Current State of Knowledge Regarding the Treatment of Cranial Bone Defects: An Overview
Abstract
1. Introduction
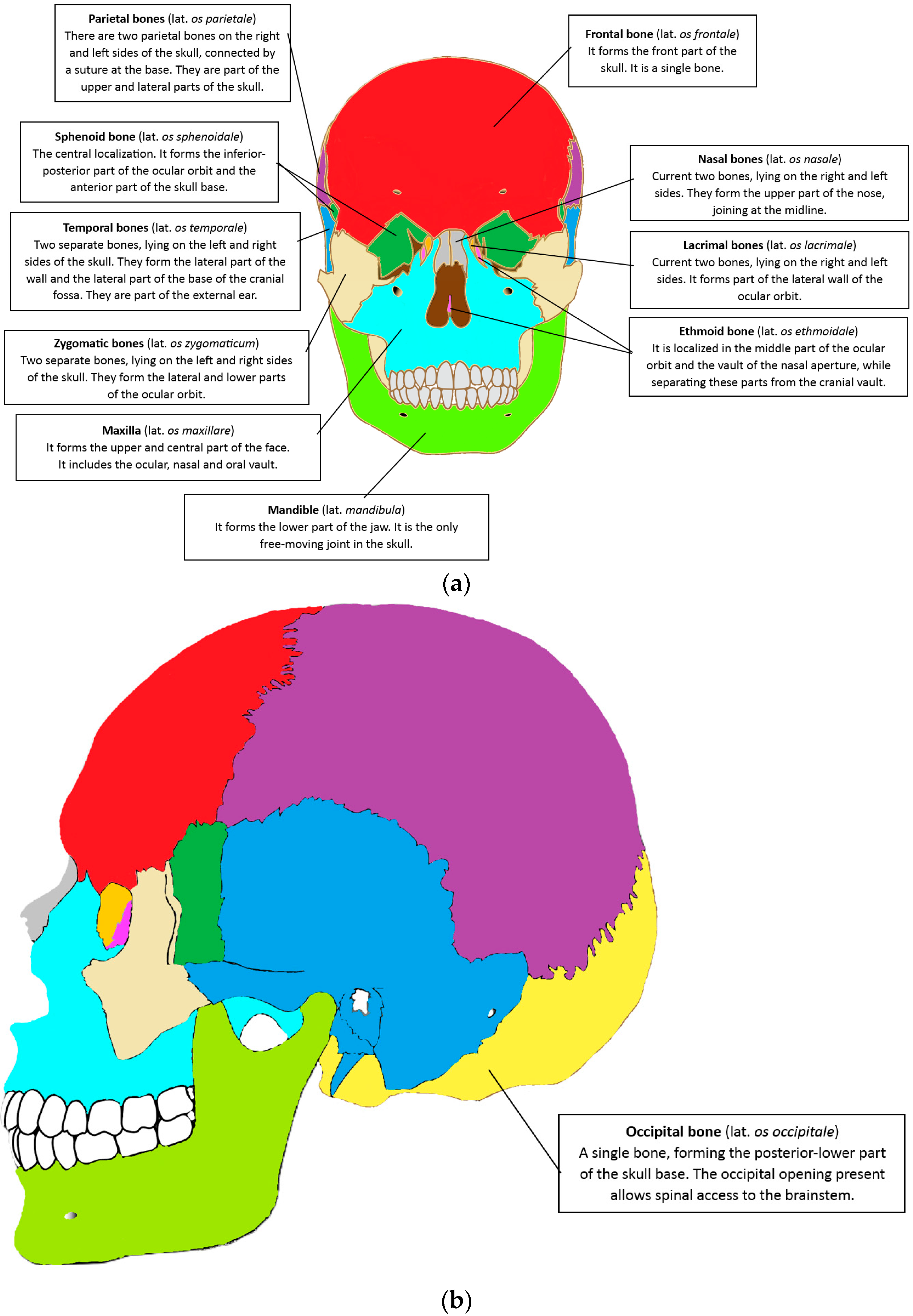
2. Anatomy of the Cranial-Maxillofacial Skeleton
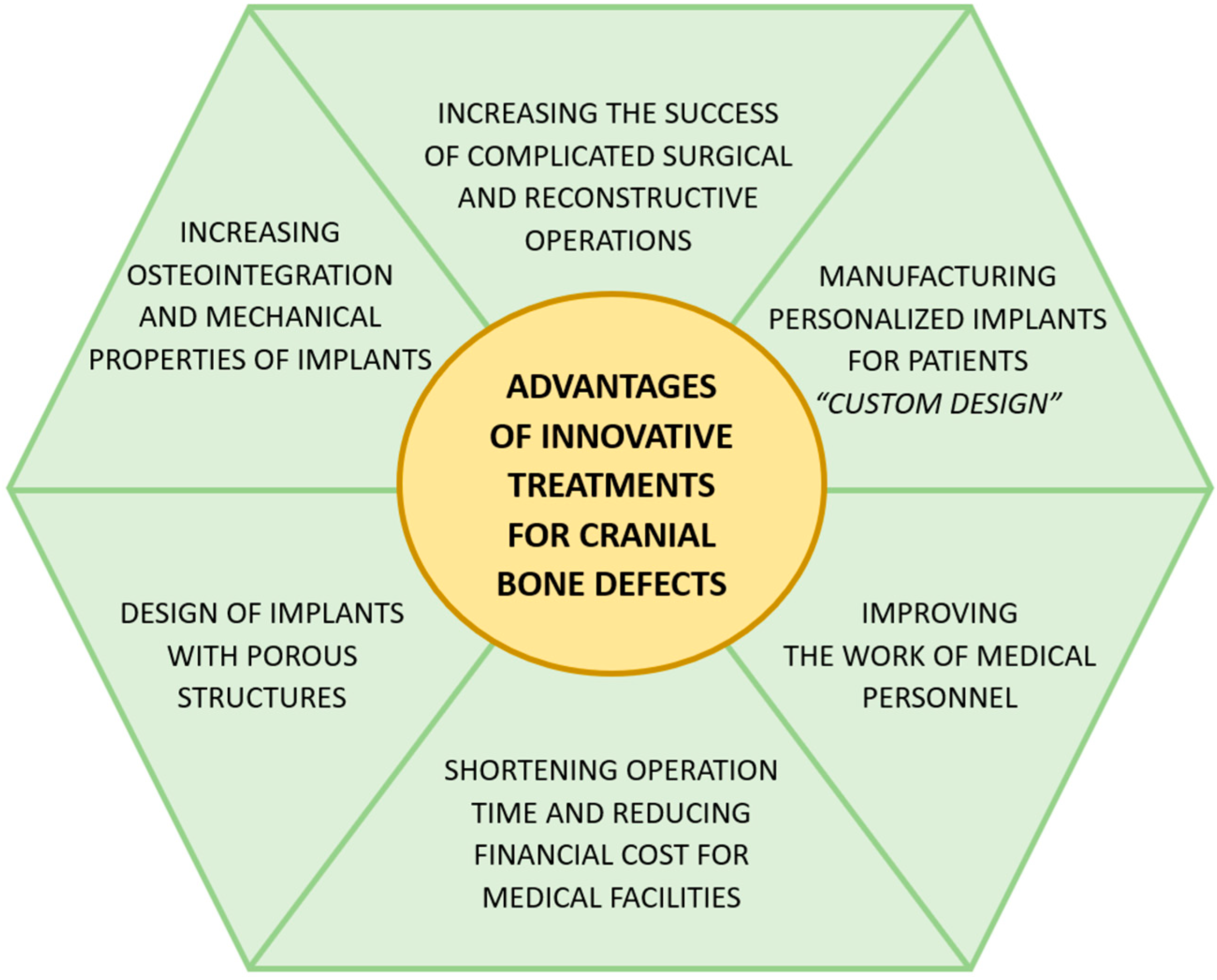
3. Innovative Methods of Medical Management of Cranial Bone Injuries
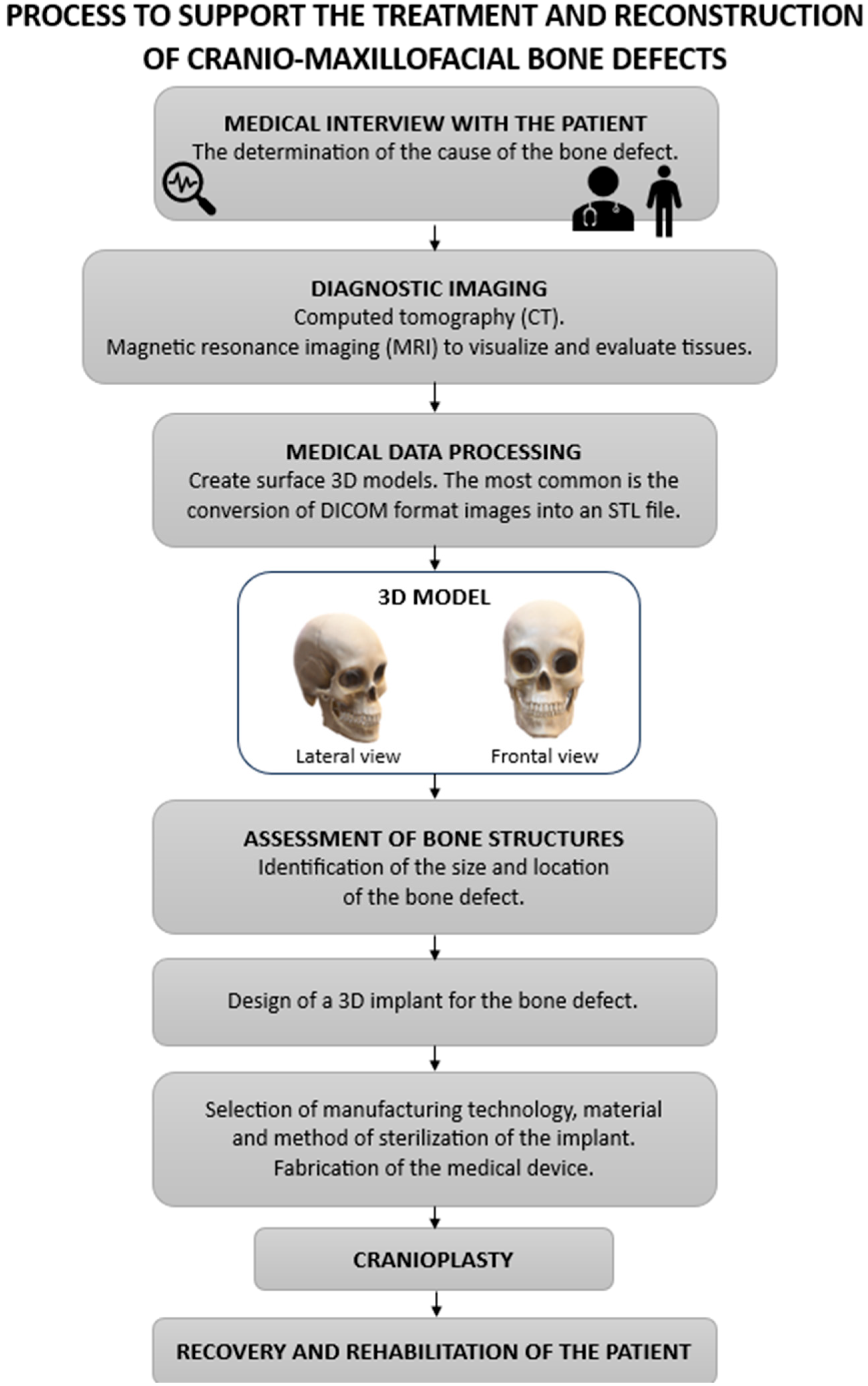
4. Methods of Treatment and Reconstruction of Cranial Bone Defects
5. Cranioplasty and Bone Defect Reconstruction
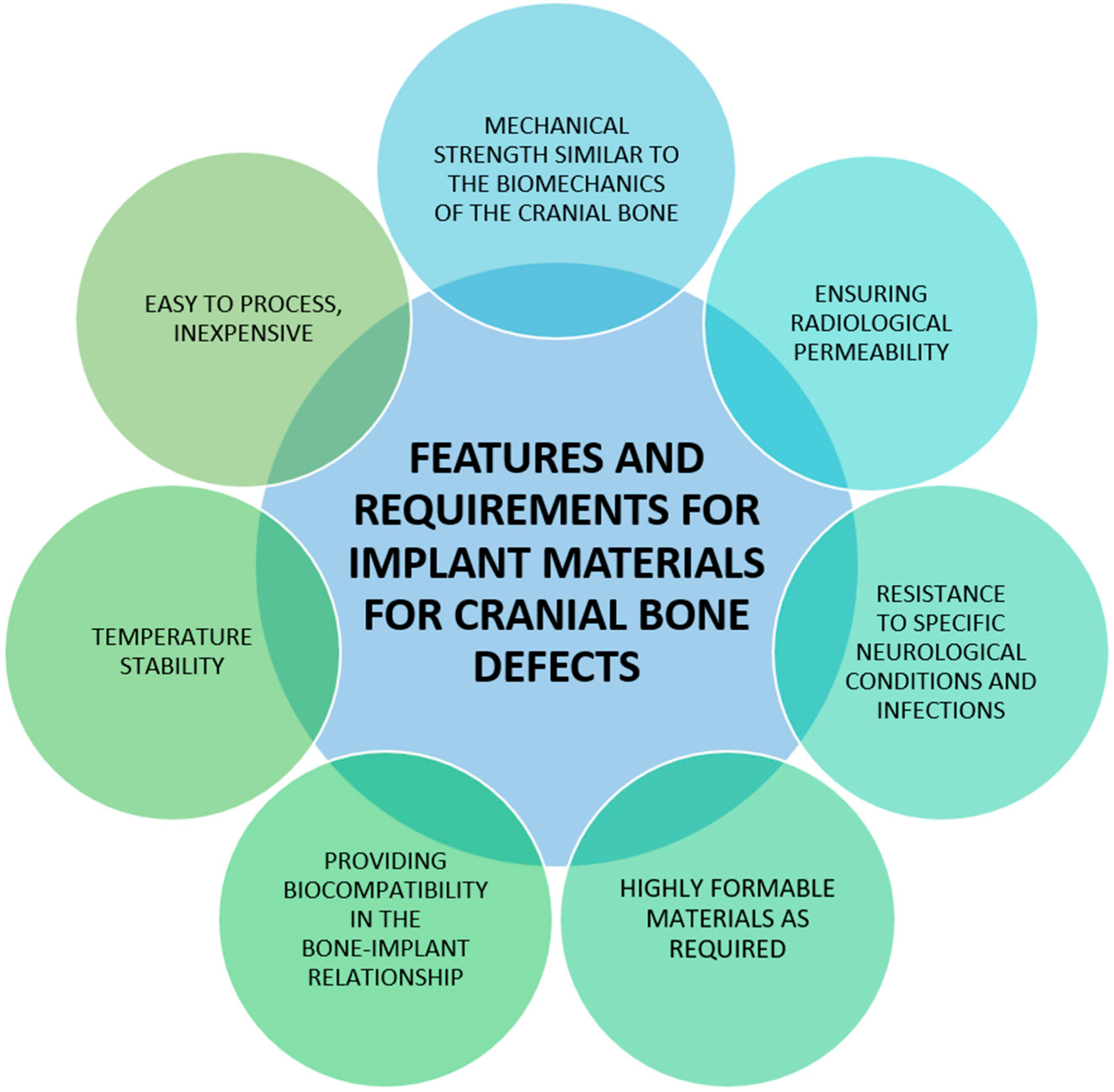
5.1. Most Popular Materials Used in Cranioplasty
5.1.1. Titanium (Ti)
5.1.2. Polyetheretherketone (PEEK)
5.1.3. Polycaprolactone (PCL)
5.1.4. Polymethyl Methacrylate (PMMA)
5.1.5. Hydroxyapatite (HA)
5.1.6. Poly(L-co-D,L-lactide) (PLDLA)
6. Innovations in Craniomaxillofacial Implants with the Addition of Hydrogel Materials
7. The Implant-Biofilm Relationship in the Perspective of Successful Treatment
7.1. Factors That Affect Biofilm Formation
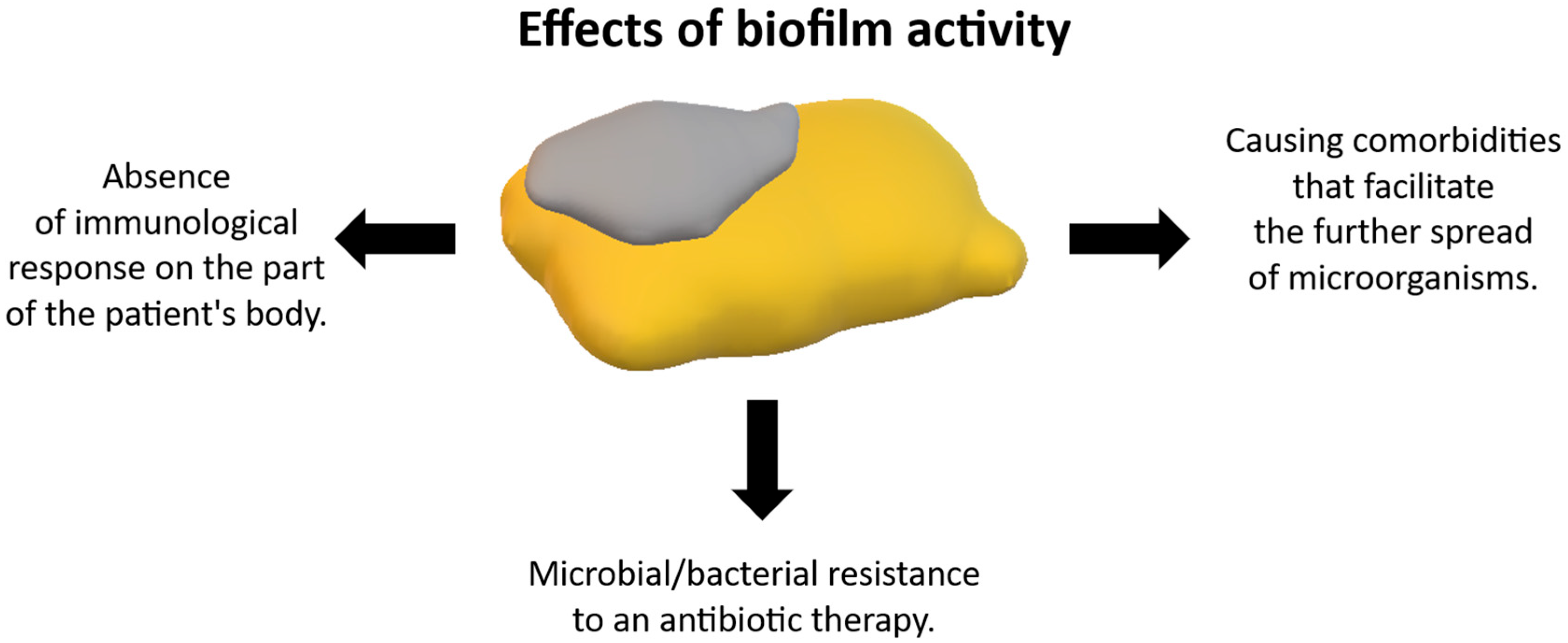
7.2. Importance of Biofilm in the Context of Treatment
8. Conclusions and Further Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lesciotto, K.M.; Richtsmeier, J.T. Craniofacial skeletal response to encephalization: How do we know what we think we know? Am. J. Phys. Anthropol. 2019, 168, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Kaucka, M.; Adameyko, I. Evolution and development of the cartilaginous skull: From a lancelet towards a human face. Semin. Cell Dev. Biol. 2019, 91, 2–12. [Google Scholar] [CrossRef]
- Kawecki, F.; Clafshenkel, W.P.; Fortin, M.; Auger, F.A.; Fradette, J. Biomimetic Tissue-Engineered Bone Substitutes for Maxillofacial and Craniofacial Repair: The Potential of Cell Sheet Technologies. Adv. Healthc. Mater. 2018, 7, 1700919. [Google Scholar] [CrossRef]
- Heuzé, Y.; Kawasaki, K.; Schwarz, T.; Schoenebeck, J.J.; Richtsmeier, J.T. Developmental and Evolutionary Significance of the Zygomatic Bone. Anat. Rec. 2016, 299, 1616–1630. [Google Scholar] [CrossRef]
