Influence of Silicate Modulus and Eggshell Powder on the Expansion, Mechanical Properties, and Thermal Conductivity of Lightweight Geopolymer Foam Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
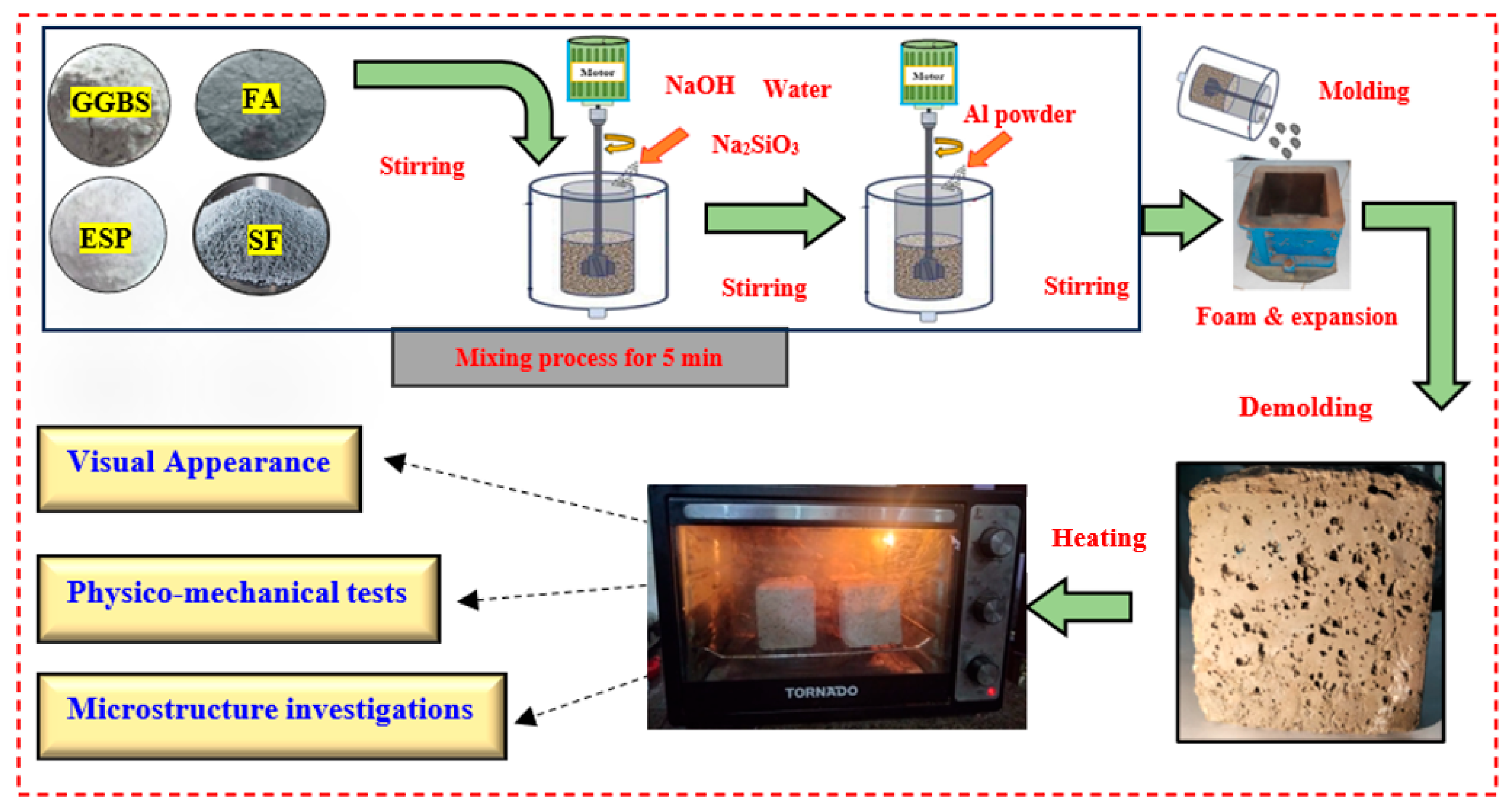
2.2. Mix Proportions and Sample Preparation
2.3. Characterization and Analytical Methods
2.3.1. Fresh and Mechanical Performance
2.3.2. Microstructure Investigation and Thermal Conductivity
3. Results and Discussion
3.1. Expansion of LWGFC
3.2. Setting Time and Density
3.3. Morphology of LWGFC
3.4. Physic-Mechanical Properties
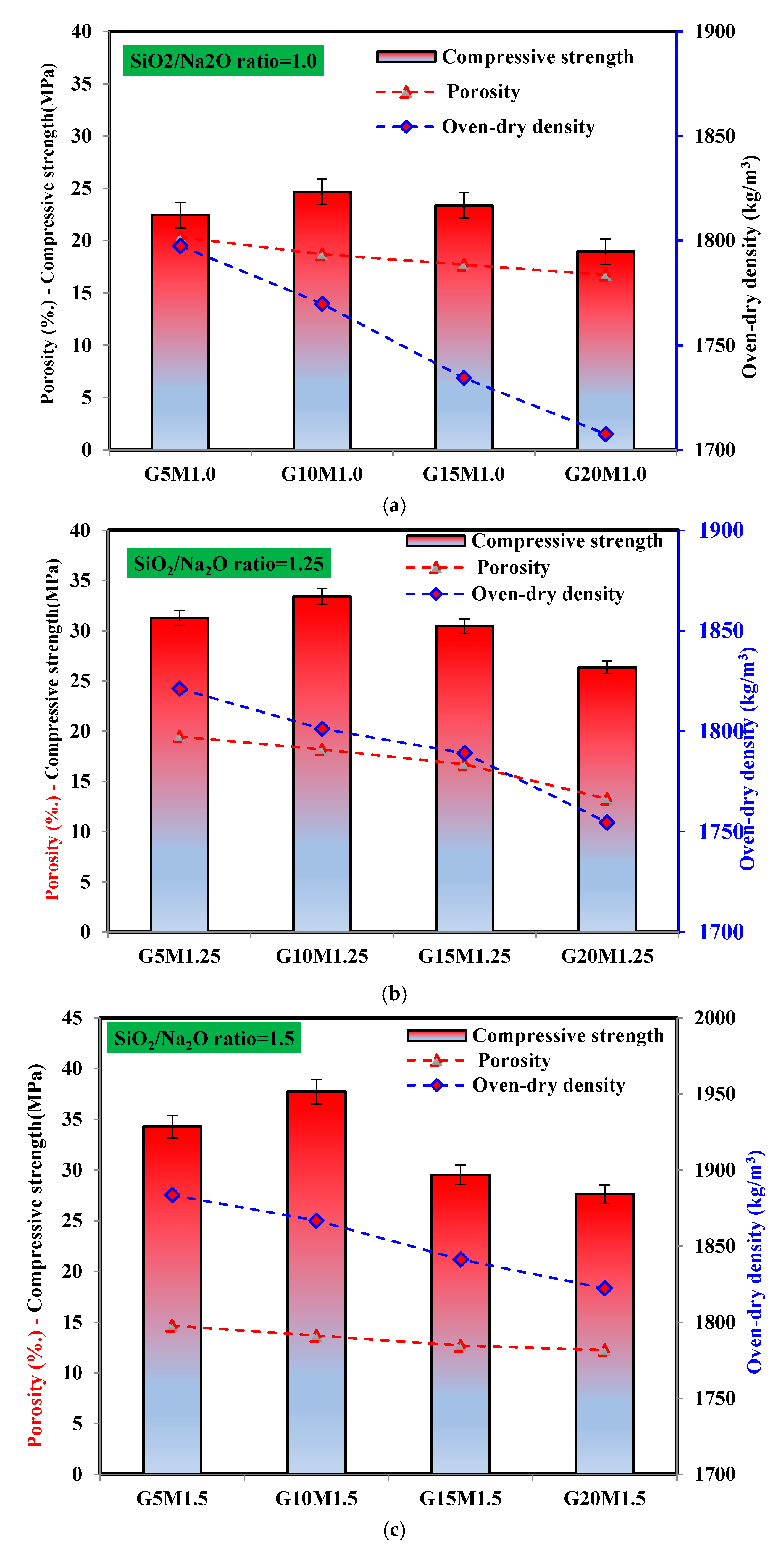
3.4.1. Correlation Between Porosity, Oven-Dry Density, and Compressive Strength
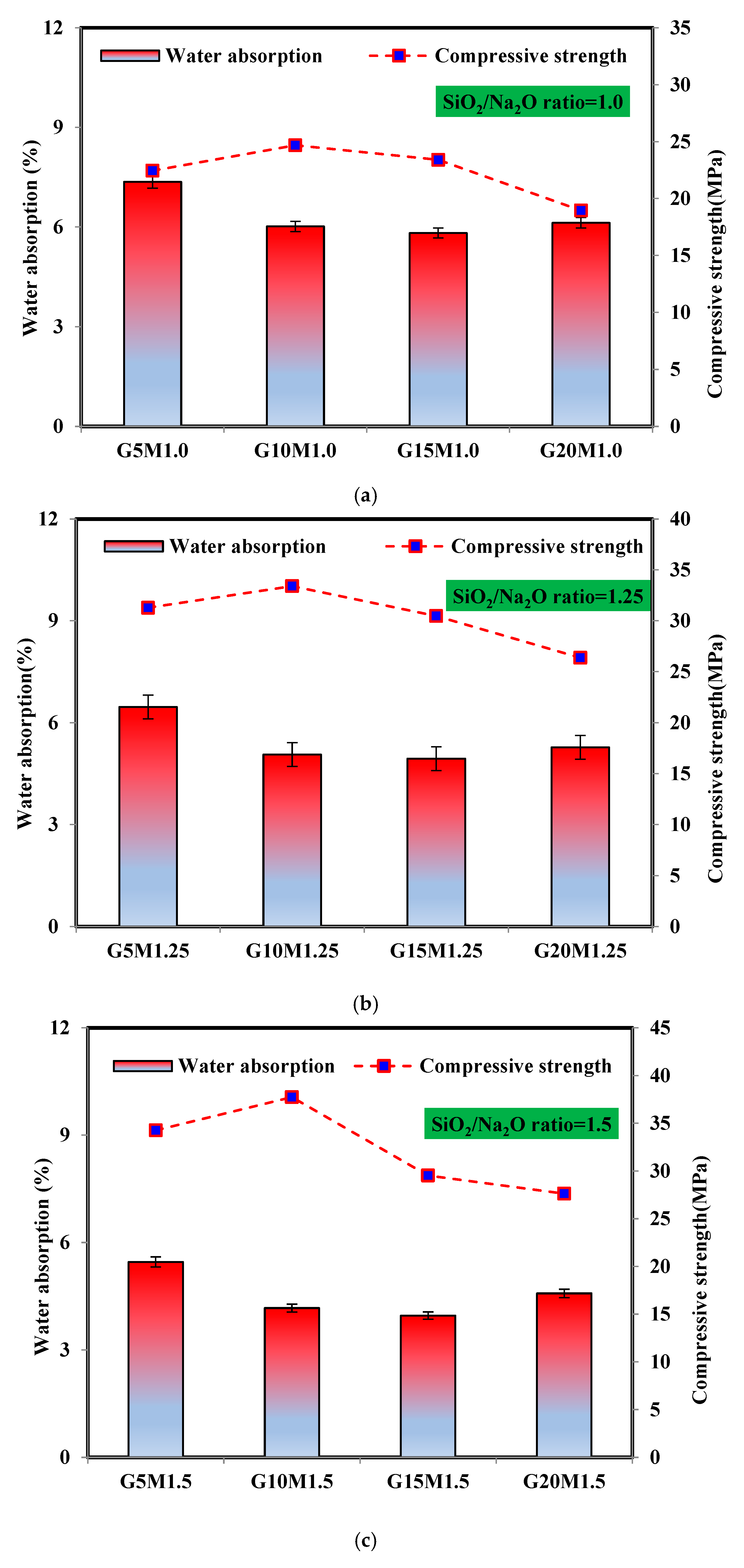
3.4.2. Water Absorption and Compressive Strength Relationship
