Critical Design Parameters of Tantalum-Based Comb Structures to Manipulate Mammalian Cell Morphology
Abstract
1. Introduction
2. Materials and Methods
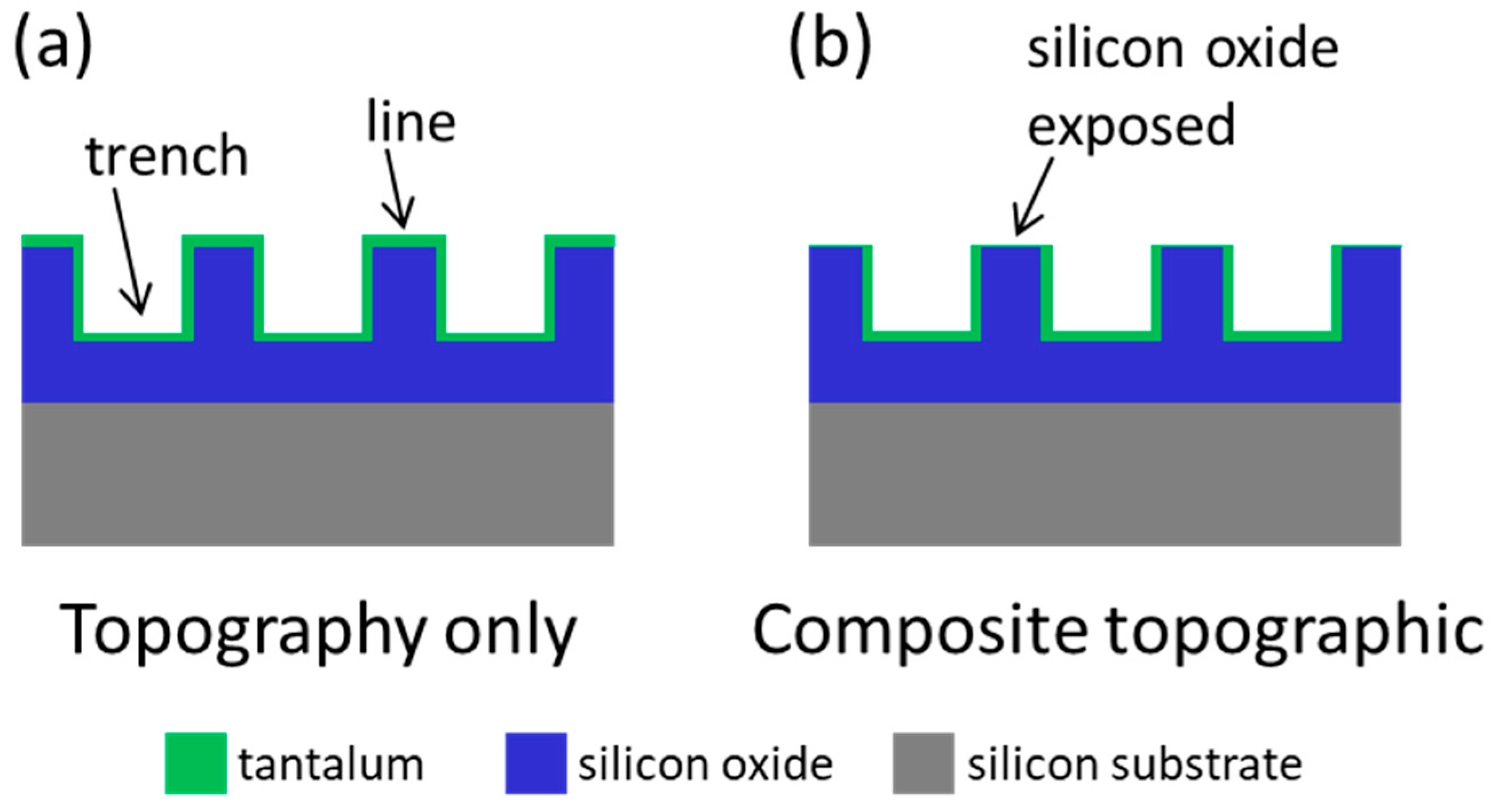
2.1. Test Structure Fabrication
2.2. Cell Culture and Deposition
2.3. Cell Fixation, Microscopy, and Measurement Parameters of Cell Alignment
2.4. Cell Staining
3. Results and Discussion
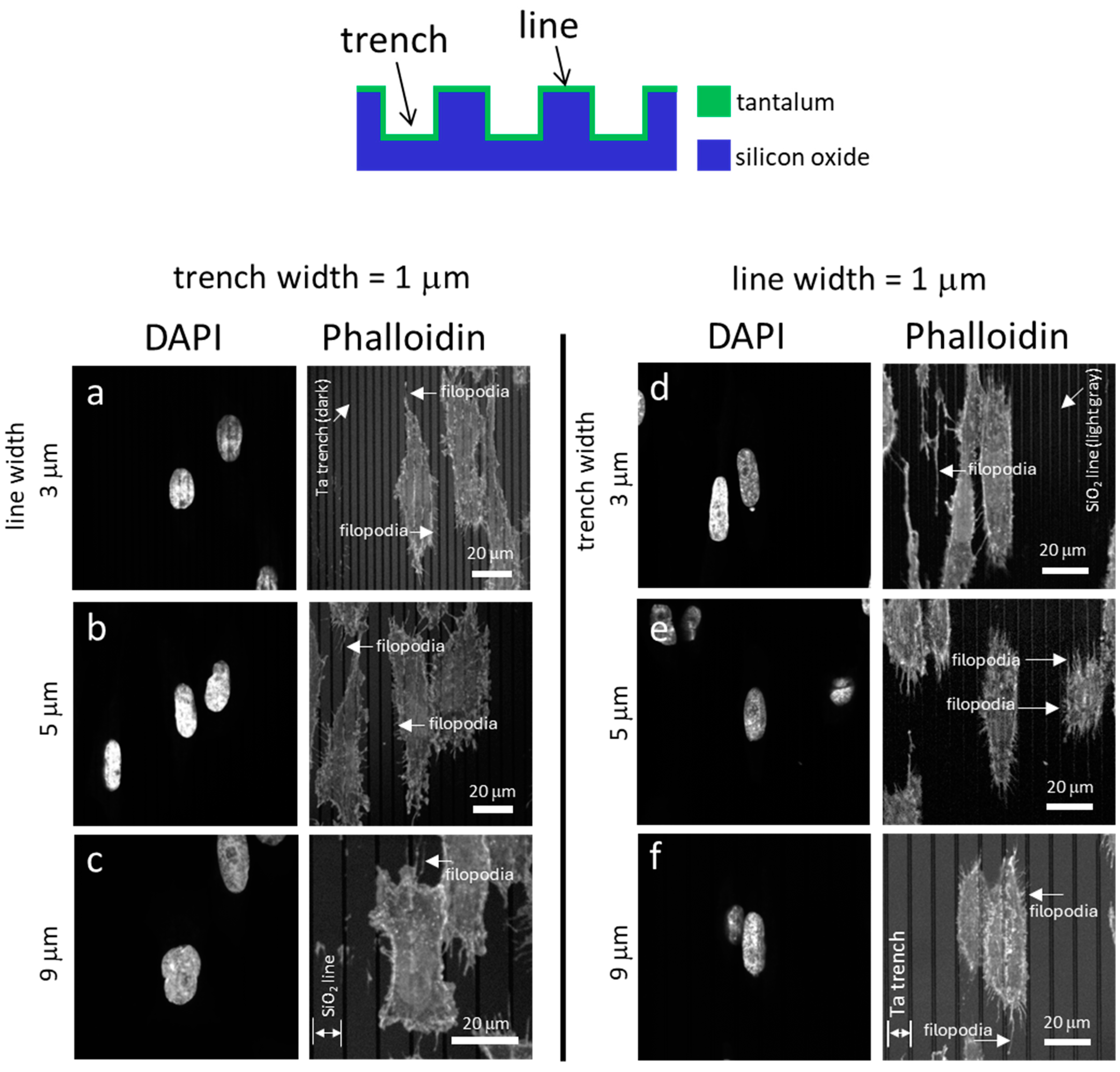
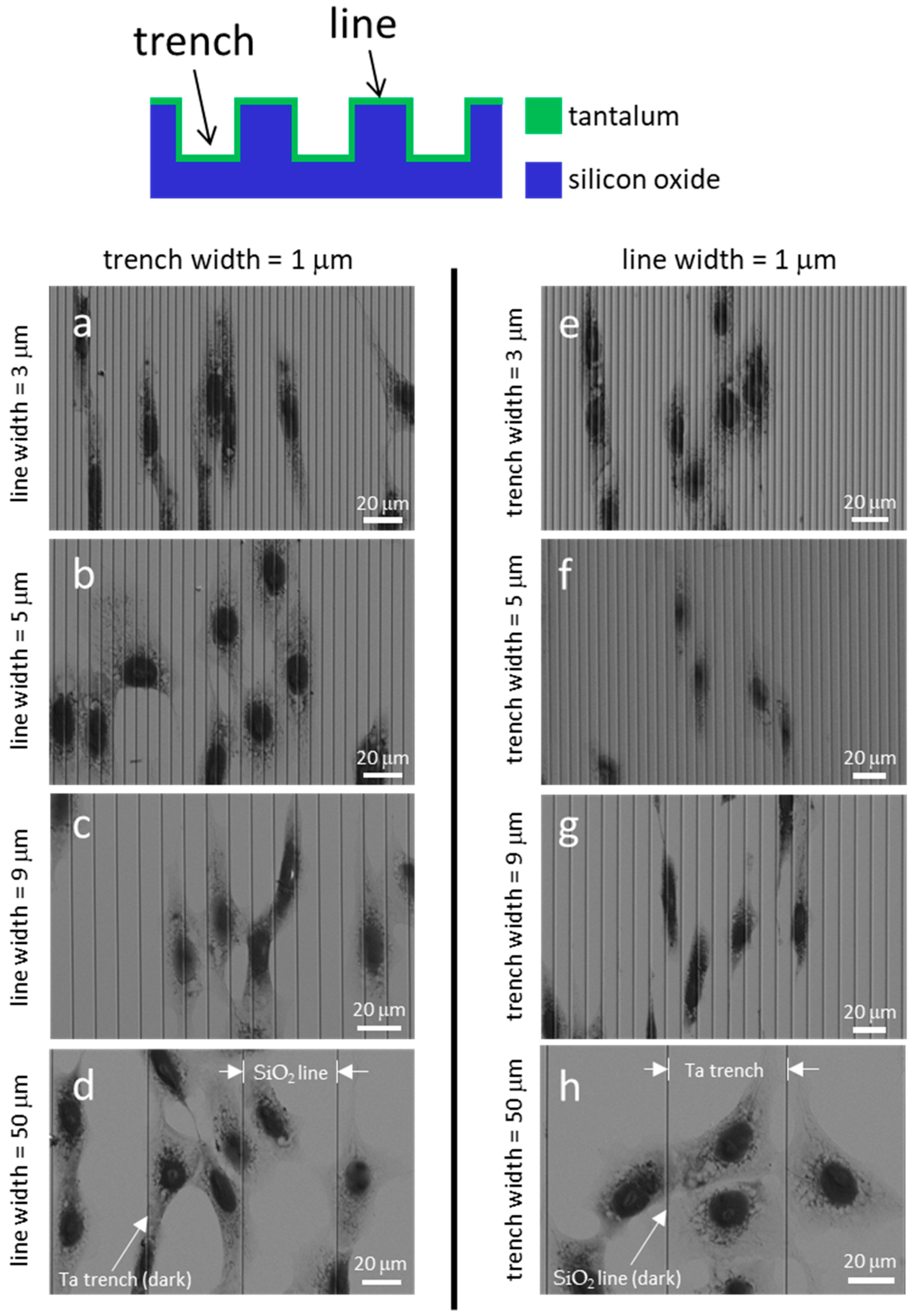
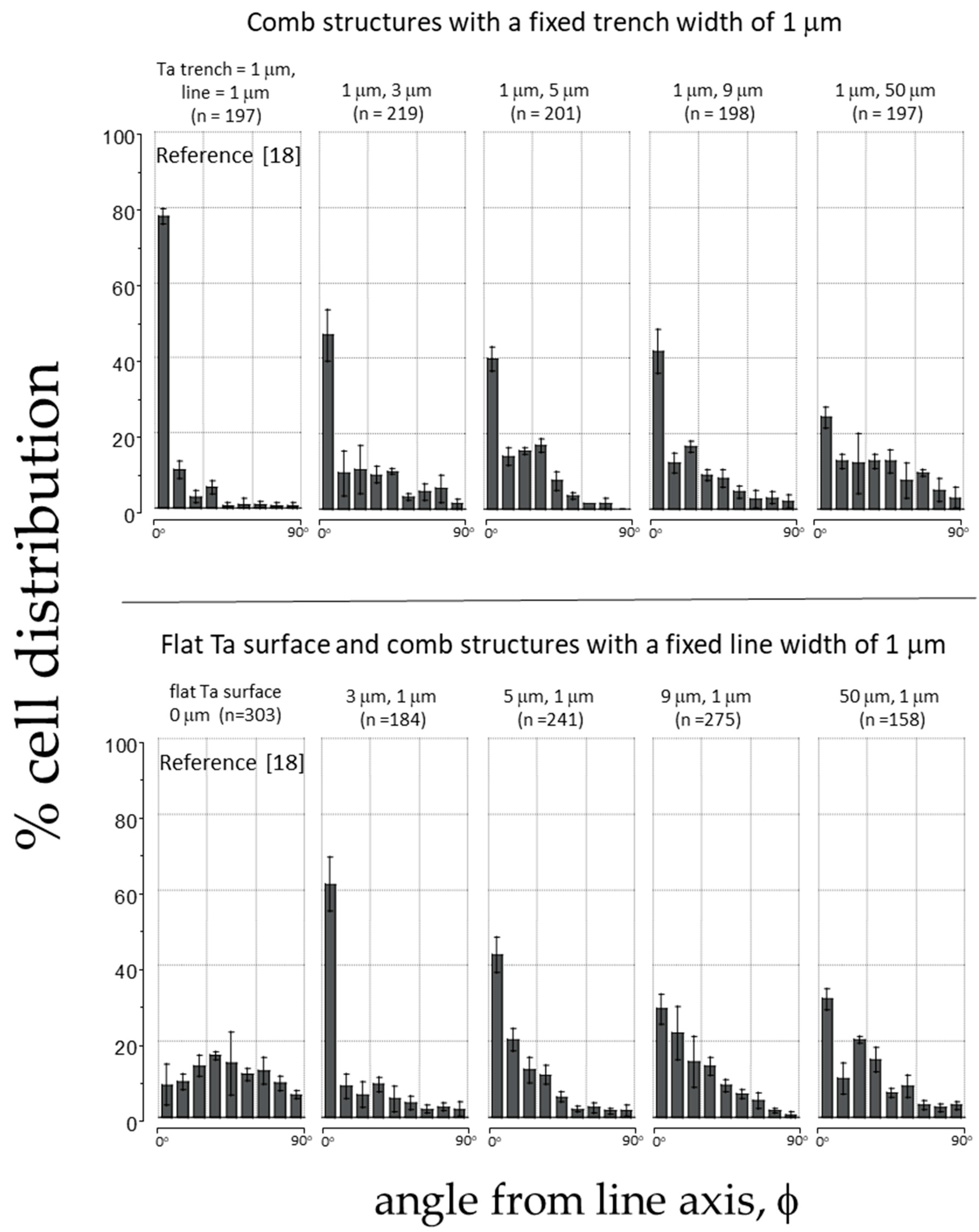
3.1. Cell Alignment on Ta Topographic Structures
3.2. Cell Alignment on Ta/SiO2 Composite Surface Structures
3.3. Pattern and Material-Dependent Cell Alignment Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aubin, H.; Nichol, J.W.; Hutson, C.B.; Bae, H.; Sieminski, A.L.; Cropek, D.M.; Akhyari, P.; Khademhosseini, A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 2010, 31, 6941–6951. [Google Scholar] [CrossRef] [PubMed]
- Gracz, A.D.; Puthoff, B.J.; Magness, S.T. Cells from Murine Intestine. In Somatic Stem Cells; Humana: Totowa, NJ, USA, 2018; pp. 89–107. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Koudouna, E.; Jester, J.; Figueiredo, F.; Connon, C.J. Template Curvature Influences Cell Alignment to Create Improved Human Corneal Tissue Equivalents. Adv. Biosyst. 2017, 1, e1700135. [Google Scholar] [CrossRef]
- Kimura, S.; Tsuchiya, A.; Ogawa, M.; Ono, M.; Suda, N.; Sekimoto, K.; Takeo, M.; Tsuji, T. Tissue-scale tensional homeostasis in skin regulates structure and physiological function. Commun. Biol. 2020, 3, 637. [Google Scholar] [CrossRef] [PubMed]
- Lenne, P.F.; Rupprecht, J.F.; Viasnoff, V. Cell Junction Mechanics beyond the Bounds of Adhesion and Tension. Dev. Cell 2021, 56, 202–212. [Google Scholar] [CrossRef]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Functionalized Stress Component onto Bio-template as a Pathway of Cytocompatibility. Sci. Rep. 2016, 6, 35425. [Google Scholar] [CrossRef] [PubMed]
- Béduer, A.; Vieu, C.; Arnauduc, F.; Sol, J.; Loubinoux, I.; Vaysse, L. Biomaterials Engineering of adult human neural stem cells differentiation through surface micropatterning. Biomaterials 2012, 33, 504–514. [Google Scholar] [CrossRef]