- Pingue, V.; Boetto, V.; Bassetto, A.; Nava, M.; Nardone, A.; Mele, C. The Role of Decompressive Craniectomy on Functional Outcome, Mortality and Seizure Onset after Traumatic Brain Injury. Brain Sci. 2023, 13, 581. [Google Scholar] [CrossRef]
- Vitali, M.; Marasco, S.; Romenskaya, T.; Elia, A.; Longhitano, Y.; Zanza, C.; Abenavoli, L.; Scarpellini, E.; Bertuccio, A.; Barbanera, A. Decompressive Craniectomy in Severe Traumatic Brain Injury: The Intensivist’s Point of View. Diseases 2023, 11, 22. [Google Scholar] [CrossRef]
- Bhumiratana, S.; Bernhard, J.C.; Alfi, D.M.; Yeager, K.; Eton, R.E.; Bova, J.; Shah, F.; Gimble, J.M.; Lopez, M.J.; Eisig, S.B.; et al. Tissue-Engineered Autologous Grafts for Facial Bone Reconstruction. Sci. Transl. Med. 2016, 8, 343ra83. [Google Scholar] [CrossRef]
- Adebusoye, F.T.; Awuah, W.A.; Alshareefy, Y.; Wellington, J.; Mani, S.; Ahmad, A.O.; Tenkorang, P.O.; Abdul-Rahman, T.; Denys, O. Craniomaxillofacial trauma in war-torn nations: Incidence, management gaps, and recommendations. Acute Med. Surg. 2023, 10, e877. [Google Scholar] [CrossRef]
- Aghali, A. Craniofacial Bone Tissue Engineering: Current Approaches and Potential Therapy. Cells 2021, 10, 2993. [Google Scholar] [CrossRef]
- Huang, X.; Lou, Y.; Duan, Y.; Liu, H.; Tian, J.; Shen, Y.; Wei, X. Biomaterial scaffolds in maxillofacial bone tissue engineering: A review of recent advances. Bioact. Mater. 2024, 33, 129–156. [Google Scholar] [CrossRef]
- Akiki, R.K.; Crozier, J.; Basta, M.; Woo, A.S. New trends and insights in facial fracture treatment in the United States. Plast. Aesthet. Res. 2023, 10, 46. [Google Scholar] [CrossRef]
