Effect of Potential-Determining Ions on Rheological Properties of Calcite Paste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Samples
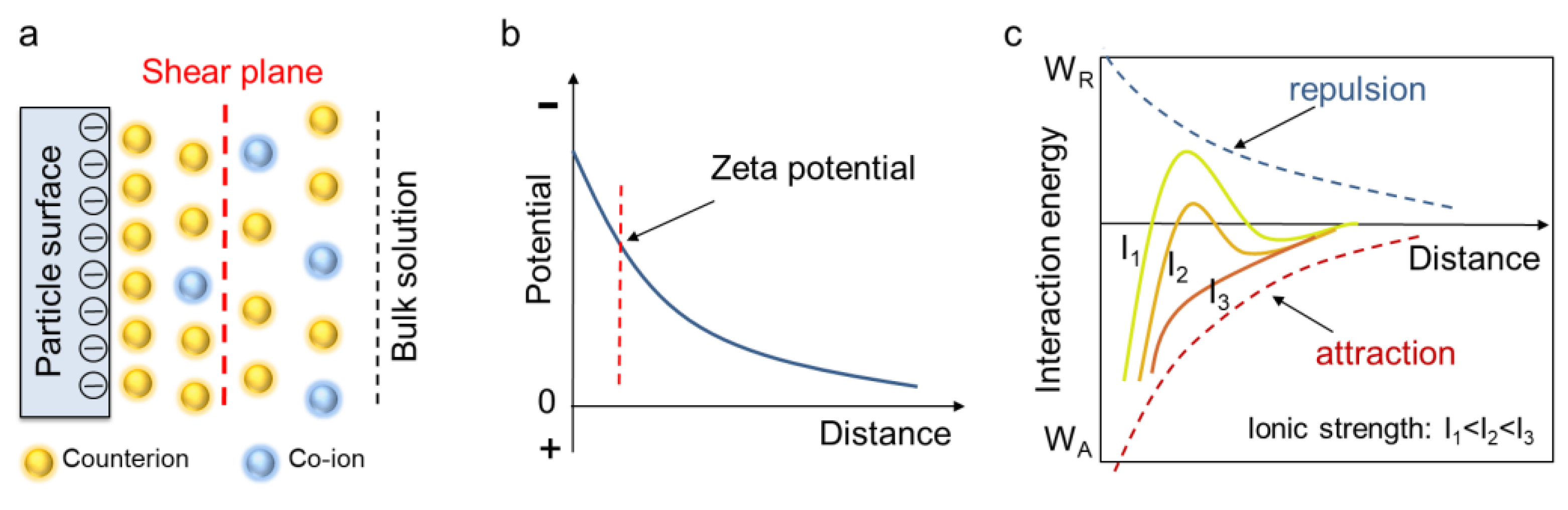
2.2. Zeta Potential Test and DLVO Theory
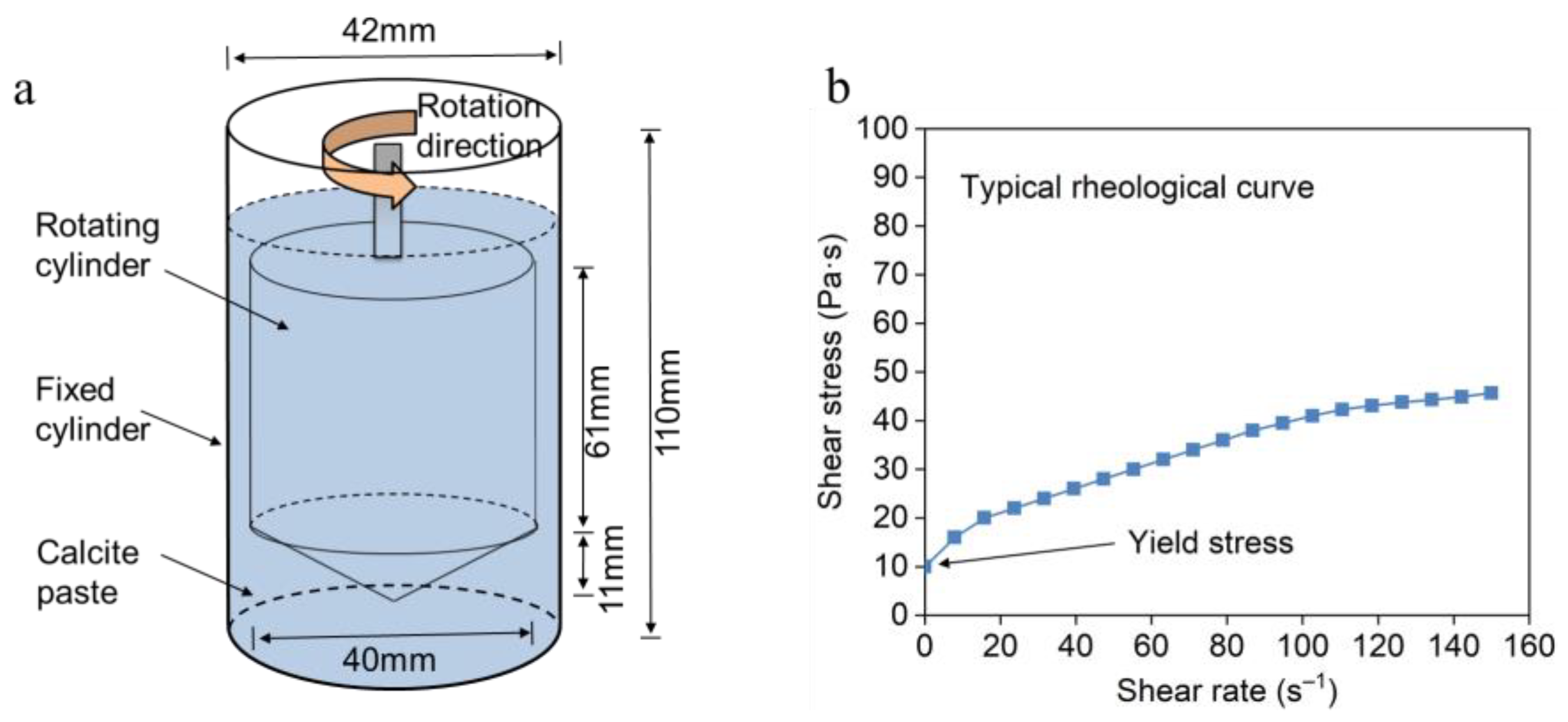
2.3. Rheology Test
