Ecofriendly Mortar with Paint Sludge Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cement
2.1.2. Paint Sludge Ash
2.1.3. Fine Aggregate
2.1.4. Mixing Water
2.2. Experimental Methods
2.2.1. Mix Proportions and Sample Preparation
2.2.2. Test Methods
3. Results and Discussion
3.1. Effects of PSA on the Fresh Properties of Mortar
3.1.1. Slump Flow
3.1.2. Consistency and Setting Time
3.1.3. Soundness
3.2. Effects of PSA on the Mechanical Properties of Mortar
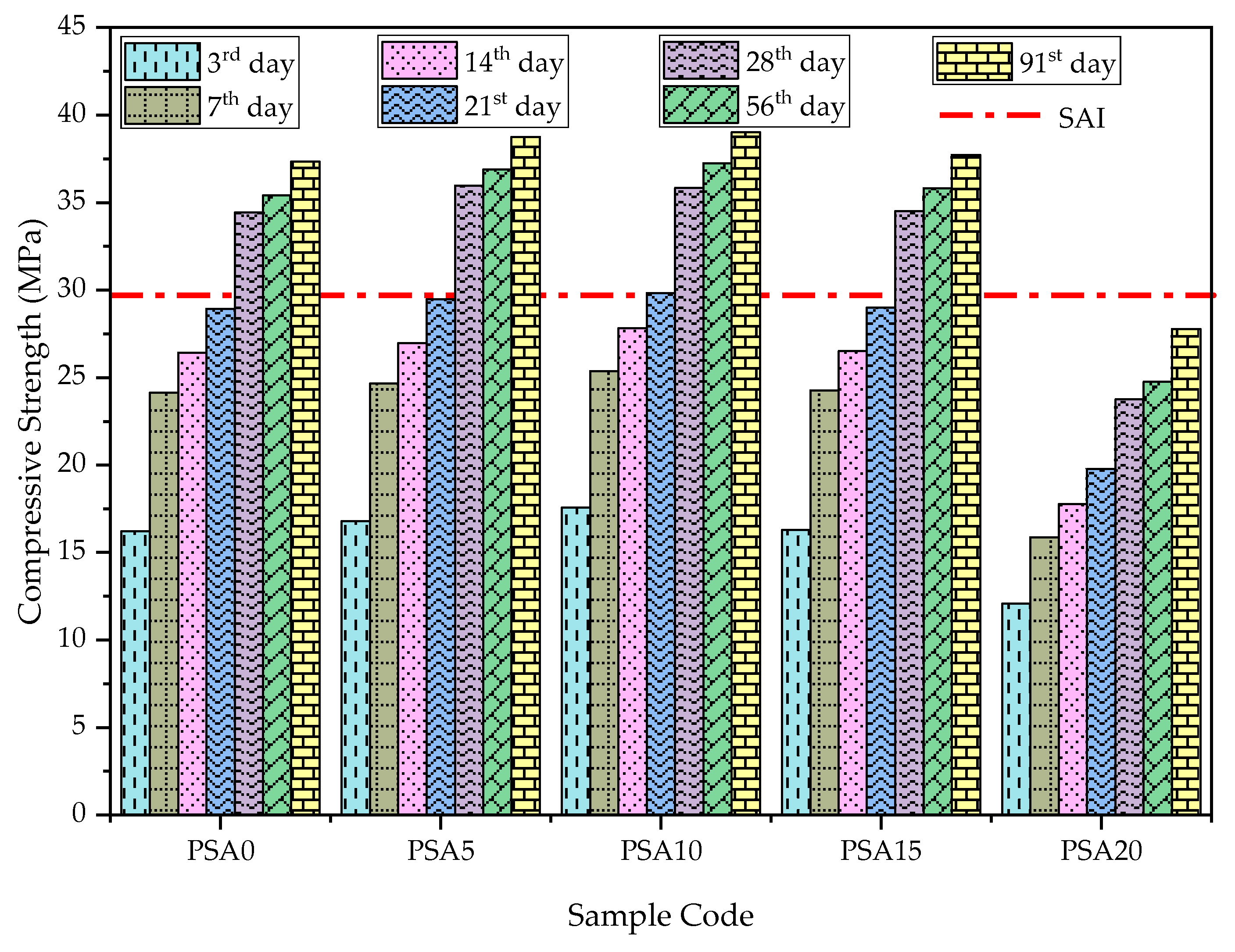