- Su, Q.; Qiao, Y.; Xiao, Y.; Yang, S.; Wu, H.; Li, J.; He, X.; Hu, X.; Yang, H.; Yong, X. Research progress of 3D printed poly (ether ether ketone) in the reconstruction of craniomaxillofacial bone defects. Front. Bioeng. Biotechnol. 2023, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ellis, D.G.; Pepe, A.; Gsaxner, C.; Aizenberg, M.R.; Kleesiek, J.; Egger, J. Back to the Roots: Reconstructing Large and Complex Cranial Defects using an Image-based Statistical Shape Model. J. Med. Syst. 2024, 48, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, J.; Jiang, Y.; Liu, S.; Zheng, J.; Li, N.; Wang, G.; Dong, F.; Chen, J.; Xie, Y.; et al. A 3D biomimetic optoelectronic scaffold repairs cranial defects. Appl. Sci. Eng. 2023, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, X.; Zhang, Z.; Zhang, X.; Wang, J.; Liu, Y.; Chen, Y.; Chen, J.; Chen, T.; Wang, Y.; et al. Biodegradable magnesium-based alloy skull repairmen (MASR) for skull bone defect: In vitro and in vivo evaluation. J. Chem. Eng. 2024, 493, 152761. [Google Scholar] [CrossRef]
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.-A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, Y.; Wu, H. Recent trends in bone defect repair and bone tissue regeneration of the two-dimensional material MXene. Ceram. Int. 2023, 49, 19578–19594. [Google Scholar] [CrossRef]
- Jie, B.; Han, B.; Yao, B.; Zhang, Y.; Liao, H.; He, Y. Automatic virtual reconstruction of maxillofacial bone defects assisted by ICP (iterative closest point) algorithm and normal people database. Clin. Oral Investig. 2022, 26, 2005–2014. [Google Scholar] [CrossRef]
- Mello-Gentil, T.; Souza-Mello, V. Contributions of anatomy to forensic sex estimation: Focus on head and neck bones. Forensci Sci. Res. 2022, 7, 11–23. [Google Scholar] [CrossRef]
- Jin, S.W.; Sim, K.B.; Kim, S.D. Development and Growth of the Normal Cranial Vault: An Embryologic Review. J. Korean Neurosurg. Soc. 2016, 59, 192–196. [Google Scholar] [CrossRef]
- Anderson, B.W.; Kortz, M.W.; Black, A.C.; Al Kharazi, K.A. Anatomy, Head and Neck, Skull. In StatePearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499834/ (accessed on 20 January 2025).