- Jang, K.J.; Kim, M.S.; Feltrin, D.; Jeon, N.L.; Suh, K.Y.; Pertz, O. Two distinct filopodia populations at the growth cone allow to sense nanotopographical extracellular matrix cues to guide neurite outgrowth. PLoS ONE 2010, 5, e15966. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Hu, W.; Crouch, A.S.; Miller, D.; Aryal, M.; Luebke, K.J. Inhibited cell spreading on polystyrene nanopillars fabricated by nanoimprinting and in situ elongation. Nanotechnology 2010, 21, 385301. [Google Scholar] [CrossRef]
- Teixeira, A.I.; McKie, G.A.; Foley, J.D.; Bertics, P.J.; Nealey, P.F.; Murphy, C.J. The effect of environmental factors on the response of human corneal epithelial cells to nanoscale substrate topography. Biomaterials 2006, 27, 3945–3954. [Google Scholar] [CrossRef]
- Santoro, F.; Zhao, W.; Joubert, L.-M.; Duan, L.; Schnitker, J.; van de Burgt, Y.; Lou, H.-Y.; Liu, B.; Salleo, A.; Cui, L.; et al. Revealing the Cell-Material Interface with Nanometer Resolution by Focused Ion Beam/Scanning Electron Microscopy. ACS Nano 2017, 11, 8320–8328. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wan, Y.; Liu, Y. Effects of nanopillar array diameter and spacing on cancer cell capture and cell behaviors. Nanoscale 2014, 6, 12482–12489. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Huang, W.-Y.; Chen, L.-H.; Liang, N.-W.; Wang, H.-C.; Lu, J.; Wang, X.; Wang, T.-W. Neural tissue engineering: The influence of scaffold surface topography and extracellular matrix microenvironment. J. Mater. Chem. B 2021, 9, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Palchesko, R.N.; Lathrop, K.L.; Funderburgh, J.L.; Feinberg, A.W. In Vitro Expansion of Corneal Endothelial Cells on Biomimetic Substrates. Sci. Rep. 2015, 5, srep07955. [Google Scholar] [CrossRef]
- Jahed, Z.; Lin, P.; Seo, B.B.; Verma, M.S.; Gu, F.X.; Tsui, T.Y.; Mofrad, M.R. Responses of Staphylococcus aureus bacterial cells to nanocrystalline nickel nanostructures. Biomaterials 2014, 35, 4249–4254. [Google Scholar] [CrossRef]
