.4. Tribological Tests
2.5. Corrosion Tests
3. Results
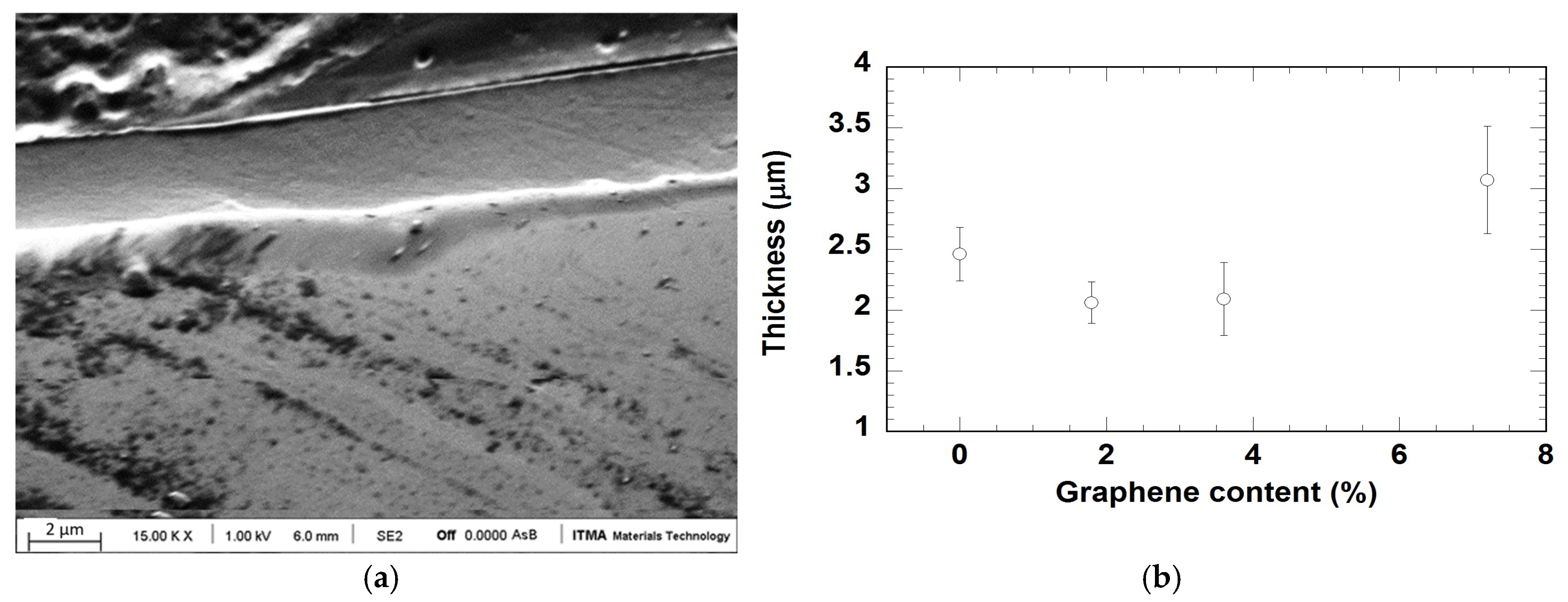
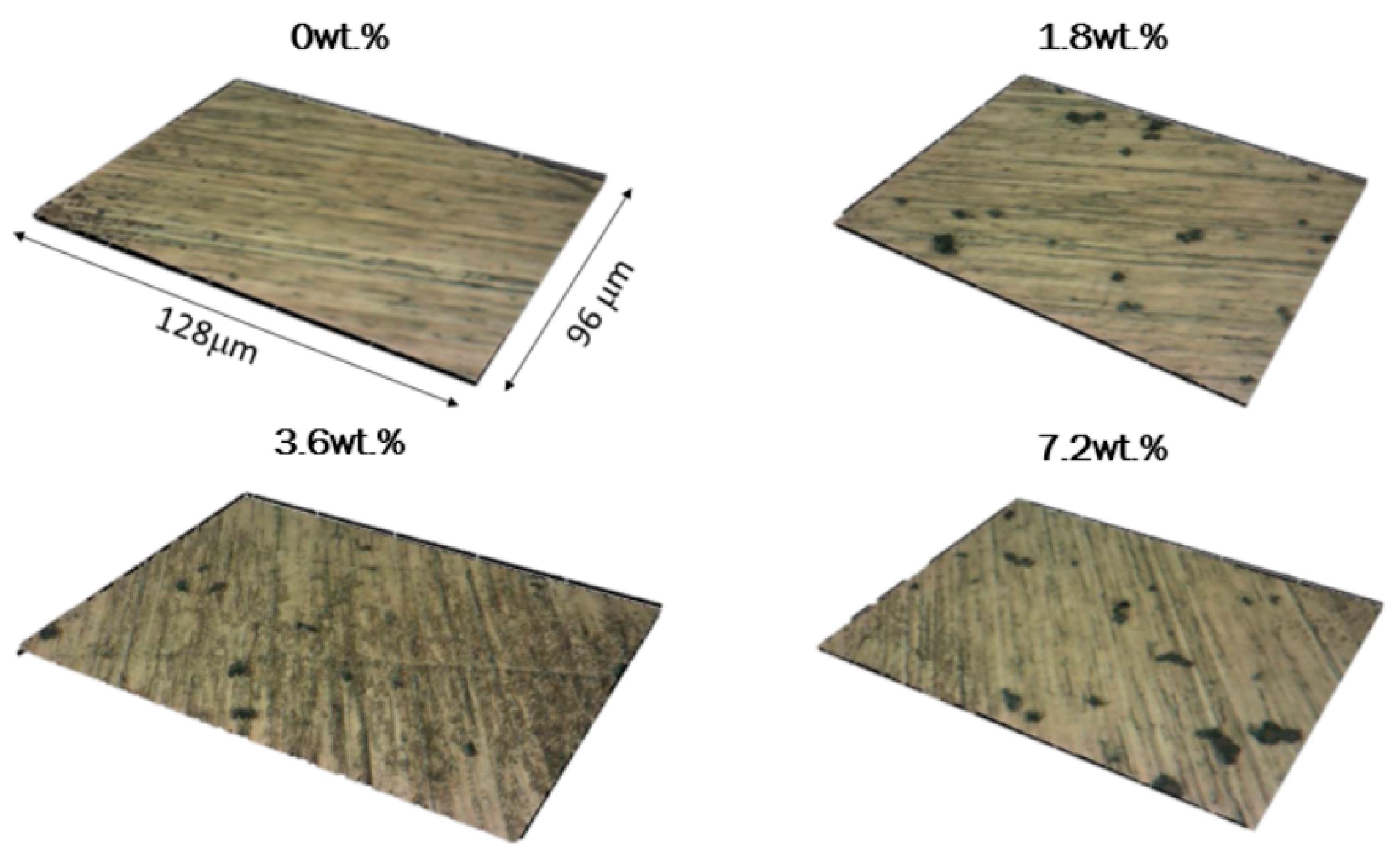
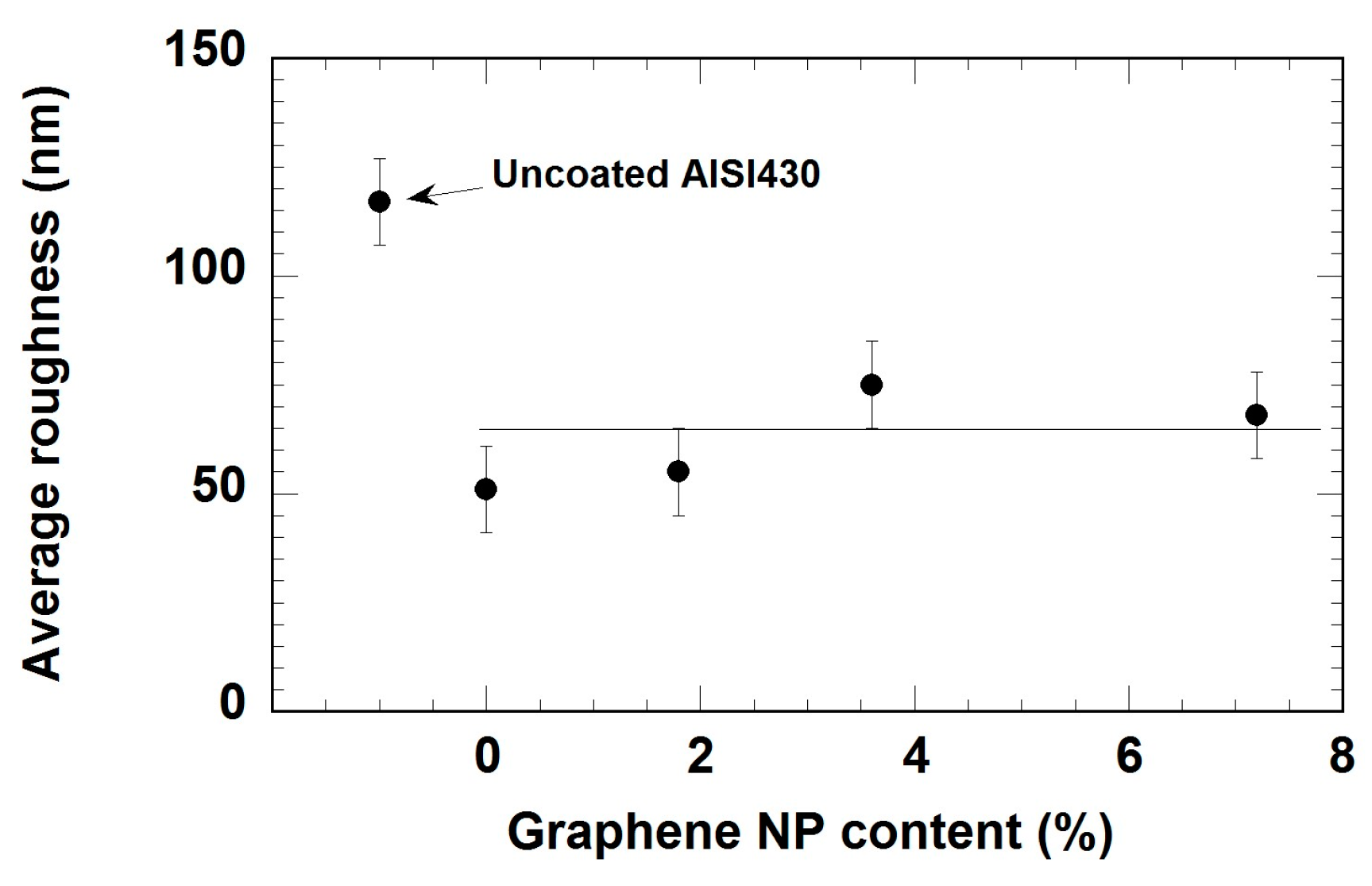
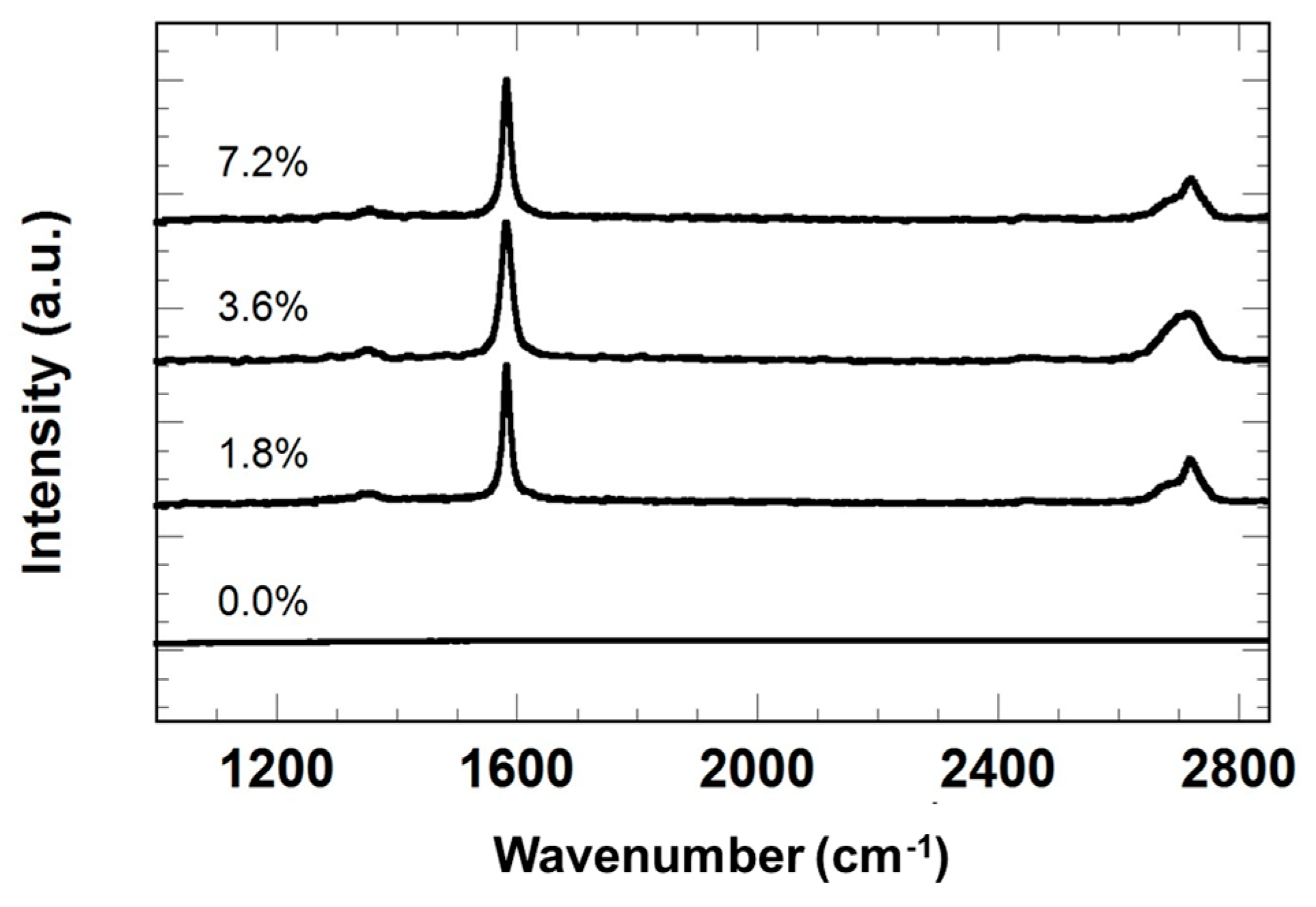
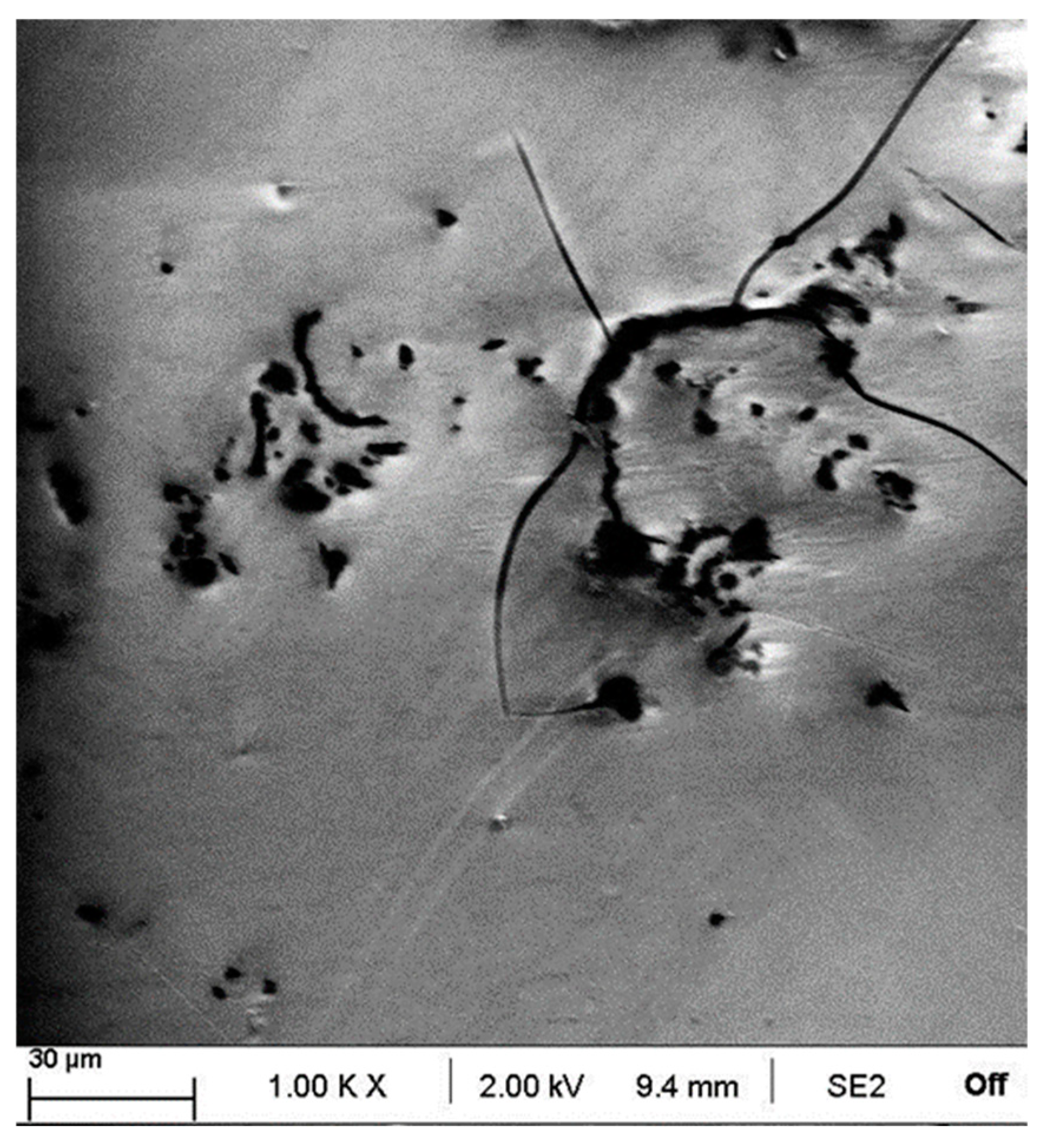
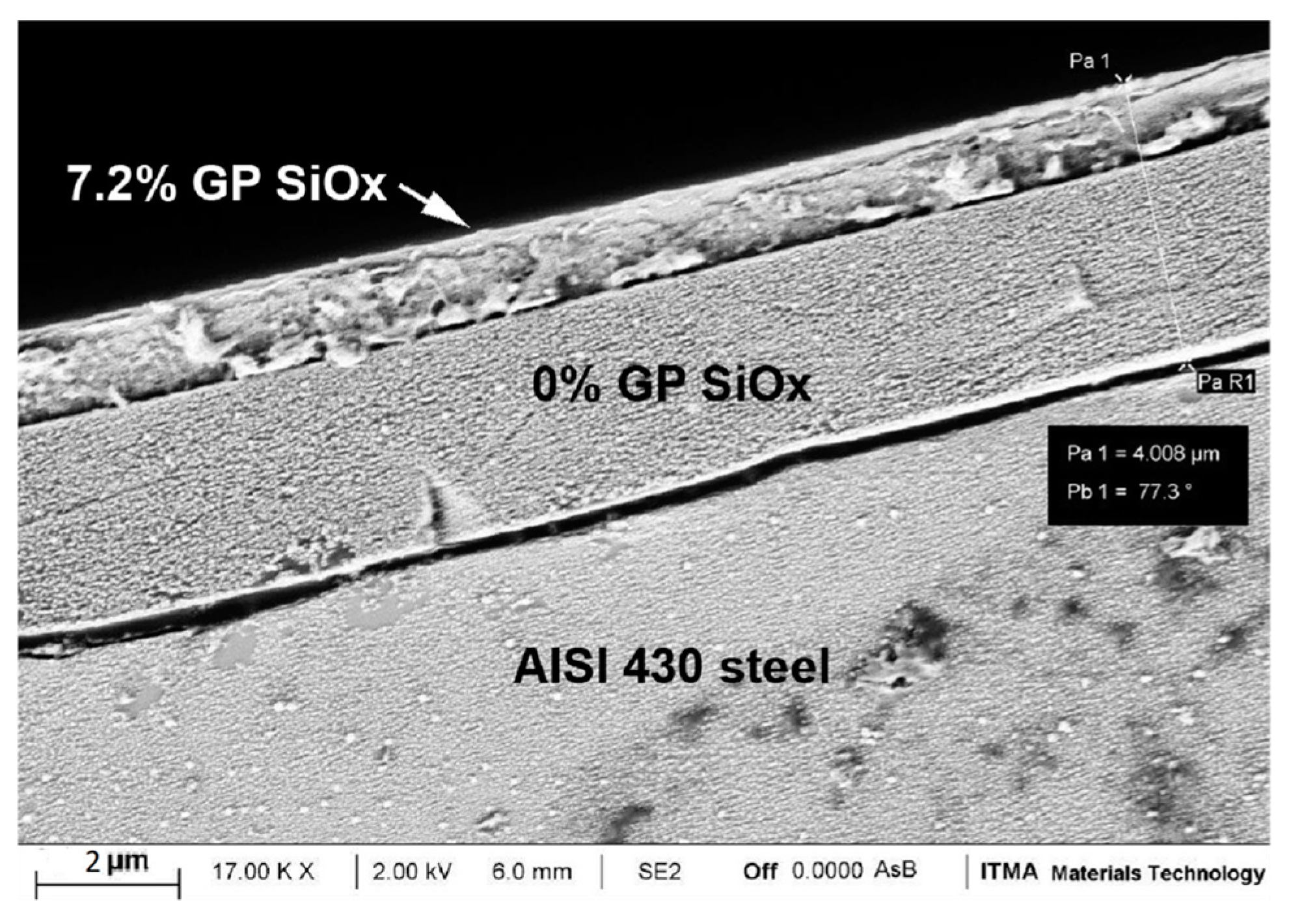
3.1. Morphology and Thickness
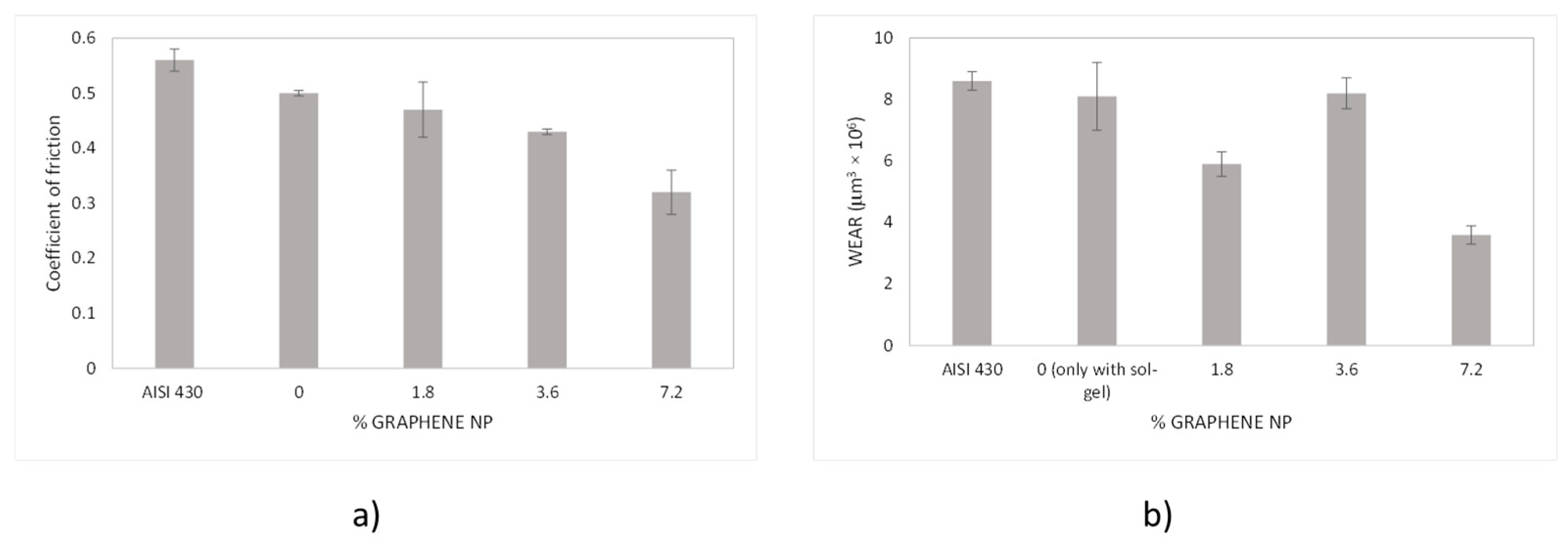
3.2. Tribology and Wear
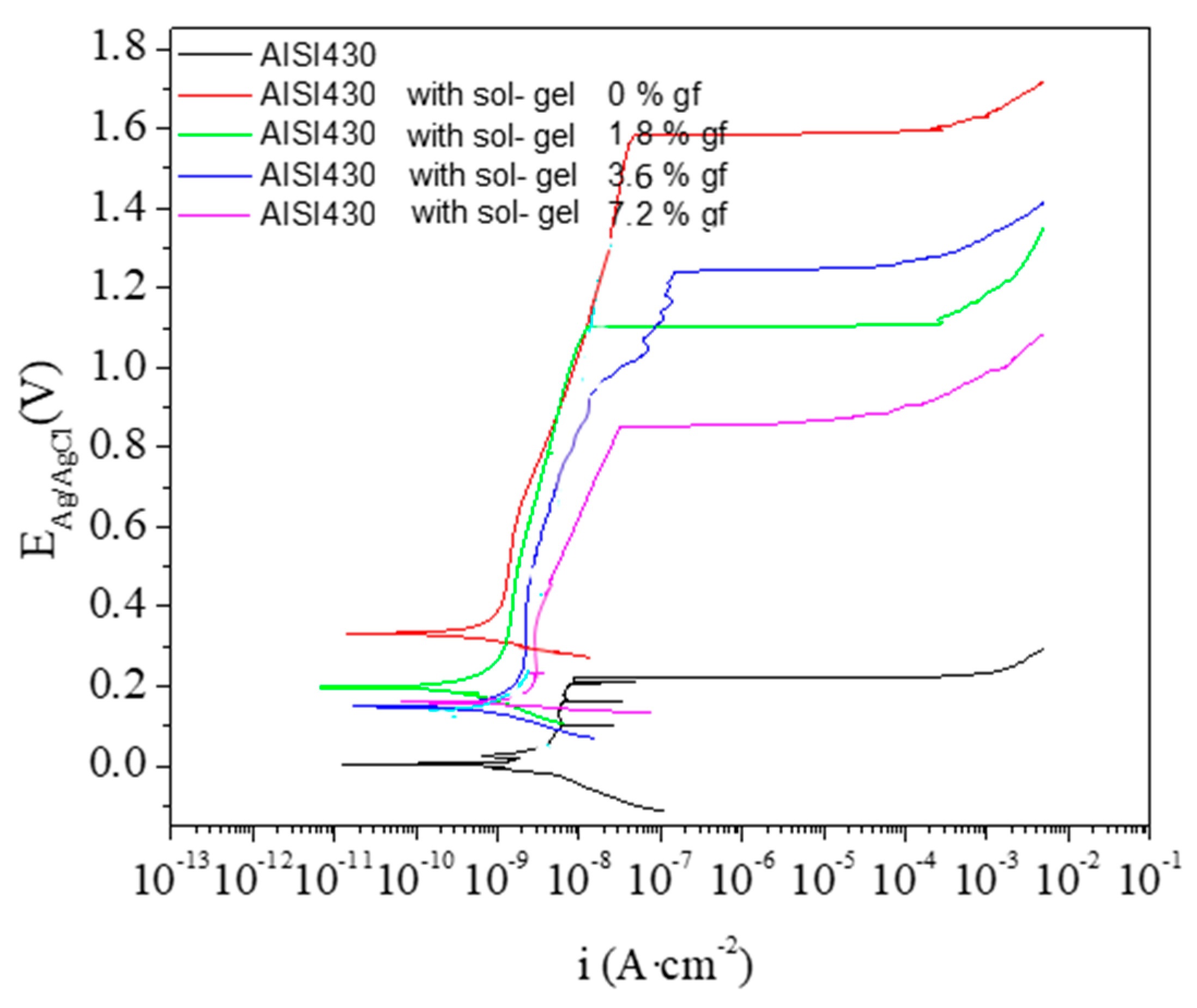
3.3. Corrosion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments

Conflicts of Interest
References
- Wu, Y.; Zhu, X.; Zhao, W.; Wang, Y.; Wang, C.; Xue, Q. Corrosion mechanism of graphene coating with different defect levels. J. Alloys Compd. 2019, 777, 135–144. [Google Scholar] [CrossRef]
- Anisur, M.R.; Chakraborty Banerjee, P.; Easton, C.D.; Singh Raman, R.K. Controlling hydrogen environment and cooling during CVD graphene growth on nickel for improved corrosion resistance. Carbon 2018, 127, 131–140. [Google Scholar] [CrossRef]