- Scarfe, W.C.; Angelopoulos, C. Maxillofacial Cone Beam Computed Tomography; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Franklin, S. The Peripheral and Central Nervous System. In Conn's Translational Neuroscience; Conn, P.M., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 113–129. [Google Scholar]
- Toneva, D.; Nikolova, S.; Tasheva-Terzieva, E.; Zlatareva, D.; Lazarov, N. A Geometric Morphometric Study on Sexual Dimorphism in Viscerocranium. Biology 2022, 11, 1333. [Google Scholar] [CrossRef]
- Dewey, M.J.; Harley, B.A.C. Biomaterial design strategies to address obstacles in craniomaxillofacial bone repair. RSC Adv. 2021, 11, 17809–17827. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Wehrhan, F.; Deschner, J.; Sander, J.; Ries, J.; Möst, T.; Bozec, A.; Gölz, L.; Kesting, M.; Lutz, R. The Special Developmental Biology of Craniofacial Tissues Enables the Understanding of Oral and Maxillofacial Physiology and Diseases. Int. J. Mol. Sci. 2021, 22, 1315. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.K.; Chen, L.W.; Ciou, J.S.; Hsiao, C.K.; Chen, Y.C. Finite Element Simulations of Bone Temperature Rise During Bone Drilling Based on a Bone Analog. J. Med. Biol. Eng. 2012, 33, 269–274. [Google Scholar] [CrossRef]
- Akhbar, M.F.A.; Yusoff, A.R. Comparison of bone temperature elevation in drilling of human, bovine and porcine bone. Procedia CIRP 2019, 82, 411–414. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, L.; Liu, Q.; Feng, L.; Wang, Z.; Ohrndorf, A.; Christ, H.J.; Xiong, J. Impact response and energy absorption of human skull cellular bones. J. Mech. Behav. Biomed. Mater. 2018, 81, 106–119. [Google Scholar] [CrossRef]
- Ferguson, J.W.; Atit, R.P. A tale of two cities: The genetic mechanisms governing calvarial bone development. Genesis 2019, 57, e23248. [Google Scholar] [CrossRef]
- Maier, W. Biomaterials in skull base surgery. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2011, 8, Doc07. [Google Scholar] [CrossRef]
- Zapata, U.; Wang, Q. Material properties of the skull layers of the primate parietal bone: A single-subject study. PLoS ONE 2020, 15, e0229244. [Google Scholar] [CrossRef]
- He, L. Biomaterials for Regenerative Cranioplasty: Current State of Clinical Application and Future Challenges. J. Funct. Biomater. 2024, 15, 84. [Google Scholar] [CrossRef]
- Jin, H.B.; Chung, J.H.; Kim, K.S.; Kim, S.H.; Choe, J.; Yang, J.Y. Reconstruction of temporal hollowing using two alloplastic materials simultaneously with titanium mesh and a silicone implant. Arch. Aesthetic Plast. Surg. 2019, 25, 37–41. [Google Scholar] [CrossRef]
- Chattopadhyay, C. Reconstruction of Acquired Frontal Bone Defects Using Titanium Mesh Implants: A Retrospective Study. Oral Surg. 2019, 18, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, S.; Ding, J.; Zhang, Y.; Deng, L.; Yao, Y.; Xiong, Y.; Chen, Y.; Wang, L.; Liu, Y.. Complex Frontal Bone Reconstruction Using Computer-designed Polyetheretherketone Implant: Case Report and Literature Review. PRS Global Open 2024, 12, e6007. [Google Scholar] [CrossRef] [PubMed]
- Fernandes da Silva, A.L.; Borba, A.M.; Simão, N.R.; Pedro, F.L.M.; Borges, A.H.; Miloro, M. Customized Polymethyl Methacrylate Implants for the Reconstruction of Craniofacial Osseous Defects. Case Rep. Surg. 2014, 2014, 358569. [Google Scholar] [CrossRef]
- Saponaro, G.; Paolantonio, C.; Barbera, G.; Foresta, E.; Gasparini, G.; Moro, A. Our problems and observations in 3D facial implant planning. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 32. [Google Scholar] [CrossRef]
- Wu, C.T.; Yang, Y.H.; Chang, Y.Z. Creating high-resolution 3D cranial implant geometry using deep learning techniques. Front. Bioeng. Biotechnol. 2023, 11, 1297933. [Google Scholar] [CrossRef]
- Safali, S.; Berk, T.; Makelov, B.; Acar, M.A.; Gueorguiev, B.; Pape, H.-C. The Possibilities of Personalized 3D Printed Implants—A Case Series Study. Medicina 2023, 59, 249. [Google Scholar] [CrossRef]
- Li, Z.; Lu, M.; Zhang, Y.; Wang, J.; Wang, Y.; Gong, T.; He, X.; Luo, Y.; Zhou, Y.; Min, L.; et al. 3D-Printed Personalized Lattice Implant as an Innovative Strategy to Reconstruct Geographic Defects in Load-Bearing Bones. Orthop. Surg. 2024, 16, 821–829. [Google Scholar] [CrossRef]
- Zhang, M.; Qi, M.; Yuan, K.; Liu, H.; Ren, J.; Liu, A.; Yao, S.; Guo, X.; Li, X.; Zhang, H. Integrated porous polyetheretherketone implants for treating skull defect. J. Mater. Res. Technol. 2023, 22, 728–734. [Google Scholar] [CrossRef]
- Vidal, L.; Kampleitner, C.; Brennan, M.Á.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef]
- Verbist, M.; Vandevelde, A.-L.; Geusens, J.; Sun, Y.; Shaheen, E.; Willaert, R. Reconstruction of Craniomaxillofacial Bone Defects with 3D-Printed Bioceramic Implants: Scoping Review and Clinical Case Series. J. Clin. Med. 2024, 13, 2805. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, J.G.; Mahmooth, Z.; Rindler, R.S.; Allen, J.W.; Grossberg, J.A.; Pradilla, G.; Ahmad, F.U. Autologous Cranioplasty is Associated with Increased Reoperation Rate: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 116, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Alkhaibary, A.; Alharbi, A.; Alnefaie, N.; Almubarak, A.O.; Aloraidi, A.; Khairy, S. Cranioplasty: A Comprehensive Review of the History, Materials, Surgical Aspects, and Complications. World Neurosurg. 2020, 139, 445–452. [Google Scholar] [CrossRef]
- Siracusa, V.; Maimone, G.; Antonelli, V. State-of-Art of Standard and Innovative Materials Used in Cranioplasty. Polymers 2021, 13, 1452. [Google Scholar] [CrossRef]
- Johnston, D.T.; Lohmeier, S.J.; Langdell, H.C.; Pyfer, B.J.; Komisarow, J.; Powers, D.B.; Erdmann, D. Current Concepts in Cranial Reconstruction: Review of Alloplastic Materials. PRS Global Open 2022, 10, e4466. [Google Scholar] [CrossRef]
- Arafat, S.W.; Ibrahim, W.H.; Shaker, S.; AlDainy, D.G.; Salama, D.; Shaheen, H.A. Reconstruction of Cranial Bone Defects Using Polyamide 12 Patient-Specific Implant: Long Term Follow Up. J. Craniofac. Surg. 2022, 33, 1825–1828. [Google Scholar] [CrossRef]
- Bečulić, H.; Spahić, D.; Begagić, E.; Pugonja, R.; Skomorac, R.; Jusić, A.; Selimović, E.; Mašović, A.; Pojskić, M. Breaking Barriers in Cranioplasty: 3D Printing in Low and Middle-Income Settings—Insights from Zenica, Bosnia and Herzegovina. Medicina 2023, 59, 1732. [Google Scholar] [CrossRef]
- Aydin, S.; Kucukyuruk, B.; Abuzayed, B.; Aydin, S.; Sanus, G.Z. Cranioplasty: Review of materials and techniques. J. Neurosci. Rural Pract. 2011, 2, 162–167. [Google Scholar] [CrossRef]