3.2.1. Compressive Strength
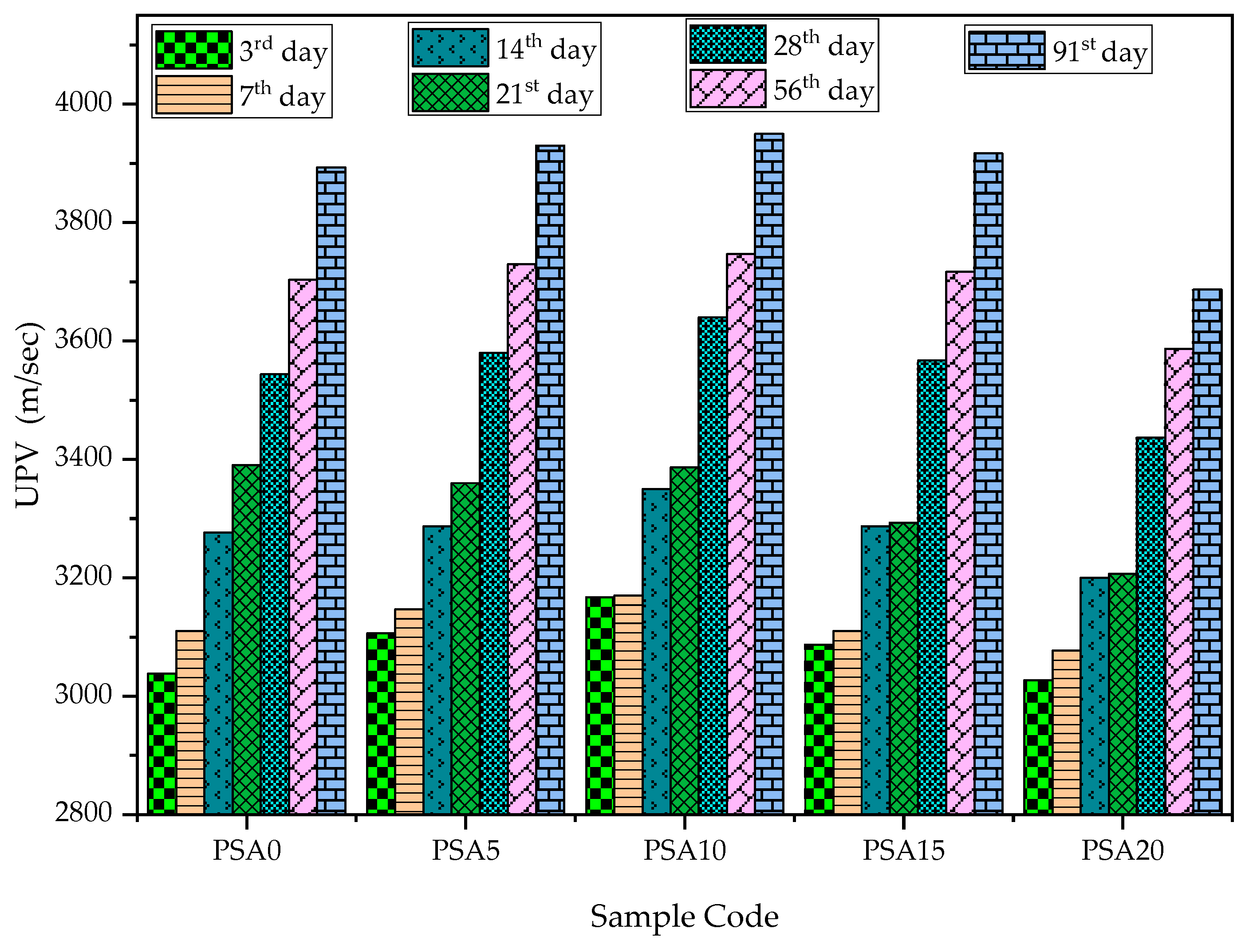
3.2.2. Ultrasonic Pulse Velocity (UPV)
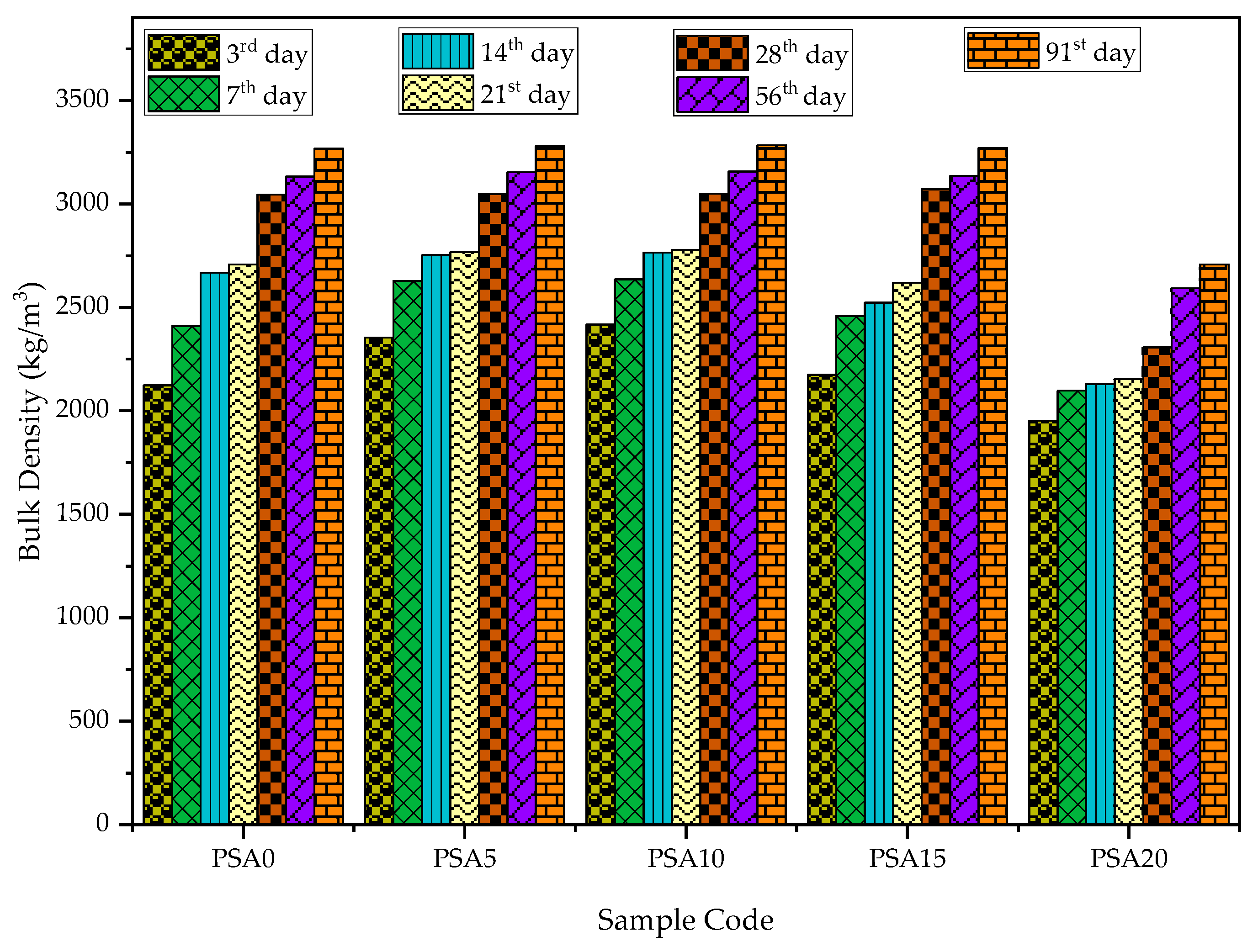
3.2.3. Bulk Density
3.3. Effects of PSA on the Durability Properties of Mortar
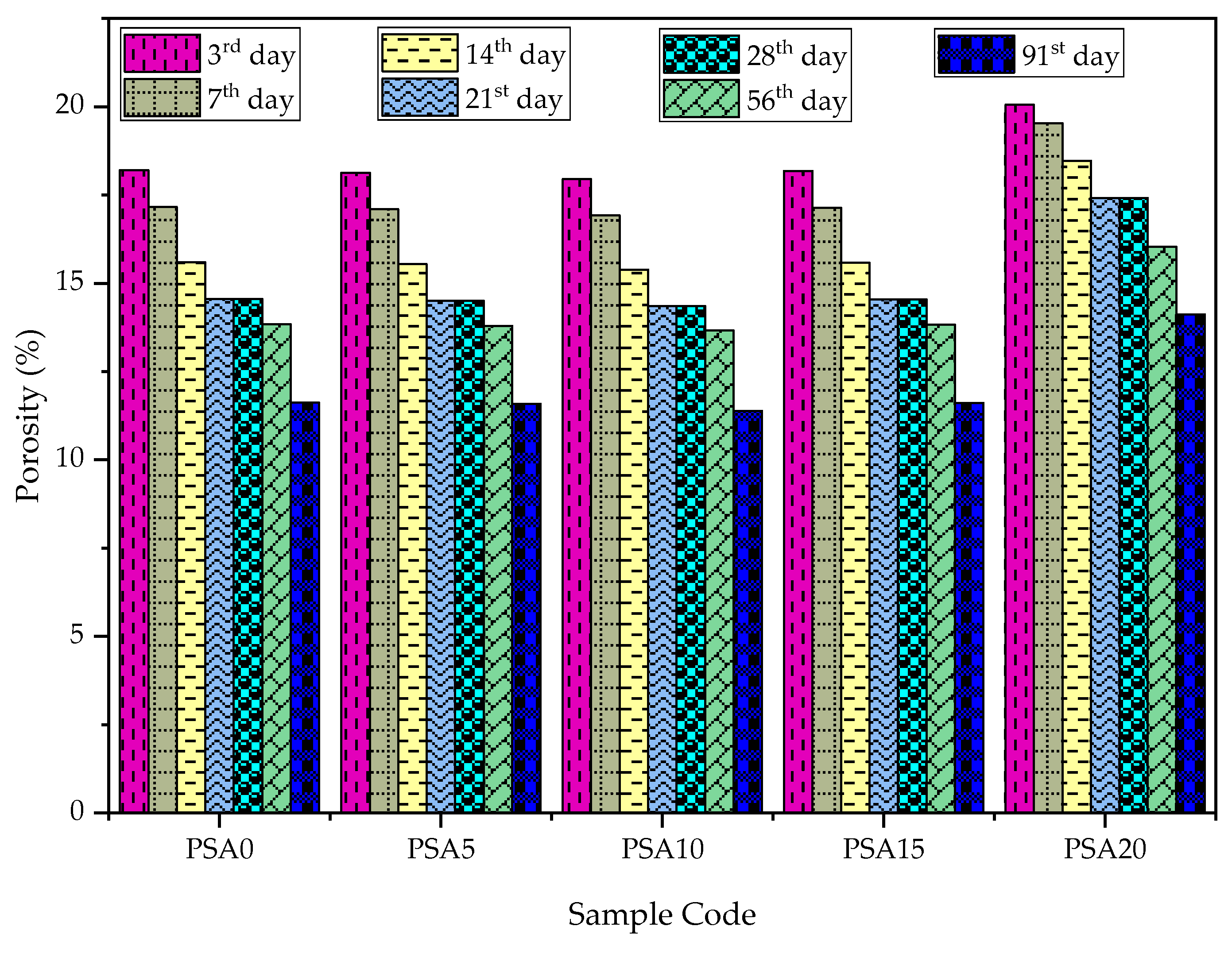
3.3.1. Water Absorption and Porosity/Voids
3.3.2. Sulfate Attack Resistance
3.4. Effects of PSA on the Microstructural Properties of Mortar
3.4.1. Scanning Electron Microscopy (SEM)