3. Results and Analysis
3.1. Effect of Indifferent Ions
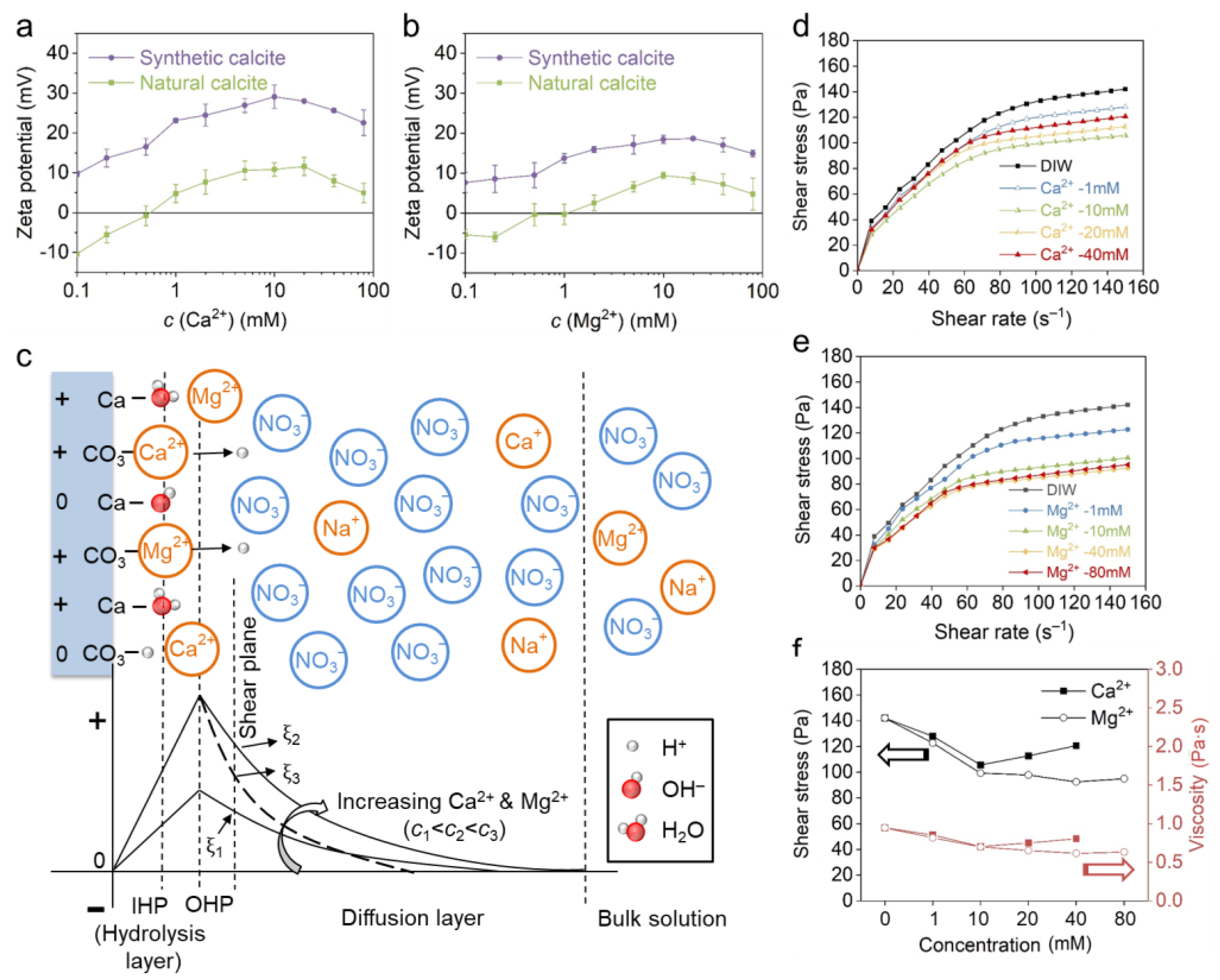
3.2. Effect of Positive PDIs
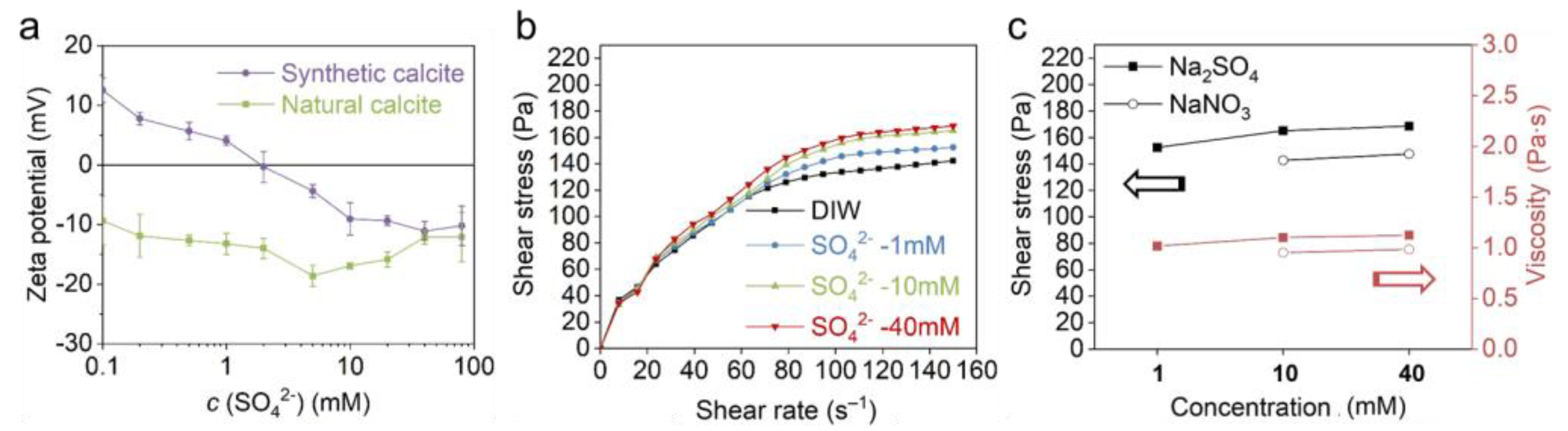
3.3. Effect of Negative PDIs
3.3.1. The Effect of OH−
3.3.2. The Effect of CO32−
3.3.3. The Effect of SO42−
