Articulating Materials Are Determinants of Survivorship of Hip Arthroplasties Performed for Nontraumatic Osteonecrosis of the Femoral Head
Abstract
1. Introduction
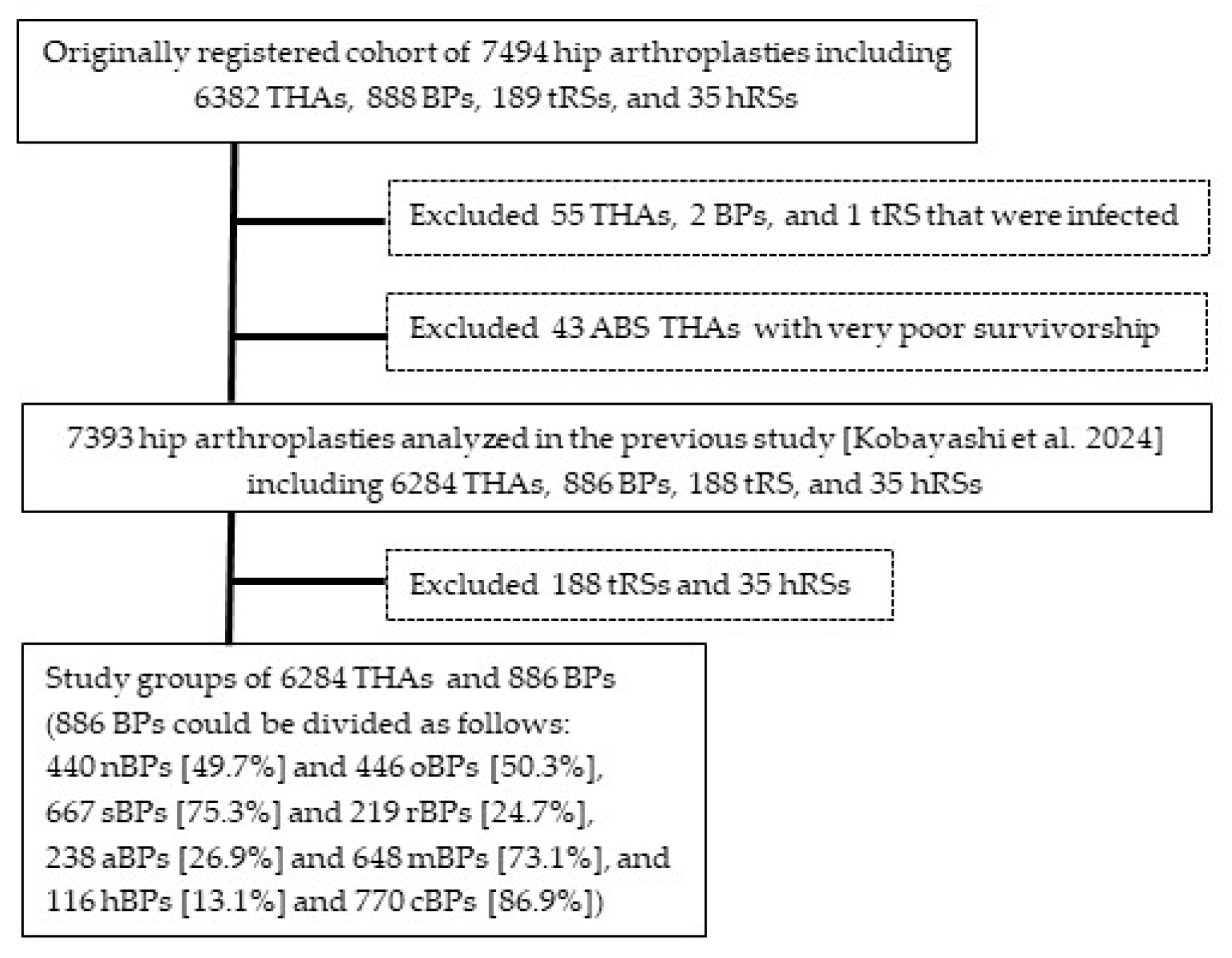
2. Materials and Methods
2.1. THAs and BPs Analyzed for Factors Related to Need for Reoperation
2.2. Statistical Analyses
2.3. Ethical Approvals
3. Results
3.1. Comparison of Demographic and Surgical Variables Between THAs and BPs
3.2. Analyses of the 6284 THAs
3.2.1. Cox Proportional-Hazard Model Analyses
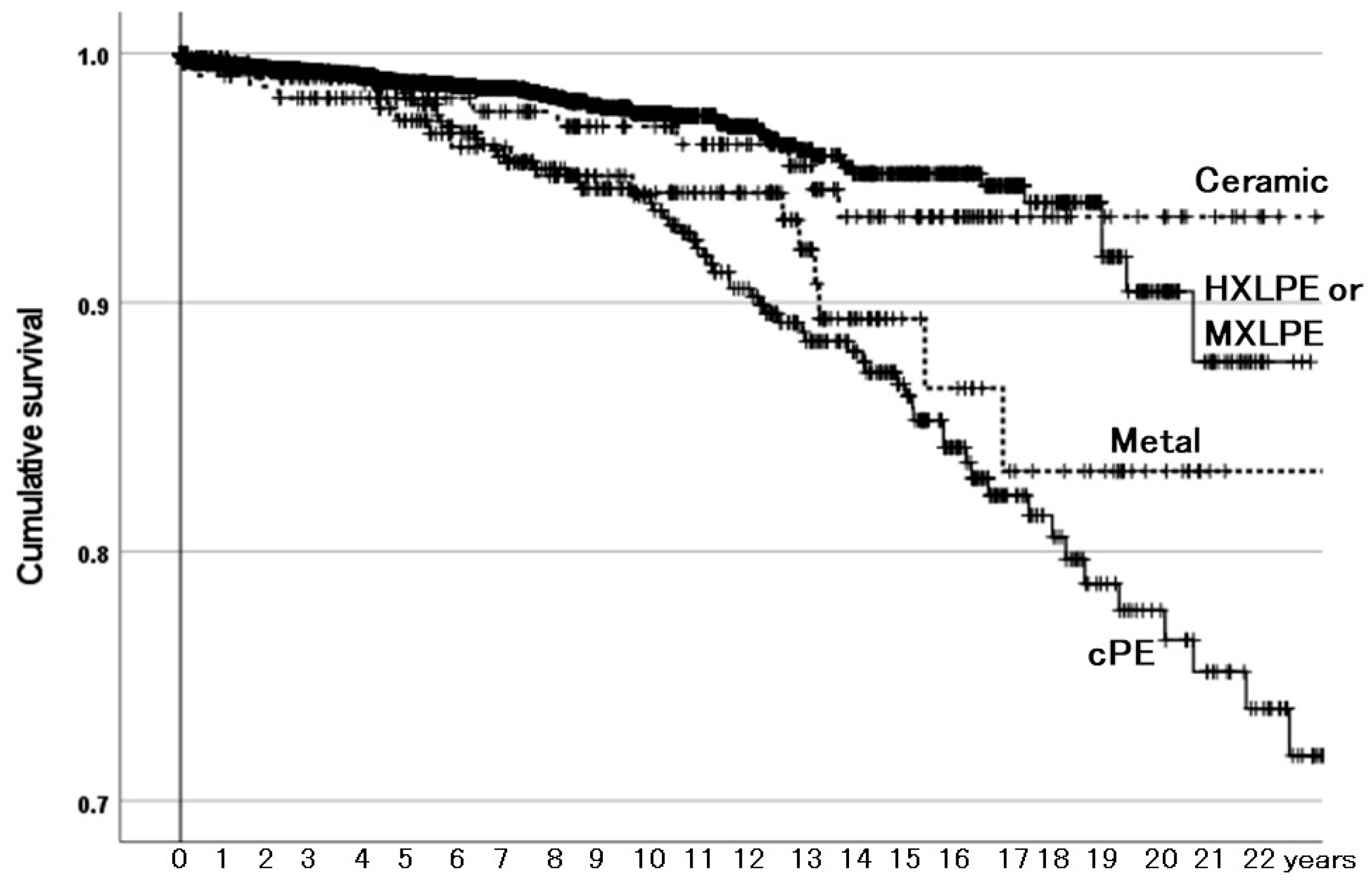
3.2.2. Survivorship Illustrated with Kaplan–Meier Estimator
3.3. Analyses of the 886 BPs
3.3.1. Cox Proportional-Hazard Model Analyses
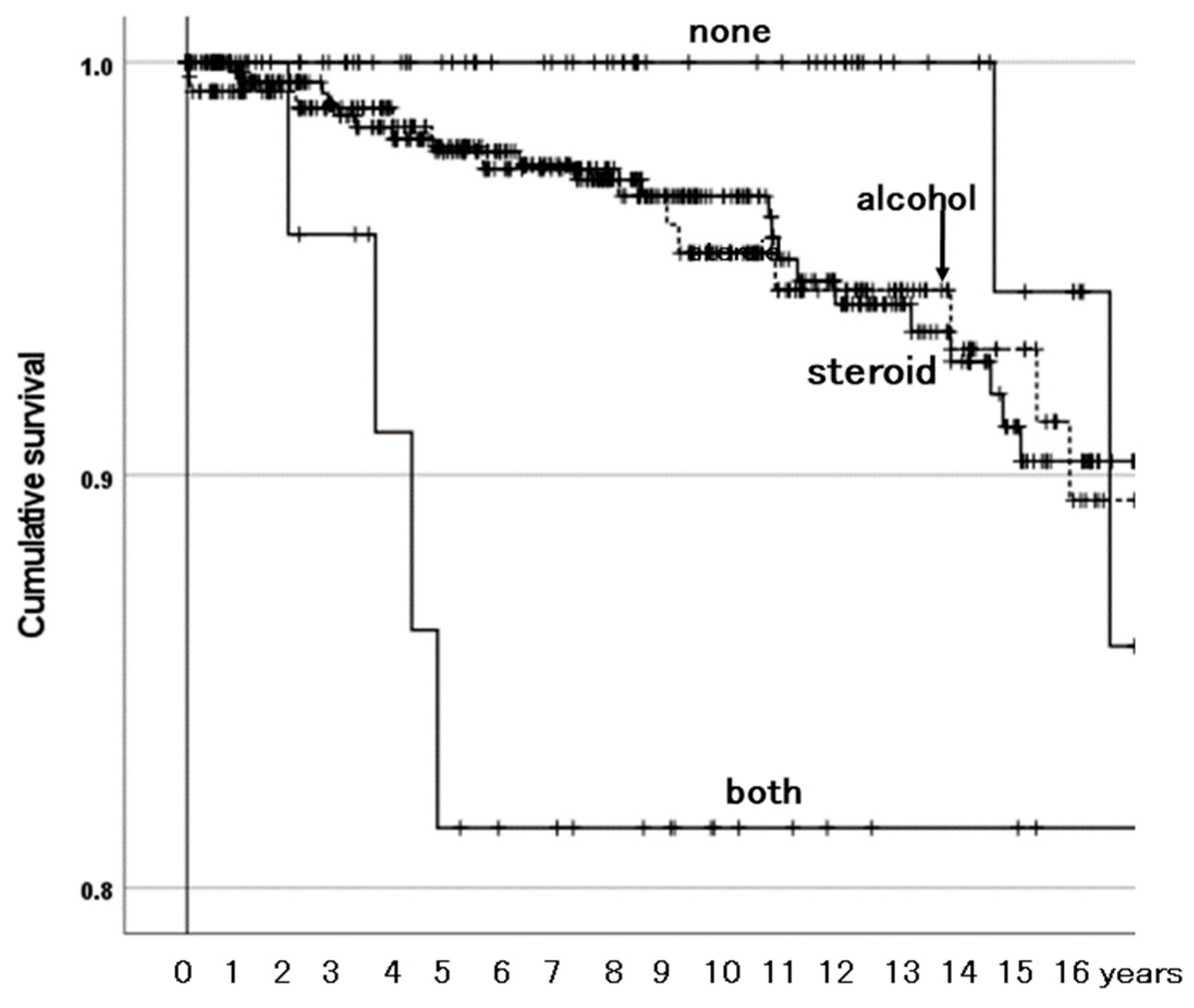
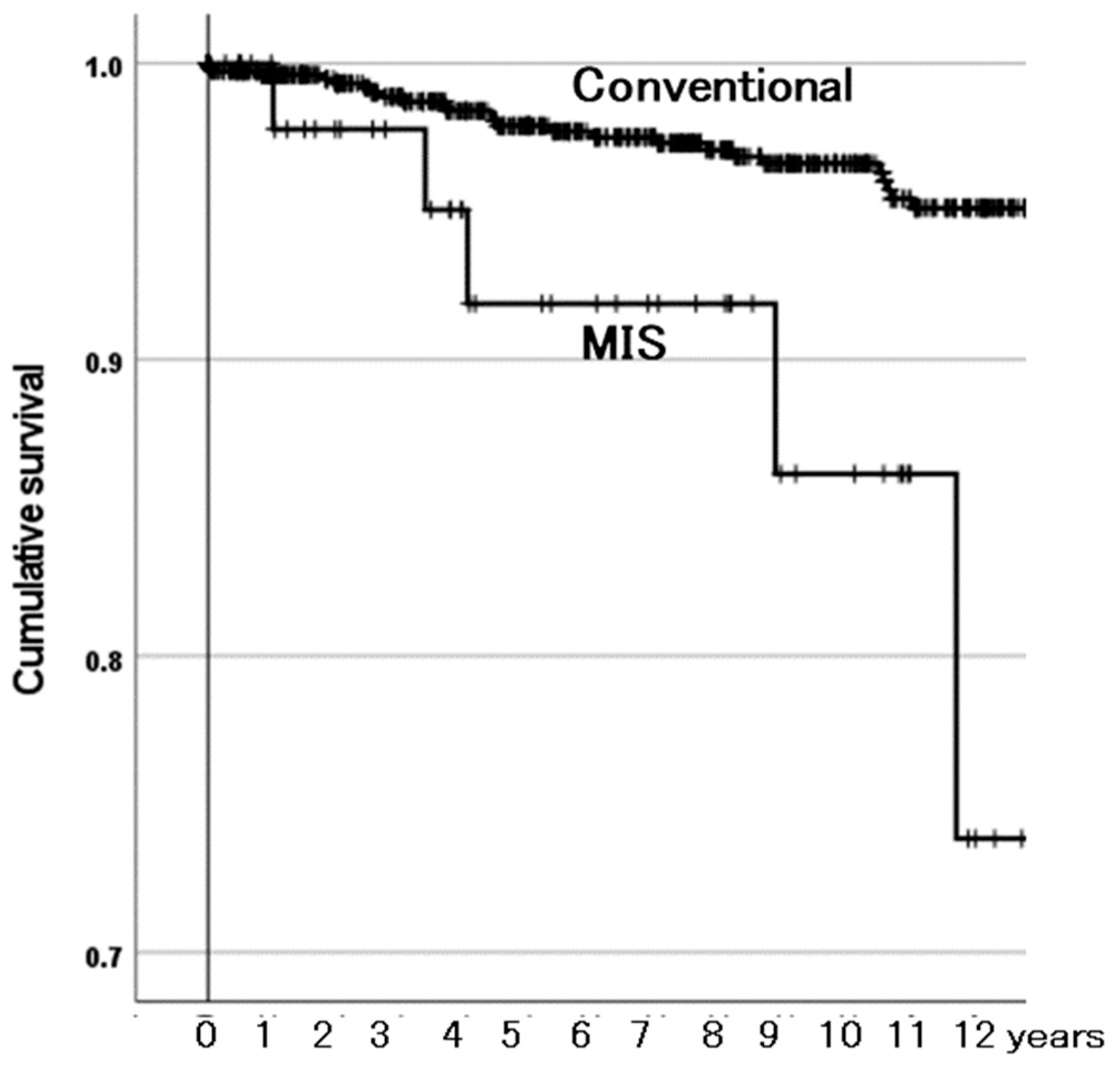
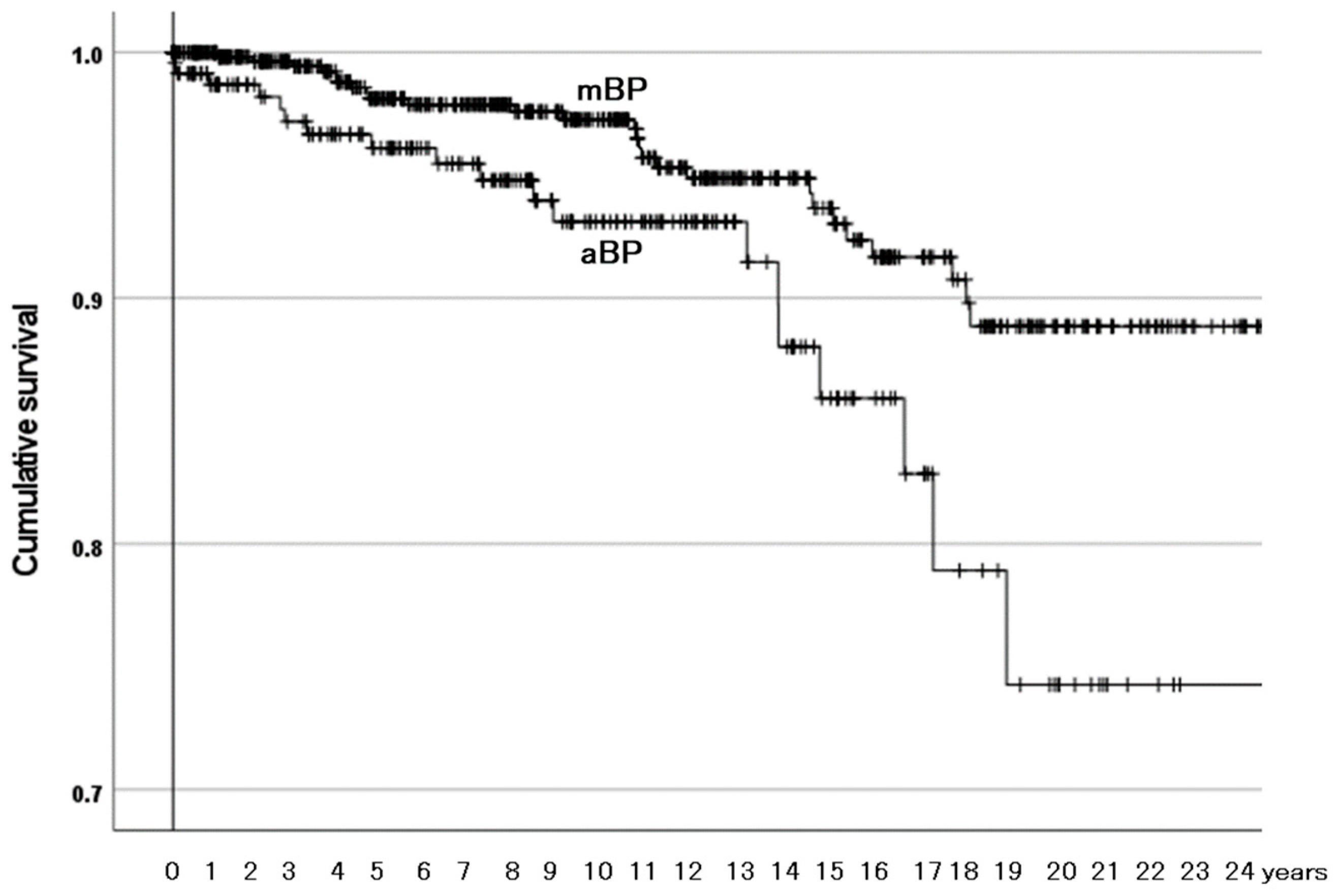
3.3.2. Survivorship Illustrated with Kaplan–Meier Estimator
4. Discussion
4.1. Acetabular-Articulating Material Is a Determinant of Survivorship of THAs
4.2. Combined Existence of Both ONFH-Associated Factors Affects Survivorship of BPs
4.3. MIS Affects Survivorship of BPs
4.4. The Material of the Outer Head Articulating with Cartilage Is a Determinant of Survivorship of BPs
4.5. Little Difference Based on Component Fixation in Survivorship of THAs
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, S.; Kubo, T.; Iwamoto, Y.; Fukushima, W.; Sugano, N. Nationwide multicenter follow-up cohort study of hip arthro-plasties performed for osteonecrosis of the femoral head. Int. Orthop. 2018, 42, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Barrett, M.C.; Beswick, A.D.; Judge, A.; Blom, A.W.; Wylde, V.; Whitehouse, M.R. Risk factors for dislocation after primary total hip replacement: A systematic review and meta-analysis of 125 studies involving approximately five million hip re-placements. Lancet Rheumatol. 