The Effect of Pulse Frequency on the Microstructure and Corrosion Resistance of an AZ31B Magnesium Alloy Composite Coating with Electron-Beam Remelting and Micro-Arc Oxidation
Abstract
1. Introduction
2. Experimental Details
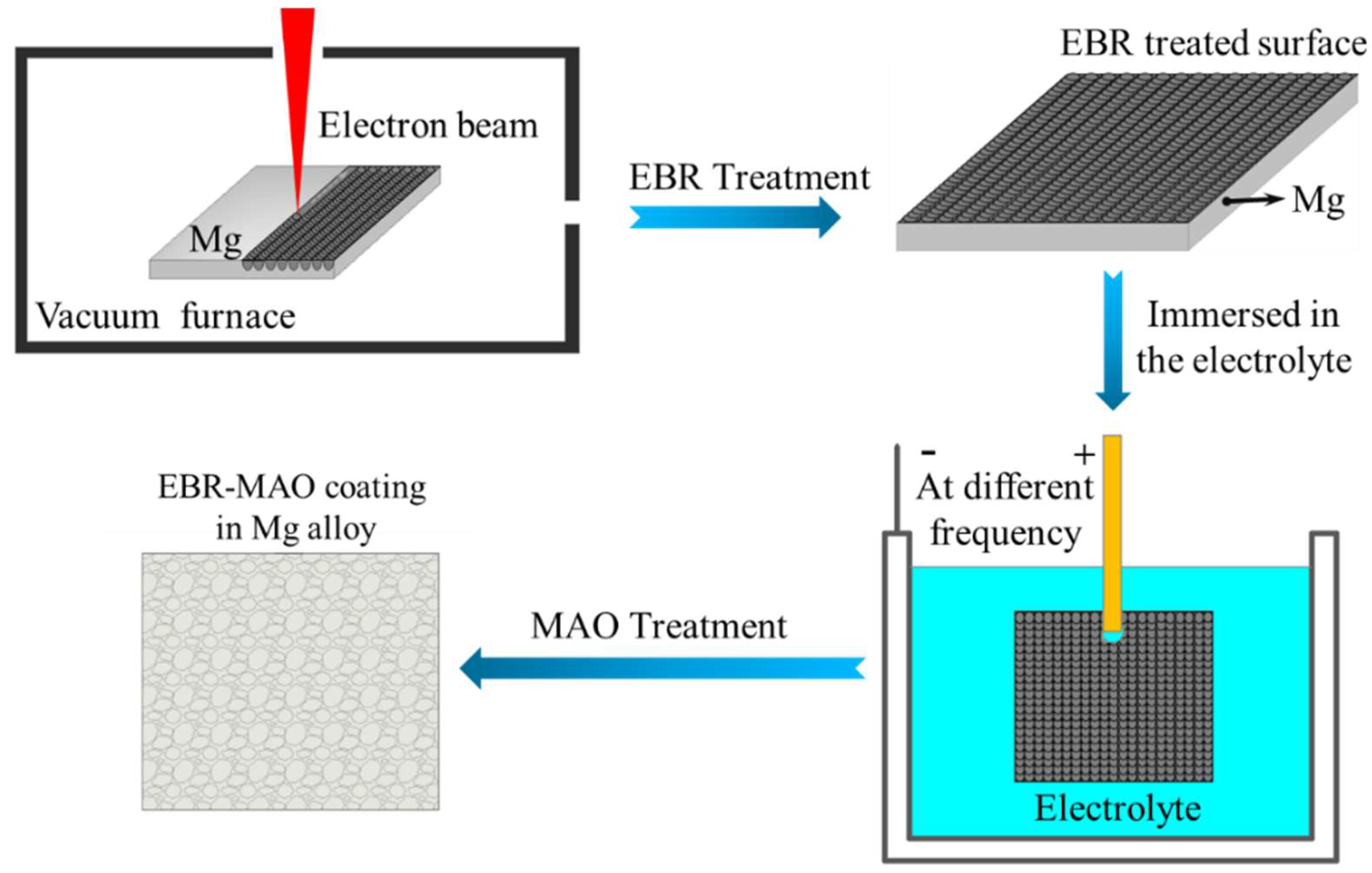
2.1. Preparation of EBR AZ31B Magnesium Alloy Samples
2.2. Preparation of EBR-MAO Composite Coatings
2.3. Characterization
3. Results and Discussion
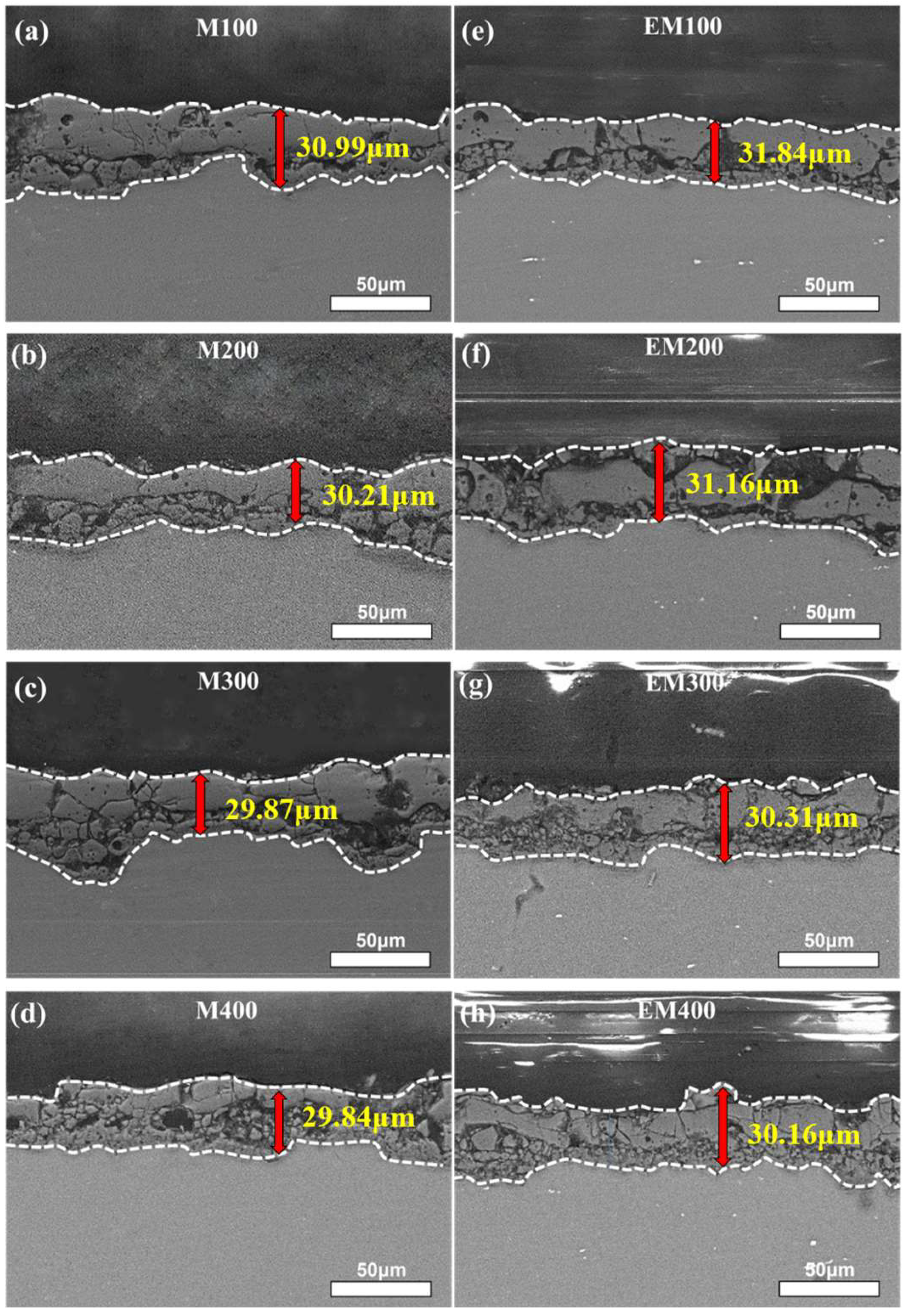
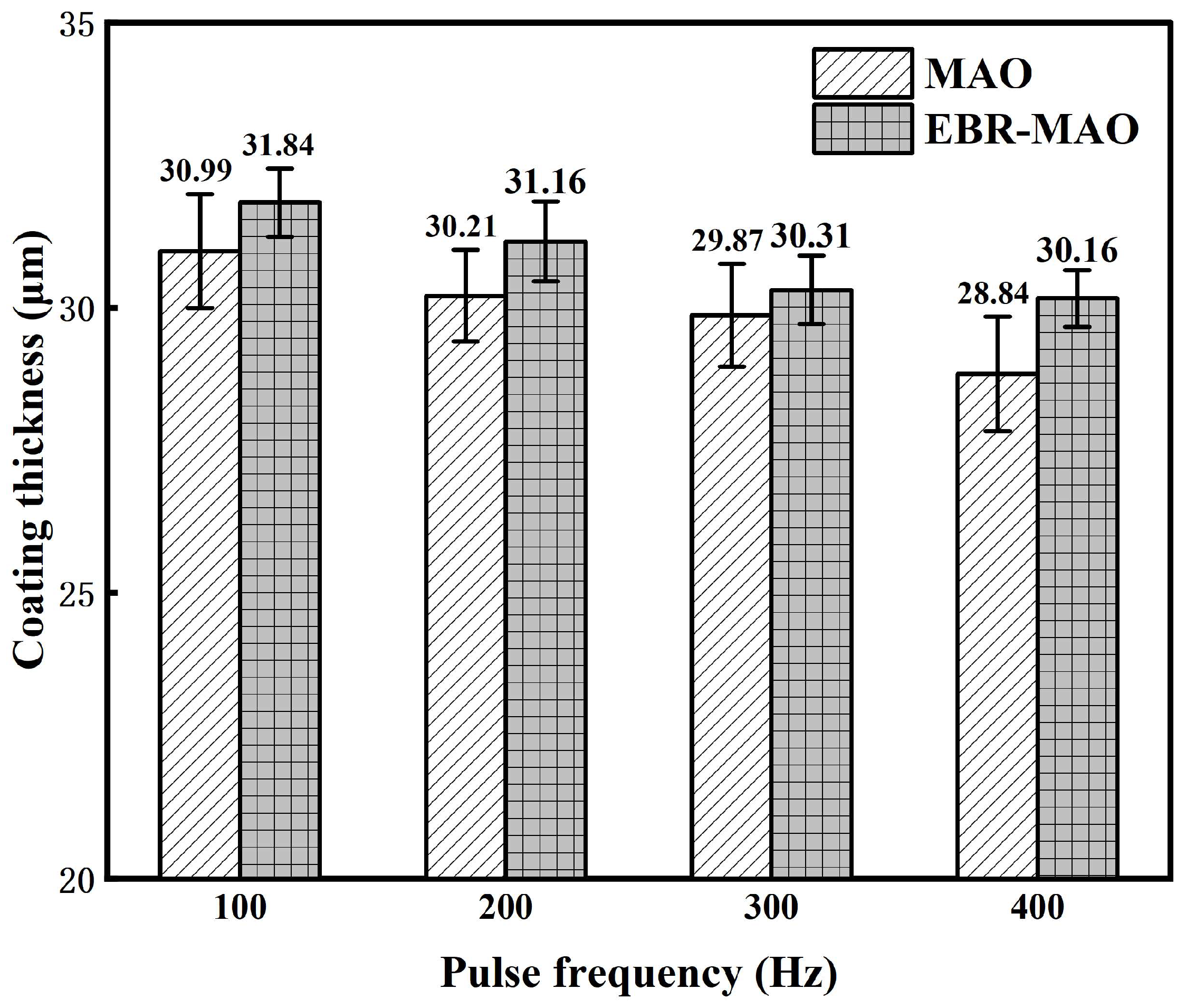
3.1. Cross-Sectional Morphology and Thickness of the Composite Coating
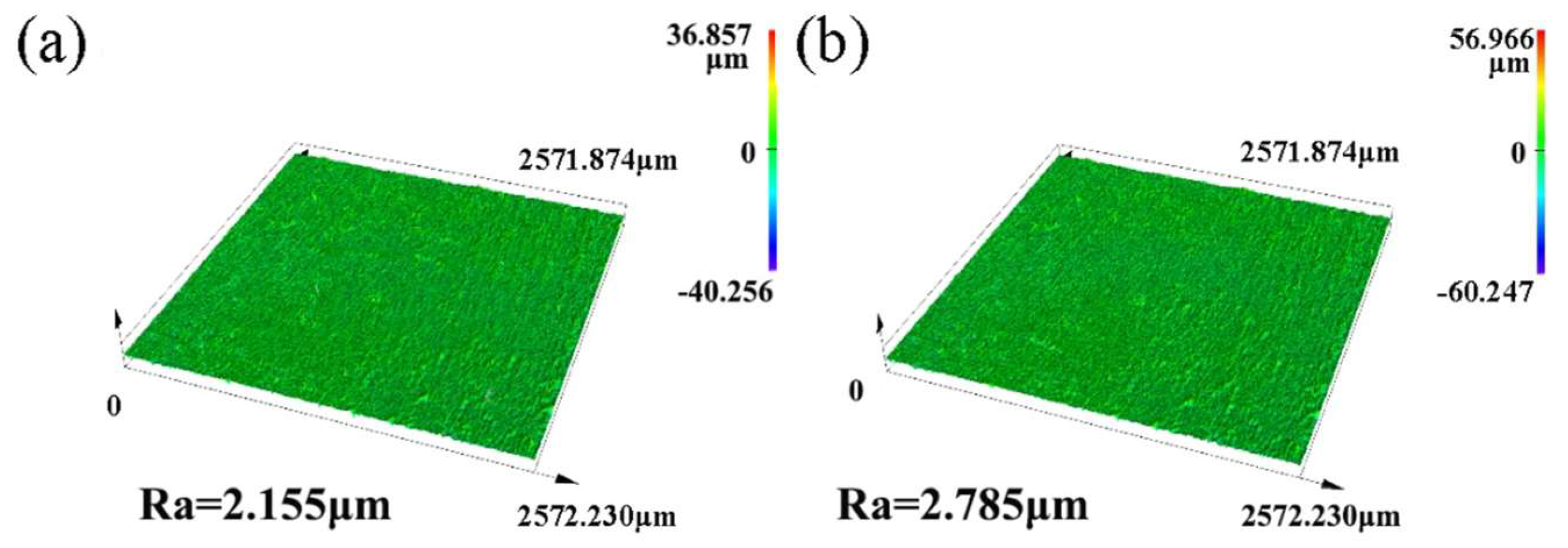
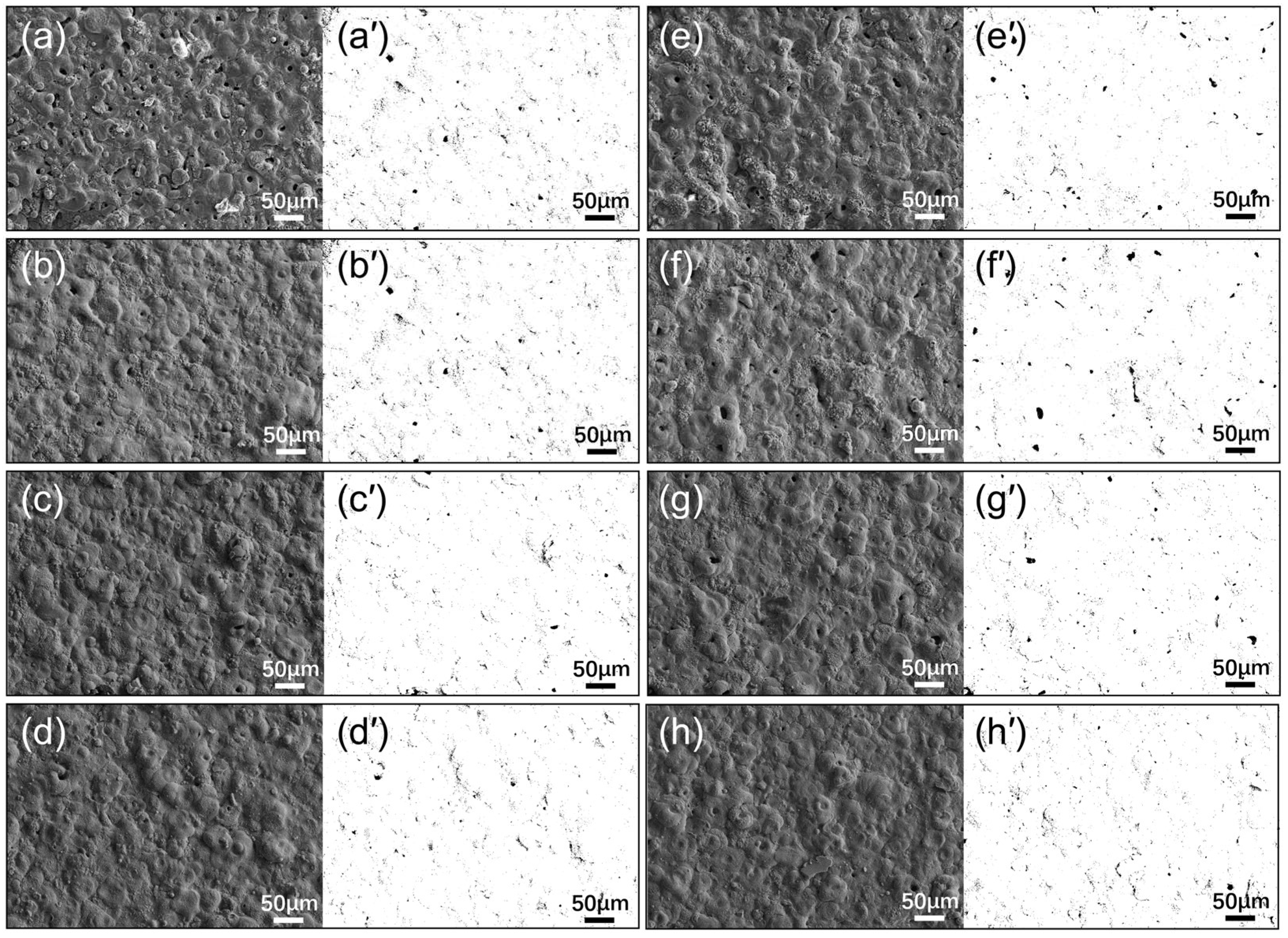
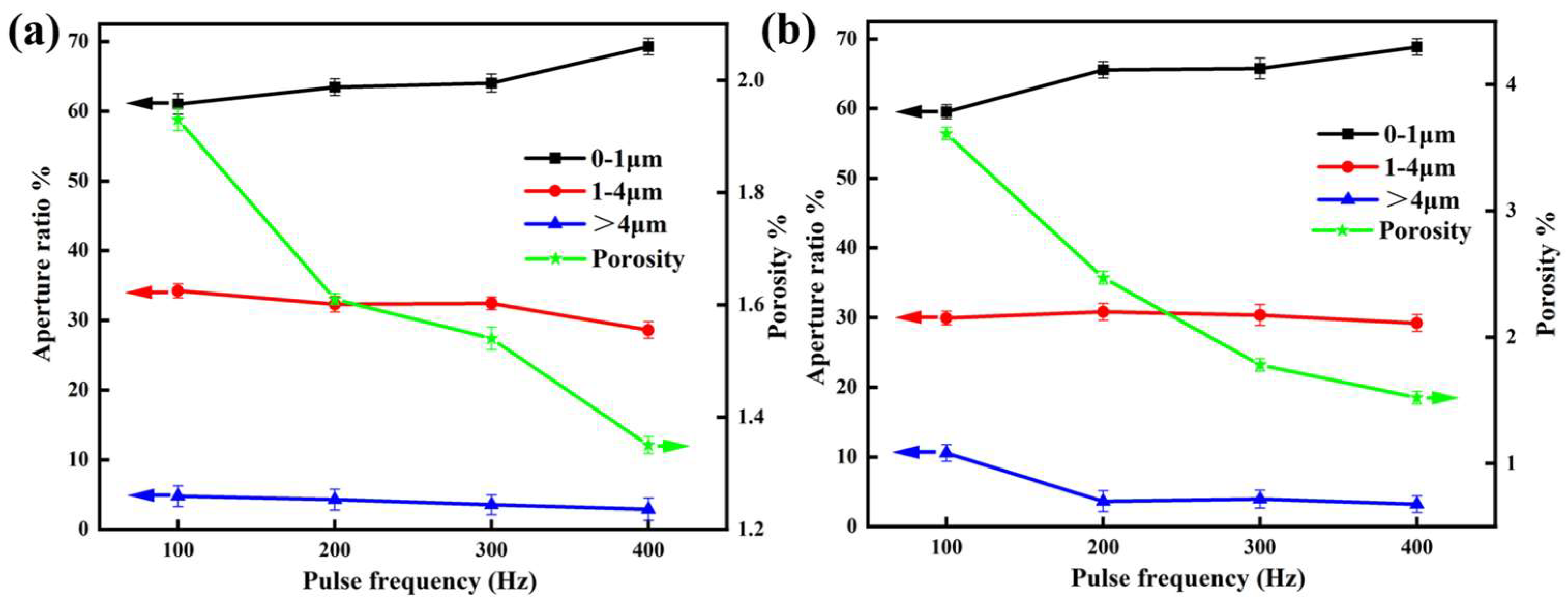
3.2. Surface Morphology of the Composite Coating
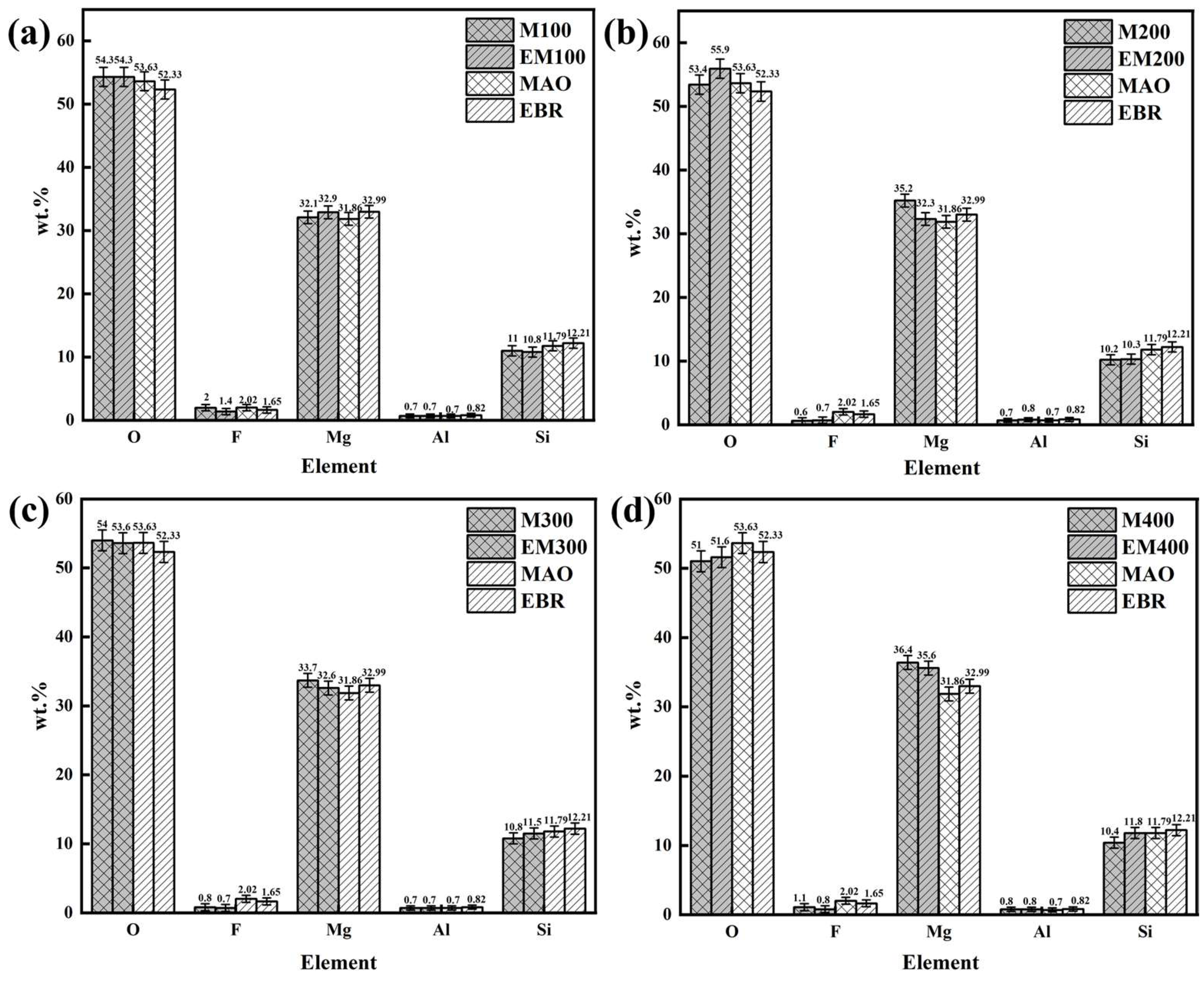
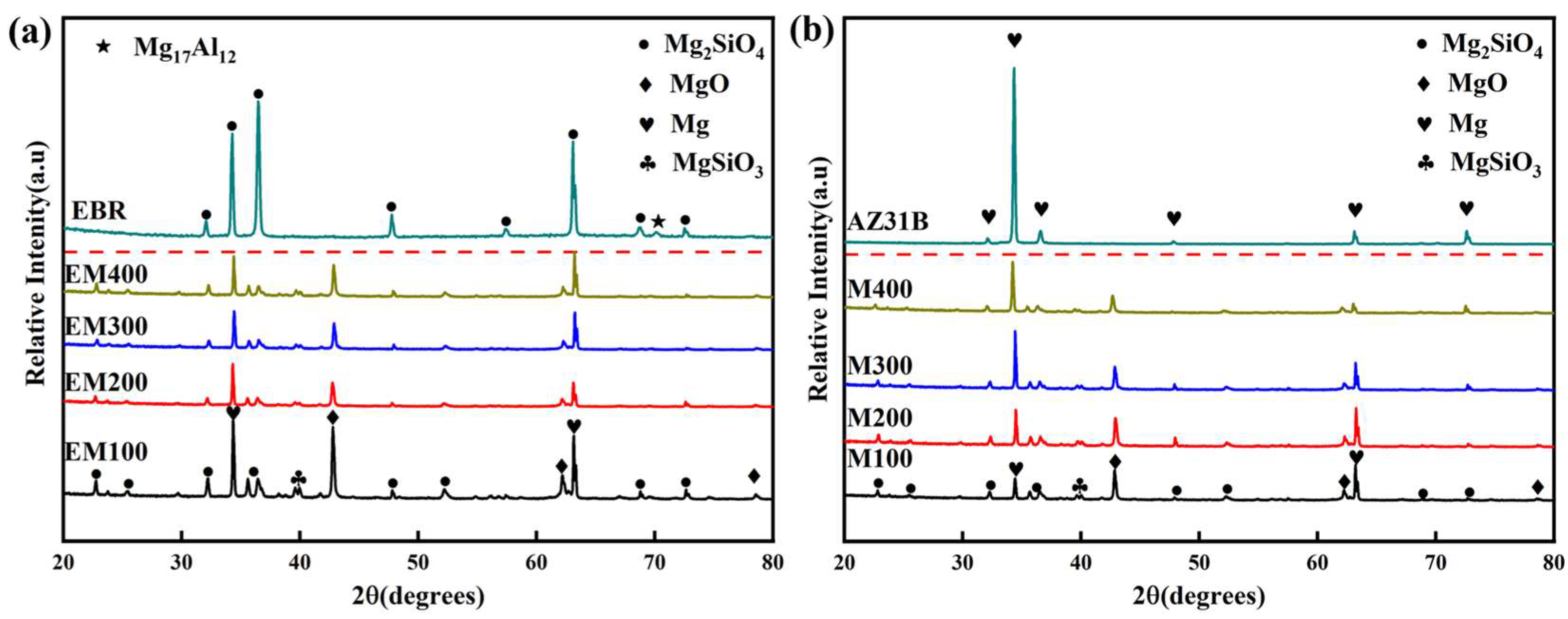
3.3. Composition of the Composite Coating
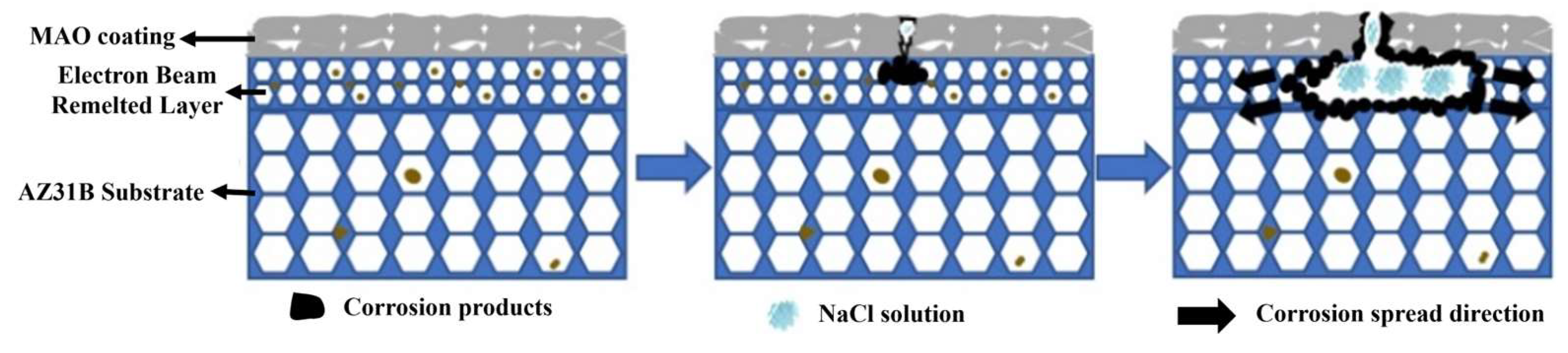
3.4. Corrosion Resistance of the Composite Coating
4. Conclusions
- (1)
