Effect of Pulpal Base, Restorative Material, and Preparation Type on Marginal and Internal Fit and Fracture Strength of Endocrowns
Abstract
1. Introduction
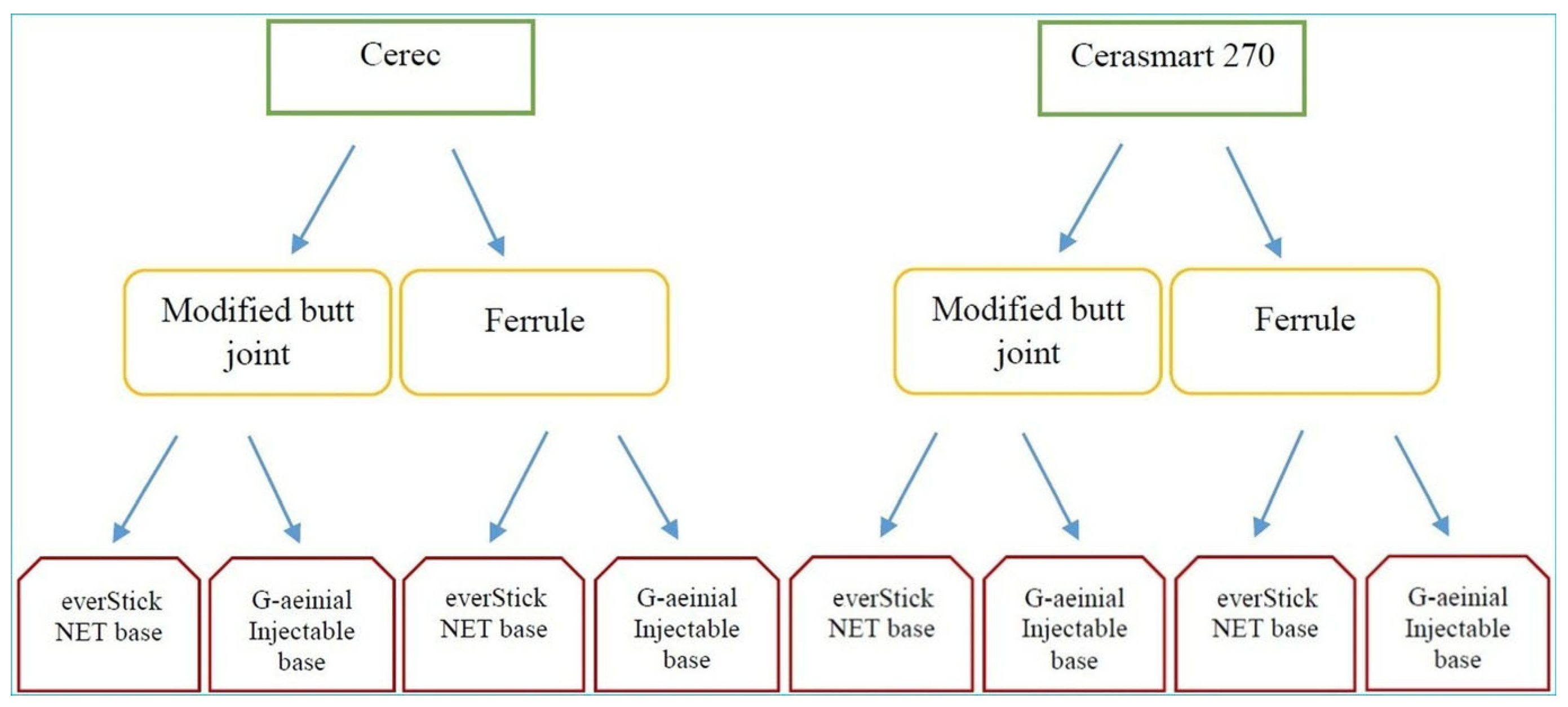
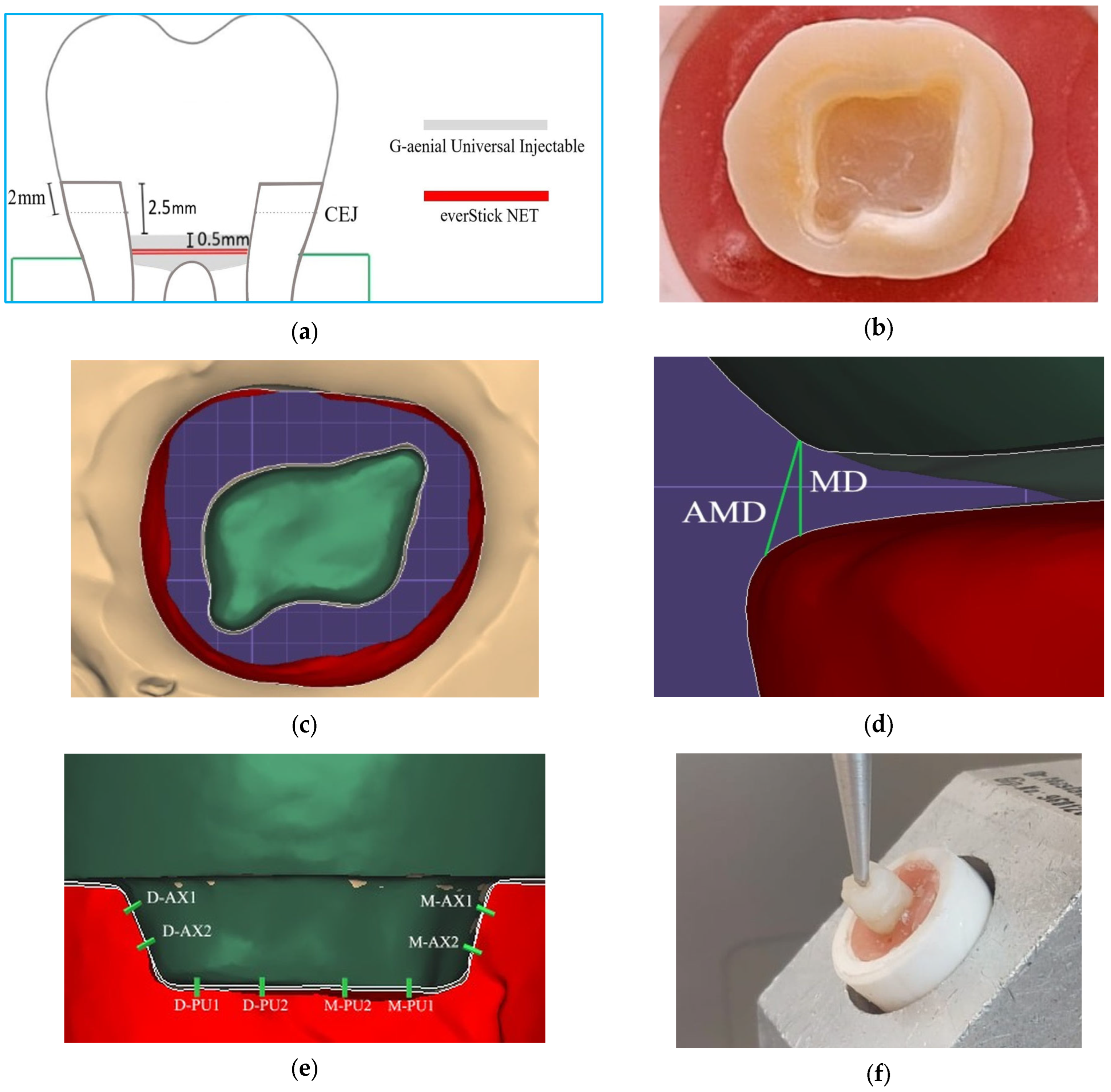
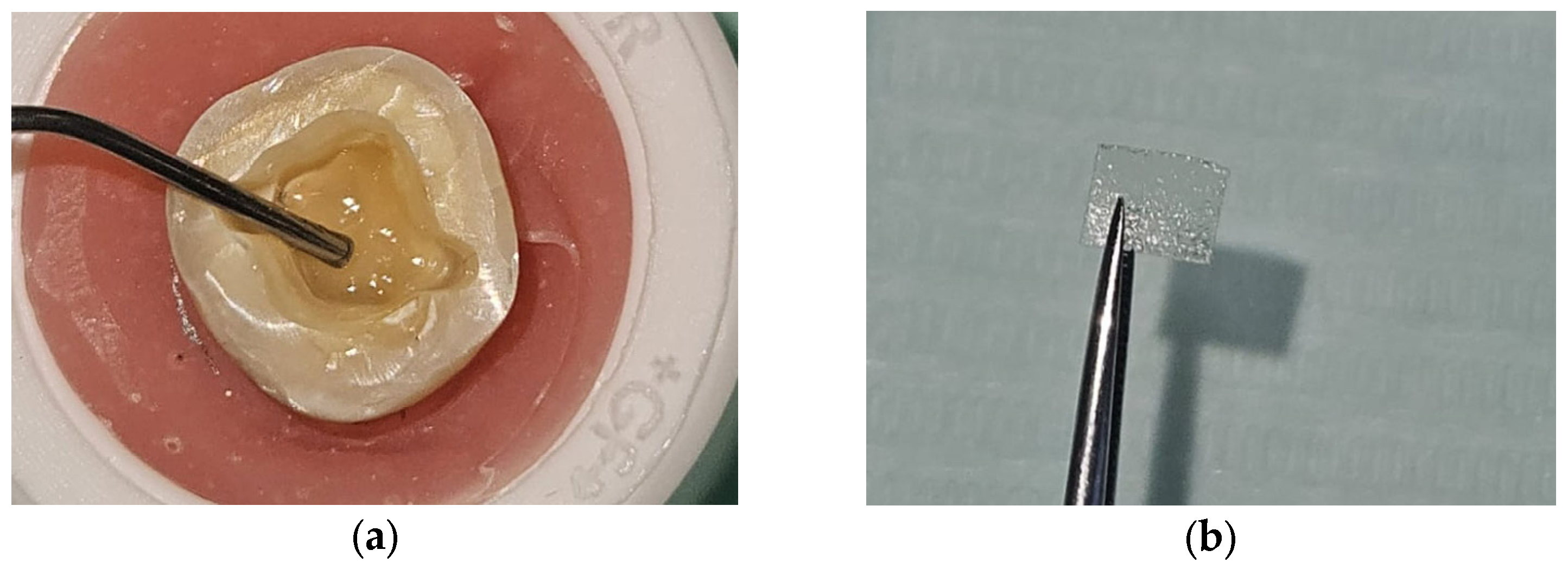
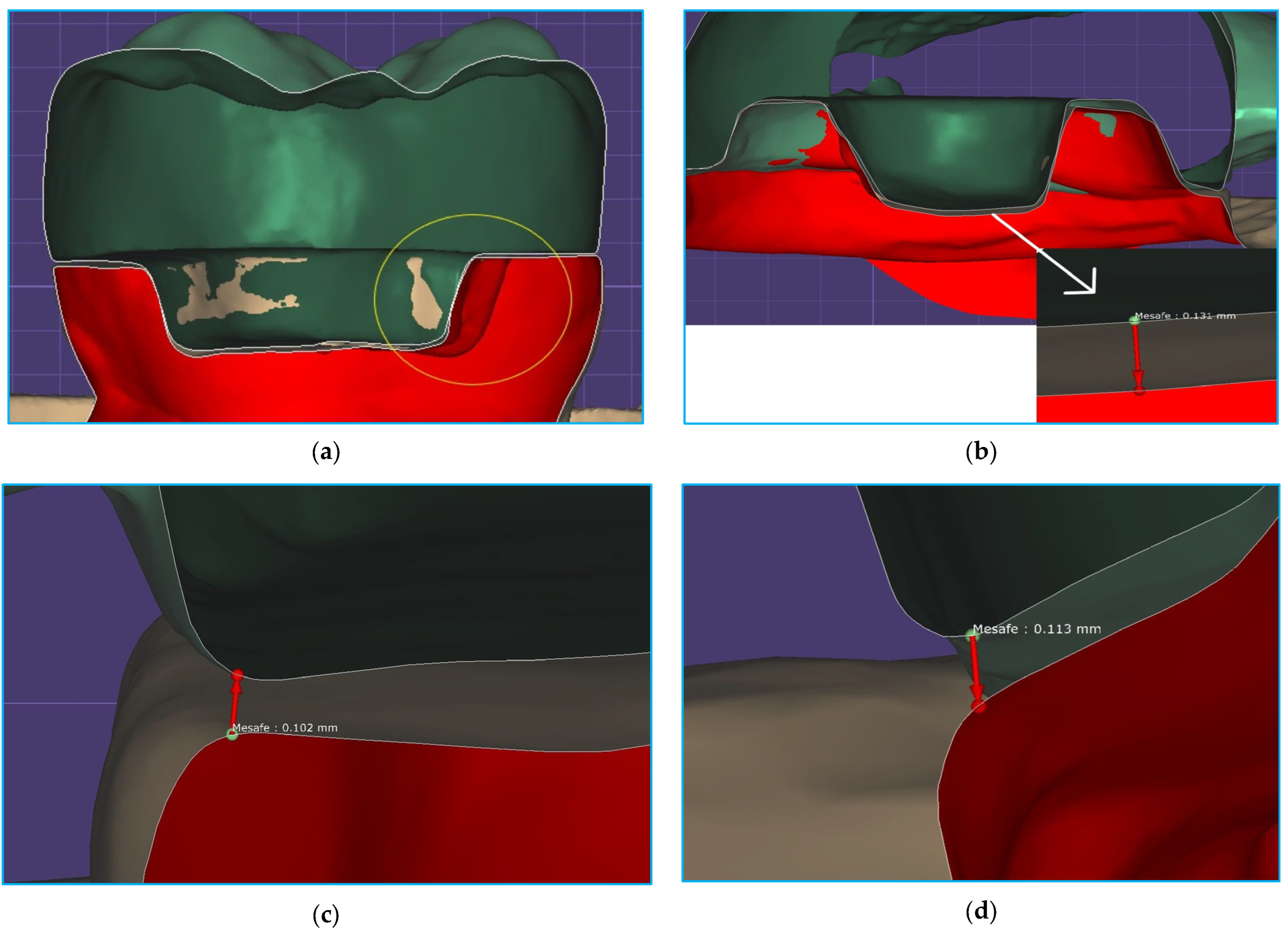
2. Materials and Methods
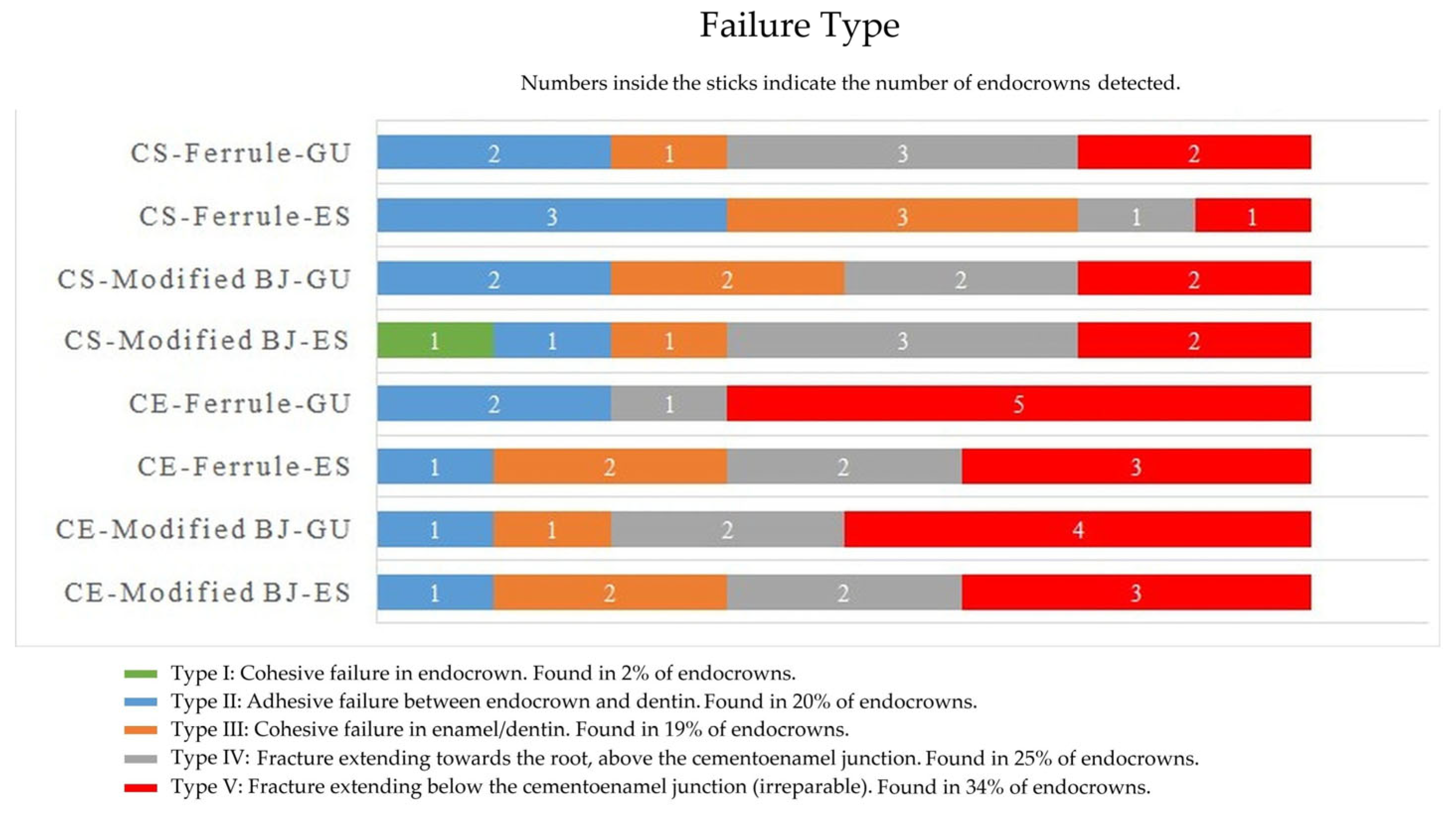
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FS | Fracture strength |
| BJ | Butt joint |
| CEJ | Cementoenamel junction |
| LD | Linear dichroism |
| MD | Marginal discrepancy |
| AMD | Absolute marginal discrepancy |
| CAD/CAM | Computer-aided design/computer-aided manufacturing |
| CE | Cerec Blocs |
| CS | Cerasmart 270 |
| N | Newton |
| ANOVA | Analysis of variance |
| Bis-GMA | Bisphenol A-glycidyl methacrylate |
| PMMA | Polymethyl methacrylate |
| TEGDMA | Triethylene glycol dimethacrylate |
References
- Rocca, G.T.; Krejci, I. Crown and post-free adhesive restorations for endodontically treated posterior teeth: From direct composite to endocrowns. Eur. J. Esthet. Dent. 2013, 8, 156–179. [Google Scholar] [PubMed]
- Bindl, A.; Mörmann, W.H. Clinical evaluation of adhesively placed Cerec endo-crowns after 2 years-preliminary results. J. Adhes. Dent. 1999, 1, 255–265. [Google Scholar] [PubMed]
- Pedrollo Lise, D.; Van Ende, A.; De Munck, J.; Umeda Suzuki, T.Y.; Cardoso Vieira, L.C.; Van Meerbeek, B. Biomechanical behavior of endodontically treated premolars using different preparation designs and CAD/CAM materials. J. Dent. 2017, 59, 54–61. [Google Scholar] [CrossRef]
- Taha, D.; Spintzyk, S.; Schille, C.; Sabet, A.; Wahsh, M.; Salah, T.; Geis-Gerstorfer, J. Fracture resistance and failure modes of polymer infiltrated ceramic endocrown restorations with variations in margin design and occlusal thickness. J. Prosthodont. Res. 2018, 62, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, M.; DuVall, N.; Wajdowicz, M.; Brewster, J.; Roberts, H. Preparation ferrule design effect on endocrown failure resistance. J. Prosthodont. 2019, 28, e237–e242. [Google Scholar] [CrossRef]
- Tarrosh, M.Y.; Moaleem, M.M.A.; Mughals, A.I.; Houmady, R.; Zain, A.A.; Moafa, A.; Darraj, M.A.; Najmi, L.E.; Bajawi, H.A.; Mohammed, S.A.; et al. Evaluation of colour change, marginal adaptation, fracture strength, and failure type in maxillary and mandibular premolar zirconia endo-crowns. BMC Oral Health 2024, 24, 970. [Google Scholar] [CrossRef]
- Jalali, S.; Jalali, H.; Kharazi Fard, M.J.; Abdolrahmani, A.; Alikhasi, M. The effect of preparation design on the fracture resistance and adaptation of the CEREC ceramic endocrowns. Clin. Exp. Dent. Res. 2023, 9, 518–525. [Google Scholar] [CrossRef]