- Marew, T.; Birhanu, G. Three dimensional printed nanostructure biomaterials for bone tissue engineering. Regen. Ther. 2021, 18, 102–111. [Google Scholar] [CrossRef]
- Toosi, S.; Javid-Naderi, M.J.; Tamayol, A.; Ebrahimzadeh, M.H.; Yaghoubian, S.; Shaegh, S.A.M. Additively manufactured porous scaffolds by design for treatment of bone defects. Front. Bioeng. Biotechnol. 2024, 11, 1252636. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Chen, S.; Siu, M.F.F.; Liu, C.; Bai, J.; Wang, M. Enhancing bone regeneration through 3D printed biphasic calcium phosphate scaffolds featuring graded pore sizes. Bioact. Mater. 2025, 46, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Samourides, A.; Browning, L.; Hearnden, V.; Chen, B. The effect of porous structure on the cell proliferation, tissue ingrowth and angiogenic properties of poly(glycerol sebacate urethane) scaffolds. Mater. Sci. Eng. C 2020, 108, 110384. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, 96–101. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Paltanea, G.; Manescu, V.; Antoniac, I.; Antoniac, A.; Nemoianu, I.V.; Robu, A.; Dura, H. A Review of Biomimetic and Biodegradable Magnetic Scaffolds for Bone Tissue Engineering and Oncology. Int. J. Mol. Sci. 2023, 24, 4312. [Google Scholar] [CrossRef]
- Shen, C.; Witek, L.; Flores, R.L.; Tovar, N.; Torroni, A.; Coelho, P.G.; Kasper, F.K.; Wong, M.; Young, S. Three-Dimensional Printing for Craniofacial Bone Tissue Engineering. Tissue Eng. Part A 2020, 26, 1303–1311. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Lin, C.T.; Yu, Y.H.; Chou, Y.C.; Liu, S.J.; Chan, E.C. Dual delivery of active antibactericidal agents and bone morphogenetic protein at sustainable high concentrations using biodegradable sheath-core-structured drug-eluting nanofibers. Int. J. Nanomedicine 2016, 11, 3927–3937. [Google Scholar] [CrossRef]
- Farazin, A.; Mahjoubi, S. Dual-functional Hydroxyapatite scaffolds for bone regeneration and precision drug delivery. J. Mech. Behav. Biomed. Mater. 2024, 157, 106661. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Y.; Tang, L.; Yin, Z.; Shi, J.; Wu, X.; Xu, Y. Dual-functional composite hydrogel platform: A “kill two birds with one stone” strategy for anti-infection and osseointegration in the treatment of infectious bone defects. Chem. J. Eng. 2024, 498, 155337. [Google Scholar] [CrossRef]
- Lai, X.; Huang, J.; Huang, S.; Wang, J.; Zheng, Y.; Luo, Y.; Tang, L.; Gao, B.; Tang, Y. Antibacterial and Osteogenic Dual-Functional Micronano Composite Scaffold Fabricated via Melt Electrowriting and Solution Electrospinning for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2024, 16, 37707–37721. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, H.; Tian, Y.; Fan, Y.; Li, S.; Wang, G.; Wang, Y.; Peng, C.; Wu, D. Dual-functional composite scaffolds for inhibiting infection and promoting bone regeneration. Mater. Today Bio 2022, 16, 100409. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhou, L.; Geng, X.; Zhang, H.; Wang, B.; Ning, B. New dual-function in situ bone repair scaffolds promote osteogenesis and reduce infection. J. Biol. Eng. 2022, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Bassous, N.; Harb, S.; Palo-Nieto, C.; Ruiz-Esparza, G.U.; Marciano, F.R.; Webster, T.J.; Furtado, A.S.A.; Lobo, A.O. Advances in dual functional antimicrobial and osteoinductive biomaterials for orthopaedic applications. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102143. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chu, L.; Yang, S.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; Tang, T. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater. 2018, 79, 265–275. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Guo, J.; Wu, H.; Wang, J.; Wu, G. Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects. Int. J. Mol. Sci. 2016, 17, 334. [Google Scholar] [CrossRef]
- Rather, H.A.; Jhala, D.; Vasita, R. Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering. Mater. Sci. Eng. C 2019, 103, 109761. [Google Scholar] [CrossRef]
- Tan, W.; Gao, C.; Feng, P.; Liu, Q.; Liu, C.; Wang, Z.; Deng, Y.; Shuai, C. Dual-functional scaffolds of poly(L-lactic acid)/nanohydroxyapatite encapsulated with metformin: Simultaneous enhancement of bone repair and bone tumor inhibition. Mater. Sci. Eng. C 2021, 120, 111592. [Google Scholar] [CrossRef]
- Selvaraj, S.; Dorairaj, J.; Shivasankar, M. 3D cranial reconstruction using titanium implant—A case report. Afr. Health Sci. 2022, 22, 383–390. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, Q.; Wang, J.; Chen, X.; Hao, Y.; Zhang, Y.; Wang, X. The unfavorable role of titanium particles released from dental implants. Nanotheranostics 2021, 5, 321–332. [Google Scholar] [CrossRef]
- Kim, C.N.T.; Binh, C.X.; Dung, V.T.; Toan, T.V. Design and mechanical evaluation of a large cranial implant and fixation parts. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2023, 31, 101676. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, C.; Luo, D.; Zhang, X.; Zhou, K. Development of a Low-Density and High-Strength Titanium Alloy. Metals 2023, 13, 251. [Google Scholar] [CrossRef]
- Yang, J.; Sun, T.; Yuan, Y.; Li, X.; Yu, H.; Guan, J. Evaluation of titanium cranioplasty and polyetheretherketone cranioplasty after decompressive craniectomy for traumatic brain injury A prospective, multicenter, non-randomized controlled trial. Medicine 2020, 99, e21251. [Google Scholar] [CrossRef] [PubMed]
- Avery, D.; Morandini, L.; Celt, N.; Bergey, L.; Simmons, J.; Martin, R.K.; Donahue, H.J.; Olivares-Navarrete, R. Immune cell response to orthopedic and craniofacial biomaterials depends on biomaterial composition. Acta Biomater. 2023, 161, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.S.; Bordbar-Khiabani, A.; Khalil-Allafi, J.; Mozafari, M.; Visai, L. Additive Manufacturing: An Opportunity for the Fabrication of Near-Net-Shape NiTi Implants. J. Manuf. Mater. Process. 2022, 6, 65. [Google Scholar] [CrossRef]
- Jahadakbar, A.; Shayesteh Moghaddam, N.; Amerinatanzi, A.; Dean, D.; Karaca, H.E.; Elahinia, M. Finite Element Simulation and Additive Manufacturing of Stiffness-Matched NiTi Fixation Hardware for Mandibular Reconstruction Surgery. Bioengineering 2016, 3, 36. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, D.; Jayamohan, J.; Sotiropoulas, S.N.; Yoon, S.H.; Cook, J.; Siviour, C.R.; Arias, A.; Jérusalem, A. On the mechanical behaviour of PEEK and HA cranial implants under impact loading. J. Mech. Behav. Biomed. Mater. 2017, 69, 342–354. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Polyetheretherketone and Its Composites for Bone Replacement and Regeneration. Polymers 2020, 12, 2858. [Google Scholar] [CrossRef]