- Llopis-Grimalt, M.A.; Amengual-Tugores, A.M.; Monjo, M.; Ramis, J.M. Oriented cell alignment induced by a nanostructured titanium surface enhances expression of cell differentiation markers. Nanomaterials 2019, 9, 1661. [Google Scholar] [CrossRef]
- Moussa, H.; Logan, M.; Wong, K.; Rao, Z.; Aucoin, M.; Tsui, T. Nanoscale-Textured Tantalum Surfaces for Mammalian Cell Alignment. Micromachines 2018, 9, 464. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, Y.; Cheng, M.; Jiang, G.; He, G.; Chen, Y. Tantalum implanted entangled porous titanium promotes surface osseointegration and bone ingrowth. Sci. Rep. 2016, 6, 26248. [Google Scholar] [CrossRef]
- Rani, V.V.D.; Vinoth-Kumar, L.; Anitha, V.C.; Manzoor, K.; Deepthy, M.; Shantikumar, V.N. Osteointegration of titanium implant is sensitive to specific nanostructure morphology. Acta Biomater. 2012, 8, 1976–1989. [Google Scholar] [CrossRef]
- Castro-Raucci, L.M.S.; Francischini, M.S.; Teixeira, L.N.; Ferraz, E.P.; Lopes, H.B.; de Oliveira, P.T.; Hassan, M.Q.; Rosa, A.L.; Beloti, M.M. Titanium with Nanotopography Induces Osteoblast Differentiation by Regulating Endogenous Bone Morphogenetic Protein Expression and Signaling Pathway. J. Cell. Biochem. 2016, 117, 1718–1729. [Google Scholar] [CrossRef]
- Jahed, Z.; Jahed, Z.; Zareian, R.; Chau, Y.Y.; Seo, B.B.; West, M.; Tsui, T.Y.; Wen, W.; Mofrad, M.R.K. Differential Collective- and Single-Cell Behaviors on Silicon Micropillar Arrays. ACS Appl. Mater. Interfaces 2016, 8, 23604–23613. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gräter, S.V.; Corbellini, F.; Rinck, S.; Bock, E.; Kemkemer, R.; Kessler, H.; Ding, J.; Spatz, J.P. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009, 9, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.P.; Hess, A.E.; Rowan, S.J.; Weder, C.; A Zorman, C.; Tyler, D.J.; Capadona, J.R. In vivo deployment of mechanically adaptive nanocomposites for intracortical microelectrodes. J. Neural Eng. 2011, 8, 46010. [Google Scholar] [CrossRef]
- Moussa, H.; Logan, M.; Chan, W.Y.; Wong, K.; Rao, Z.; Aucoin, M.G.; Tsui, T.Y. Pattern-Dependent Mammalian Cell (Vero) Morphology on Tantalum/Silicon Oxide 3D Nanocomposites. Materials 2018, 11, 1306. [Google Scholar] [CrossRef]