4. Discussion
4.1. Zeta Potential and PDI Determination
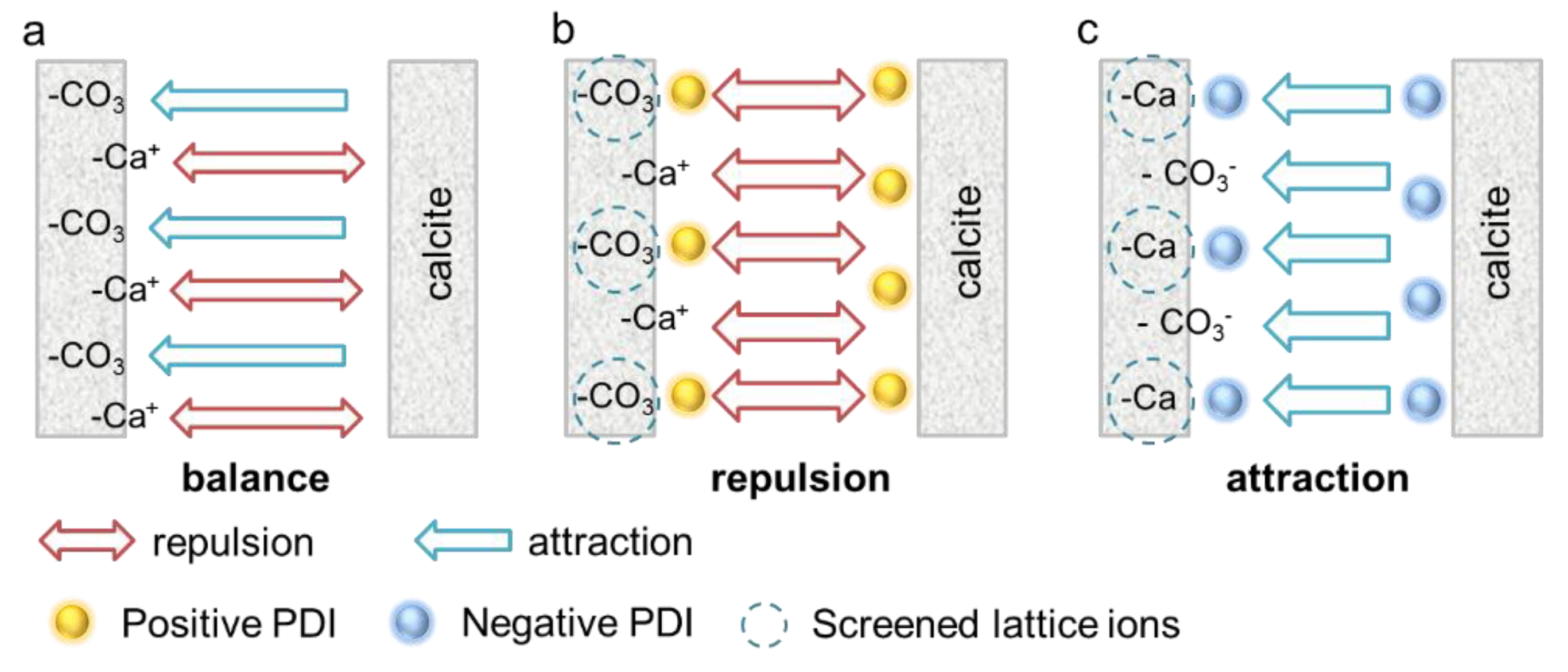
4.2. The Non-DLVO Behavior of Calcite Paste
5. Conclusions
- (1)
- The adsorption of positive PDIs elevates the positive charge of calcite, whereas the negative PDIs make the calcite more negatively charged. Calcite exhibits higher zeta potential in Ca2+ than Mg2+, and in negative PDI solutions follows the order of . Indifferent ions such as Na+, K+ and NO32− slightly change the zeta potential by tuning the ionic strength and Debye length.