2019, 1, e111–e121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chi, J.; Driskill, E.; Mont, M.; Jones, L.C.; Cui, Q. Effect of patient age on total hip arthroplasty outcomes in patients who have osteonecrosis of the femoral head compared to patients who have hip osteoarthritis. J. Arthroplast. 2024, 39, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Australian Orthopaedic Association National Joint Replacement Registry. AOANJRR 2024 Annual Report. Available online: https://aoanjrr.sahmri.com/annual-reports-2024 (accessed on 9 April 2025).
- Bergh, C.; Fenstad, A.M.; Furnes, O.; Garellick, G.; Havelin, L.I.; Overgaard, S.; Pedersen, A.B.; Mäkelä, K.T.; Pulkkinen, P.; Mohaddes, M.; et al. Increased risk of revision in patients with non-traumatic femoral head necrosis. Acta Orthop. 2014, 85, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.; Janz, V.; Trousdale, R.T.; Sierra, R.J.; Berry, D.J.; Abdel, M.P. Long-term survivorship of total hip arthroplasty with highly cross-linked polyethylene for osteonecrosis. J. Bone Joint Surg. Am. 2019, 101, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Radl, R.; Hungerford, M.; Materna, W.; Rehak, P.; Windhager, R. Higher failure rate and stem migration of an uncemented femoral component in patients with femoral head osteonecrosis than in patients with osteoarthrosis. Acta Orthop. 2005, 76, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Salman, L.A.; Hantouly, A.T.; Khatkar, H.; Al-Ani, A.; Abudalou, A.; Al-Juboori, M.; Ahmed, G. The outcomes of total hip replacement in osteonecrosis versus osteoarthritis: A systematic review and meta-analysis. Int. Orthop. 2023, 47, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Sugano, N.; Nakata, K.; Matsui, M.; Ohzono, K. Comparison between bipolar hemiarthroplasty and THA for osteonecrosis of the femoral head. Clin. Orthop. Relat. Res. 2004, 424, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Kim, Y.H.; Kim, Y.S.; Choi, I.Y. Is bipolar hemiarthroplasty a reliable option for Ficat stage III