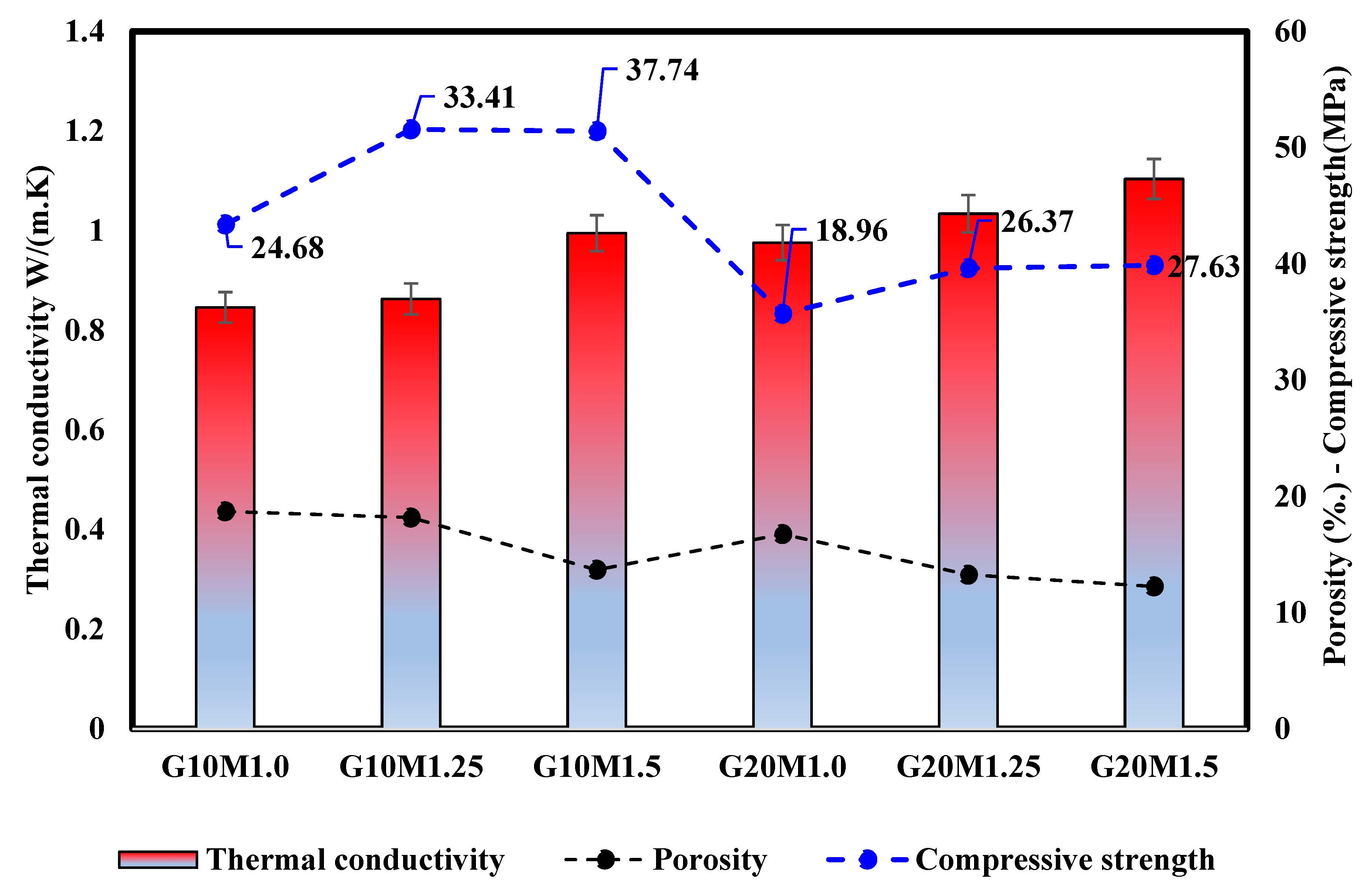
3.4.3. Correlation Between the Thermal Conductivity Porosity and Compressive Strength
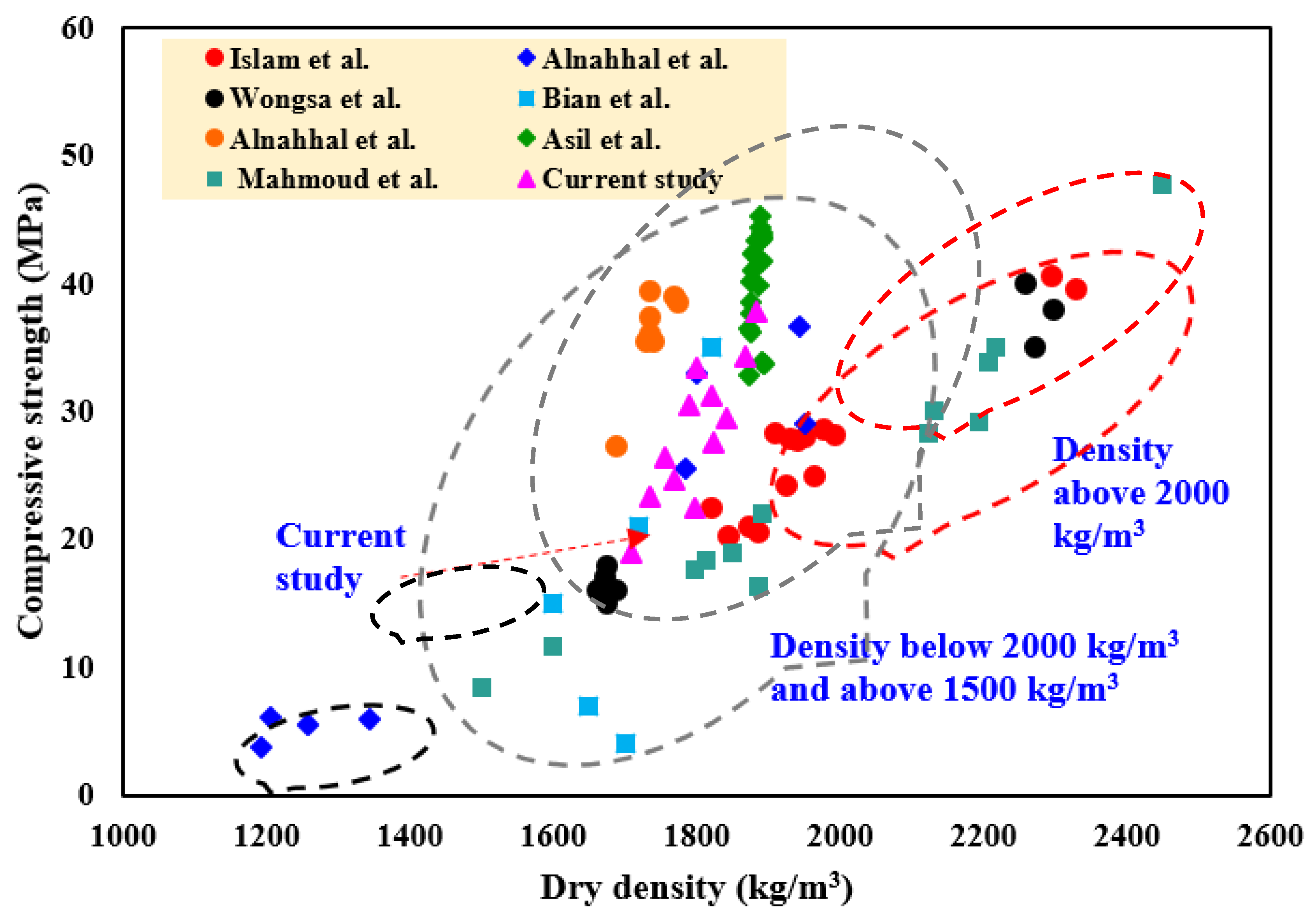
3.4.4. Comparison with LWGFC Properties in Previous Studies
4. Conclusions
- The inclusion of ESP as a partial replacement for GGBS accelerated the setting time due to its high CaCO3 content and high-water absorption. Further, by increasing the SiO2/Na2O ratio to 1.25 and 1.5, the setting time declined by 12.07% and 27.59%, respectively, compared to the SiO2/Na2O ratio of 1.0.
- At all SiO2/Na2O ratios, the optimum content of ESP in LWGFC was 10%, which reduced the porosity by 2.89% and 26.94% and enhanced the compressive strength by 35.37% and 52.92% at SiO2/Na2O ratios