- Pulse frequency significantly influences the morphology of MAO and EBR-MAO coatings. As the pulse frequency increases, the proportion of micropores (0–1 μm) on the surface of EBR-MAO coatings rises from 61.03% to 69.27%, while the porosity gradually decreases from 1.93% to 1.35%. EBR-MAO coatings are denser especially at high frequency 400 Hz.
- (2)
- The thickness of both MAO and EBR-MAO coatings decreases with increasing pulse frequency. Compared to MAO coatings, the thickness of the EBR-MAO coatings decreases slowly.
- (3)
- The corrosion resistance of EBR-MAO coatings is consistently higher than that of MAO coatings at different pulse frequencies. At 400 Hz, Icorr of the EBR-MAO coating reaches its lowest value of 2.897 × 10−8 A·cm−2, and R2 reaches the highest value 4.57 × 105 Ω·cm2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dou, J.H.; Chen, Y.; Yu, H.J.; Chen, C.Z. Research status of magnesium alloys by micro-arc oxidation: A review. Surf. Eng. 2017, 33, 731–738. [Google Scholar] [CrossRef]
- Kaya, A.A. A Review on Developments in Magnesium Alloys. Front. Mater. 2020, 7, 198. [Google Scholar] [CrossRef]
- Lin, Z.S.; Wang, T.L.; Yu, X.M.; Sun, X.T.; Yang, H.Z. Functionalization treatment of micro-arc oxidation coatings on magnesium alloys: A review. J. Alloys Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- She, J.; Chen, J.; Xiong, X.M.; Yang, Y.; Peng, X.D.; Chen, D.L.; Pan, F.S. Research advances of magnesium and magnesium alloys globally in 2023. J. Magnes. Alloys 2024, 12, 3441–3475. [Google Scholar] [CrossRef]
- Bai, J.Y.; Yang, Y.; Wen, C.; Chen, J.; Zhou, G.; Jiang, B.; Peng, X.D.; Pan, F.S. Applications of magnesium alloys for aerospace: A review. J. Magnes. Alloys 2023, 11, 3609–3619. [Google Scholar] [CrossRef]
- Chen, H.L.; Wang, Y.; He, L.; Zhang, X.Y.; Mei, Y.N.; Wu, T.; Wang, J.; Zheng, Y.; Tang, H. Surface engineering of biodegradable magnesium alloys as orthopedic implant materials: Recent developments and future prospects. Coatings 2025, 15, 191. [Google Scholar] [CrossRef]