- Noel, S.; Baraton, L.; Alamarguy, D.; Jaffre, A.; Hauquier, F.; Viel, P. Graphene films for corrosion protection of gold coated cuprous substrates in view of an application to electrical contacts. In Proceedings of the IEEE 58th Holm Conference on Electrical Contacts, Portland, OR, USA, 23–26 September 2012. [Google Scholar]
- Mondal, J.; Kozlova, J.; Sammelselg, V. Graphene Nanoplatelets Based Protective and Functionalizing Coating for Stainless Steel. J. Nanosci. Nanotechnol. 2015, 9, 6747–6750. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Marques, A.; Aarik, L.; Kozlova, J.; Simoes, A.; Sammelselg, V. Development of a thin ceramic-graphene nanolaminate coating for corrosion protection of stainless steel. Corros. Sci. 2016, 105, 161–169. [Google Scholar] [CrossRef]
- Clauss, F.J. (Ed.) Solid Lubricants and Self-Lubricating Solids; Elsevier: Amsterdam, The Netherlands, 1972. [Google Scholar]
- Donnet, C.; Erdemir, A. Historical developments and new trends in tribological and solid lubricant coatings. Surf. Coat. Technol. 2004, 180, 76–84. [Google Scholar] [CrossRef]
- Wang, H.D.; Xu, B.S.; Liu, J.J.; Zhuang, D.M. The tribological properties of solid lubrication graphite coatings prepared by a sol–gel method. Carbon 2005, 43, 2017–2020. [Google Scholar] [CrossRef]
- Wang, M.; Wang, A.; Meng, X.; Peng, X.; Wang, J. Effect of interlayer bonding and stacking structure on the tribological properties of graphene oxide coatings. Tribol. Int. 2025, 27, 110636. [Google Scholar] [CrossRef]