- Hayes, A.; Duvall, N.; Wajdowicz, M.; Roberts, H. Effect of endocrown pulp chamber extension depth on molar fracture resistance. Oper. Dent. 2017, 42, 327–334. [Google Scholar] [CrossRef]
- Shin, Y.; Park, S.; Park, J.W.; Kim, K.M.; Park, Y.B.; Roh, B.D. Evaluation of the marginal and internal discrepancies of CAD-CAM endocrowns with different cavity depths: An in vitro study. J. Prosthet. Dent. 2017, 117, 109–115. [Google Scholar] [CrossRef]
- Dartora, N.R.; de Conto Ferreira, M.B.; Moris, I.C.M.; Brazão, E.H.; Spazin, A.O.; Sousa-Neto, M.D.; Silva-Sousa, Y.T.; Gomes, E.A. Effect of Intracoronal Depth of Teeth Restored with Endocrowns on Fracture Resistance: In Vitro and 3-dimensional Finite Element Analysis. J. Endod. 2018, 44, 1179–1185. [Google Scholar] [CrossRef]
- Gaintantzopoulou, M.D.; El-Damanhoury, H.M. Effect of Preparation Depth on the Marginal and Internal Adaptation of Computer-aided Design/Computer-assisted Manufacture Endocrowns. Oper. Dent. 2019, 41, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Tribst, J.P.M.; Dal Piva, A.M.O.; Madruga, C.F.L.; Valera, M.C.; Borges, A.L.S.; Bresciani, E.; de Melo, R.M. Endocrown restorations: Influence of dental remnant and restorative material on stress distribution. Dent. Mater. 2018, 34, 1466–1473. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, D.; Rong, Q.; Qian, J.; Wang, X. Effect of central retainer shape and abduction angle during preparation of teeth on dentin and cement layer stress distributions in endocrown-restored mandibular molars. Dent. Mater. J. 2020, 39, 464–470. [Google Scholar] [CrossRef]
- Mittal, N.; Thangamuthu, T.; Gupta, S.; Gupta, S.; Aggarwal, H.; Kharat, S. Comparative evaluation of resin-based sealers and bioceramic sealers for postoperative pain after endodontic treatment: A systematic review. Dent. Med. Probl. 2024, 61, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Anton, Y.; Otero, C.; Bijelic-Donova, J.; Saratti, C.M.; Vallittu, P.K.; di Bella, E.; Krejci, I.; Rocca, G.T. The influence of FRC base and bonded CAD/CAM resin composite endocrowns on fatigue behavior of cracked endodontically-treated molars. J. Mech. Behav. Biomed. Mater. 2021, 121, 104647. [Google Scholar] [CrossRef]
- Rocca, G.T.; Saratti, C.M.; Cattani-Lorente, M.; Feilzer, A.J.; Scherrer, S.; Krejci, I. The effect of a fiber reinforced cavity configuration on load bearing capacity and failure mode of endodontically treated molars restored with CAD/CAM resin composite overlay restorations. J. Dent. 2015, 43, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Saker, S.; Alqutaibi, A.Y.; Alghauli, M.A.; Hashem, D.; Borzangy, S.; Farghal, A.E.; Alnazzawi, A.A.; Ainoosah, S.; AbdElaziz, M.H. The Influence of Ferrule Design and Pulpal Extensions on the Accuracy of Fit and the Fracture Resistance of Zirconia-Reinforced Lithium Silicate Endocrowns. Materials 2024, 17, 1411. [Google Scholar] [CrossRef]