- Chi, J.W.; Zhang, H.L.; Song, S.Y.; Zhang, W.S.; He, X.Y.; Nong, Z.S.; Cui, X.; Liu, T.; Man, T.N. The impact of pre- and post-treatment processes on corrosion resistance of micro-arc oxidation coatings on mg alloys: A systematic review. Materials 2025, 18, 723. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, K.M. Corrosion and protection of magnesium alloys: Recent advances and future perspectives. Coatings 2023, 13, 1533. [Google Scholar] [CrossRef]
- Jiang, J.H.; Geng, X.; Zhang, X.B. Stress corrosion cracking of magnesium alloys: A review. J. Magnes. Alloys 2023, 11, 1906–1930. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar]
- Yao, W.H.; Wu, L.; Wang, J.F.; Jiang, B.; Zhang, D.F.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F.S. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Chen, C.A.; Lee, J.L.; Jian, S.Y.; Lee, C.Y.; Ger, M.D. Effect of Surface Activation on the Corrosion Resistance of a MAO/Ni-P Composite Coating on LZ91 Magnesium Alloy. J. Taiwan Inst. Chem. Eng. 2023, 147, 104881. [Google Scholar] [CrossRef]
- Xie, Z.H.; Teng, Y.H.; Huang, Z.F.; Feng, P.C.; Yong, Q.W.; Jiang, X.; Wu, L. Corrosion-resistant and superhydrophobic nickel-based composite coating on magnesium alloy. Surf. Coat. Technol. 2025, 496, 131638. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Yang, J.W.; Zhang, Z.C.; Yang, G. Effect of laser surface melting pretreatment on wear and corrosion properties of micro-arc oxidation coatings on magnesium alloys. Ceram. Int. 2025, 51, 3037–3052. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, Y.; Jiang, H.T. Effect of grain size uniformity and crystallographic orientation on the corrosion behavior of Mg-2Zn-1Al bar. Mater. Charact. 2021, 179, 111374. [Google Scholar] [CrossRef]