osteonecrosis of the femoral head? 15- to 24-year follow-up study. Arch. Orthop. Trauma Surg. 2012, 132, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Takaoka, K.; Tsukada, A.; Ueno, M. Polyethylene wear from femoral bipolar neck-cup impingement as a cause of femoral prosthetic loosening. Arch. Orthop. Trauma Surg. 1998, 117, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Moriya, M.; Uchiyama, K.; Takahira, N.; Fukushima, K.; Yamamoto, T.; Hoshi, K.; Itoman, M.; Takaso, M. Evaluation of bipolar hemiarthroplasty for the treatment of steroid-induced osteonecrosis of the femoral head. Int. Orthop. 2012, 36, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Sugano, N.; Ando, W.; Fukushima, W.; Kondo, K.; Sakai, T. Concerns with alumina bipolar hemiarthroplasties compared to metal bipolar hemiarthroplasties when performed for nontraumatic osteonecrosis of the femoral head. Int. Orthop. 2024, 48, 2535–2543. [Google Scholar] [CrossRef]
- Holappa, E.; Kettunen, J.; Miettinen, H.; Kröger, H.; Miettinen, S. Long-term survival analysis of cementless large-diameter head metal-on-metal total hip arthroplasty. Arch. Orthop. Trauma Surg. 2023, 143, 4437–4446. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, D.Y.; Pitta, M.; Carroll, K.M.; Alexiades, M. Hip arthroplasty for osteonecrosis of the femoral head secondary to alcohol abuse. Arthroplast. Today 2018, 5, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Taunton, M.J.; Trousdale, R.T. Total hip arthroplasty for alcoholic osteonecrosis of the femoral head. Orthopedics 2009, 32, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Mjaaland, K.E.; Svenningsen, S.; Fenstad, A.M.; Havelin, L.I.; Furnes, O.; Nordsletten, L. Implant survival after minimally invasive anterior or anterolateral vs. conventional posterior or direct lateral approach: An analysis of 21,860 total hip arthroplasties from the Norwegian Arthroplasty Register (2008 to 2013). J. Bone