- Taha, D.; Spintzyk, S.; Sabet, A.; Wahsh, M.; Salah, T. Assessment of marginal adaptation and fracture resistance of endocrown restorations utilizing different machinable blocks subjected to thermomechanical aging. J. Esthet. Restor. Dent. 2018, 30, 319–328. [Google Scholar] [CrossRef]
- Yang, M.S.; Kim, S.K.; Heo, S.J.; Koak, J.Y.; Park, J.M. Investigation of the marginal fit of a 3D-printed three-unit resin prosthesis with different build orientations and layer thicknesses. J. Adv. Psthodont. 2022, 14, 250–261. [Google Scholar] [CrossRef]
- Dolev, E.; Bitterman, Y.; Meirowitz, A. Comparison of marginal fit between CAD-CAM and hot-press lithium disilicate crowns. J. Prosthet. Dent. 2019, 121, 124–128. [Google Scholar] [CrossRef]
- Holmes, J.R.; Bayne, S.C.; Holland, G.A.; Sulik, W.D. Considerations in measurement of marginal fit. J. Prosthet. Dent. 1989, 62, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Abualsaud, R.; Alalawi, H. Fit, Precision, and Trueness of 3D-Printed Zirconia Crowns Compared to Milled Counterparts. Dent. J. 2022, 10, 215. [Google Scholar] [CrossRef]
- Nagi, N.; Fouda, A.M.; Bourauel, C. Comparative evaluation of internal fit and marginal gap of endocrowns using lithium disilicate and polyether ether ketone materials—An in vitro study. BMC Oral Health 2023, 23, 207. [Google Scholar] [CrossRef] [PubMed]
- Kassis, C.; Mehanna, C.; Khoury, P.; Tohme, H.; Cuevas-Suárez, C.E.; Bourgi, R.; Lukomska-Szymanska, M.; Hardan, L. Triple scan evaluation of internal and marginal adaptation of overlays using different restorative materials. J. Esthet. Restor. Dent. 2023, 35, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Sağlam, G.; Cengiz, S.; Karacaer, Ö. Marginal adaptation and fracture strength of endocrowns manufactured with different restorative materials: SEM and mechanical evaluation. Microsc. Res. Tech. 2021, 84, 284–290. [Google Scholar] [CrossRef]
- Mertsöz, B.; Ongun, S.; Ulusoy, M. In-Vitro Investigation of Marginal Adaptation and Fracture Resistance of Resin Matrix Ceramic Endo-Crown Restorations. Materials 2023, 16, 2059. [Google Scholar] [CrossRef]
- Ferrairo, B.M.; Piras, F.F.; Lima, F.F.; Honório, H.M.; Duarte, M.A.H.; Borges, A.F.S.; Rubo, J.H. Comparison of marginal adaptation and internal fit of monolithic lithium disilicate crowns produced by 4 different CAD/CAM systems. Clin. Oral Investig. 2021, 25, 2029–2036. [Google Scholar] [CrossRef]
- Alexopoulou, E.; Polychronis, G.; Konstantonis, D.; Sifakakis, I.; Zinelis, S.; Eliades, T. A study of the mechanical properties of as-received and intraorally exposed single-crystal and polycrystalline orthodontic ceramic brackets. Eur. J. Orthod. 2020, 42, 72–77. [Google Scholar] [CrossRef]