- Zhang, J.; Su, Y.; Rao, X.; Pang, H.; Zhu, H.; Liu, L.; Chen, L.; Li, D.; He, J.; Peng, J.; et al. Additively manufactured polyether ether ketone (PEEK) skull implant as an alternative to titanium mesh in cranioplasty. Int. J. Bioprint. 2023, 9, 173–180. [Google Scholar] [CrossRef]
- Moiduddin, K.; Mian, S.H.; Elseufy, S.M.; Alkhalefah, H.; Ramalingam, S.; Sayeed, A. Polyether-Ether-Ketone (PEEK) and Its 3D-Printed Quantitate Assessment in Cranial Reconstruction. J. Funct. Biomater. 2023, 14, 429. [Google Scholar] [CrossRef]
- Mulliken, G.H.; Bichot, N.P.; Ghadooshahy, A.; Sharma, J.; Kornblith, S.; Philcock, M.; Desimone, R. Custom-fit radiolucent cranial implants for neurophysiological recording and stimulation. J. Neurosci. Methods. 2016, 15, 146–154. [Google Scholar] [CrossRef]
- Jindal, P.; Chaitanya; Bharadwaja, S.S.S.; Rattra, S.; Pareek, D.; Gupta, V.; Breedon, P.; Reinwald, Y.; Juneja, M. Optimizing cranial implant and fixture design using different materials in cranioplasty. Proc. Inst. Mech. Eng. Pt. L J. Mater. Des. Appl. 2023, 237, 107–121. [Google Scholar] [CrossRef]
- Cárdenas-Serres, C.; Almeida-Parra, F.; Simón-Flors, A.M.; de Leyva-Moreno, P.; Ranz-Colio, Á.; Ley-Urzaiz, L.; Acero-Sanz, J. Custom CAD/CAM Peek Implants for Complex Orbitocranial Reconstruction: Our Experience with 15 Patients. J. Clin. Med. 2024, 13, 695. [Google Scholar] [CrossRef] [PubMed]
- Hashim, H.b.; Emran, N.A.A.b.; Isono, T.; Katsuhara, S.; Ninoyu, H.; Matsushima, T.; Yamamoto, T.; Borsali, R.; Satoh, T.; Tajima, K. Improving the mechanical properties of polycaprolactone using functionalized nanofibrillated bacterial cellulose with high dispersibility and long fiber length as a reinforcement material. Compos.-A Appl. Sci. Manuf. 2022, 158, 106978. [Google Scholar] [CrossRef]
- Kurowiak, J. Comparison of two polymers PDO and PLLA/PCL in application of urological stent for the treatment of male urethral stenosis. Acta Bioeng Biomech. 2024, 26, 3–12. [Google Scholar] [CrossRef]
- Alharbi, N.; Guthold, M. Mechanical properties of hydrated electrospun polycaprolactone (PCL) nanofibers. J. Mech. Behav. Biomed. Mater. 2024, 155, 106564. [Google Scholar] [CrossRef]
- Volokhova, A.A.; Kudryavtseva, V.L.; Spiridonova, T.I.; Kolesnik, I.; Goreninskii, S.I.; Sazonova, R.V.; Remnev, G.E.; Tverdokhlebov, S.I. Controlled drug release from electrospun PCL non-woven scaffolds via multi-layering and e-beam treatment. Mater. Today Commun. 2021, 26, 102134. [Google Scholar] [CrossRef]
- Abdullah, T.; Gauthaman, K.; Mostafavi, A.; Alshahrie, A.; Salah, N.; Morganti, P.; Chianese, A.; Tamayol, A.; Memic, A. Sustainable drug release from polycaprolactone coated chitin-lignin gel fibrous scaffolds. Sci. Rep. 2020, 10, 20428. [Google Scholar] [CrossRef]
- Gharibshahian, M.; Salehi, M.; Beheshtizadeh, N.; Kamalabadi-Farahani, M.; Atashi, A.; Nourbakhsh, M.S.; Alizadeh, M. Recent advances on 3D-printed PCL-based composite scaffolds for bone tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1168504. [Google Scholar] [CrossRef]
- Emadi, H.; Baghani, M.; Masoudi Rad, M.; Hoomehr, B.; Baniassadi, M.; Lotfian, S. 3D-Printed Polycaprolactone-Based Containing Calcium Zirconium Silicate: Bioactive Scaffold for Accelerating Bone Regeneration. Polymers 2024, 16, 1389. [Google Scholar] [CrossRef]
- Kim, S.-C.; Heo, S.-Y.; Oh, G.-W.; Yi, M.; Jung, W.-K. A 3D-Printed Polycaprolactone/Marine Collagen Scaffold Reinforced with Carbonated Hydroxyapatite from Fish Bones for Bone Regeneration. Mar. Drugs 2022, 20, 344. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Koons, G.L.; Mikos, A.G.; Qiu, Z.; Song, T.; Cui, F.; Wang, X. Tuning pore features of mineralized collagen/PCL scaffolds for cranial bone regeneration in a rat model. Mater. Sci. Eng. C 2020, 106, 110186. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.-S.; Kim, Y.-C.; Min, J.-C.; Park, H.-J.; Lee, E.-J.; Shim, J.-H.; Choi, J.-W. Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects. Polymers 2022, 14, 740. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, D.; Shim, J.H.; Nam, W. Efficacy of three-dimensionally printed polycaprolactone/beta tricalcium phosphate scaffold on mandibular reconstruction. Sci. Rep. 2020, 10, 4979. [Google Scholar] [CrossRef]
- Abd El-Ghani, W.M.A. Cranioplasty with polymethyl methacrylate implant: Solutions of pitfalls. Egypt. J. Neurosurg. 2018, 33, 7. [Google Scholar] [CrossRef][Green Version]
- Moncayo-Matute, F.P.; Torres-Jara, P.B.; Vázquez-Silva, E.; Peña-Tapia, P.G.; Moya-Loaiza, D.P.; Abad-Farfán, G. Finite element analysis of a customized implant in PMMA coupled with the cranial bone. J. Mech. Behav. Biomed. Mater. 2023, 146, 106046. [Google Scholar] [CrossRef]
- Leão, R.S.; Maior, J.R.S.; Lemos, C.A.A.; Vasconcelos, B.C.D.E.; Montes, M.A.J.R.; Pellizzer, E.P.; Moraes, S.L.D. Complications with PMMA compared with other materials used in cranioplasty: A systematic review and meta-analysis. Braz. Oral Res. 2018, 32, e31. [Google Scholar] [CrossRef]
- Shash, Y.H. Assessment of cranial reconstruction utilizing various implant materials: Finite element study. J. Mater. Sci: Mater. Med. 2024, 35, 50. [Google Scholar] [CrossRef]
- Bruno, Z.; Angelo, N.; Riccardo, S.; Nicola, Z.; Stefano, P.; Camillo, P.P.; Federico, N.; Carlotta, M. Custom-made Hydroxyapatite Cranioplasty: Radiological and Histological Evidence of Bone-Biomaterial Osteointegration in Five Patients. Asian J. Neurosurg. 2020, 15, 198–203. [Google Scholar] [CrossRef]
- Zaed, I.; Cardia, A.; Stefini, R. From Reparative Surgery to Regenerative Surgery: State of the Art of Porous Hydroxyapatite in Cranioplasty. Int. J. Mol. Sci. 2022, 23, 5434. [Google Scholar] [CrossRef]
- Mannella, F.C.; Faedo, F.; Fumagalli, M.; Norata, G.D.; Zaed, I.; Servadei, F. Long-Term Follow-Up of Custom-Made Porous Hydroxyapatite Cranioplasties: Analysis of Infections in Adult and Pediatric Patients. J. Clin. Med. 2024, 13, 1133. [Google Scholar] [CrossRef]
- Messias, A.D.; Martins, K.F.; Motta, A.C.; Aparecida de Rezende Duek, E. Synthesis, Characterization, and Osteoblastic Cell Culture of Poly(L-co-D,L-lactide-co-trimethylene carbonate) Scaffolds. Int. J. Biomater. 2014, 2014, 501789. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, F.; Gomes, R.C.; Moraes, A.S.; Antunes, B.S.L.; Motta, A.C.; Dávila, J.L.; Hausen, M.A.; Komatsu, D.; Aparecida de Rezende Duek, E. Poly(L-co-D,L-lactic acid-co-trimethylene carbonate) for extraction-based 3D printing: Comprehensive characterization and cytocompatibility assessment. Polymer 2024, 290, 126585. [Google Scholar] [CrossRef]