- Reza, M.N.; Mohammadpour, M.; Saremi-Yarahmadi, S. Graphene oxide as an additive in aqueous lubricants for electric drive units: Synthesis, preparation, and tribological performance. Tribol. Int. 2025, 205, 110554. [Google Scholar]
- Brinker, C.J.; Haurd, A.J.; Schunk, P.R.; Frye, G.C.; Ashley, C.S. Review of Sol-Gel Thin Film Formation. J. Non-Cryst. Solids 1992, 147, 424–436. [Google Scholar] [CrossRef]
- Tlili, B.; Barkaoui, A.; Walock, M. Tribology and wear resistance of the stainless steel. The sol–gel coating impact on the friction and damage. Tribol. Int. 2016, 102, 348–354. [Google Scholar] [CrossRef]
- Kossman, S.; Coelho, L.B.; Montagne, A.; Mejias, A.; Gorp, A.; Coorevits, T.; Touzin, M.; Druart, A.; Mariana, H.; Staia, M.H.; et al. The effect of the substrate surface state on the morphology, topography and tribocorrosion behavior of Si/Zr sol-gel coated 316L stainless steel. Surf. Coat. Technol. 2021, 406, 126666. [Google Scholar] [CrossRef]
- Parhizkar, N.; Ramezanzadeh, B.; Shahrabi, T. Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a sol-gel based silane coating filled with amino and isocyanate silane functionalized graphene oxide nanosheets. Appl. Surf. Sci. 2018, 439, 45–59. [Google Scholar] [CrossRef]
- Xue, B.; Yu, M.; Liu, J.; Liu, J.; Li, S.; Xiong, L. Corrosion protection of AA2024-T3 by sol-gel film modified with graphene oxide. J. Alloys Compd. 2017, 725, 84–95. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Qi, J.; Chen, F. Corrosion protection of Ti6Al4V by a composite coating with a plasma electolytic oxidation layer and sol-gel layer filled with graphene oxide. Prog. Org. Coat. 2020, 144, 105632. [Google Scholar] [CrossRef]