- Hassouneh, L.; Jum’ah, A.A.; Ferrari, M.; Wood, D.J. Post-fatigue fracture resistance of premolar teeth restored with endocrowns: An in vitro investigation. J. Dent. 2020, 100, 103426. [Google Scholar] [CrossRef]
- Ghoul, W.E.; Özcan, M.; Tribst, J.P.M.; Salameh, Z. Fracture resistance, failure mode and stress concentration in a modified endocrown design. Biomater. Investig. Dent. 2020, 7, 110–119. [Google Scholar] [CrossRef]
- de Paula Silveira, A.C.; Chaves, S.B.; Hilgert, L.A.; Ribeiro, A.P. Marginal and internal fit of CAD-CAM-fabricated composite resin and ceramic crowns scanned by 2 intraoral cameras. J. Prosthet. Dent. 2017, 117, 386–392. [Google Scholar] [CrossRef]
- Sanchez-Lara, A.; Hosney, S.; Lampraki, E.; Conejo, J.; Blatz, M.B.; Barmak, A.B.; Ercoli, C.; Chochlidakis, K. Evaluation of marginal and internal fit of single crowns manufactured with an analog workflow and three CAD-CAM systems: A prospective clinical study. J. Prosthodont. 2023, 32, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Dauti, R.; Cvikl, B.; Lilaj, B.; Heimel, P.; Moritz, A.; Schedle, A. Micro-CT evaluation of marginal and internal fit of cemented polymer infiltrated ceramic network material crowns manufactured after conventional and digital impressions. J. Prosthodont. Res. 2019, 63, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Fayed, A.K.; Azer, A.S.; AboElhassan, R.G. Fit accuracy and fracture resistance evaluation of advanced lithium disilicate crowns (in-vitro study). BMC Oral Health 2025, 25, 58. [Google Scholar] [CrossRef]
- Dauti, R.; Lilaj, B.; Heimel, P.; Moritz, A.; Schedle, A.; Cvikl, B. Influence of two different cement space settings and three different cement types on the fit of polymer-infiltrated ceramic network material crowns manufactured using a complete digital workflow. Clin. Oral Investig. 2020, 24, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Svanborg, P.; Andersson, M.; Reinedahl, T.; Alstad, T. Comparison of the 3D triple-scan protocol and the impression replica technique for 3-unit tooth-supported fixed dental prostheses. Biomater. Investig. Dent. 2019, 6, 32–34. [Google Scholar] [CrossRef]
- Gomes, R.S.; Souza, C.M.C.; Bergamo, E.T.P.; Bordin, D.; Del Bel Cury, A.A. Misfit and fracture load of implant-supported monolithic crowns in zirconia-reinforced lithium silicate. J. Appl. Oral Sci. 2017, 25, 282–289. [Google Scholar] [CrossRef]
- Dahl, B.E.; Rønold, H.J.; Dahl, J.E. Internal fit of single crowns produced by CAD-CAM and lost-wax metal casting technique assessed by the triple-scan protocol. J. Prosthet. Dent. 2017, 117, 400–404. [Google Scholar] [CrossRef]