of 1.25 and 1.5, respectively, compared to the low SiO2/Na2O ratio, which was highly associated to the geopolymerization reaction of LWGFC samples containing optimum ESP.
- The thermal conductivity was negatively affected by the ESP incorporation SiO2/Na2O ratio, which related to the decreasing porosity and increased oven-dry density. For instance, the thermal conductivity of samples with 20% ESP increased by 15.3% compared to 10% ESP samples at a low SiO2/Na2O ratio. Further, increasing the SiO2/Na2O ratio could improve the microstructure of LWGFC, thus leading to increased thermal conductivity. This enhancement may be due to the high dissolution of rich-calcium materials (GGBS and ESP) in the high alkaline-activation solution, which generates more geopolymerization reactions and consequently tends to reduce LWGFC porosity.
- Firstly, while ESP incorporation up to 10% enhances the formation of N-(C)-A-S-H gels, improving compressive strength and reducing porosity, higher ESP contents (e.g., 20%) lead to a significant strength reduction (up to 26.2%) due to its high calcium content, weak pozzolanic activity, and high-water absorption. Future studies should explore hybrid additives, such as combining ESP with other pozzolanic materials like silica fume or nano-silica, to mitigate the negative effects of high ESP replacement while maintaining its sustainability benefits. This approach could enhance the pozzolanic reactivity and reduce microcracking, potentially achieving a better balance between strength and density.