- Nikkhah, M.; Eshak, N.; Zorlutuna, P.; Annabi, N.; Castello, M.; Kim, K.; Dolatshahi-Pirouz, A.; Edalat, F.; Bae, H.; Yang, Y.; et al. Biomaterials Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials 2012, 33, 9009–9018. [Google Scholar] [CrossRef]
- Moussa, H.I.; Logan, M.; Siow, G.C.; Phann, D.L.; Rao, Z.; Aucoin, M.G.; Tsui, T.Y. Manipulating mammalian cell morphologies using chemical-mechanical polished integrated circuit chips. Sci. Technol. Adv. Mater. 2017, 18, 839–856. [Google Scholar] [CrossRef]
- Arnold, M.; Schwieder, M.; Blümmel, J.; Cavalcanti-Adam, E.A.; López-Garcia, M.; Kessler, H.; Geiger, B.; Spatz, J.P. Cell interactions with hierarchically structured nano-patterned adhesive surfaces. Soft Matter 2009, 5, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.I.; Chan, W.Y.; Logan, M.; Aucoin, M.G.; Tsui, T.Y. Limitation in controlling the morphology of mammalian vero cells induced by cell division on asymmetric tungsten-silicon oxide nanocomposite. Materials 2020, 13, 335. [Google Scholar] [CrossRef]
- Moussa, H.I.; Kim, G.; Tong, J.; Glerum, D.M.; Tsui, T.Y. Influence of antimycin a, a bacterial toxin, on human dermal fibroblast cell adhesion to tungsten-silicon oxide nanocomposites. J. Exp. Nanosci. 2019, 14, 69–88. [Google Scholar] [CrossRef]
- Bajaj, P.; Millet, L.R., Jr.; Wei, C. Quantitative biosciences from nano to macro Integrative Biology Patterning the differentiation of C2C12 skeletal myoblasts. Integr. Biol. 2011, 3, 897–909. [Google Scholar] [CrossRef]
- Shrirao, A.B.; Kung, F.H.; Yip, D.; Firestein, B.L.; Cho, C.H.; Townes-Anderson, E. A versatile method of patterning proteins and cells. J. Vis. Exp. 2017, 2017, e55513. [Google Scholar] [CrossRef]
- Humenik, M.; Winkler, A.; Scheibel, T. Patterning of protein-based materials. Biopolymers 2021, 112, e23412. [Google Scholar] [CrossRef] [PubMed]