- Goujat, A.; Abouelleil, H.; Colon, P.; Jeannin, C.; Pradelle, N.; Seux, D.; Grosgogeat, B. Marginal and internal fit of CAD-CAM inlay/onlay restorations: A systematic review of in vitro studies. J. Prosthet. Dent. 2019, 121, 590–597.e3. [Google Scholar] [CrossRef]
- Gurpinar, B.; Tak, O. Effect of pulp chamber depth on the accuracy of endocrown scans made with different intraoral scanners versus an industrial scanner: An in vitro study. J. Prosthet. Dent. 2022, 127, 430–437. [Google Scholar] [CrossRef]
- Nawafleh, N.A.; Mack, F.; Evans, J.; Mackay, J.; Hatamleh, M.M. Accuracy and reliability of methods to measure marginal adaptation of crowns and FDPs: A literature review. J. Prosthodont. 2013, 22, 419–428. [Google Scholar] [CrossRef]
- Contrepois, M.; Soenen, A.; Bartala, M.; Laviole, O. Marginal adaptation of ceramic crowns: A systematic review. J. Prosthet. Dent. 2013, 110, 447–454.e10. [Google Scholar] [CrossRef] [PubMed]
- Temizci, T.; Bozoğulları, H.N. Effect of thermocycling on the mechanical properties of permanent composite-based CAD-CAM restorative materials produced by additive and subtractive manufacturing techniques. BMC Oral Health 2024, 24, 334. [Google Scholar] [CrossRef]
- Ruse, N.D.; Sadoun, M.J. Resin-composite blocks for dental CAD/CAM applications. J. Dent. Res. 2014, 93, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, O.; Iwasaki, M.; Saito, M.; Morimoto, T. Influence of clenching intensity on bite force balance, occlusal contact area, and average bite pressure. J. Dent. Res. 1999, 78, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Dikici, B.; Can, E.; Türkeş Başaran, E.; Barut, G.; Dönmez, N. Fracture strength of endocrowns after thermomechanical aging. Odontology 2024, 112, 884–894. [Google Scholar] [CrossRef]
- Zheng, Z.; He, Y.; Ruan, W.; Ling, Z.; Zheng, C.; Gai, Y.; Yan, W. Biomechanical behavior of endocrown restorations with different CAD-CAM materials: A 3D finite element and in vitro analysis. J. Prosthet. Dent. 2021, 125, 890–899. [Google Scholar] [CrossRef]
- Davis, D.M.; Waters, N.E. Fractography of a bis-GMA resin. J. Dent. Res. 1989, 68, 1194–1198. [Google Scholar] [CrossRef]
- Monaco, C.; Bortolotto, T.; Arena, A.; Krejci, I. Restoring Nonvital Premolars with Composite Resin Onlays: Effect of Different Fiber-reinforced Composite Layers on Marginal Adaptation and Fracture Load. J. Adhes. Dent. 2015, 17, 567–574. [Google Scholar] [CrossRef]
- Fildisi, M.A.; Eliguzeloglu Dalkilic, E. The effect of fiber insertion on fracture strength and fracture modes in endocrown and overlay restorations. Microsc. Res. Tech. 2022, 85, 1799–1807. [Google Scholar] [CrossRef]