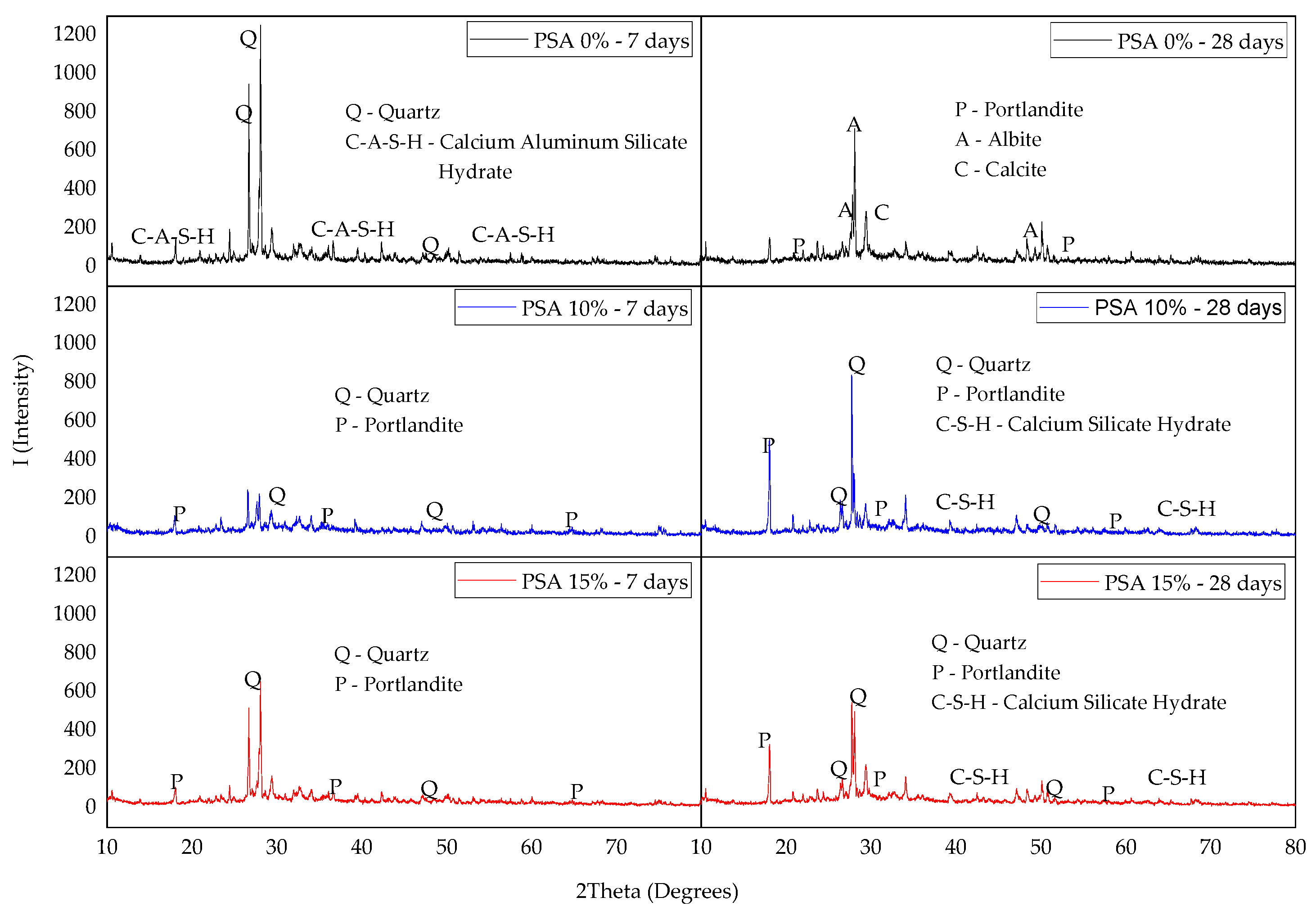
3.4.2. X-Ray Diffraction (XRD)
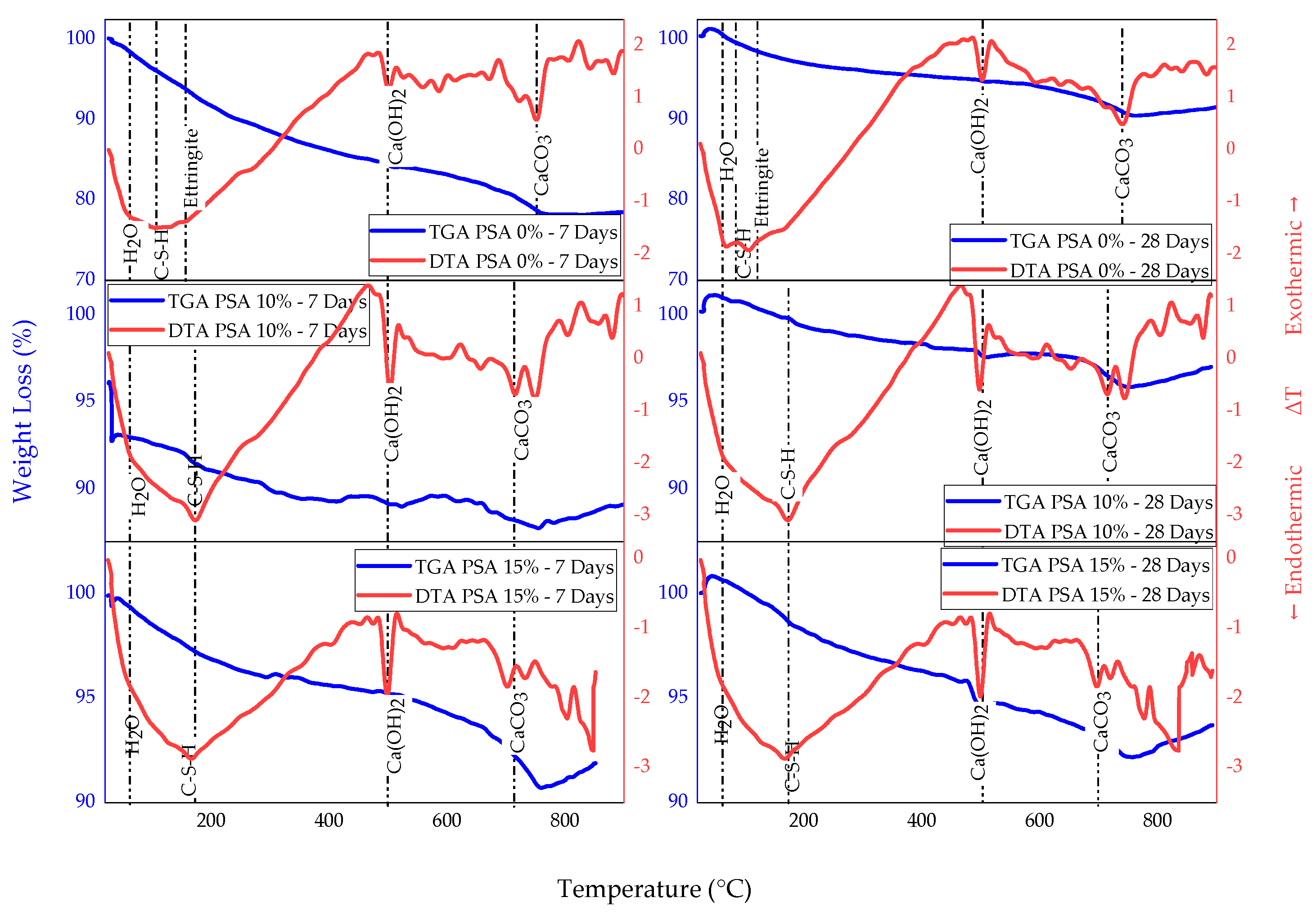
3.4.3. Thermogravimetric Analysis (TGA) and Differential Thermal Analysis (DTA)
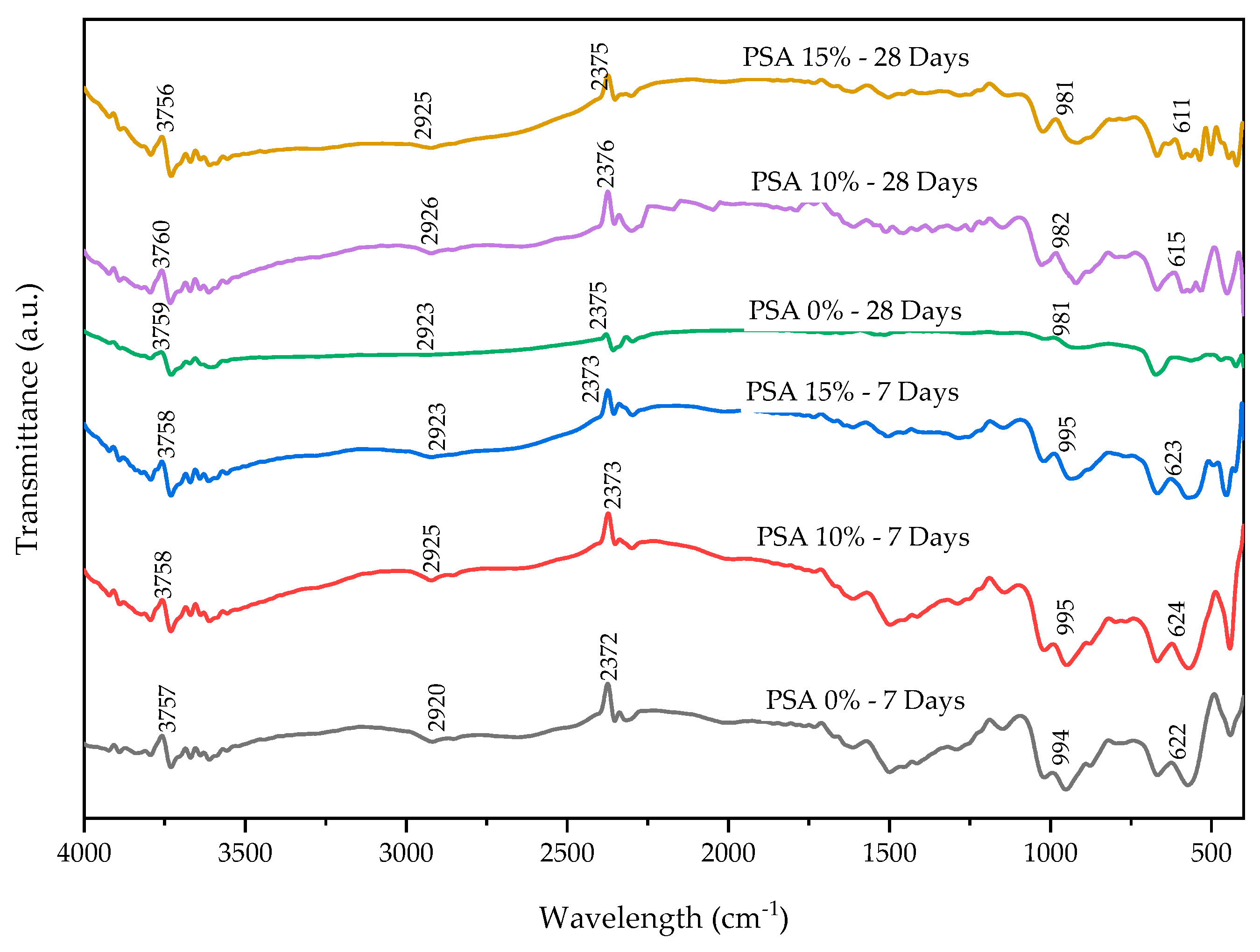
3.4.4. Fourier-Transform Infrared Spectroscopy (FTIR)
4. Conclusions
- Material Characterization: PSA powder was categorized as Class N pozzolan material according to ASTM C618. Its angular, irregular, and porous surface influences its behavior in cementitious mixtures.