Joint Surg. Am. 2017, 99, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.B.; Zhu, M.; Frampton, C.; Vane, A.; Poutawera, V. Comparing uncemented, hybrid and cemented primary total hip arthroplasty in young patients, a New Zealand Joint Registry study. Arch Orthop Trauma Surg. 2022, 142, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
| Variable | THAs | BPs | p-Value | |
|---|---|---|---|---|
| Patient age (years) | 52.0 ± 14.34 | 49.2 ± 14.21 | <0.001 | |
| Male gender (%) | 54.0 | 58.1 | 0.024 | |
| Height (cm) | 162.1 ± 9.26 | 163.0 ± 9.10 | 0.011 | |
| Weight (kg) | 61.3 ± 12.84 | 60.8 ± 11.83 | 0.242 | |
| BMI (body mass index) (kg/m2) | 23.23 ± 3.951 | 22.80 ± 3.589 | 0.002 | |
| ONFH-associated factor (%) | Systemic steroid use | 59.9 | 53.9 | <0.001 |
| Excessive alcohol consumption | 27.0 | 33.5 | ||
| None of them | 10.8 | 9.3 | ||
| Both of them | 2.3 | 3.3 | ||
| ONFH stage (%) | 2 (without collapse of FH) | 2.7 | 4.6 | <0.001 |
| 3 (collapse of FH) | 49.8 | 89.6 | ||
| 4 (osteoarthrosis) | 47.5 | 5.8 | ||
| Previous surgery in index joint (%) | Yes (joint-preserving surgery) | 8.1 | 4.3 | <0.001 |
| Surgical approach (%) | Posterior | 58.5 | 87.6 | <0.001 |
| Anterior or anterolateral | 22.3 | 1.9 | ||
| Lateral | 19.2 | 10.5 | ||
| Incision length (%) | Conventional | 68.9 | 94.0 | <0.001 |
| MIS | 31.1 | 6.0 | ||
| Cemented fixation (%) | Acetabular component | 2.3 | NA | NA |
| Femoral component | 14.4 | 8.2 | <0.001 | |
| Surface finish of femoral stem | Grid blast (for bone on growth) | 7.8 | 4.3 | <0.001 |
| Porous with/without coating | 69.9 | 83.6 | ||
| Cement with/without polish | 13.9 | 7.1 | ||
| Others | 8.4 | 5.0 | ||
| Acetabular-articulating material in THAs and inner articulating surface of outer head in BPs (%) | HXLPE | 61.4 | 13.1 | NA |
| MXLPE | 21.3 | 0 | ||
| cPE | 9.3 | 86.9 | ||
| Cobalt–chrome | 4.3 | 0 | ||
| Ceramic | 3.7 | 0 | ||