- (2)
- The natural calcite is constantly more negative than synthetic calcite due to the existence of impurities, indicating that the detection of an IEP by zeta potential test is insufficient for PDI identification. Fajans’s rule, denoting the correlation between ionic adsorption and solubility of precipitates, provides a practical method for PDI identification. In addition, the rheology test also indicates a feasible approach, since the calcite paste rheology is insensitive to indifferent ions such as Na+, K+ and NO3−.
- (3)
- The incorporation of positive PDIs significantly increases the positive charge of calcite and enhances the suspension flow, which basically follows the DLVO model. Ca2+ has a higher capability of improving calcite charge than Mg2+, whereas the Mg2+ is more capable in improving the suspension flow. The secondary differences may result from a different covalency index and affinity to the calcite surface, where Ca2+ is more strongly bound by calcite and the Mg2+ bond is relatively weak.
- (4)
- The increasing negative PDIs make the calcite negatively charged leading to a more viscous paste. Calcite paste exhibits non-DLVO behavior with negative PDI (OH−, CO32− and SO42−) solutions, where negatively charged calcite paste exhibits much higher viscosity. Specific attraction and lattice site screening induced by negative PDIs may be the reason for this phenomenon. The deprotonation reaction by OH− generates the highest specific attraction in calcite paste, thus inducing the greatest rigidity and viscosity, while the weakly attracted SO42− induces a much smaller attraction. The interaction model, denoting an extended DLVO theory, provides a rational explanation for the non-DLVO behavior.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hofmann, S.; Voïtchovsky, K.; Spijker, P.; Schmidt, M.; Stumpf, T. Visualising the molecular alteration of the calcite (104)—Water interface by sodium nitrate. Sci. Rep. 2016, 6, 21576. [Google Scholar] [CrossRef] [PubMed]
- Knauss, K.G.; Johnson, J.W.; Steefel, C.I. Evaluation of the impact of CO2, co-contaminant gas, aqueous fluid and reservoir rock interactions on the geologic sequestration of CO2. Chem. Geol. 2005, 217, 339–350. [Google Scholar] [CrossRef]
- Guo, H.; Kovscek, A.R. Investigation of the effects of ions on short-range non-DLVO forces at the calcite/brine interface and implications for low salinity oil-recovery processes. J. Colloid Interface Sci. 2019, 552, 295–311. [Google Scholar] [CrossRef]
- Sadatshojaei, E.; Jamialahmadi, M.; Esmaeilzadeh, F.; Wood, D.A.; Ghazanfari, M.H. The impacts of silica nanoparticles coupled with low-salinity water on wettability and interfacial tension: Experiments on a carbonate core. J. Dispers. Sci. Technol. 2019, 41, 1159–1173. [Google Scholar] [CrossRef]
- Heberling, F.; Bosbach, D.; Eckhardt, J.-D.; Fischer, U.; Glowacky, J.; Haist, M.; Kramar, U.; Loos, S.; Müller, H.S.; Neumann, T.; et al. Reactivity of the calcite–water-interface, from molecular scale processes to geochemical engineering. Appl. Geochem. 2014, 45, 158–190. [Google Scholar] [CrossRef]
- Papo, A.; Piani, L. Rheological Behavior of Calcite Slurries: Effect of Deflocculant Addition. Part. Sci. Technol. 2005, 23, 85–91. [Google Scholar] [CrossRef]
- Liberto, T.; Le Merrer, M.; Barentin, C.; Bellotto, M.; Colombani, J. Elasticity and yielding of a calcite paste: Scaling laws in a dense colloidal suspension. Soft Matter 2017, 13, 2014–2023. [Google Scholar] [CrossRef]