- Oskoee, P.A.; Ajami, A.A.; Navimipour, E.J.; Oskoee, S.S.; Sadjadi, J. The effect of three composite fiber insertion techniques on fracture resistance of root-filled teeth. J. Endod. 2009, 35, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Dyer, S.R.; Lassila, L.V.; Jokinen, M.; Vallittu, P.K. Effect of fiber position and orientation on fracture load of fiber-reinforced composite. Dent. Mater. 2004, 20, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Abduljawad, D.E.; Rayyan, M.R. Marginal and internal fit of lithium disilicate endocrowns fabricated using conventional, digital, and combination techniques. J. Esthet. Restor. Dent. 2022, 34, 707–714. [Google Scholar] [CrossRef] [PubMed]


| Intended Use | Code | Name | Feature | Content (% in Weight) | Manufacturer |
|---|---|---|---|---|---|
| Pulpal base | GU | G-ænial Universal Injectable | Light-cured injectable composite | 31% matrix (methacrylate monomer), 69% fillers (silica, barium glass) | GC Corp, Tokyo, Japan |
| ES | everStick NET | Fiber ribbon | Bidirectional glass fibers, polymer/resin gel matrix (PMMA, Bis-GMA) | GC Corp, Tokyo, Japan | |
| Restorative material | CE | Cerec Blocs | Feldspathic ceramic blocs | 56–64% SiO2, 20–23% Al2O3, 6–9% Na2O, 6–8% K2O, 0.3–0.6% other oxides | Sirona Dental, Bensheim, Germany |
| CS | Cerasmart 270 | Hybrid ceramic blocs | 29% matrix (UDMA, Bis-MEPP, DMA), 71% fillers (300 nm barium glass; 20 nm SiO2 nanoparticles) | GC Corp, Tokyo, Japan |
| Source | Sum of Squares | df | F | p-Value | Partial Eta Squared | |
|---|---|---|---|---|---|---|
| AMD | Material | 2626.563 | 1 | 4.596 | 0.036 | 0.076 |
| Preparation | 156.25 | 1 | 0.273 | 0.603 | 0.005 | |
| Base | 12.25 | 1 | 0.021 | 0.884 | 0.001 | |
| Material–preparation | 27.563 | 1 | 0.048 | 0.827 | 0.001 | |
| Material–base | 612.563 | 1 | 1.072 | 0.305 | 0.019 | |
| Preparation–base | 1156 | 1 | 2.023 | 0.16 | 0.035 | |
| Material–preparation–base | 248.063 | 1 | 0.434 | 0.513 | 0.008 | |
| MD | Material | 756.25 | 1 | 5.589 | 0.022 | 0.091 |
| Preparation | 34.516 | 1 | 0.255 | 0.616 | 0.005 | |
| Base | 54.391 | 1 | 0.402 | 0.529 | 0.007 | |
| Material–preparation | 2.641 | 1 | 0.02 | 0.889 | 0.001 | |
| Material–base | 102.516 | 1 | 0.758 | 0.388 | 0.013 | |
| Preparation–base | 60.063 | 1 | 0.444 | 0.508 | 0.008 | |
| Material–preparation–base | 18.063 | 1 | 0.133 | 0.716 | 0.002 | |
| Axial fit | Material | 650.25 | 1 | 6.197 | 0.016 | 0.1 |
| Preparation | 240.25 | 1 | 2.29 | 0.136 | 0.039 | |
| Base | 22.563 | 1 | 0.215 | 0.645 | 0.004 | |
| Material–preparation | 6.25 | 1 | 0.06 | 0.808 | 0.001 | |
| Material–base | 52.563 | 1 | 0.501 | 0.482 | 0.009 | |
| Preparation–base | 39.063 | 1 | 0.372 | 0.544 | 0.007 | |
| Material–preparation–base | 5.063 | 1 | 0.048 | 0.827 | 0.001 | |
| Pulpal fit | Material | 2588.266 | 1 | 13.491 | <0.001 | 0.094 |
| Preparation | 1113.891 | 1 | 0.281 | 0.598 | 0.005 | |
| Base | 3150.016 | 1 | 0.281 | 0.598 | 0.005 | |