| Femoral head material in THAs and inner head material in BPs (%) | Ceramic | 64.4 | 40.2 | NA |
| Cobalt–chrome | 28.2 | 53.4 | ||
| Oxidized zirconium | 5.6 | 1.8 | ||
| Stainless steel | 1.8 | 4.6 | ||
| Head diameter in THAs and inner head diameter in BPs (%) | ≥36 mm | 21.4 | 0 | NA |
| 32 mm | 36.3 | 0.6 | ||
| 28 mm | 23.9 | 18.2 | ||
| 26 mm | 14.5 | 32.5 | ||
| 22 mm | 3.9 | 48.7 | ||
| Variable | Factor | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|
| Patient age (years) | Increment of 1 | 1.01 (0.99–1.02) | 0.146 |
| Gender | Female | Reference | |
| Male | 1.02 (0.75–1.37) | 0.920 | |
| Height (cm) | Increment of 1 | 1.00 (0.98–1.02) | 0.997 |
| Weight (kg) | Increment of 1 | 0.99 (0.98–1.01) | 0.333 |
| BMI (body mass index) (kg/m2) | Increment of 1 | 0.98 (0.94–1.02) | 0.247 |
| ONFH-associated factor | Systemic steroid use | 0.94 (0.57–1.55) | 0.807 |
| Excessive alcohol consumption | 1.31 (0.76–2.25) | 0.326 | |
| None of them | Reference | ||
| Both of them | 1.82 (0.72–4.60) | 0.203 | |
| ONFH stage | 2 or 3 (before osteoarthrosis) | 0.84 (0.61–1.14) | 0.253 |
| 4 (osteoarthrosis) | Reference | ||
| Previous hip surgery in index joint | No | Reference | |
| Yes | 1.16 (0.72–1.86) | 0.554 | |
| Surgical approach | Posterior | 0.71 (0.50–1.01 | 0.054 |
| Anterior or anterolateral | 0.42 (0.23–0.79) | 0.007 | |
| Lateral | Reference | ||
| Incision length | Conventional | Reference | |
| MIS | 0.53 (0.33–0.83) | 0.005 | |
| Acetabular component fixation | Uncemented | Reference | |
| Cemented | 1.71 (0.84–3.49) | 0.137 | |
| Femoral component fixation | Uncemented | Reference | |
| Cemented | 1.04 (0.67–1.62) | 0.860 | |
| Surface finish of femoral stem | Grid blast (for bone on growth) | Reference | |
| Porous with/without coating | 0.81 (0.048–1.36) | 0.429 | |
| Cement with/without polish | 0.73 (0.38–1.42) | 0.353 | |
| Others | 0.59 (0.29–1.24) | 0.164 | |
| Acetabular-articulating material | HXLPE or MXLPE | Reference | |