- Huang, S.Y.; Chu, Y.R.; Yang, S.H.; Lee, Y.L. Effect of pause time on microstructure and corrosion resistance in AZ31 magnesium Alloy’s micro-arc oxidation coating. Surf. Coat. Technol. 2023, 475, 130164. [Google Scholar] [CrossRef]
- Dong, H.R.; Li, Q.; Xie, D.B.; Jiang, W.G.; Ding, H.J.; Wang, S.; An, L.Y. Forming characteristics and mechanisms of micro-arc oxidation coatings on magnesium alloys based on different types of single electrolyte solutions. Ceram. Int. 2023, 49, 32271–32281. [Google Scholar] [CrossRef]
- Bai, L.J.; Dong, B.X.; Chen, G.T.; Xin, T.; Wu, J.N.; Sun, X.D. Effect of positive pulse voltage on color value and corrosion property of magnesium alloy black micro-arc oxidation ceramic coating. Surf. Coat. Technol. 2019, 374, 402–408. [Google Scholar] [CrossRef]
- Wang, D.Y.; Yuan, J.; Liu, Q.B. Biodegradable behavior of the micro-arc oxidation coating with micro-nano structure formed by femtosecond laser on magnesium alloy AZ31B. Ceram. Int. 2025, 51, 9864–9876. [Google Scholar] [CrossRef]