- Secondly, the increase in thermal conductivity with higher ESP content (e.g., from 0.84 W/m·K at 10% ESP to 0.97 W/m·K at 20% ESP) and SiO2/Na2O ratios (e.g., a 17.7% increase from SiO2/Na2O = 1.0 to 1.5) indicates a trade-off between mechanical strength and thermal insulation properties. To address this, future research should investigate the incorporation of additional lightweight fillers or foaming agents that can maintain low thermal conductivity while preserving the strength gains achieved at higher SiO2/Na2O ratios. For instance, materials like perlite or expanded polystyrene beads could be tested to further reduce density and thermal conductivity, making LWGFC more suitable for energy-efficient building applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dylewski, R.; Adamczyk, J. Economic and environmental benefits of thermal insulation of building external walls. Build. Environ. 2011, 46, 2615–2623. [Google Scholar] [CrossRef]
- Dombaycı, Ö.A. The environmental impact of optimum insulation thickness for external walls of buildings. Build. Environ. 2007, 42, 3855–3859. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Mourad, A.-H.; Hittini, W.; Hassan, M.; Hameedi, S. Traditional, state-of-the-art and renewable thermal building insulation materials: An overview. Constr. Build. Mater. 2019, 214, 709–735. [Google Scholar] [CrossRef]
- Elfadaly, E.; Othman, A.M.; Aly, M.H.; Elgarhy, W.A.; Abdellatief, M. Assessing performance and environmental benefits of high-performance geopolymer mortar incorporating pumice and rice straw ash. Sustain. Chem. Pharm. 2025, 44, 101918. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Hu, Z.; Li, J.; Yao, X.; Zhang, C.; Wu, C.; Zhang, J.; Wang, W. Recent advances in sustainable lightweight foamed concrete incorporating recycled waste and byproducts: A review. Constr. Build. Mater. 2023, 403, 133083. [Google Scholar] [CrossRef]
- Tran, N.P.; Nguyen, T.N.; Ngo, T.D.; Le, P.K.; Le, T.A. Strategic progress in foam stabilisation towards high-performance foam concrete for building sustainability: A state-of-the-art review. J. Clean. Prod. 2022, 375, 133939. [Google Scholar] [CrossRef]
- Xiang, Q.; Pan, H.; Ma, X.; Yang, M.; Lyu, Y.; Zhang, X.; Shui, W.; Liao, W.; Xiao, Y.; Wu, J.; et al. Impacts of energy-saving and emission-reduction on sustainability of cement production. Renew. Sustain. Energy Rev. 2024, 191, 114089. [Google Scholar] [CrossRef]
- Tahwia, A.M.; Abdellatief, M.; Salah, A.; Youssf, O. Valorization of recycled concrete powder, clay brick powder, and volcanic pumice powder in sustainable geopolymer concrete. Sci. Rep. 2025, 15, 11049. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Rathnayaka, M.; Karunasinghe, D.; Gunasekara, C.; Wijesundara, K.; Lokuge, W.; Law, D.W. Machine learning approaches to predict compressive strength of fly ash-based geopolymer concrete: A comprehensive review. Constr. Build. Mater. 2024, 419, 135519. [Google Scholar] [CrossRef]
- Chelluri, S.; Hossiney, N. Performance evaluation of ternary blended geopolymer binders comprising of slag, fly ash and brick kiln rice husk ash. Case Stud. Constr. Mater. 2024, 20, e02918. [Google Scholar] [CrossRef]
- Yan, D.; Shi, Y.; Zhang, Y.; Wang, W.; Qian, H.; Chen, S.; Liu, Y.; Ruan, S. A comparative study of porous geopolymers synthesized by pre-foaming and H2O2 foaming methods: Strength and pore structure characteristics. Ceram. Int. 2024, 50, 17807–17817. [Google Scholar] [CrossRef]
- Nematollahi, B.; Ranade, R.; Sanjayan, J.; Ramakrishnan, S. Thermal and mechanical properties of sustainable lightweight strain hardening geopolymer composites. Arch. Civil Mech. Eng. 2017, 17, 55–64. [Google Scholar] [CrossRef]
- Dang, J.; Tang, X.; Xiao, J.; Duan, Z.; Han, A. Role of recycled brick powder and alkaline solution on the properties of eco-friendly alkali-activated foam concrete. J. Clean. Prod. 2024, 436, 140381. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, G.; Liang, K. Development of fly ash and slag based high-strength alkali-activated foam concrete. Cem. Concr. Compos. 2022, 128, 104447. [Google Scholar] [CrossRef]
- Raj, S.; Ramamurthy, K. Physical; hydrolytic, and mechanical stability of alkali-activated fly ash-slag foam concrete. Cem. Concr. Compos. 2023, 142, 105223. [Google Scholar] [CrossRef]
- Majeed, S.S.; Mydin, M.A.O.; Bahrami, A.; Dulaimi, A.; Özkılıç, Y.O.; Omar, R.; Jagadesh, P. Development of ultralightweight foamed concrete modified with silicone dioxide (SiO2) nanoparticles: Appraisal of transport, mechanical, thermal, and microstructural properties. J. Mater. Res. Technol. 2024, 30, 3308–3327. [Google Scholar] [CrossRef]
- Waheed, M.; Yousaf, M.; Shehzad, A.; Inam-Ur-Raheem, M.; Khan, M.K.I.; Khan, M.R.; Ahmad, N.; Abdullah; Aadil, R.M. Channelling eggshell waste to valuable and utilizable products: A comprehensive review. Trends Food Sci. Technol. 2020, 106, 78–90. [Google Scholar] [CrossRef]