| cPE | 2.82 (2.00–3.98) | <0.001 | |
| Metal (metal-on-metal THA) | 2.12 (1.25–3.59) | 0.005 | |
| Ceramic (ceramic-on-ceramic THA) | 1.11 (0.57–2.15) | 0.765 | |
| Femoral head material | Metal | Reference | |
| Ceramic | 0.50 (0.37–0.68) | <0.001 | |
| Head diameter | ≥36 mm | 0.38 (0.21–0.67) | <0.001 |
| 32 mm | 0.30 (0.17–0.51) | <0.001 | |
| 28 mm | 0.36 (0.22–0.58) | <0.001 | |
| 26 mm | 0.41 (0.26–0.66) | <0.001 | |
| 22 mm | Reference |
| Variable | Factor | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|
| Surgical approach | Posterior | 0.69 (0.47–1.00) | 0.050 |
| Anterior or anterolateral | 0.52 (0.27–1.00) | 0.051 | |
| Lateral | Reference | ||
| Acetabular-articulating material | HXLPE or MXLPE | Reference | |
| cPE | 2.55 (1.69–3.84) | <0.001 | |
| Metal (metal-on-metal THA) | 2.15 (1.08–4.28) | 0.028 | |
| Ceramic (ceramic-on-ceramic THA) | 1.27 (0.63–2.57) | 0.503 | |
| Head diameter | ≥36 mm | 0.62 (0.28–1.36) | 0.233 |
| 32 mm | 0.68 (0.35–1.32) | 0.250 | |
| 28 mm | 0.60 (0.34–1.03) | 0.065 | |
| 26 mm | 0.68 (0.40–1.13) | 0.138 | |
| 22 mm | Reference |
| Reasons for Need for Reoperation (n) | HXLPE or MXLPE (5194) | cPE (584) | Metal (274) | Ceramic (232) | Total (6284) |
|---|---|---|---|---|---|
| Recurrent dislocation | 35 | 13 | 3 | 6 | 57 |
| Periprosthetic femoral fracture | 20 | 5 | 0 | 1 | 26 |
| Osteolysis | 6 | 18 | 2 | 0 | 26 |
| Polyethylene wear and/or breakage | 3 | 17 | 1 | 0 | 21 |
| Stem loosening | 9 | 8 | 0 | 1 | 18 |
| Socket loosening | 9 | 2 | 2 | 1 | 14 |
| Adverse reaction to metal debris | 1 | 0 | 8 | 0 | 9 |
| Others (n ≤ 3 for each) | 10 | 5 | 2 | 1 | 18 |
| Total (%) | 93 (1.8%) | 68 (11.6%) | 18 (6.6%) | 10 (4.3%) | 189 (3.0%) |
| Variable | Factor | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|
| Patient age (years) | Increment of 1 | 0.99 (0.97–1.01) | 0.304 |
| Gender | Female | Reference | |
| Male | 1.05 (0.59–1.88) | 0.868 | |