- Fresh Properties: As PSA content increased, workability decreased, and setting time was shortened due to its higher fineness, irregular rough texture, and porous structure.
- Mechanical Properties: PSA incorporation enhanced compressive strength, UPV, and bulk density up to a 15% replacement of PSA. However, the 15% PSA replacement exhibited a slight decline compared to the 5% and 10% PSA replacements, with the 10% PSA replacement achieving the highest strength and densification.
- Durability Performance: Mortars with up to 15% PSA replacement showed improved water absorption, sulfate resistance, and lower porosity, primarily due to the pore-filling effect of the finer PSA particles.
- Microstructural Analysis: Mortars with 10% PSA exhibited higher matrix density, greater thermal stability, and minimal mass loss at elevated temperatures, indicating enhanced hydration product formation and structural integrity.
- Sustainability Impact: The effective use of PSA in mortar production helps mitigate environmental issues associated with paint sludge disposal and contributes to reducing the carbon footprint of cement manufacturing. Additionally, replacing cement with PSA can lower production costs and eliminate expenses related to sludge management, including transportation, landfill use, and land acquisition.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, M. The cement industry on the way to a low-carbon future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Walsh, P.P.; Banerjee, A.; Murphy, E. The UN 2030 Agenda for Sustainable Development. In Partnerships and the Sustainable Development Goals; Sustainable Development Goals Series; Springer: Cham, Switzerland, 2022; Volume Part F2740, pp. 1–12. [Google Scholar] [CrossRef]
- Helms, A. Kyoto protocol. In Geography Today An Encyclopedia of Concepts, Issues, and Technology; Bloomsbury Publishing: London, UK, 2019; pp. 296–298. [Google Scholar]
- Moriconi, G. Environmental-friendly durable concrete made with recycled materials for sustainable concrete construction. In Proceedings of the International Symposium on Sustainable Development of Cement, Concrete and Concrete Structures, Toronto, ON, Canada, 5–7 October 2005. [Google Scholar]
- Plessis, C. Du A strategic framework for sustainable construction in developing countries. Constr. Manag. Econ. 2007, 25, 67–76. [Google Scholar] [CrossRef]
- Baloi, D. Sustainable construction: Challenges and opportunities. Assoc. Res. Constr. Manag. 2003, 1, 289–297. Available online: www.arcom.ac.uk/-docs/proceedings/ar2003-289-297_Baloi.pdf (accessed on 20 January 2025).
- Salihoglu, G.; Salihoglu, N.K. A review on paint sludge from automotive industries: Generation, characteristics and management. J. Environ. Manag. 2016, 169, 223–235. [Google Scholar] [CrossRef]
- Ruffino, B.; Campo, G.; Idris, S.S.; Salihoğlu, G.; Zanetti, M. Automotive Paint Sludge: A Review of Pretreatments and Recovery Options. Resources 2023, 12, 45. [Google Scholar] [CrossRef]
- Ruffino, B.; Farina, A.; Dalmazzo, D.; Blengini, G.; Zanetti, M.; Santagata, E. Cost analysis and environmental assessment of recycling paint sludge in asphalt pavements. Environ. Sci. Pollut. Res. 2020, 28, 24628–24638. [Google Scholar] [CrossRef]
- Basak, B.; Arsh, K.; Masum, M.H.; Pal, S.K.; Hoque, A.; Hasan, M.; Shahidullah, M.; Biswas, A. Evaluating Opportunities of Transforming Waste Paint Sludge (PS) to an Asset in Alternative Uses Based on SEN and EDX Analyses. In Proceedings of the 6th International Conference on Advances in Civil Engineering (ICACE-2022), CUET, Chattogram, Bangladesh, 21–23 December 2022. [Google Scholar]
- Hossain, R.; Mrinmoy, H.S.; Arsh, K.; Hoque, A.; Masum, M.M.H. Partial Replacement of Cement with Paint Sludge in Concrete Block & its Environmental Impacts; Atlantis Press International BV: Dordrecht, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Firdissa, B.; Solomon, Y.; Soromessa, T. Assessment of the Status of Industrial Waste Water Effluent for Selected Industries in Addis Ababa, Ethiopia. J. Nat. Sci. Res. 2016, 6, 1–10. [Google Scholar]
- Tian, Y.; Chen, L.; Gao, L.; Michel, F.C.; Keener, H.M.; Klingman, M.; Dick, W.A. Composting of waste paint sludge containing melamine resin and the compost’s effect on vegetable growth and soil water quality. J. Hazard. Mater. 2012, 243, 28–36. [Google Scholar] [CrossRef]
- Islam, A.; Dey, N. Performance of Cement Concrete (CC) Block with Partial Replacement of Cement by Paint Sludge in Coastal Environment. In Proceedings of the 7th International Conference on Advances in Civil Engineering (ICACE-2024), CUET, Chattogram, Bangladesh, 12–14 December 2024. [Google Scholar]
- Devi, S.K.; Gopalakrishnan, A.N. Feasibility of making construction material using hazardous paint sludge. Int. J. Emerg. Technol. Comput. Sci. Electron. 2016, 22, 2–4. [Google Scholar]
- Ruffino, B.; Dalmazzo, D.; Santagata, E.; Zanetti, M.C. Preliminary Evaluation of the Potential Use of Paint Sludge in Bituminous Binders. In Proceedings of the Sardinia 2011, XIII International Waste Management and Landfill Symposium, Cagliari, Italy, 3–7 October 2011. [Google Scholar]
- Zanetti, M.C.; Ruffino, B.; Vercelli, A.; Dalmazzo, D.; Santagata, E. Reuse of paint sludge in road pavements: Technological and environmental issues. Waste Manag. Res. 2018, 36, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Avci, H.; Ghorbanpoor, H.; Topcu, I.B.; Nurbas, M. Investigation and recycling of paint sludge with cement and lime for producing lightweight construction mortar. J. Environ. Chem. Eng. 2017, 5, 861–869. [Google Scholar] [CrossRef]
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A review of sludge-to-energy recovery methods. Energies 2019, 12, 60. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, L.; Gao, L.; Michel, F.C.; Wan, C.; Li, Y.; Dick, W.A. Composting of waste paint sludge containing melamine resin as affected by nutrients and gypsum addition and microbial inoculation. Environ. Pollut. 2012, 162, 129–137. [Google Scholar] [CrossRef]
- Endale, S.A.; Taffese, W.Z.; Vo, D.H.; Yehualaw, M.D. Rice Husk Ash in Concrete. Sustainability 2023, 15, 137. [Google Scholar] [CrossRef]
- Alemu, M.Y.; Yehualaw, M.D.; Nebiyu, W.M.; Nebebe, M.D. Marble and Glass Waste Powder in Cement Mortar Marble and Glass Waste Powder in Cement Mortar. Appl. Sci. 2025, 15, 3930. [Google Scholar] [CrossRef]
- Getachew, E.M.; Yifru, B.W.; Taffese, W.Z.; Yehualaw, M.D. Enhancing Mortar Properties through Thermoactivated Recycled Concrete Cement. Buildings 2023, 13, 2209. [Google Scholar] [CrossRef]
- Gedefaw, A.; Worku Yifru, B.; Endale, S.A.; Habtegebreal, B.T.; Yehualaw, M.D. Experimental Investigation on the Effects of Coffee Husk Ash as Partial Replacement of Cement on Concrete Properties. Adv. Mater. Sci. Eng. 2022, 2022, 4175460. [Google Scholar] [CrossRef]
- Worku, M.A.; Taffese, W.Z.; Hailemariam, B.Z.; Yehualaw, M.D. Cow Dung Ash in Mortar: An Experimental Study. Appl. Sci. 2023, 13, 6218. [Google Scholar] [CrossRef]
- Yehualaw, M.D.; Alemu, M.; Hailemariam, B.Z.; Vo, D.H.; Taffese, W.Z. Aquatic Weed for Concrete Sustainability. Sustainability 2022, 14, 15501. [Google Scholar] [CrossRef]
- Almesfer, N.; Ingham, J. Effect of waste latex paint on concrete. Cem. Concr. Compos. 2014, 46, 19–25. [Google Scholar] [CrossRef]
- Nehdi, M.; Sumner, J. Recycling waste latex paint in concrete. Cem. Concr. Res. 2003, 33, 857–863. [Google Scholar] [CrossRef]
- Almesfer, N.; Haigh, C.; Ingham, J. Waste paint as an admixture in concrete. Cem. Concr. Compos. 2012, 34, 627–633. [Google Scholar] [CrossRef]
- Fitrah, N.; Bakar, A.; Norhasri, M.; Sidek, M.; Mariam, S.; Abd, N. Materials Today: Proceedings Characterization and evaluation of dried automotive paint sludge as cement-based composite. Mater. Today Proc. 2022, 63, S301–S305. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ahmed, Z.B.; Ahmed, T. Case Studies in Construction Materials Feasibility of sludge generated in water-based paint industries as cement replacement material. Case Stud. Constr. Mater. 2024, 16, e01119. [Google Scholar] [CrossRef]
- Mohammed, A.; Nehdi, M.L.; Adawi, A. Recycling Waste Latex Paint in Concrete with Added Value Prediction of the Mechanical Properties of Concrete Using Machine Learning View Project. Available online: https://www.researchgate.net/publication/285841983 (accessed on 15 January 2025).