- Pesen, D.; Haviland, D.B. Modulation of Cell Adhesion Complexes by Surface Protein Patterns. ACS Appl. Mater. Interfaces 2009, 1, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Leduc, P.R. Micropatterning polyvinyl alcohol as a biomimetic material through soft lithography with cell culture. Mol. Biosyst. 2006, 2, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Poudel, I.; Lee, J.S.; Tan, L.; Lim, J.Y. Micropatterning—Retinoic acid co-control of neuronal cell morphology and neurite outgrowth. Acta Biomater. 2013, 9, 4592–4598. [Google Scholar] [CrossRef]
- Bugga, P.; Mrksich, M. Dynamic substrates for cell biology. Curr. Opin. Colloid Interface Sci. 2018, 38, 80–87. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Corbin, E.A.; Caliari, S.R.; Ouyang, L.; Vega, S.L.; Truitt, R.; Han, L.; Margulies, K.B.; Burdick, J.A. Mechanically dynamic PDMS substrates to investigate changing cell environments. Biomaterials 2017, 145, 23–32. [Google Scholar] [CrossRef]
- Feinberg, A.W.; Wilkerson, W.R.; Seegert, C.A.; Gibson, A.L.; Hoipkemeier-Wilson, L.; Brennan, A.B. Systematic variation of microtopography, surface chemistry and elastic modulus and the state dependent effect on endothelial cell alignment. J. Biomed. Mater. Res. Part A 2007, 86A, 522–534. [Google Scholar] [CrossRef]
- Song, S.; Kim, E.J.; Bahney, C.S.; Miclau, T.; Marcucio, R.; Roy, S. The synergistic effect of micro-topography and biochemical culture environment to promote angiogenesis and osteogenic differentiation of human mesenchymal stem cells. Acta Biomater. 2015, 18, 100–111. [Google Scholar] [CrossRef]
- Tang, Z.; Xie, Y.; Yang, F.; Huang, Y.; Wang, C.; Dai, K.; Zheng, X.; Zhang, X. Porous Tantalum Coatings Prepared by Vacuum Plasma Spraying Enhance BMSCs Osteogenic Differentiation and Bone Regeneration In Vitro and In Vivo. PLoS ONE 2013, 8, e66263. [Google Scholar] [CrossRef]
- Eskandari, A.; Glerum, D.M.; Tsui, T.Y. Influence of Indium (III) Chloride on Human Dermal Fibroblast Cell Adhesion on Tantalum/Silicon Oxide Nano-Composites. Materials 2022, 15, 3577. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Ohshima, M.; Iwata, H. Time-lapse observation of cell alignment on nanogrooved patterns. J. R. Soc. Interface 2009, 6, S269–S277. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Ting, Y.-C.; Lai, J.-Y.; Liu, H.-L.; Fang, H.-W.; Tsai, W.-B. Quantitative analysis of osteoblast-like cells (MG63) morphology on nanogrooved substrata with various groove and ridge dimensions. J. Biomed. Mater. Res. A 2009, 90, 629–640. [Google Scholar] [CrossRef]