- Liberto, T.; Barentin, C.; Colombani, J.; Costa, A.; Gardini, D.; Bellotto, M.; Le Merrer, M. Simple ions control the elasticity of calcite gels via interparticle forces. J. Colloid Interface Sci. 2019, 553, 280–288. [Google Scholar] [CrossRef]
- Benachour, Y.; Davy, C.A.; Skoczylas, F.; Houari, H. Effect of a high calcite filler addition upon microstructural, mechanical, shrinkage and transport properties of a mortar. Cem. Concr. Res. 2008, 38, 727–736. [Google Scholar] [CrossRef]
- Pourchet, S.; Pochard, I.; Brunel, F.; Perrey, D. Chemistry of the calcite/water interface: Influence of sulfate ions and consequences in terms of cohesion forces. Cem. Concr. Res. 2013, 52, 22–30. [Google Scholar] [CrossRef]
- Goergens, J.; Manninger, T.; Goetz-Neunhoeffer, F.J.C.; Research, C. In-situ XRD study of the temperature-dependent early hydration of calcium aluminate cement in a mix with calcite. Cem. Concr. Res. 2020, 136, 106160. [Google Scholar] [CrossRef]
- Gulmez, N. Roles of aluminium shavings and calcite on engineering properties of cement-based composites. J. Clean. Prod. 2020, 277, 124104. [Google Scholar] [CrossRef]
- Mikanovic, N.; Jolicoeur, C. Influence of superplasticizers on the rheology and stability of limestone and cement pastes. Cem. Concr. Res. 2008, 38, 907–919. [Google Scholar] [CrossRef]
- Deraguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solution of electrolytes. Prog. Surf. Sci. 1941, 14, 633–662. [Google Scholar]
- Verwey, E.J.W.; Overbeek, J.T.G.; Van Nes, K. Theory of the Stability of Lyophobic Colloids: The Interaction of Sol Particles Having an Electric Double Layer; Elsevier Publishing Company: Amsterdam, The Netherlands, 1948. [Google Scholar]
- Thompson, D.W.; Pownall, P.G. Surface electrical properties of calcite. J. Colloid Interface Sci. 1989, 131, 74–82. [Google Scholar] [CrossRef]
- Cicerone, D.S.; Regazzoni, A.E.; Blesa, M.A. Electrokinetic properties of the calcite/water interface in the presence of magnesium and organic matter. J. Colloid Interface Sci. 1992, 154, 423–433. [Google Scholar] [CrossRef]
- Nyström, R.; Lindén, M.; Rosenholm, J.B. The Influence of Na+, Ca2+, Ba2+, and La3+ on the ζ Potential and the Yield Stress of Calcite Dispersions. J. Colloid Interface Sci. 2001, 242, 259–263. [Google Scholar] [CrossRef]
- Pierre, A.; Lamarche, J.M.; Mercier, R.; Foissy, A.; Persello, J. Calcium as Potential Determining Ion in Aqueous Calcite Suspensions. J. Dispers. Sci. Technol. 1990, 11, 611–635. [Google Scholar] [CrossRef]
- Huang, Y.C.; Fowkes, F.M.; Lloyd, T.B.; Sanders, N.D. Adsorption of calcium ions from calcium chloride solutions onto calcium carbonate particles. Langmuir 1991, 7, 1742–1748. [Google Scholar] [CrossRef]
- Eriksson, R.; Merta, J.; Rosenholm, J.B. The calcite/water interface: I. Surface charge in indifferent electrolyte media and the influence of low-molecular-weight polyelectrolyte. J. Colloid Interface Sci. 2007, 313, 184–193. [Google Scholar] [CrossRef]
- Moulin, P.; Roques, H. Zeta potential measurement of calcium carbonate. J. Colloid Interface Sci. 2003, 261, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Foxall, T.; Peterson, G.C.; Rendall, H.M.; Smith, A.L. Charge determination at calcium salt/aqueous solution interface. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1979, 75, 1034–1039. [Google Scholar] [CrossRef]
- Douglas, H.W.; Walker, R.A. The electrokinetic behaviour of iceland spar against aqueous electrolyte solutions. Trans. Faraday Soc. 1950, 46, 559–568. [Google Scholar] [CrossRef]
- Song, J.; Zeng, Y.; Wang, L.; Duan, X.; Puerto, M.; Chapman, W.G.; Biswal, S.L.; Hirasaki, G.J. Surface complexation modeling of calcite zeta potential measurements in brines with mixed potential determining ions (Ca2+, CO32−, Mg2+, SO42−) for characterizing carbonate wettability. J. Colloid Interface Sci. 2017, 506, 169–179. [Google Scholar] [CrossRef]