| Material–preparation | 446.266 | 1 | 0.052 | 0.821 | 0.001 | |
| Material–base | 293.266 | 1 | 0.206 | 0.651 | 0.004 | |
| Preparation–base | 43.891 | 1 | 0.116 | 0.735 | 0.002 | |
| Material–preparation–base | 54.391 | 1 | 0.323 | 0.572 | 0.006 | |
| Overall fit | Material | 1602.501 | 1 | 18.398 | <0.001 | 0.247 |
| Preparation | 78.766 | 1 | 0.904 | 0.346 | 0.016 | |
| Base | 126.563 | 1 | 1.453 | 0.233 | 0.025 | |
| Material–preparation | 6.25 | 1 | 0.072 | 0.79 | 0.001 | |
| Material–base | 183.941 | 1 | 2.112 | 0.152 | 0.036 | |
| Preparation–base | 153.915 | 1 | 1.767 | 0.189 | 0.031 | |
| Material–preparation–base | 1.806 | 1 | 0.021 | 0.886 | 0.001 | |
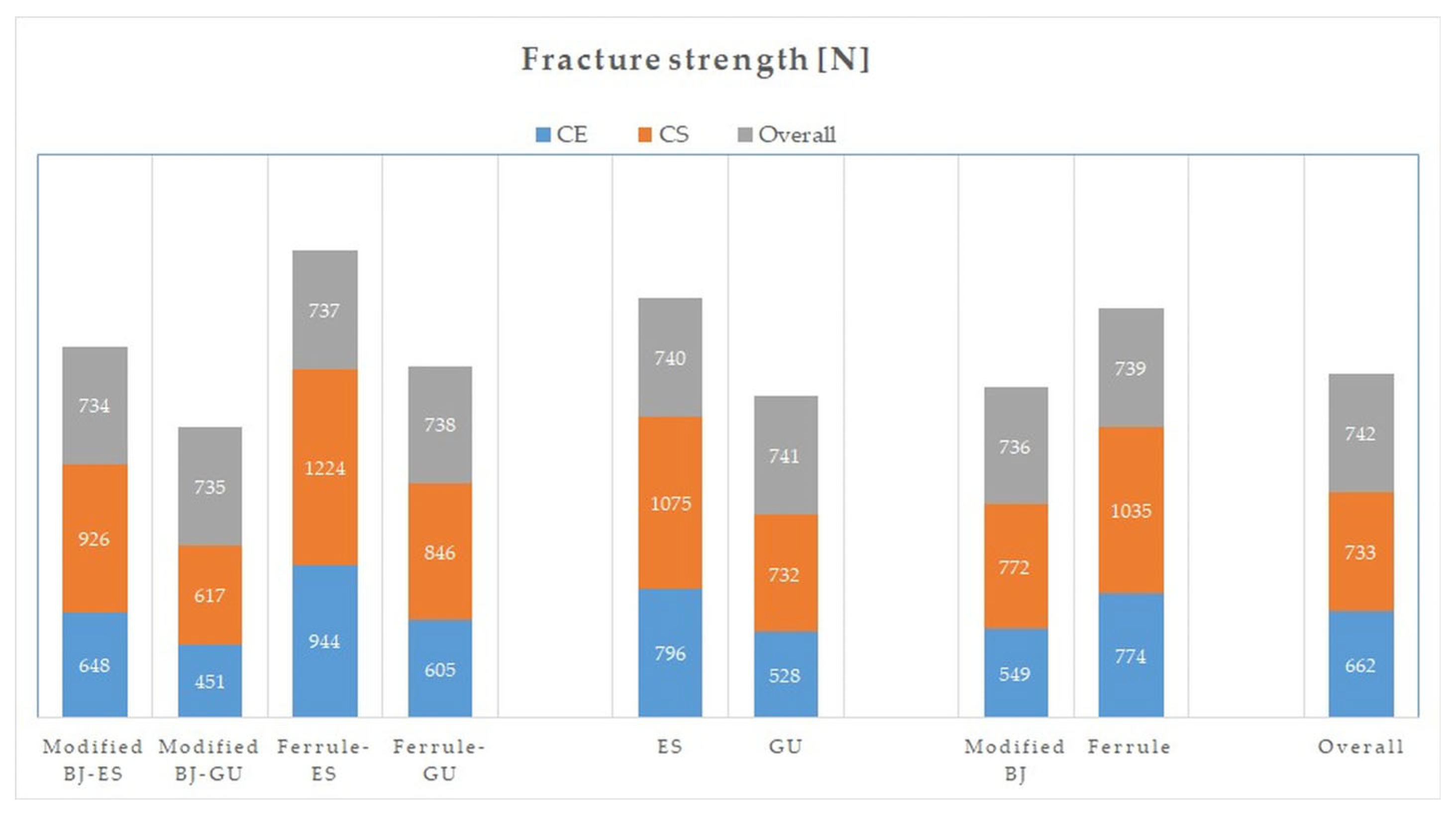
| Fracture strength | Material | 932,575.214 | 1 | 87.424 | <0.001 | 0.61 |
| Preparation | 955,052.36 | 1 | 89.531 | <0.001 | 0.615 | |
| Base | 1,493,630.413 | 1 | 140.02 | <0.001 | 0.714 | |
| Material–preparation | 5878.626 | 1 | 0.551 | 0.461 | 0.01 | |
| Material–base | 22,564.026 | 1 | 2.115 | 0.151 | 0.036 | |
| Preparation–base | 45,216.506 | 1 | 4.239 | 0.044 | 0.07 | |
| Material–preparation–base | 5402.61 | 1 | 0.506 | 0.48 | 0.009 |
| Material | Preparation | N | Base | Means ± Standard Deviations | |||||
|---|---|---|---|---|---|---|---|---|---|
| AMD (µm) | MD (µm) | Axial (µm) | Pulpal (µm) | Overall (µm) | FS (N) | ||||
| CE | Modified BJ | 8 | ES | 136 ± 18 | 112 ± 7 | 106 ± 9 | 146 ± 22 | 124 ± 6 | 648 ± 148 |
| 8 | GU | 119 ± 15 | 108 ± 8 | 102 ± 7 | 128 ± 30 | 114 ± 3 | 451 ± 67 | ||
| 16 | Total | 128 ± 18 | 110 ± 7 | 104 ± 8 | 137 ± 27 | 119 ± 7 | 549 ± 150 | ||
| Ferrule | 8 | ES | 122 ± 25 | 107 ± 10 | 109 ± 13 | 149 ± 31 | 122 ± 6 | 944 ± 117 | |
| 8 | GU | 129 ± 32 | 109 ± 11 | 107 ± 6 | 131 ± 33 | 119 ± 6 | 605 ± 86 | ||
| 16 | Total | 125 ± 28 | 108 ± 10 | 108 ± 10 | 140 ± 32 | 120 ± 6 | 774 ± 201 | ||
| Total | 16 | ES | 129 ± 22 | 109 ± 9 | 107 ± 11 | 148 ± 26 | 123 ± 6 | 796 ± 200 | |
| 16 | GU | 124 ± 25 | 108 ± 9 | 104 ± 7 | 129 ± 30 | 117 ± 5 | 528 ± 109 | ||
| 32 | Total | 127 ± 23 | 108 ± 9 | 106 ± 9 | 139 ± 29 | 120 ± 6 | 662 ± 209 | ||
| CS | Modified BJ | 8 | ES | 140 ± 22 | 115 ± 8 | 111 ± 11 | 151 ± 23 | 129 ± 13 | 926 ± 106 |
| 8 | GU | 143 ± 18 | 118 ± 11 | 110 ± 8 | 138 ± 20 | 127 ± 11 | 617 ± 72 | ||
| 16 | Total | 142 ± 19 | 116 ± 10 | 111 ± 10 | 145 ± 22 | 128 ± 11 | 772 ± 182 | ||
| Ferrule | 8 | ES | 131 ± 27 | 113 ± 16 | 112 ± 11 | 161 ± 13 | 129 ± 8 | 1224 ± 105 | |
| 8 | GU | 143 ± 25 | 118 ± 15 | 115 ± 11 | 155 ± 17 | 133 ± 12 | 846 ± 98 | ||
| 16 | Total | 137 ± 26 | 115 ± 15 | 114 ± 10 | 158 ± 15 | 131 ± 10 | 1035 ± 218 | ||
| Total | 16 | ES | 136 ± 24 | 114 ± 12 | 112 ± 11 | 156 ± 19 | 129 ± 10 | 1075 ± 185 | |
| 16 | GU | 143 ± 21 | 118 ± 13 | 113 ± 10 | 147 ± 20 | 130 ± 12 | 732 ± 144 | ||