- Paruthi, S.; Khan, A.H.; Kumar, A.; Kumar, F.; Hasan, M.A.; Magbool, H.M.; Manzar, M.S. Sustainable cement replacement using waste eggshells: A review on mechanical properties of eggshell concrete and strength prediction using artificial neural network. Case Stud. Constr. Mater. 2023, 18, e02160. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Doh, S.I.; Chin, S.C. Eggshell as a partial cement replacement in concrete development. Mag. Concr. Res. 2018, 70, 662–670. [Google Scholar] [CrossRef]
- Shekhawat, P.; Sharma, G.; Singh, R.M. Microstructural and morphological development of eggshell powder and flyash-based geopolymers. Constr. Build. Mater. 2020, 260, 119886. [Google Scholar] [CrossRef]
- Katha, P.S.; Ahmed, Z.; Alam, R.; Saha, B.; Acharjee, A.; Rahman, M.S. Efficiency analysis of eggshell and tea waste as Low cost adsorbents for Cr removal from wastewater sample. S. Afr. J. Chem. Eng. 2021, 37, 186–195. [Google Scholar] [CrossRef]
- Kalbarczyk, M.; Szcześ, A. Potential biomedical application of calcium phosphates obtained using eggshells as a biosource of calcium at different initial pH values. Ceram. Int. 2021, 47, 33687–33696. [Google Scholar] [CrossRef]
- Adamu, M.; Alanazi, H.; Ibrahim, Y.E.; Abdellatief, M. Mechanical, microstructural characteristics and sustainability analysis of concrete incorporating date palm ash and eggshell powder as ternary blends cementitious materials. Constr. Build. Mater. 2024, 411, 134753. [Google Scholar] [CrossRef]
- Abdellatief, M.; Ahmed, Y.M.; Taman, M.; Elfadaly, E.; Tang, Y.; Abadel, A.A. Physico-mechanical; thermal insulation properties, and microstructure of geopolymer foam concrete containing sawdust ash and egg shell. J. Build. Eng. 2024, 90, 109374. [Google Scholar] [CrossRef]
- Zaid, O.; Hashmi, S.R.Z.; El Ouni, M.H.; Martínez-García, R.; de Prado-Gil, J.; Yousef, S.E.A.S. Experimental and analytical study of ultra-high-performance fiber-reinforced concrete modified with egg shell powder and nano-silica. J. Mater. Res. Technol. 2023, 24, 7162–7188. [Google Scholar] [CrossRef]
- Tiong, H.Y.; Lim, S.K.; Lee, Y.L.; Ong, C.F.; Yew, M.K. Environmental impact and quality assessment of using eggshell powder incorporated in lightweight foamed concrete. Constr. Build. Mater. 2020, 244, 118341. [Google Scholar] [CrossRef]
- Wattanasiriwech, D.; Yomthong, K.; Wattanasiriwech, S. Characterisation and properties of class C-fly ash based geopolymer foams: Effects of foaming agent content, aggregates, and surfactant. Constr. Build. Mater. 2021, 306, 124847. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P. How does aluminium foaming agent impact the geopolymer formation mechanism? Cem. Concr. Compos. 2017, 80, 277–286. [Google Scholar] [CrossRef]
- Saif, M.S.; El-Hariri, M.O.R.; Sarie-Eldin, A.I.; Tayeh, B.A.; Farag, M.F. Impact of Ca+ content and curing condition on durability performance of metakaolin-based geopolymer mortars. Case Stud. Constr. Mater. 2022, 16, e00922. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Dhasindrakrishna, K.; Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Progress, current thinking and challenges in geopolymer foam concrete technology. Cem. Concr. Compos. 2021, 116, 103886. [Google Scholar] [CrossRef]
- Abdellatief, M.; Elrahman, M.A.; Alanazi, H.; Abadel, A.A.; Tahwia, A. A state-of-the-art review on geopolymer foam concrete with solid waste materials: Components, characteristics, and microstructure. Innov. Infrastruct. Solut. 2023, 8, 230. [Google Scholar] [CrossRef]
- Tekin, I. Properties of NaOH activated geopolymer with marble, travertine and volcanic tuff wastes. Constr. Build. Mater. 2016, 127, 607–617. [Google Scholar] [CrossRef]
- Babalola, B.M.; Wilson, L.D. Valorization of Eggshell as Renewable Materials for Sustainable Biocomposite Adsorbents—An Overview. J. Compos. Sci. 2024, 8, 414. [Google Scholar] [CrossRef]
- ASTM C403/C403M-16; Standard Test Method for Time of Setting of Concrete Mixtures by Penetration Resistance. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM C567/C567M-19; Standard Test Method for Determining Density of Structural Lightweight Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- Beghoura, I.; Castro-Gomes, J. Design of alkali-activated aluminium powder foamed materials for precursors with different particle sizes. Constr. Build. Mater. 2019, 224, 682–690. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P. Enhancing the strength of pre-made foams for foam concrete applications. Cem. Concr. Compos. 2018, 87, 164–171. [Google Scholar] [CrossRef]
- ASTM C1585-20; Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM C138/C138M-17a; Standard Test Method for Density (Unit Weight), Yield, and Air Content (Gravimetric) of Concrete. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM C109/C109M-21; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM E1952-17; Standard Test Method for Thermal Conductivity and Thermal Diffusivity by Modulated Temperature Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2017.