- Van Cappellen, P.; Charlet, L.; Stumm, W.; Wersin, P. A surface complexation model of the carbonate mineral-aqueous solution interface. Geochim. Cosmochim. Acta 1993, 57, 3505–3518. [Google Scholar] [CrossRef]
- Heberling, F.; Trainor, T.P.; Lutzenkirchen, J.; Eng, P.; Denecke, M.A.; Bosbach, D. Structure and reactivity of the calcite-water interface. J. Colloid Interface Sci. 2011, 354, 843–857. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Schott, J. Surface chemistry and dissolution kinetics of divalent metal carbonates. Environ. Sci. Technol. 2002, 36, 426–432. [Google Scholar] [CrossRef]
- Alroudhan, A.; Vinogradov, J.; Jackson, M.D. Zeta potential of intact natural limestone: Impact of potential-determining ions Ca, Mg and SO4. Colloids Surf. A Physicochem. Eng. Asp. 2016, 493, 83–98. [Google Scholar] [CrossRef]
- Smallwood, P.V. Some aspects of the surface chemistry of calcite and aragonite Part I: An electrokinetic study. Colloid Polym. Sci. 1977, 255, 881–886. [Google Scholar] [CrossRef]
- Zhang, P.; Austad, T. Wettability and oil recovery from carbonates: Effects of temperature and potential determining ions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 279, 179–187. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic press: Cambridge, MA, USA, 2011. [Google Scholar]
- Røyne, A.; Dalby, K.N.; Hassenkam, T. Repulsive hydration forces between calcite surfaces and their effect on the brittle strength of calcite-bearing rocks. Geophys. Res. Lett. 2015, 42, 4786–4794. [Google Scholar] [CrossRef]
- Diao, Y.; Espinosa-Marzal, R.M. Molecular insight into the nanoconfined calcite-solution interface. Proc. Natl. Acad. Sci. USA 2016, 113, 12047–12052. [Google Scholar] [CrossRef] [PubMed]
- Grasso, D.; Subramaniam, K.; Butkus, M.; Strevett, K.; Bergendahl, J. A review of non-DLVO interactions in environmental colloidal systems. Rev. Environ. Sci. Bio/Technol. 2002, 1, 17–38. [Google Scholar] [CrossRef]
- Javadi, S.; Røyne, A. Adhesive forces between two cleaved calcite surfaces in NaCl solutions: The importance of ionic strength and normal loading. J. Colloid Interface Sci. 2018, 532, 605–613. [Google Scholar] [CrossRef]
- Marchuk, A.; Rengasamy, P. Clay behaviour in suspension is related to the ionicity of clay–cation bonds. Appl. Clay Sci. 2011, 53, 754–759. [Google Scholar] [CrossRef]
- Huang, J.; Xu, W.; Chen, H.; Xu, G. Elucidating how ionic adsorption controls the rheological behavior of quartz and cement-quartz paste. Constr. Build. Mater. 2021, 272, 121957. [Google Scholar] [CrossRef]
- Zhu, Y.; Ali, A.; Dang, A.; Wandel, A.P.; Bennett, J.M. Re-examining the flocculating power of sodium, potassium, magnesium and calcium for a broad range of soils. Geoderma 2019, 352, 422–428. [Google Scholar] [CrossRef]
- Allen, J.B.; Larry, R.F. Electrochemical Methods Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA,, 2001. [Google Scholar]
- Wolthers, M.; Charlet, L.; Van Cappellen, P. The surface chemistry of divalent metal carbonate minerals; a critical assessment of surface charge and potential data using the charge distribution multi-site ion complexation model. Am. J. Sci. 2008, 308, 905–941. [Google Scholar] [CrossRef]
- Al Mahrouqi, D.; Vinogradov, J.; Jackson, M.D. Zeta potential of artificial and natural calcite in aqueous solution. Adv. Colloid Interface Sci. 2017, 240, 60–76. [Google Scholar] [CrossRef]