- Xiao, N.; Dong, X.; Song, L.; Liu, D.; Yan Tay, Y.; Wu, S.; Li, L.-J.; Zhao, Y.; Yu, T.; Zhang, H.; et al. Enhanced thermopower of graphene films with oxygen plasma treatment. ACS Nano 2011, 5, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Zin, V.; Barison, S.; Agresti, F.; Colla, L.; Paguraa, C.; Fabrizio, M. Improved tribological and thermal properties of lubricants by graphene based nano-additives. RSC Adv. 2016, 6, 59477. [Google Scholar] [CrossRef]
- Kumar, D.S.A.; Viswanath, B.A.; Raghul, P. A Review of Preparation and Characterization of Sol-Gel coating for Corrosion Mitigation. Int. J. Eng. Technol. Manag. Sci. 2024, 8, 11–24. [Google Scholar]
- Shokrieh, M.; Ghoreishi, S.; Esmkhani, M.; Zhao, Z. Effects of graphene nanoplatelets and graphene nanosheets on fracture toughness of epoxy nanocomposites. Fatigue Fract. Eng. Mater. Struct. 2014, 37, 1116–1123. [Google Scholar] [CrossRef]


| Chemical Composition % (wt.) | ||||||
|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | Cr | |
| AISI 430 | ≤0.08 | ≤1.0 | ≤1.0 | ≤0.04 | ≤0.03 | 16.0–18.0 |
| Materials | Ecorr (mV) | Ebd (mV) | Ebd-Ecorr (mV) | Icorr (mA/cm2) |
|---|---|---|---|---|
| AISI430 (uncoated) | 4 | 220 | 216 | 5.077 × 10−7 |
| AISI430 + 0 wt.% GP | 334 | 1571 | 1237 | 1.198 × 10−7 |
| AISI430 + 1.8 wt.% GP | 201 | 1104 | 903 | 3.122 × 10−7 |
| AISI430 + 3.6 wt.% GP | 151 | 1235 | 1084 | 3.057 × 10−7 |
| AISI430 + 7.2 wt.% GP | 161 | 841 | 680 | 5.990 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menéndez Suárez, M.F.; Sanchez, P.; Martínez Díez, A.L.; Roman, B.M.; Mohedano Sánchez, M. Development of Thin Carbon-Ceramic Based Coatings in Roll-to-Roll Mode: Tribological and Corrosion Results on Stainless Steel. Materials 2025, 18, 2159. https://doi.org/10.3390/ma18092159
Menéndez Suárez MF, Sanchez P, Martínez Díez AL, Roman BM, Mohedano Sánchez M. Development of Thin Carbon-Ceramic Based Coatings in Roll-to-Roll Mode: Tribological and Corrosion Results on Stainless Steel. Materials. 2025; 18(9):2159. https://doi.org/10.3390/ma18092159
Chicago/Turabian StyleMenéndez Suárez, Mª Fe, Pascal Sanchez, Ana L. Martínez Díez, Beatriz Mingo Roman, and Marta Mohedano Sánchez. 2025. "Development of Thin Carbon-Ceramic Based Coatings in Roll-to-Roll Mode: Tribological and Corrosion Results on Stainless Steel" Materials 18, no. 9: 2159. https://doi.org/10.3390/ma18092159
APA StyleMenéndez Suárez, M. F., Sanchez, P., Martínez Díez, A. L., Roman, B. M., & Mohedano Sánchez, M. (2025). Development of Thin Carbon-Ceramic Based Coatings in Roll-to-Roll Mode: Tribological and Corrosion Results on Stainless Steel. Materials, 18(9), 2159. https://doi.org/10.3390/ma18092159