- Ballesteros, F.; Manila, A.A.; Choi, A.E.S.; Lu, M.C. Electroplating sludge handling by solidification/stabilization process: A comprehensive assessment using kaolinite clay, waste latex paint and calcium chloride cement additives. J. Mater. Cycles Waste Manag. 2019, 21, 1505–1517. [Google Scholar] [CrossRef]
- Feng, E.; Sun, J.; Feng, L. Regeneration of paint sludge and reuse in cement concrete. E3S Web Conf. 2018, 38, 02021. [Google Scholar] [CrossRef]
- ASTM C618-22; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use. ASTM: West Conshohocken, PA, USA, 2010.
- Ramezanianpour, A.A. Cement Replacement Materials: Properties, Durability, Sustainability (Springer Geochemistry/Mineralogy), 2014th ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Claisse, P. Cements and cement replacement materials. In Civil Engineering Materials; Butterworth-Heinemann: Oxford, UK, 2016; pp. 163–176. [Google Scholar] [CrossRef]
- Gautam, S.P.; Bundela, P.S.; Murumkar, M. Paint sludge waste co-processing at the ACC Wadi Cement Works in Karnataka, India. WIT Trans. Ecol. Environ. 2010, 140, 57–66. [Google Scholar] [CrossRef]
- Saleh, A.M.; Rahmat, M.N.; Ismail, N. SWPS ECO Bricks: Development of Sustainable Brick Utilising Solid Waste Fly Ash and Paint Sludge. IOP Conf. Ser. Earth Environ. Sci. 2021, 641, 012025. [Google Scholar] [CrossRef]
- Karadumpa, C.S.; Pancharathi, R.K. Study on energy use and carbon emission from manufacturing of OPC and blended cements in India. Environ. Sci. Pollut. Res. 2024, 31, 5364–5383. [Google Scholar] [CrossRef]
- Salihoglu, N.K.; Ucaroglu, S.; Salihoglu, G. Bioconversion of industrial wastes: Paint sludge from automotive manufacturing. J. Mater. Cycles Waste Manag. 2018, 20, 2100–2109. [Google Scholar] [CrossRef]
- Dave, N.; Misra, A.K.; Srivastava, A.; Sharma, A.K.; Kaushik, S.K. Green quaternary concrete composites: Characterization and evaluation of the mechanical properties. Struct. Concr. 2018, 19, 1280–1289. [Google Scholar] [CrossRef]
- Mymrin, V.; Praxedes, P.B.; Alekseev, K.; Avanci, M.A.; Rolim, P.H.B.; Povaluk, A.E.; Aibuldinov, Y.K.; Catai, R.E. Manufacturing of sustainable ceramics with improved mechanical properties from hazardous car paint waste to prevent environment pollution. Int. J. Adv. Manuf. Technol. 2019, 105, 2357–2367. [Google Scholar] [CrossRef]
- Dalmazzo, D.; Vercelli, A.; Santagata, E.; Ruffino, B.; Zanetti, M.C. Rheological characterization and performance-related evaluation of paint sludge modified binders. Mater. Struct. 2017, 50, 74. [Google Scholar] [CrossRef]
- ASTM C150/C150M-22; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2017; pp. 1–8.
- Vaccarezza, V.; Anderson, C.G. The Use of Waterborne Automotive Paint Sludge as an Alternative Binder for Magnetite Ore Pellets. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2023. [Google Scholar]
- Nakouzi, S.; Mielewski, D.; Ball, J.C.; Kim, B.R.; Salmeen, I.T.; Bauer, D.; Narula, C.K. A novel approach to paint sludge recycling: Reclaiming of paint sludge components as ceramic composites and their applications in reinforcement of metals and polymers. J. Mater. Res. 1998, 13, 53–60. [Google Scholar] [CrossRef]
- ASTM C136; Standard Test Method for Sieve Analysis of Fine and Coarse Aggregates. ASTM: West Conshohocken, PA, USA, 2009; pp. 1–5.
- ASTM C29; Standard Test Method for Bulk Density (“Unit Weight”) and Voids in Aggregate. ASTM: West Conshohocken, PA, USA, 2009; pp. 1–5.
- ASTM C128; Standard Test Method for Density, Relative Density (Specific Gravity), and Absorption of Fine Aggregate. ASTM: West Conshohocken, PA, USA, 2009; pp. 1–7.
- ASTM C566-97; Standard Test Method for Total Evaporable Moisture Content of Aggregate by Drying. Annual Book of ASTM Standards. ASTM: West Conshohocken, PA, USA, 1997; Volume 97, pp. 5–7.
- ASTM C40-04; Standard Test Method for Organic Impurities in Fine Aggregates for Concrete. ASTM: West Conshohocken, PA, USA, 2009.
- ASTM C305/C305-20; Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency. ASTM International: West Conshohocken, PA, USA, 2009. Available online: https://www.astm.org/c0305-20.html (accessed on 1 February 2025).
- ASTM C192/C 192M-07; Standard Practice for Making and Curing Concrete Test Specimens in the Laboratory. ASTM: West Conshohocken, PA, USA, 2009.
- ASTM C1437; Standard Test Method and Flow of Hydraulic Cement Mortar. ASTM: West Conshohocken, PA, USA, 2009.
- ASTM C187; Standard Test Method for Normal Consistency of Hydraulic Cement. ASTM: West Conshohocken, PA, USA, 2021.
- ASTMC191-08; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2009.
- ASTM C151; Standard Test Method for Autoclave Expansion of Hydraulic Cement. ASTM: West Conshohocken, PA, USA, 2009.
- ASTM C109; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM: West Conshohocken, PA, USA, 2009.
- ASTM C597; Standard Test Method for Ultrasonic Pulse Velocity Through Concrete. ASTM: West Conshohocken, PA, USA, 2016.
- ASTM C138/C138M-13; Standard Test Method for Density (Unit Weight), Yield, and Air Content (Gravimetric). ASTM (American Society for Testing and Materials): West Conshohocken, PA, USA, 2013.
- ASTM C 642; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete C642-97. ASTM International: West Conshohocken, PA, USA, 1997.
- ASTM C 1012; Lenght Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution. ASTM: West Conshohocken, PA, USA, 2009.