| 32 | Total | 139 ± 23 | 116 ± 13 | 112 ± 10 | 151 ± 20 | 130 ± 11 | 903 ± 238 | ||
| Overall | Modified BJ | 16 | ES | 138 ± 19 | 114 ± 7 | 108 ± 10 | 149 ± 19 | 127 ± 10 | 787 ± 190 |
| 16 | GU | 131 ± 20 | 113 ± 11 | 106 ± 8 | 133 ± 25 | 121 ± 10 | 534 ± 109 | ||
| 32 | Total | 135 ± 20 | 113 ± 9 | 107 ± 9 | 141 ± 25 | 124 ± 10 | 660 ± 199 | ||
| Ferrule | 16 | ES | 127 ± 16 | 110 ± 13 | 110 ± 12 | 155 ± 24 | 126 ± 8 | 1084 ± 180 | |
| 16 | GU | 136 ± 29 | 113 ± 14 | 112 ± 9 | 143 ± 28 | 126 ± 12 | 725 ± 153 | ||
| 32 | Total | 131 ± 27 | 112 ± 13 | 111 ± 10 | 149 ± 26 | 126 ± 10 | 905 ± 245 | ||
| Total | 32 | ES | 133 ± 23 | 111 ± 11 | 110 ± 11 | 152 ± 23 | 126 ± 9 | 935 ± 236 | |
| 32 | GU | 134 ± 25 | 113 ± 12 | 109 ± 9 | 138 ± 27 | 123 ± 11 | 630 ± 163 | ||
| 64 | Total | 133 ± 24 | 112 ± 11 | 109 ± 10 | 145 ± 26 | 125 ± 10 | 783 ± 253 | ||
| Material–Preparation | ||||
| Mean | 95% Confidence Interval | |||
| Lower Bound | Upper Bound | |||
| CE | Modified BJ | 549 | 497 | 601 |
| Ferrule | 774 | 722 | 826 | |
| CS | Modified BJ | 771 | 719 | 823 |
| Ferrule | 1035 | 98 | 1086 | |
| Material–base | ||||
| Mean | 95% Confidence interval | |||
| Lower bound | Upper bound | |||
| CE | ES | 795 | 744 | 847 |
| GU | 527 | 476 | 579 | |
| CS | ES | 1074 | 102 | 1126 |
| GU | 731 | 680 | 783 | |
| Preparation–base | ||||
| Mean | 95% Confidence interval | |||
| Lower bound | Upper bound | |||
| Modified BJ | ES | 786 | 734 | 838 |
| GU | 534 | 482 | 586 | |
| Ferrule | ES | 1084 | 1032 | 1135 |
| GU | 725 | 673 | 777 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yılmaz, K.; Aydın, H.; Gönüldaş, F.; Kara, S.; Çiloğlu, Ö.; Özdemir, E.; Bilen, Z. Effect of Pulpal Base, Restorative Material, and Preparation Type on Marginal and Internal Fit and Fracture Strength of Endocrowns. Materials 2025, 18, 2137. https://doi.org/10.3390/ma18092137
Yılmaz K, Aydın H, Gönüldaş F, Kara S, Çiloğlu Ö, Özdemir E, Bilen Z. Effect of Pulpal Base, Restorative Material, and Preparation Type on Marginal and Internal Fit and Fracture Strength of Endocrowns. Materials. 2025; 18(9):2137. https://doi.org/10.3390/ma18092137
Chicago/Turabian StyleYılmaz, Kerem, Hakan Aydın, Fehmi Gönüldaş, Sukan Kara, Özge Çiloğlu, Erdem Özdemir, and Zeynep Bilen. 2025. "Effect of Pulpal Base, Restorative Material, and Preparation Type on Marginal and Internal Fit and Fracture Strength of Endocrowns" Materials 18, no. 9: 2137. https://doi.org/10.3390/ma18092137
APA StyleYılmaz, K., Aydın, H., Gönüldaş, F., Kara, S., Çiloğlu, Ö., Özdemir, E., & Bilen, Z. (2025). Effect of Pulpal Base, Restorative Material, and Preparation Type on Marginal and Internal Fit and Fracture Strength of Endocrowns. Materials, 18(9), 2137. https://doi.org/10.3390/ma18092137