| Height (cm) | Increment of 1 | 1.01 (0.98–1.05) | 0.452 |
| Weight (kg) | Increment of 1 | 1.01 (0.99–1.04) | 0.351 |
| BMI (kg/m2) | Increment of 1 | 1.03 (0.95–1.13) | 0.480 |
| ONFH-associated factor | Systemic steroid use | 1.96 (0.46–8.28) | 0.363 |
| Excessive alcohol consumption | 2.16 (0.49–9.42) | 0.308 | |
| None of them | Reference | ||
| Both of them | 10.34 (2.00–53.57) | 0.005 | |
| ONFH stage | 2 or 3 (before osteoarthrosis) | 0.51 (0.20–1.30) | 0.161 |
| 4 (osteoarthrosis) | Reference | ||
| Previous hip surgery in index joint | No | Reference | |
| Yes | 0.98 (0.30–3.18) | 0.976 | |
| Surgical approach | Posterior | 0.77 (0.32–1.81 | 0.546 |
| Anterior or anterolateral | UC1 | 0.974 | |
| Lateral | Reference | ||
| Incision length | Conventional | Reference | |
| MIS | 5.07 (2.07–12.39) | <0.001 | |
| New type of BPs (nBPs) or others (oBPs) | nBPs | Reference | |
| oBPs | 0.83 (0.46–1.51) | 0.551 | |
| Surface of prosthetic neck: smooth (sBPs) or rough (rBPs) | sBPs | Reference | |
| rBPs | 1.40 (0.75–2.60) | 0.292 | |
| Outer surface of outer head: alumina ceramic (aBPs) or metal (mBPs) | aBPs | 2.17 (1.20–3.92) | 0.010 |
| mBPs | Reference | ||
| Inner articulating surface of outer head: HXLPE (hBPs) or cPE (cBPs) | hBPs | Reference | |
| cBPs | 0.56 (0.24–1.35) | 0.200 | |
| Femoral component fixation | Uncemented | Reference | |
| Cemented | 0.94 (0.29–3.03) | 0.911 | |
| Surface finish of femoral stem | Grid blast (for bone on growth) | Reference | |
| Porous with/without coating | 0.57 (0.14–2.37) | 0.435 | |
| Cement with/without polish | 0.66 (0.11–3.98) | 0.653 | |
| Others | 0.16 (0.01–1.78) | 0.135 | |
| Material of inner head | Metal | Reference | |
| Ceramic | 1.63 (0.91–2.92) | 0.101 | |
| Inner head diameter | 32 mm | UC2 | 0.975 |
| 28 mm | 0.80 (0.33–1.96) | 0.630 | |
| 26 mm | 1.20 (0.62–2.31) | 0.597 | |
| 22 mm | Reference |