- Hamada, H.M.; Tayeh, B.A.; Al-Attar, A.; Yahaya, F.M.; Muthusamy, K.; Humada, A.M. The present state of the use of eggshell powder in concrete: A review. J. Build. Eng. 2020, 32, 101583. [Google Scholar] [CrossRef]
- Kaze, C.R.; Djobo, J.N.Y.; Nana, A.; Tchakoute, H.K.; Kamseu, E.; Melo, U.C.; Leonelli, C.; Rahier, H. Effect of silicate modulus on the setting, mechanical strength and microstructure of iron-rich aluminosilicate (laterite) based-geopolymer cured at room temperature. Ceram. Int. 2018, 44, 21442–21450. [Google Scholar] [CrossRef]
- Ouyang, X.; Ma, Y.; Liu, Z.; Liang, J.; Ye, G. Effect of the Sodium Silicate Modulus and Slag Content on Fresh and Hardened Properties of Alkali-Activated Fly Ash/Slag. Minerals 2020, 10, 15. [Google Scholar] [CrossRef]
- Abdellatief, M.; Wong, L.S.; Din, N.M.; Ahmed, A.N.; Hassan, A.M.; Ibrahim, Z.; Murali, G.; Mo, K.H.; El-Shafie, A. Sustainable foam glass property prediction using machine learning: A comprehensive comparison of predictive methods and techniques. Results Eng. 2025, 25, 104089. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Dedecek, J.; Jirglova, H.; Benito, P.; Vaccari, A.; Landi, E. Effect of metallic Si addition on polymerization degree of in situ foamed alkali-aluminosilicates. Ceram. Int. 2013, 39, 7657–7668. [Google Scholar] [CrossRef]
- Jaya, N.A.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Hussin, K. Correlation between pore structure, compressive strength and thermal conductivity of porous metakaolin geopolymer. Constr. Build. Mater. 2020, 247, 118641. [Google Scholar] [CrossRef]
- Alnahhal, A.M.; Alengaram, U.J.; Yusoff, S.; Darvish, P.; Srinivas, K.; Sumesh, M. Engineering performance of sustainable geopolymer foamed and non-foamed concretes. Constr. Build. Mater. 2022, 316, 125601. [Google Scholar] [CrossRef]
- Ivanović, M.; Knežević, S.; Mirković, M.M.; Kljajević, L.; Bučevac, D.; Pavlović, V.B.; Nenadović, M. Structural Characterization of Geopolymers with the Addition of Eggshell Ash. Sustainability 2023, 15, 5419. [Google Scholar] [CrossRef]
- Rathinavel, N.; Kannadasan, K.; Ismail, A.A.M.; Mammo, W.D.; Alagar, M. Impact of Eggshell Powder on the Mechanical and Thermal Properties of Lightweight Geopolymer. Adv. Civil. Eng. 2023, 2023, 6655921. [Google Scholar] [CrossRef]
- Bian, Z.; Lu, J.X.; Huang, Y.; Xuan, D.; Ou, G.; Poon, C.S. Recycling of waste glass in lightweight geopolymer using incineration bottom ash as a foaming agent: Towards energy conservation. Constr. Build. Mater. 2023, 400, 132632. [Google Scholar] [CrossRef]
- Yuan, X.; Dai, M.; Gao, Y.; Zhou, Y.; Liu, F. Effect on mechanical properties and microstructure of high-strength eco-friendly concrete with waste glass powder-eggshell particles. J. Build. Eng. 2023, 79, 107871. [Google Scholar] [CrossRef]
- Liu, M.Y.J.; Alengaram, U.J.; Jumaat, M.Z.; Mo, K.H. Evaluation of thermal conductivity, mechanical and transport properties of lightweight aggregate foamed geopolymer concrete. Energy Build. 2014, 72, 238–245. [Google Scholar] [CrossRef]
- Islam, A.; Alengaram, U.J.; Jumaat, M.Z.; Ghazali, N.B.; Yusoff, S.; Bashar, I.I. Influence of steel fibers on the mechanical properties and impact resistance of lightweight geopolymer concrete. Constr. Build. Mater. 2017, 152, 964–977. [Google Scholar] [CrossRef]
- Alnahhal, A.M.; Alengaram, U.J.; Ibrahim, M.S.I.; Yusoff, S.; Metselaar, H.S.C.; Johnson, P.G. Synthesis of ternary binders and sand-binder ratio on the mechanical and microstructural properties of geopolymer foamed concrete. Constr. Build. Mater. 2022, 349, 128682. [Google Scholar] [CrossRef]