- Fujita, S.; Ono, D.; Ohshima, M.; Iwata, H. Supercritical CO2-assisted embossing for studying cell behaviour on microtextured surfaces. Biomaterials 2008, 29, 4494–4500. [Google Scholar] [CrossRef]
- Solis-Castro, M.E.; Jaramillo-Corrales, A.; Seminario, R.V.G.; Grados, N.J.; Pilco, I.E.M.; Quispe, K.E.V.; Rosillo, L.Y.L.T.; Dominguez, M.N.V.; Cusi, D.T.E.; Minaya, P.; et al. Effectiveness of the Inactivated SARS-CoV-2 (Vero Cell) Vaccine in Peruvian Health Workers. Life 2022, 12, 1318. [Google Scholar] [CrossRef]
- Ren, B.; Zhai, Z.; Guo, K.; Liu, Y.; Hou, W.; Zhu, Q.; Zhu, J. The application of porous tantalum cylinder to the repair of comminuted bone defects: A study of rabbit firearm injuries. Int. J. Clin. Exp. Med. 2015, 8, 5055–5064. [Google Scholar] [PubMed]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef]
- Patil, N.; Lee, K.; Goodman, S.B. Review Porous Tantalum in Hip and Knee Reconstructive Surgery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89B, 242–251. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, D.; Wang, B.; Wang, W.; Kang, K.; Xie, H.; Liu, B.; Zhang, X.; Zhang, J.; Yang, Z. Tantalum coating of porous carbon scaffold supplemented with autologous bone marrow stromal stem cells for bone regeneration in vitro and in vivo. Exp. Biol. Med. 2016, 241, 592–602. [Google Scholar] [CrossRef]
- Huo, W.T.; Zhao, L.Z.; Yu, S.; Yu, Z.T.; Zhang, P.X.; Zhang, Y.S. Significantly enhanced osteoblast response to nano-grained pure tantalum. Sci. Rep. 2017, 7, 40868. [Google Scholar] [CrossRef]
- Mani, G.; Porter, D.; Grove, K.; Collins, S.; Ornberg, A.; Shulfer, R. A comprehensive review of biological and materials properties of Tantalum and its alloys. J. Biomed. Mater. Res. Part A 2022, 110, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
- Tran, R.; Xu, Z.; Radhakrishnan, B.; Winston, D.; Sun, W. Data Descriptor: Surface energies of elemental crystals. Sci. Data 2016, 3, 160080. [Google Scholar] [CrossRef]