| Variable | Factor | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|
| ONFH-associated factor | Systemic steroid use | 1.98 (0.47–8.40) | 0.354 |
| Excessive alcohol consumption | 2.14 (0.49–9.39) | 0.311 | |
| None of them | Reference | ||
| Both of them | 9.13 (1.76–47.33) | 0.008 | |
| Incision length | Conventional | Reference | |
| MIS | 5.61 (2.25–13.97) | <0.001 | |
| Outer surface of outer head | Alumina ceramic (aBPs) | 2.40 (1.32–4.38) | 0.004 |
| Metal (mBPs) | Reference |
| Reasons for Need for Reoperation (n) | mBP (648) | aBP (238) | Total (886) |
|---|---|---|---|
| Proximal migration of the outer head | 17 | 13 | 30 |
| Pain | 5 | 2 | 7 |
| Others (n ≤ 3 for each) | 5 | 5 | 10 |
| Total (%) | 27 (4.2%) | 20 (8.4%) | 47 (5.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, S.; Sugano, N.; Ando, W.; Fukushima, W.; Kondo, K.; Sakai, T. Articulating Materials Are Determinants of Survivorship of Hip Arthroplasties Performed for Nontraumatic Osteonecrosis of the Femoral Head. Materials 2025, 18, 2125. https://doi.org/10.3390/ma18092125
Kobayashi S, Sugano N, Ando W, Fukushima W, Kondo K, Sakai T. Articulating Materials Are Determinants of Survivorship of Hip Arthroplasties Performed for Nontraumatic Osteonecrosis of the Femoral Head. Materials. 2025; 18(9):2125. https://doi.org/10.3390/ma18092125
Chicago/Turabian StyleKobayashi, Seneki, Nobuhiko Sugano, Wataru Ando, Wakaba Fukushima, Kyoko Kondo, and Takashi Sakai. 2025. "Articulating Materials Are Determinants of Survivorship of Hip Arthroplasties Performed for Nontraumatic Osteonecrosis of the Femoral Head" Materials 18, no. 9: 2125. https://doi.org/10.3390/ma18092125
APA StyleKobayashi, S., Sugano, N., Ando, W., Fukushima, W., Kondo, K., & Sakai, T. (2025). Articulating Materials Are Determinants of Survivorship of Hip Arthroplasties Performed for Nontraumatic Osteonecrosis of the Femoral Head. Materials, 18(9), 2125. https://doi.org/10.3390/ma18092125