- Kasha, A.; Al-Hashim, H.; Abdallah, W.; Taherian, R.; Sauerer, B. Effect of Ca2+, Mg2+ and SO42− ions on the zeta potential of calcite and dolomite particles aged with stearic acid. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 290–299. [Google Scholar] [CrossRef]
- Sondi, I.; Bišćan, J.; Vdović, N.; Škapin, S.D.J.C. The electrokinetic properties of carbonates in aqueous media revisited. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 342, 84–91. [Google Scholar] [CrossRef]
- Mahani, H.; Keya, A.L.; Berg, S.; Nasralla, R. Electrokinetics of carbonate/brine interface in low-salinity waterflooding: Effect of brine salinity, composition, rock type, and pH on?-potential and a surface-complexation model. SPE J. 2018, 22, 53–68. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, G.; Wang, L.; Wu, W.; Ge, J.J.C.; Physicochemical, S.A.; Aspects, E. Zeta potential of limestone in a large range of salinity. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 450, 1–8. [Google Scholar] [CrossRef]
- Vdović, N.; Bišćan, J.J.C.; Physicochemical, S.A.; Aspects, E. Electrokinetics of natural and synthetic calcite suspensions. Colloids Surf. A Physicochem. Eng. Asp. 1998, 137, 7–14. [Google Scholar] [CrossRef]
- Vdović, N. Electrokinetic behaviour of calcite—The relationship with other calcite properties. Chem. Geol. 2001, 177, 241–248. [Google Scholar] [CrossRef]
- Zhang, Y.; Dawe, R. Influence of Mg2+ on the kinetics of calcite precipitation and calcite crystal morphology. Chem. Geol. 2000, 163, 129–138. [Google Scholar] [CrossRef]
- Zarga, Y.; Boubaker, H.B.; Ghaffour, N.; Elfil, H. Study of calcium carbonate and sulfate co-precipitation. Chem. Eng. Sci. 2013, 96, 33–41. [Google Scholar] [CrossRef]
- Curti, E. Coprecipitation of radionuclides with calcite: Estimation of partition coefficients based on a review of laboratory investigations and geochemical data. Appl. Geochem. 1999, 14, 433–445. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; MacNevin, W.M. The Adsorption of Barium Salts on Barium Sulfate from Solutions in 50% Ethanol. J. Am. Chem. Soc. 2002, 58, 1543–1546. [Google Scholar] [CrossRef]
- Stone, H.E.N. Valency relations between alkali and alkali earth elements and elements of second and third long periods. Mater. Sci. Technol. 2013, 3, 171–175. [Google Scholar] [CrossRef]




| CaCO3 | MgCO3 | SiO2 | Al2O3 | Fe2O3 | SO3 | K2O | SrO | NiO | MnO | Cr2O3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Syntheticcalcite | >99 | 0.05 | - | - | 0.001 | 0.01 | 0.005 | 0.05 | - | - | - |
| Natural calcite | 91.05 | 7.19 | 1.21 | 0.30 | 0.11 | 0.05 | 0.03 | 0.02 | 0.02 | 0.01 | 0.01 |
| pH | 1 mM | 10 mM | 20 mM | 40 mM |
|---|---|---|---|---|
| NaOH | 10.8 | 12.0 | 12.3 | 12.6 |
| Na2CO3 | 10.3 | 11.1 | 11.3 | 11.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Li, R.; Cai, J.; Wang, Y.; Chen, J.; Zheng, H. Effect of Potential-Determining Ions on Rheological Properties of Calcite Paste. Materials 2025, 18, 2020. https://doi.org/10.3390/ma18092020
Huang J, Li R, Cai J, Wang Y, Chen J, Zheng H. Effect of Potential-Determining Ions on Rheological Properties of Calcite Paste. Materials. 2025; 18(9):2020. https://doi.org/10.3390/ma18092020
Chicago/Turabian StyleHuang, Jizhi, Ruyu Li, Jiacheng Cai, Yu Wang, Jiansheng Chen, and Hengbin Zheng. 2025. "Effect of Potential-Determining Ions on Rheological Properties of Calcite Paste" Materials 18, no. 9: 2020. https://doi.org/10.3390/ma18092020
APA StyleHuang, J., Li, R., Cai, J., Wang, Y., Chen, J., & Zheng, H. (2025). Effect of Potential-Determining Ions on Rheological Properties of Calcite Paste. Materials, 18(9), 2020. https://doi.org/10.3390/ma18092020