- Hallab, N.J.; Bundy, K.J.; Connor, K.O.; Moses, R.L.; Jacobs, J.J. Evaluation of Metallic and Polymeric Biomaterial Surface Energy and Surface Roughness Characteristics for Directed Cell Adhesion. Tissue Eng. 2001, 7, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Balla, V.K.; Bodhak, S.; Bose, S.; Bandyopadhyay, A. Porous Tantalum Structures for Bone Implants: Fabrication, Mechanical and In vitro Biological Properties. Acta Biomater. 2011, 6, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qin, Y.; Wang, Z.; Yu, T.; Chen, Y.; Ge, Z. Recent advance in cell patterning techniques: Approaches, applications and future prospects. Sens. Actuators A Phys. 2022, 333, 113229. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yan, C.; Liu, P.; Ding, J. Fabrication of RGD Micro/Nanopattern and Corresponding Study of Stem Cell Di ff erentiation. Nano Lett. 2015, 15, 1457–1467. [Google Scholar] [CrossRef]
- Tsui, T.Y.; Logan, M.; Moussa, H.I.; Aucoin, M.G. What’s happening on the other side? Revealing nano-meter scale features of mammalian cells on engineered textured tantalum surfaces. Materials 2018, 12, 114. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, J.; Hu, J.; Chen, Y. Cells cultured on microgrooves with or without surface coating: Correlation between cell alignment, spreading and local membrane deformation. Mater. Sci. Eng. C 2013, 33, 855–863. [Google Scholar] [CrossRef]
- Bencharit, S.; Morelli, T.; Barros, S.; Seagroves, J.T.; Kim, S.; Yu, N.; Byrd, K.; Brenes, C.; Offenbacher, S. Comparing Initial Wound Healing and Osteogenesis of Porous Tantalum Trabecular Metal and Titanium Alloy Materials. J. Oral Implant. 2019, 45, 173–180. [Google Scholar] [CrossRef]
- Leven, R.M.; Virdi, A.S.; Sumner, D.R. Patterns of gene expression in rat bone marrow stromal cells cultured on titanium alloy discs of different roughness. J. Biomed. Mater. Res. Part A 2004, 70, 391–401. [Google Scholar] [CrossRef]


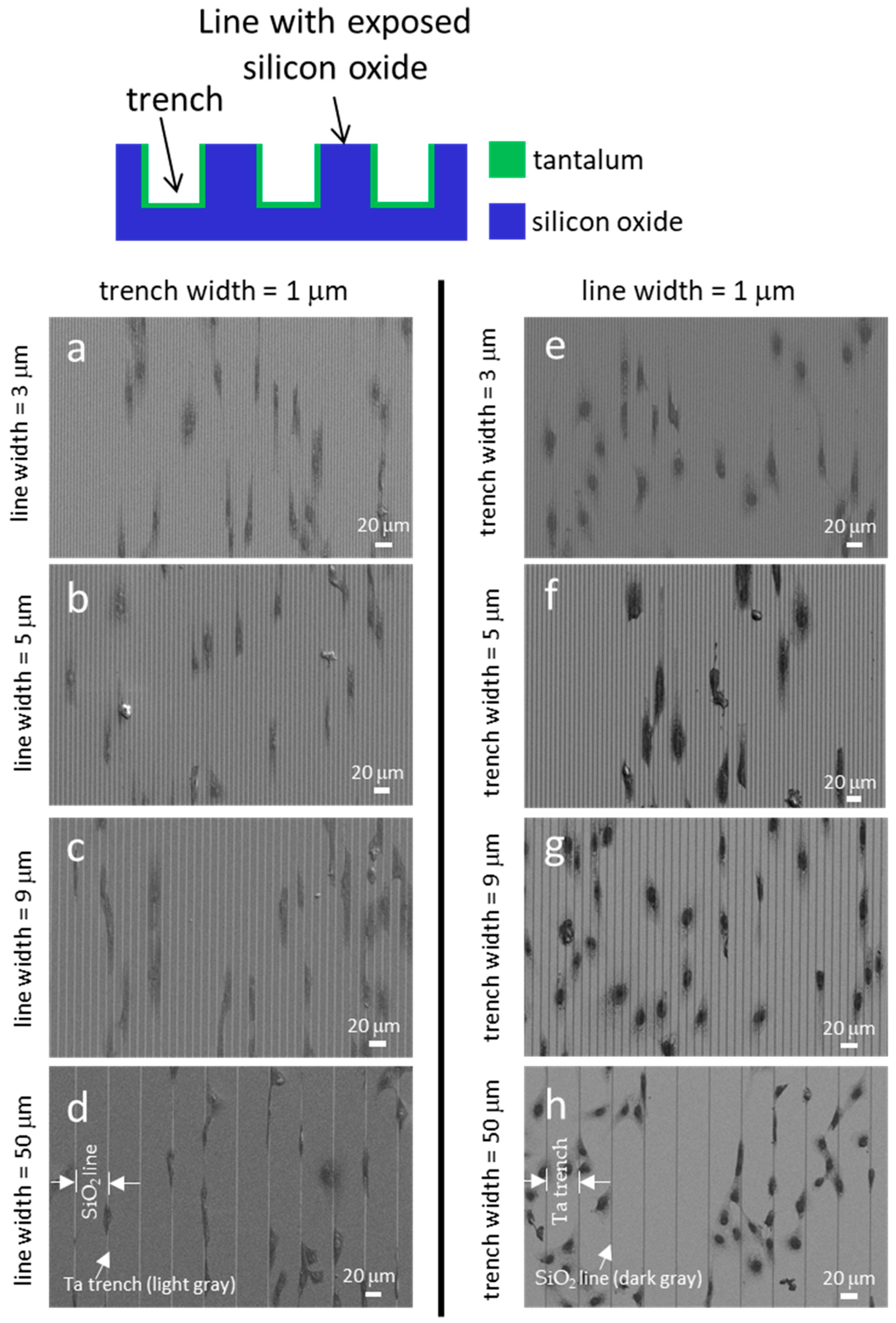

| Specimen Number | Substrate Surface Material | Width (μm) | Total Cell Count (n) | % Cell Aligned ±10° from Line Axes | |
|---|---|---|---|---|---|
| Trench | Line | ||||