- Más, B.A.; Coutinho de Luna Freire, D.; Mara de Melo Cattani, S.; Motta, A.C.; Barbo, M.L.P.; Aparecida de Rezende Duek, E. Biological Evaluation of PLDLA Polymer Synthesized as onstruct on Bone Tissue Engineering Application. Mater. Res. 2016, 19, 300–307. [Google Scholar] [CrossRef]
- Silmani, M.; Baus, A.; Bich, C.S.; de Rousiers, A.; Duhoux, A.; Brachet, M.; Duhamel, P.; Bey, E. Methylmetacrylate (PMMA) cranioplasty technique: Technical interest of intraoperative modeling and review of the literature. Ann. Chir. Plast. Esthet. 2023, 68, 99–105. [Google Scholar] [CrossRef]
- Koo, H.T.; Oh, J.; Heo, C.Y. Cranioplasty Using Three-Dimensional–Printed Polycaprolactone Implant and Free Latissimus Dorsi Musculocutaneous Flap in a Patient with Repeated Wound Problem following Titanium Cranioplasty. Arch. Plast. Surg. 2022, 49, 740–744. [Google Scholar] [CrossRef]
- Hwang, K.; Villavicencio, J.B.; Agdamag, A.M.P. Tissue Engineering and Regenerative Medicine Cranioplasty Using Polycaprolactone-Tricalcium Phosphate: Management and Treatment Outcomes. Open Neurosurg. 2021, 2, okab027. [Google Scholar] [CrossRef]
- Fuchs, A.; Bartolf-Kopp, M.; Böhm, H.; Straub, A.; Kübler, A.C.; Linz, C.; Gbureck, U. Composite grafts made of polycaprolactone fiber mats and oil-based calcium phosphate cement pastes for the reconstruction of cranial and maxillofacial defects. Clin. Oral Investig. 2023, 27, 3199–3209. [Google Scholar] [CrossRef]
- Chmal-Fudali, E.; Basińska, D.; Kucharska-Jastrząbek, A.; Struszczyk, M.H.; Muzalewska, M.; Wyleżoł, M.; Wątrobiński, M.; Andrzejewski, J.; Tarzyńska, N.; Gzyra-Jagieła, K. Effect of the Advanced Cranial and Craniofacial Implant Fabrication on Their Degradation Affinity. Materials 2023, 16, 6070. [Google Scholar] [CrossRef]
- Hong, S.-O.; Pyo, J.-Y.; On, S.-W.; Seo, J.-Y.; Choi, J.-Y. The Biocompatibility and the Effect of Titanium and PEKK on the Osseointegration of Customized Facial Implants. Materials 2024, 17, 4435. [Google Scholar] [CrossRef]
- Gugliotta, Y.; Zavattero, E.; Ramieri, G.; Borbon, C.; Gerbino, G. Cranio-Maxillo-Facial Reconstruction with Polyetheretherketone Patient-Specific Implants: Aesthetic and Functional Outcomes. J. Pers. Med. 2024, 14, 849. [Google Scholar] [CrossRef]
- Chakranarayan, A.; Kumari, P.; Nagori, S.A.; Sudhan, M.D.; Menon, P.S.; Kapro, A. Effectiveness of Additive Manufactured Titanium Implants in the Reconstruction of Large Cranial Defects: Case Series and Review of Literature. J. Maxillofac. Oral Surg. 2023, 23, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Moiduddin, K.; Mian, S.H.; Umer, U.; Alkhalefah, H.; Ahmed, F.; Hashmi, F.H. Design, Analysis, and 3D Printing of a Patient-Specific Polyetheretherketone Implant for the Reconstruction of Zygomatic Deformities. Polymers 2023, 15, 886. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Ghorbani, M.; Khatoonabad, M.J.; Aghazadeh, M. A novel injectable hydrogel containing polyetheretherketone for bone regeneration in the craniofacial region. Sci. Rep. 2023, 13, 864. [Google Scholar] [CrossRef] [PubMed]
- González-Díaz, E.C.; Varghese, S. Hydrogels as Extracellular Matrix Analogs. Gels 2016, 2, 20. [Google Scholar] [CrossRef]
- Stowers, R.S. Advances in Extracellular Matrix-Mimetic Hydrogels to Guide Stem Cell Fate. Cells Tissues Organs 2022, 211, 703–720. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Gao, R.; Liu, X.; Feng, Z.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. Biomimetic glycopeptide hydrogel coated PCL/nHA scaffold for enhanced cranial bone regeneration via macrophage M2 polarization-induced osteo-immunomodulation. Biomaterials 2022, 285, 121538. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, J.; Lun, P.; Wu, Z.; Deng, W.; Sun, P. Dynamic Cross-Linking, Self-Healing, Antibacterial Hydrogel for Regenerating Irregular Cranial Bone Defects. ACS Appl. Mater. Interfaces 2024, 16, 39035–39050. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Yang, F.; Chen, X.; He, X.; Liu, Z.; Xiao, J. Bioactive poly(ethylene glycol)-chondroitin sulfate-triple helical recombinant collagen hydrogel for enhanced cranial defect repair. Collagen and Leather 2024, 6, 26. [Google Scholar] [CrossRef]
- Rozis, M.; Evangelopoulos, D.S.; Pneumaticos, S.G. Orthopedic Implant-Related Biofilm Pathophysiology: A Review of the Literature. Cureus 2021, 13, e15634. [Google Scholar] [CrossRef]
- Gajula, B.; Munnamgi, S.; Basu, S. How bacterial biofilms affect chronic wound healing: A narrative review. Int. J. Surg. Global Health 2020, 3, e16. [Google Scholar] [CrossRef]
- Gulati, K.; Ding, C.; Guo, T.; Guo, H.; Yu, H.; Liu, Y. Craniofacial therapy: Advanced local therapies from nanoengineered titanium implants to treat craniofacial conditions. Int. J. Oral Sci. 2023, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, A.; Nguyen Ngoc, D. Risks to the body caused by bacterial biofilm on biomaterials. In Biomaterials the Hope of the Future; Wessely-Szponder, J., Osmęcka, D., Domańska, A., Eds.; Publishing House of the University of Life Sciences in Lublin: Lublin, Poland, 2024; pp. 56–63. [Google Scholar]
- Fischer, H.; Steffen, C.; Schmidt-Bleek, K.; Duda, G.N.; Heiland, M.; Rendenbach, C.; Raguse, J.-D. Histological Processing of CAD/CAM Titanium Scaffold after Long-Term Failure in Cranioplasty. Materials 2022, 15, 982. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Miyata, K.; Komatsu, K.; Yamashita, K.; Wanibuchi, M.; Mikuni, N. Exposure of titanium implants after cranioplasty: A matter of long-term consequences. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2017, 8, 64–67. [Google Scholar] [CrossRef]
- de Morais, S.D.B.; Kak, G.; Menousek, J.P.; Kielian, T. Immunopathogenesis of Craniotomy Infection and Niche-Specific Immune Responses to Biofilm. Front. Immunol. 2021, 12, 625467. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Cłapa, T.; Selwet, M.; Narożna, D. Community Life—Conditions for biofilm formation. Cosmos. Problems of the biological sciences. Cosmos. Probl. Biol. Sci. 2016, 65, 463–468. [Google Scholar]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- De-la-Pinta, I.; Cobos, M.; Ibarretxe, J.; Montoya, E.; Eraso, E.; Guraya, T.; Quindós, G. Effect of biomaterials hydrophobicity and roughness on biofilm development. J. Mater. Sci. Mater. Med. 2019, 30, 77. [Google Scholar] [CrossRef]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef]
- Bowden, G.H.W.; Li, Y.H. Nutritional influences on biofilm development. Adv. Dent. Res. 1997, 11, 81–99. [Google Scholar] [CrossRef]