- Asil, M.B.; Ranjbar, M.M. Hybrid effect of carbon nanotubes and basalt fibers on mechanical, durability, and microstructure properties of lightweight geopolymer concretes. Constr. Build. Mater. 2022, 357, 129352. [Google Scholar] [CrossRef]
- Wongsa, A.; Zaetang, Y.; Sata, V.; Chindaprasirt, P. Properties of lightweight fly ash geopolymer concrete containing bottom ash as aggregates. Constr. Build. Mater. 2016, 111, 637–643. [Google Scholar] [CrossRef]
- Mahmoud, H.A.; Tawfik, T.A.; El-razik, M.M.A.; Faried, A.S. Mechanical and acoustic absorption properties of lightweight fly ash/slag-based geopolymer concrete with various aggregates. Ceram. Int. 2023, 49, 21142–21154. [Google Scholar] [CrossRef]
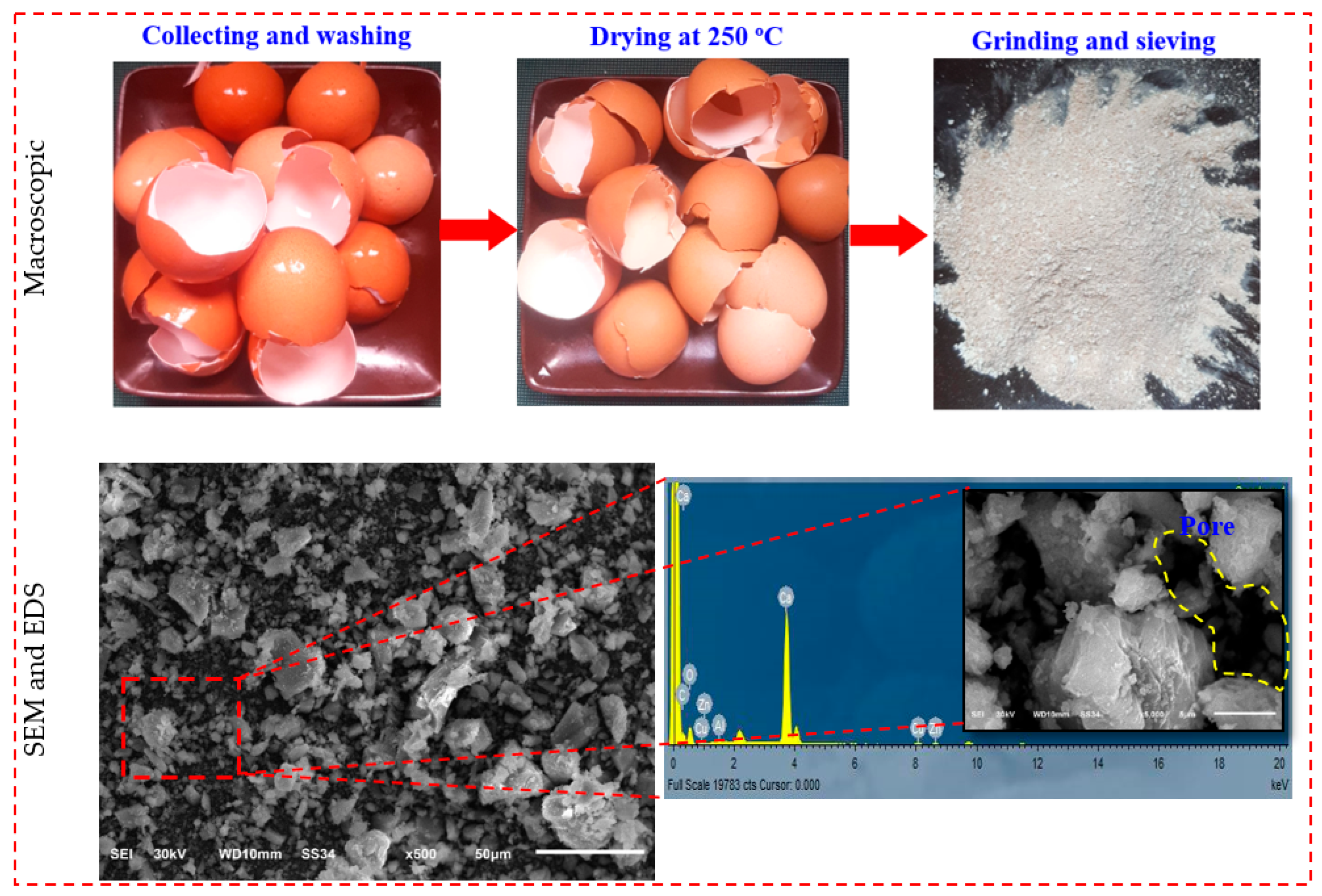









| Oxides (%) | GGBS | FA | SF | ESP |
|---|---|---|---|---|
| CaO | 36.83 | 7.6 | 0.35 | 94.04 |
| SiO2 | 39.6 | 46.44 | 96.29 | 0.342 |
| Al2O3 | 13.79 | 38.10 | 0.2 | - |
| TiO2 | 0.58 | 1.17 | - | - |
| Fe2O3 | 1.69 | 3.12 | 0.53 | - |
| P2O5 | - | 0.76 | - | 2.09 |
| MgO | 5.79 | 0.23 | 0.66 | 1.22 |
| SrO | - | - | - | 0.48 |
| SO3 | - | 0.69 | 0.17 | 1.15 |
| K2O | 0.97 | 0.88 | 0.56 | 0.2 |
| Na2O | 0.48 | 0.4 | 0.38 | 0.4 |
| L.O.I | 0.32 | - | - | 2.4 |
| Basicity Index | 0.98 | - | - | - |
| Mix Type | kg/m3 | (%) | SiO2/Na2O | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GGBS | SF | FA | ESP | Sand | Dolomite | NaOH | Na2SiO3 | Extra Water | Al Powder | ||
| G5M1.0 | 295 | 57 | 76 | 4.8 | 459 | 846 | 83 | 195 | 108 | 0.9 | 1.0 |
| G10M1.0 | 278 | 9.6 | 82 | 195 | |||||||
| G15M1.0 | 264 | 14.4 | 82 | 195 | |||||||
| G20M1.0 | 249 | 19.2 | 82 | 195 | |||||||
| G5M1.25 | 295 | 4.8 | 79 | 242 | 86 | 1.25 | |||||
| G10M1.25 | 278 | 9.6 | 77 | 242 | 85 | ||||||
| G15M1.25 | 264 | 14.4 | 77 | 242 | 83 | ||||||
| G20M1.25 | 249 | 19.2 | 77 | 242 | 81 | ||||||
| G5M1.5 | 295 | 4.8 | 62 | 273 | 72 | 1.5 | |||||
| G10M1.5 | 278 | 9.6 | 60 | 273 | |||||||
| G15M1.5 | 264 | 14.4 | 59 | 273 | 71 | ||||||
| G20M1.5 | 249 | 19.2 | 58 | 273 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdellatief, M.; Mortagi, M.; Hamouda, H.; Skrzypkowski, K.; Zagórski, K.; Zagórska, A. Influence of Silicate Modulus and Eggshell Powder on the Expansion, Mechanical Properties, and Thermal Conductivity of Lightweight Geopolymer Foam Concrete. Materials 2025, 18, 2088. https://doi.org/10.3390/ma18092088
Abdellatief M, Mortagi M, Hamouda H, Skrzypkowski K, Zagórski K, Zagórska A. Influence of Silicate Modulus and Eggshell Powder on the Expansion, Mechanical Properties, and Thermal Conductivity of Lightweight Geopolymer Foam Concrete. Materials. 2025; 18(9):2088. https://doi.org/10.3390/ma18092088
Chicago/Turabian StyleAbdellatief, Mohamed, Mohamed Mortagi, Hassan Hamouda, Krzysztof Skrzypkowski, Krzysztof Zagórski, and Anna Zagórska. 2025. "Influence of Silicate Modulus and Eggshell Powder on the Expansion, Mechanical Properties, and Thermal Conductivity of Lightweight Geopolymer Foam Concrete" Materials 18, no. 9: 2088. https://doi.org/10.3390/ma18092088
APA StyleAbdellatief, M., Mortagi, M., Hamouda, H., Skrzypkowski, K., Zagórski, K., & Zagórska, A. (2025). Influence of Silicate Modulus and Eggshell Powder on the Expansion, Mechanical Properties, and Thermal Conductivity of Lightweight Geopolymer Foam Concrete. Materials, 18(9), 2088. https://doi.org/10.3390/ma18092088