| 1 | Ta | 1 | 1 | 197 [18] | 78 ± 2 [18] |
| 2 | Ta | 3 | 1 | 184 | 61 ± 7 |
| 3 | Ta | 5 | 1 | 241 | 43 ± 5 |
| 4 | Ta | 9 | 1 | 275 | 28 ± 4 |
| 5 | Ta | 50 | 1 | 158 | 31 ± 3 |
| 6 | Ta | 1 | 3 | 219 | 46 ± 7 |
| 7 | Ta | 1 | 5 | 201 | 40 ± 3 |
| 8 | Ta | 1 | 9 | 198 | 42 ± 6 |
| 9 | Ta | 1 | 50 | 197 | 24 ± 3 |
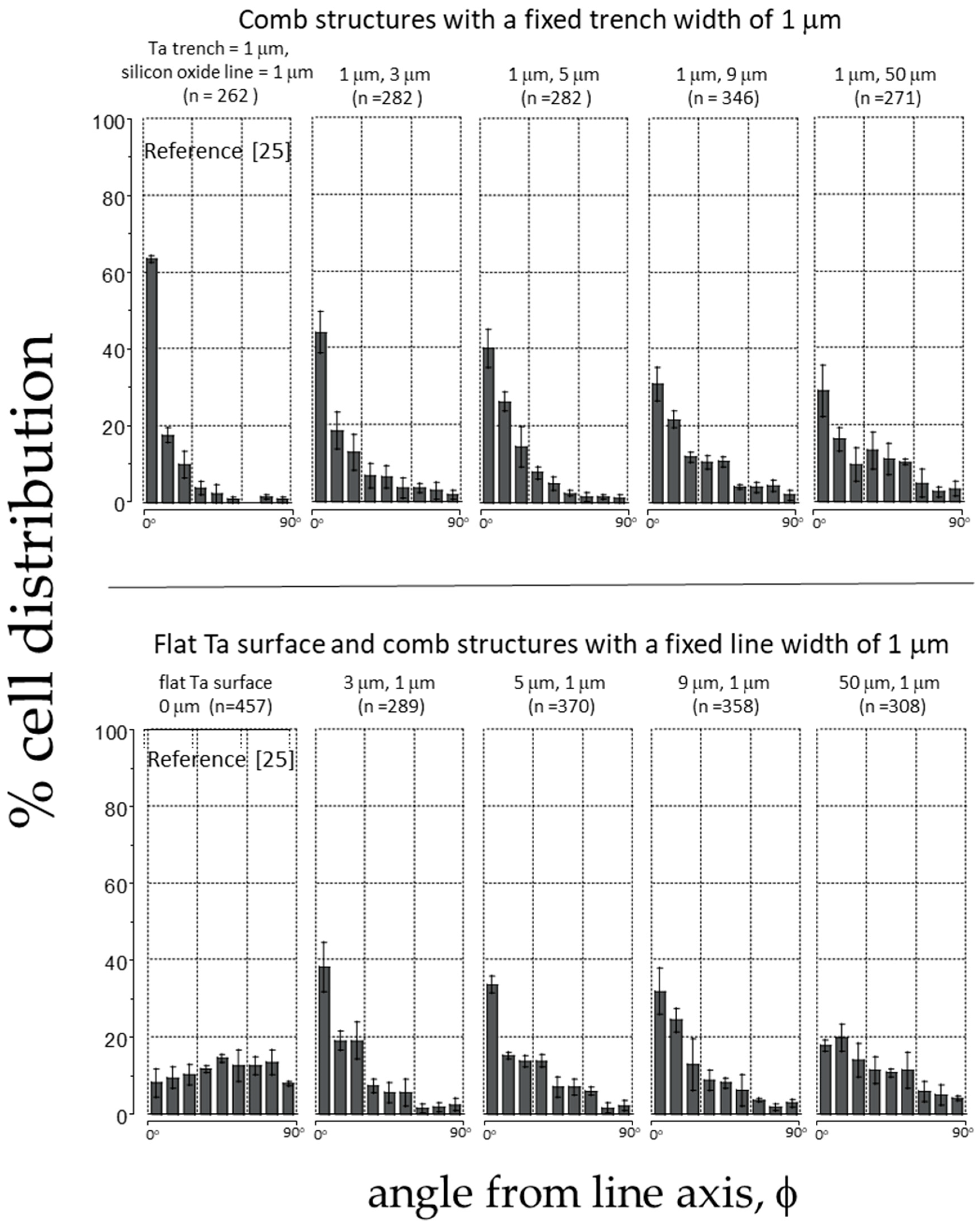
| 10 | Ta/SiO2 | 1 | 1 | 262 [25] | 63 ± 1 [25] |
| 11 | Ta/SiO2 | 1 | 3 | 282 | 44 ± 5 |
| 12 | Ta/SiO2 | 1 | 5 | 282 | 40 ± 5 |
| 13 | Ta/SiO2 | 1 | 9 | 346 | 31 ± 4 |
| 14 | Ta/SiO2 | 1 | 50 | 271 | 29 ± 7 |
| 15 | Ta/SiO2 | 3 | 1 | 289 | 38 ± 6 |
| 16 | Ta/SiO2 | 5 | 1 | 370 | 34 ± 2 |
| 17 | Ta/SiO2 | 9 | 1 | 358 | 32 ± 6 |
| 18 | Ta/SiO2 | 50 | 1 | 308 | 18 ± 1 |
| Trench Width (μm) | Line Width (μm) | p-Value | Significant Different |
|---|---|---|---|
| 1 | 1 | 0.003 | Yes |
| 1 | 3 | 0.688 | No |
| 1 | 5 | 0.941 | No |
| 1 | 9 | 0.058 | No |
| 1 | 50 | 0.367 | No |
| 1 | 1 | 0.003 | Yes |
| 3 | 1 | 0.013 | Yes |
| 5 | 1 | 0.052 | No |
| 9 | 1 | 0.463 | No |
| 50 | 1 | 0.006 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, H.I.; Logan, M.; Eskandari, A.; Glerum, D.M.; Aucoin, M.G.; Tsui, T.Y. Critical Design Parameters of Tantalum-Based Comb Structures to Manipulate Mammalian Cell Morphology. Materials 2025, 18, 2099. https://doi.org/10.3390/ma18092099
Moussa HI, Logan M, Eskandari A, Glerum DM, Aucoin MG, Tsui TY. Critical Design Parameters of Tantalum-Based Comb Structures to Manipulate Mammalian Cell Morphology. Materials. 2025; 18(9):2099. https://doi.org/10.3390/ma18092099
Chicago/Turabian StyleMoussa, Hassan I., Megan Logan, Ali Eskandari, D. Moira Glerum, Marc G. Aucoin, and Ting Y. Tsui. 2025. "Critical Design Parameters of Tantalum-Based Comb Structures to Manipulate Mammalian Cell Morphology" Materials 18, no. 9: 2099. https://doi.org/10.3390/ma18092099
APA StyleMoussa, H. I., Logan, M., Eskandari, A., Glerum, D. M., Aucoin, M. G., & Tsui, T. Y. (2025). Critical Design Parameters of Tantalum-Based Comb Structures to Manipulate Mammalian Cell Morphology. Materials, 18(9), 2099. https://doi.org/10.3390/ma18092099