- Goller, C.C.; Romeo, T. Environmental influences on biofilm development. Curr. Top. Microbiol. Immunol. 2008, 322, 37–66. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Pereira, J.E.; Maltez, L.; Poeta, P.; Igrejas, G. Influence of Environmental Factors on Biofilm Formation of Staphylococci Isolated from Wastewater and Surface Water. Pathogens 2022, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Minasyan, H. Sepsis: Mechanisms of bacterial injury to the patient. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 19. [Google Scholar] [CrossRef] [PubMed]
- Azevado, M.M.; Lisboa, C.; Cobrado, L.; Pina-Vaz, C.; Rodrigues, A. Hard-to-heal wounds, biofilm and wound healing: An intricate interrelationship. Br. J. Nurs. 2020, 29, S6–S13. [Google Scholar] [CrossRef]
- Huang, D.N.; Wang, J.; Ren, K.F.; Ji, J. Functionalized Biomaterials to Combat Biofilm. Biomater. Sci. 2020, 8, 4052–4066. [Google Scholar] [CrossRef]
| Research Type | Application | Material | Conclusions | Ref. |
|---|---|---|---|---|
| Original article: a case series. | Reconstruction of bone defects in the cranial vault. | Methyl methacrylate (PMMA). | Cranioplasty—intraoperative PMMA cement patterning. | [107] |
| Original article: a case report. | Reconstruction of a large skull bone defect after a complication of cranioplasty performed with titanium mesh. | Three-dimensional 3D polycaprolactone (PCL) implant. | The implant was biocompatible, well established at the implant site with successful osteointegration. After a one-year follow-up, the implant was shown to perform both protective and esthetic functions. No complications were observed. | [108] |
| Original article: a case series. | Cranioplasty of large skull bone defects after decompressive craniectomy. | The implant is based on polycaprolactone (PCL) and tricalcium phosphate (TCP) with the addition of autologous biological material in the form of bone marrow. | The (PCL-TCP) implant with the addition of stem cells and active growth factors had a positive effect on the patients’ health. The imaging studies performed after 8 and 20 months showed ossification in the defect area. | [109] |
| Original article: research. | The composite implants for the reconstruction of cranial and maxillofacial defects. | The scaffolds based on polycaprolactone (PCL) fiber mats and calcium phosphate cement (CPC) paste. The scaffolds manufactured using 3D printing (FDM—Fused Deposition Modelling technology). | High strength with simultaneous satisfactory flexibility of the (PCL-CPC) material was demonstrated. The proposed solution can be used for facial bone substitute implants. | [110] |
| Original article: research. | The design and numerical analysis of an implant for major cranial bone defects. | The cranial implant made of (PEEK) polyetheretherketone. | The numerical and clinical studies conducted for the proposed (PEEK) implant solution showed that the scaffold fulfills its function by providing durability, reconstruction and protection of the brain, and improved esthetics. | [73] |
| Original article: research. | The effect of the used manufacturing technology on craniofacial implants including degradation time. | The implants manufactured by 3D printing method (FDM—Fused Deposition Modelling technology), based on poly(L-co-D,L-lactide) (PLDLA) with the addition of hydroxyapatite nanoparticles and implants manufactured by injection molding based on (PLDLA). | The research showed that material degradation time is significantly affected by the choice of implant manufacturing technology. It was observed that 3D printed implants degraded faster than those that were manufactured by injection molding. | [111] |
| Original article: research. | The implants dedicated to mandibular bone defects. | Implants based on titanium and polyetheretherketone (PEEK). | The manufactured implants demonstrated biocompatibility and promoted osteointegration. The proposed treatment method enables bone regeneration, which, in retrospect, may have a positive impact on preventing mandibular bone atrophy caused by aging patients. | [112] |
| Original article: a case report. | The treatment of cranio-maxillofacial defects tailored to the individual patient’s functional and esthetic needs. High percentage of defects caused by excision of tumors. | The implants are based on polyetheretherketone (PEEK). | The high success rate of implanted treatment of bone defects with (PEEK) implants after tumor resection. This study showed no implant failures (ruptures, dislocations). | [113] |
| Original article: a case series and review of literature. | Reconstruction of Large Cranial Defects. | Titanium implants manufactured using the additive method. | The use of additively manufactured titanium implants to treat large cranial bone defects is considered a good solution. The titanium implants are appropriate for bone reconstruction. | [114] |
| Original article: research. | The reconstruction of a zygomatic bone defect with an incrementally manufactured implant. | The implant based on polyetheretherketone (PEEK), manufactured using method 3D printing (FFF—Fused Filament Fabrication technology). | The experimental studies have shown that the proposed implant solution, together with the appropriate choice of material, is capable of handling heavy loads as well. | [115] |
| Original article: research. | Development of a porous NiTi fixation plate for mandibular bone reconstruction. | Implant based on NiTi manufactured by selective laser melting. | The developed finite element model for the porous NiTi fixation plate showed, proper stress distribution and proper fit of the plate to the bone graft. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurowiak, J.; Piesik, K.; Klekiel, T. Current State of Knowledge Regarding the Treatment of Cranial Bone Defects: An Overview. Materials 2025, 18, 2021. https://doi.org/10.3390/ma18092021
Kurowiak J, Piesik K, Klekiel T. Current State of Knowledge Regarding the Treatment of Cranial Bone Defects: An Overview. Materials. 2025; 18(9):2021. https://doi.org/10.3390/ma18092021
Chicago/Turabian StyleKurowiak, Jagoda, Krystian Piesik, and Tomasz Klekiel. 2025. "Current State of Knowledge Regarding the Treatment of Cranial Bone Defects: An Overview" Materials 18, no. 9: 2021. https://doi.org/10.3390/ma18092021
APA StyleKurowiak, J., Piesik, K., & Klekiel, T. (2025). Current State of Knowledge Regarding the Treatment of Cranial Bone Defects: An Overview. Materials, 18(9), 2021. https://doi.org/10.3390/ma18092021