- Getachew, E.M.; Yifru, B.W.; Habtegebreal, B.T.; Yehualaw, M.D. Performance evaluation of mortar with ground and thermo-activated recycled concrete cement. Cogent. Eng. 2024, 11, 2357726. [Google Scholar] [CrossRef]
- Arvaniti, E.C.; Juenger, M.C.G.; Bernal, S.A.; Duchesne, J.; Courard, L.; Leroy, S.; Provis, J.L.; Klemm, A.; De Belie, N. Determination of particle size, surface area, and shape of supplementary cementitious materials by different techniques. Mater. Struct. Constr. 2015, 48, 3687–3701. [Google Scholar] [CrossRef]
- Nega, D.M.; Yifru, B.W.; Taffese, W.Z.; Ayele, Y.K.; Yehualaw, M.D. Impact of Partial Replacement of Cement with a Blend of Marble and Granite Waste Powder on Mortar. Appl. Sci. 2023, 13, 8998. [Google Scholar] [CrossRef]
- Anderson, C. Using Automotive Paint Sludge as a Binder for Pelletizing Magnetite Ore. Asp. Min. Miner. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Wang, W.C. Feasibility of stabilizing expanding property of furnace slag by autoclave method. Constr. Build. Mater. 2014, 68, 552–557. [Google Scholar] [CrossRef]
- BS 1881-125; Testing Concrete Part 203. Recommendations for the Measurement of Velocity of Ultrasonic Pulses in Concrete. British Standards Institution: London, UK, 1986.
- Yehualaw, M.D.; Hwang, C.L.; Vo, D.H.; Koyenga, A. Effect of alkali activator concentration on waste brick powder-based ecofriendly mortar cured at ambient temperature. J. Mater. Cycles Waste Manag. 2021, 23, 727–740. [Google Scholar] [CrossRef]
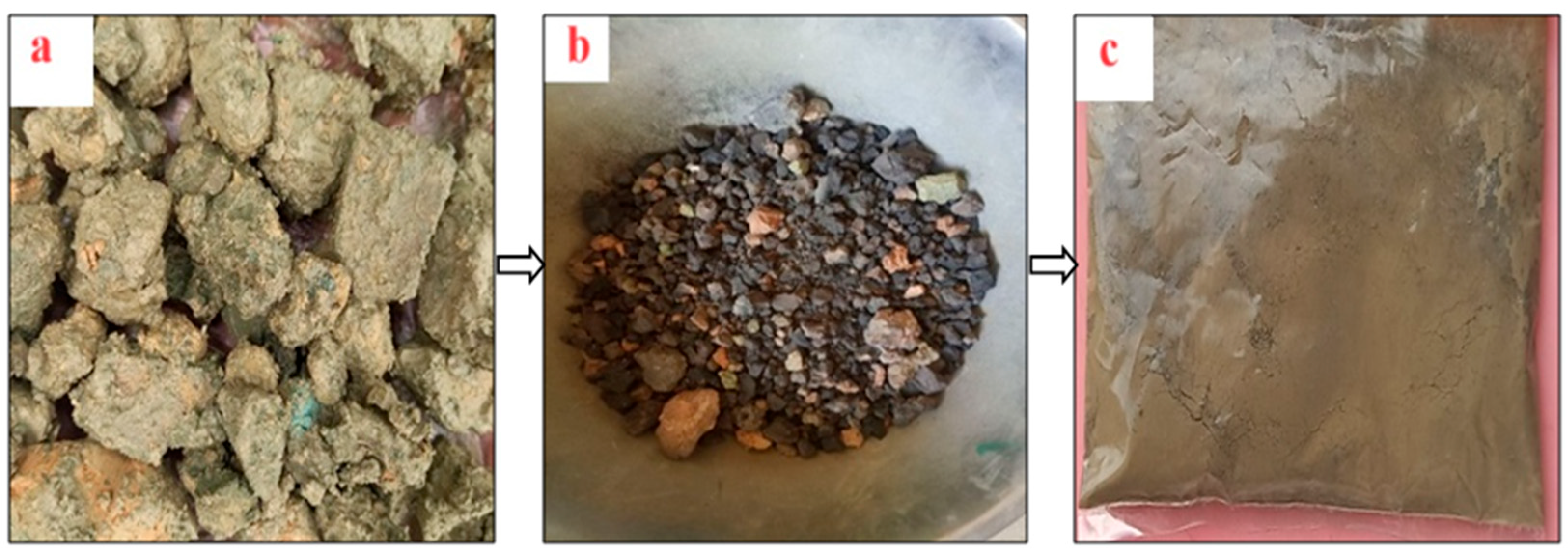
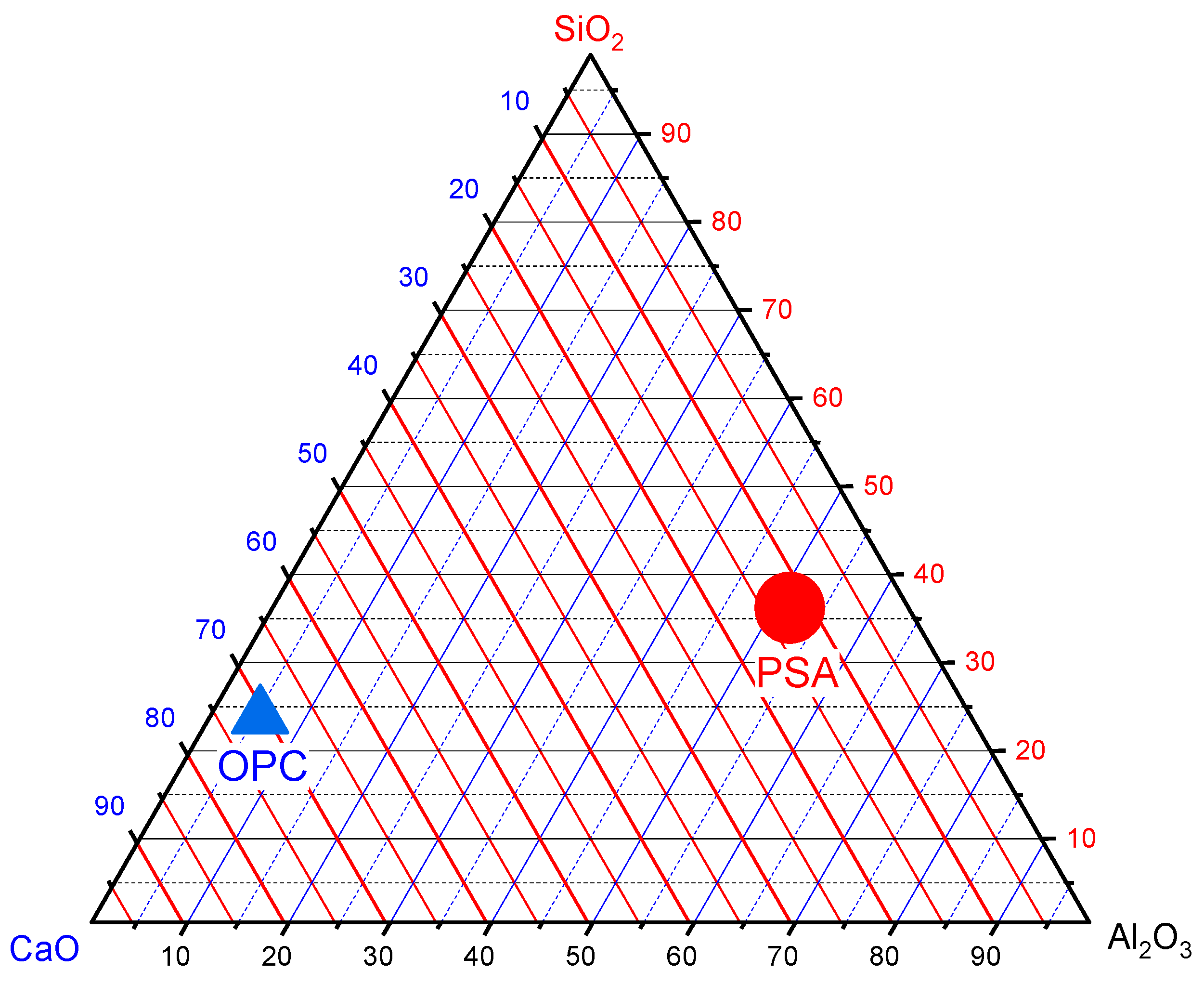
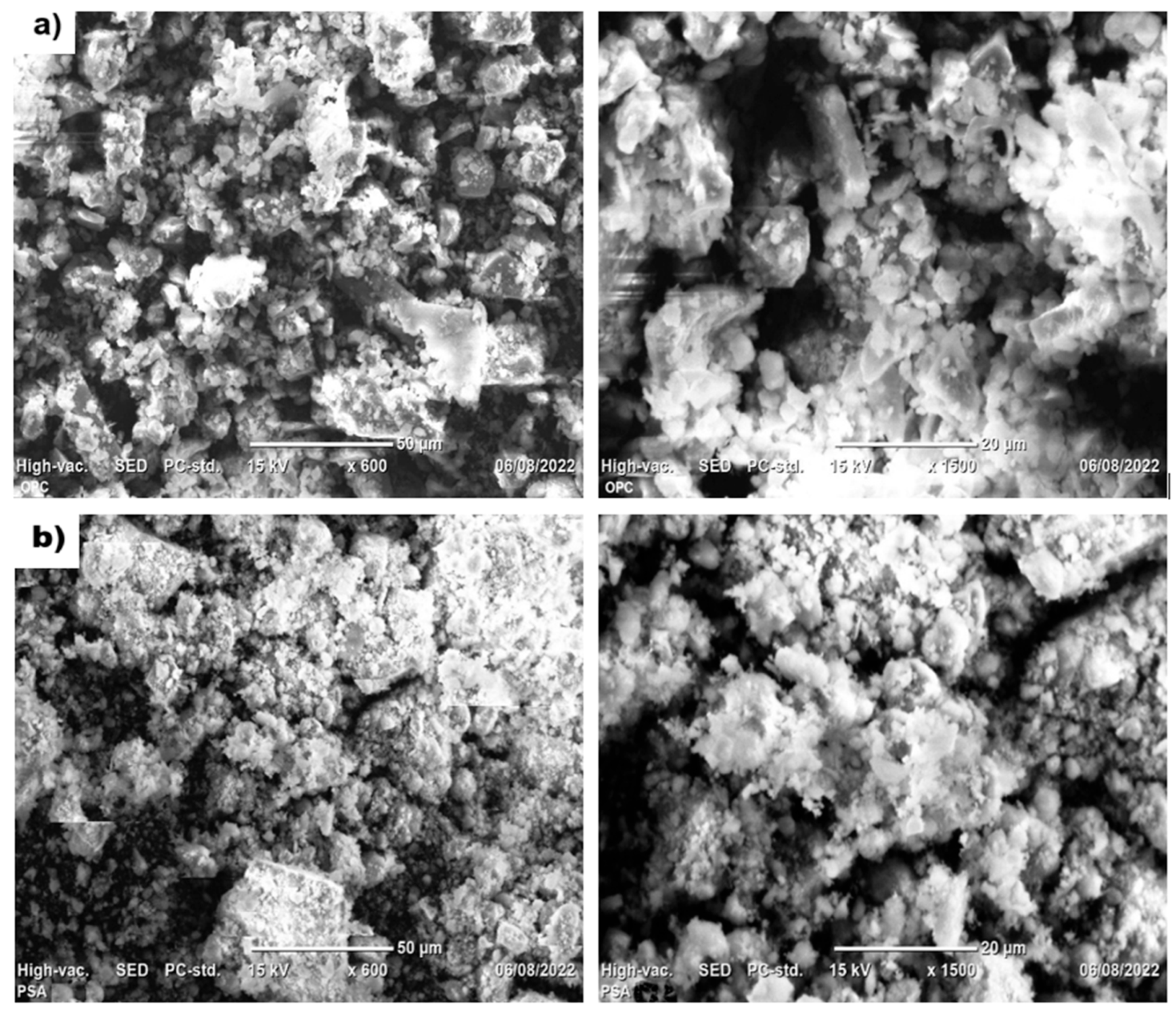
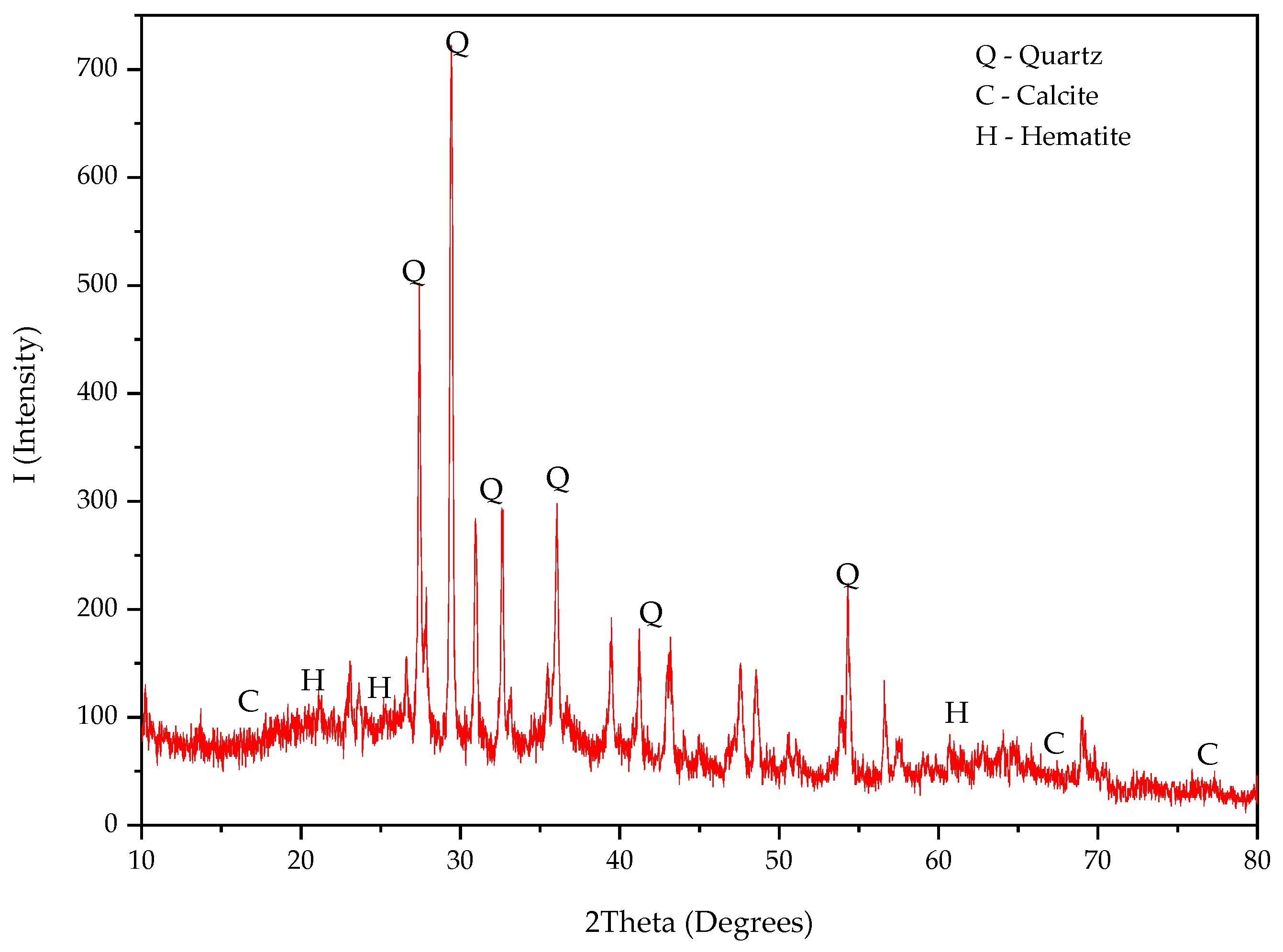



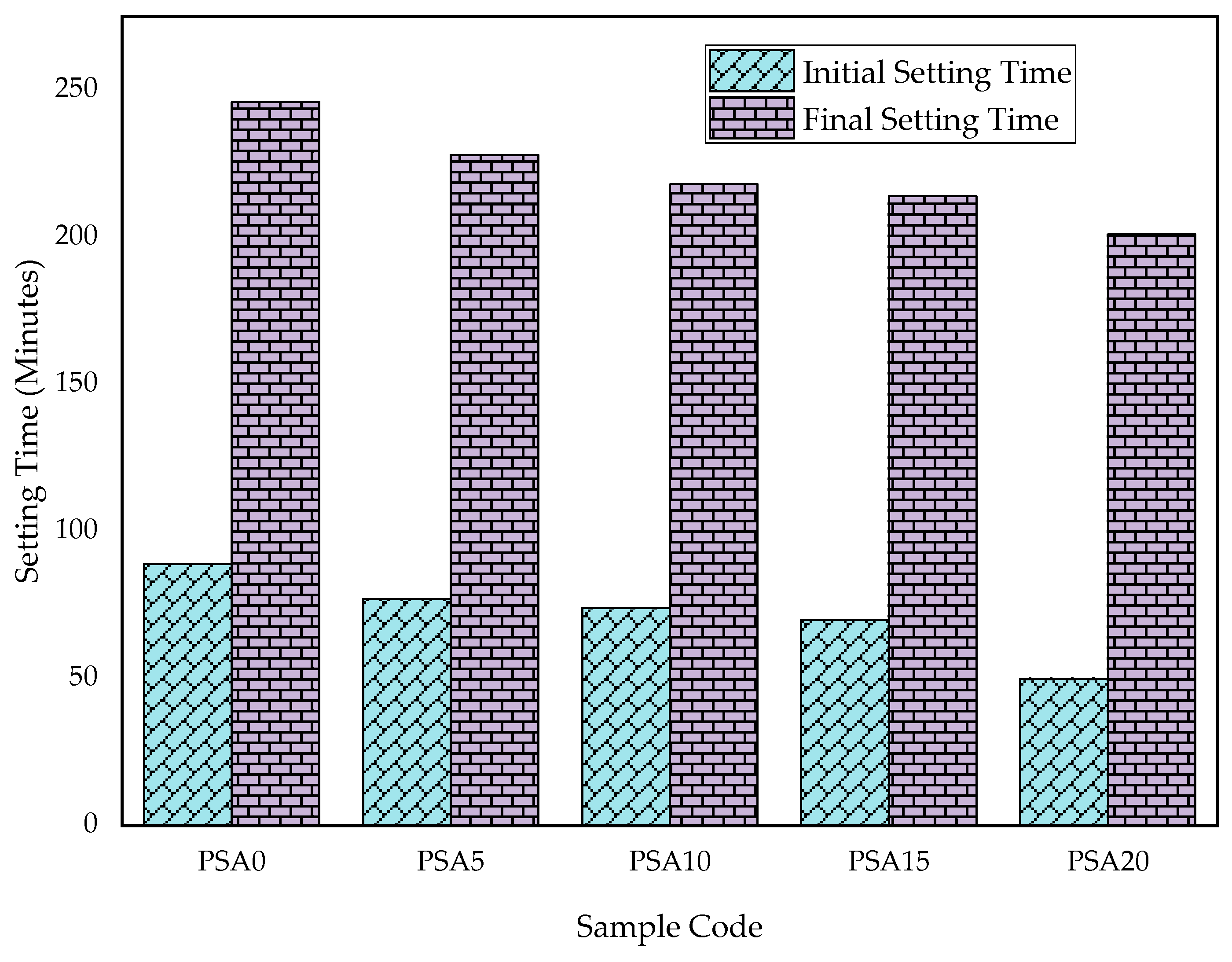






| Oxides | OPC | PSA-700 °C | PSA-750 °C | PSA-800 °C |
|---|---|---|---|---|
| SiO2 | 21.74 | 19.73 | 17.65 | 16.48 |
| Al2O3 | 4.98 | 21.69 | 25.19 | 19.85 |
| Fe2O3 | 3.99 | 26.84 | 29.49 | 30.13 |
| CaO | 64.5 | 2.92 | 5.98 | 7.09 |
| MgO | 1.67 | 1.52 | 1.77 | 1.81 |
| Na2O | 0.19 | 0.12 | 0.12 | 0.12 |
| K2O | 0.52 | 0.54 | 0.53 | 0.56 |
| Cr2O3 | - | 3.76 | 1.97 | 3.98 |
| TiO2 | - | 6.39 | 3.20 | 5.5 |
| MnO | - | 1.3 | 1.03 | 1.1 |
| P2O5 | - | 3.08 | 1.15 | 2.17 |
| LOI | 0.6 | 5.04 | 5.28 | 6.47 |
| Others | 1.81 | 7.07 | 6.64 | 4.74 |
| Physical Properties | OPC | PSA |
|---|---|---|
| Bulk density (kg/m3) | 1440 | 2157 |
| Specific gravity (g/cm3) | 3.15 | 2.76 |
| BET surface area m2/g | 340 | 490 |
| Color | Light gray | Darker light gray |
| Physical Properties | Results | Test Methods and Standards | ASTM Limit | Compliance |
|---|---|---|---|---|
| Unit weight (kg/m3) | 1659.33 | ASTM C29 [50] | 1200–1760 | ✓ |
| Relative density | 2.76 | ASTM C128 [51] | 2.3–2.9 | ✓ |
| Fineness modulus | 2.7 | ASTM C136 [49] | 2.3–3.2 | ✓ |
| Moisture content (%) | 1.48 | ASTM C566 [52] | 0–10 | ✓ |
| Absorption capacity (%) | 2 | ASTM C128 [51] | 0.2–2 | ✓ |
| Silt content (%) | 2.14 | ASTM C40 [53] | <5 | ✓ |
| Mix Code (%) | OPC (g) | PSA (g) | Sand (g) | Water (mL) |
|---|---|---|---|---|
| PSA0 | 104.5 | 0.0 | 234.1 | 50.1 |
| PSA5 | 99.2 | 5.2 | 234.1 | 50.1 |
| PSA10 | 94.0 | 10.4 | 234.1 | 50.1 |
| PSA15 | 88.8 | 15.7 | 234.1 | 50.1 |
| PSA20 | 83.6 | 20.9 | 234.1 | 50.1 |
| Test Categories | Test Properties | Test Standards | Examined Samples | CURING Ages |
|---|---|---|---|---|
| Fresh properties | Slump flow | ASTM C1437 [56] | All mortar mix | _ |
| Consistency | ASTM C187 [57] | |||
| Setting time | ASTM C191 [58] | |||
| Soundness | ASTM C151 [59] | |||
| Mechanical properties | Compressive strength | ASTM C109 [60] | All mortar cube specimens | 3, 7, 14, 21, 28, 56, and 91 days |
| UPV | ASTM C597 [61] | |||
| Bulk density | ASTM C138 [62] | |||
| Durability properties | Water absorption | ASTM C642 [63] | All mortar cube specimens | 3, 7, 14, 21, 28, 56, and 91 days |
| Porosity | ASTM C642 [63] | |||
| Sulfate attack resistance | ASTM C1012 [64] | |||
| Microstructural analysis | SEM | _ | Samples taken from the mortar cube test specimens | 7 and 28 days |
| XRD | ||||
| TGA/DTA | ||||
| FTIR |
| Sample Code | 3rd Day | 7th Day | 14th Day | 21st Day | 28th Day | 56th Day | 91st Day |
|---|---|---|---|---|---|---|---|
| PSA5 | 1.03 | 1.02 | 1.02 | 1.02 | 1.04 | 1.04 | 1.04 |
| PSA10 | 1.08 | 1.05 | 1.05 | 1.03 | 1.04 | 1.05 | 1.05 |
| PSA15 | 1.00 | 1.01 | 1.00 | 1.00 | 1.00 | 1.01 | 1.01 |
| PSA20 | 0.74 | 0.66 | 0.67 | 0.68 | 0.69 | 0.70 | 0.74 |
| Sample Code | Weight Loss | |||
|---|---|---|---|---|
| 7 Days | 28 Days | |||
| (mg) | (%) | (mg) | (%) | |
| PSA0 | 0.22 | 21.75 | 0.10 | 9.5 |
| PSA10 | 0.08 | 8.32 | 0.04 | 4.13 |
| PSA15 | 0.09 | 9.19 | 0.08 | 7.82 |
| Thermal Event | Temperature Range |
|---|---|
| Evaporation of absorbed water, interlayer water, and capillary moisture | 25–100 °C |
| Dehydration of C-S-H gel and ettringite | 100–450 °C |
| Decomposition of portlandite Ca(OH)2 | 480–520 °C |
| Decarbonization of calcite (CaCO3) | 740–760 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endale, S.A.; Yehualaw, M.D.; Taffese, W.Z.; Vo, D.-H. Ecofriendly Mortar with Paint Sludge Ash. Materials 2025, 18, 2080. https://doi.org/10.3390/ma18092080
Endale SA, Yehualaw MD, Taffese WZ, Vo D-H. Ecofriendly Mortar with Paint Sludge Ash. Materials. 2025; 18(9):2080. https://doi.org/10.3390/ma18092080
Chicago/Turabian StyleEndale, Solomon Asrat, Mitiku Damtie Yehualaw, Woubishet Zewdu Taffese, and Duy-Hai Vo. 2025. "Ecofriendly Mortar with Paint Sludge Ash" Materials 18, no. 9: 2080. https://doi.org/10.3390/ma18092080
APA StyleEndale, S. A., Yehualaw, M. D., Taffese, W. Z., & Vo, D.-H. (2025). Ecofriendly Mortar with Paint Sludge Ash. Materials, 18(9), 2080. https://doi.org/10.3390/ma18092080