- Pan, H.K.; Hu, H.H.; Wei, Z.H.; Yang, F.; Fan, Z.S.; Lu, H.L. Investigation on surface properties of AZ31 magnesium alloy modified by micro-arc oxidation and cathodic deposition techniques. Surf. Coat. Technol. 2025, 496, 131599. [Google Scholar] [CrossRef]
- Sun, S.; Shang, J. Improvement of corrosion resistance of AZ31 magnesium alloy by laser remelting/microarc oxidation/LDH composite layer. Mater. Lett. 2024, 356, 135531. [Google Scholar] [CrossRef]
- Zhao, G.H.; Zhang, P.; Li, J.; Zhang, Z.; Li, H.Y.; Ma, L.F. Effects of different scanning speeds on microstructure evolution and tribological properties of Inconel 718 alloy vacuum electron beam surface modification. Mater. Today Sustain. 2024, 25, 100613. [Google Scholar] [CrossRef]
- Niu, S.Q.; Yin, L.X.; You, Q.F.; You, X.G.; Wang, Y.N.; Tan, Y. The alloying elements dispersion and its mechanisms in a Ni-based superalloy during electron beam remelting. Vacuum 2019, 166, 107–113. [Google Scholar] [CrossRef]
- Zhao, G.H.; Sun, M.X.; Zhao, H.; Li, J.; Li, H.Y.; Ma, L.F. Study on modification of 253 MA austenitic stainless steel by electron beam surface remelting treatment. Surf. Coat. Technol. 2023, 470, 129834. [Google Scholar] [CrossRef]
- Yao, J.Y.; Li, J.; Zhao, G.H.; Li, H.Y.; Ma, L.F.; Liu, J. Microstructure Evolution and Mechanical Properties of TiC Coating over Inconel 625 Obtained by Vacuum Electron Beam Surface Alloying. Metals 2023, 13, 275. [Google Scholar] [CrossRef]
- Ma, Y.H.; Mei, J.H.; Ouyang, J.X.; Wu, P.; Wang, S.; Yang, J.G.; He, Y.M.; Zheng, W.J.; Li, H.X.; Lu, C.Y.; et al. Structure and corrosion resistance of electron-beam-strengthened and micro-arc oxidized coatings on magnesium alloy AZ31. J. Vac. Sci. Technol. A 2023, 41, 53101. [Google Scholar] [CrossRef]
- Zhao, J.H.; Wen, F.L.; Gu, C.; Gao, L.S.; Zhang, J.Y. Effect of vacuum induction heat treatment on the formation of intermetallic compounds between electroplated Ni coating and TC4 alloy. Vacuum 2021, 191, 110380. [Google Scholar] [CrossRef]
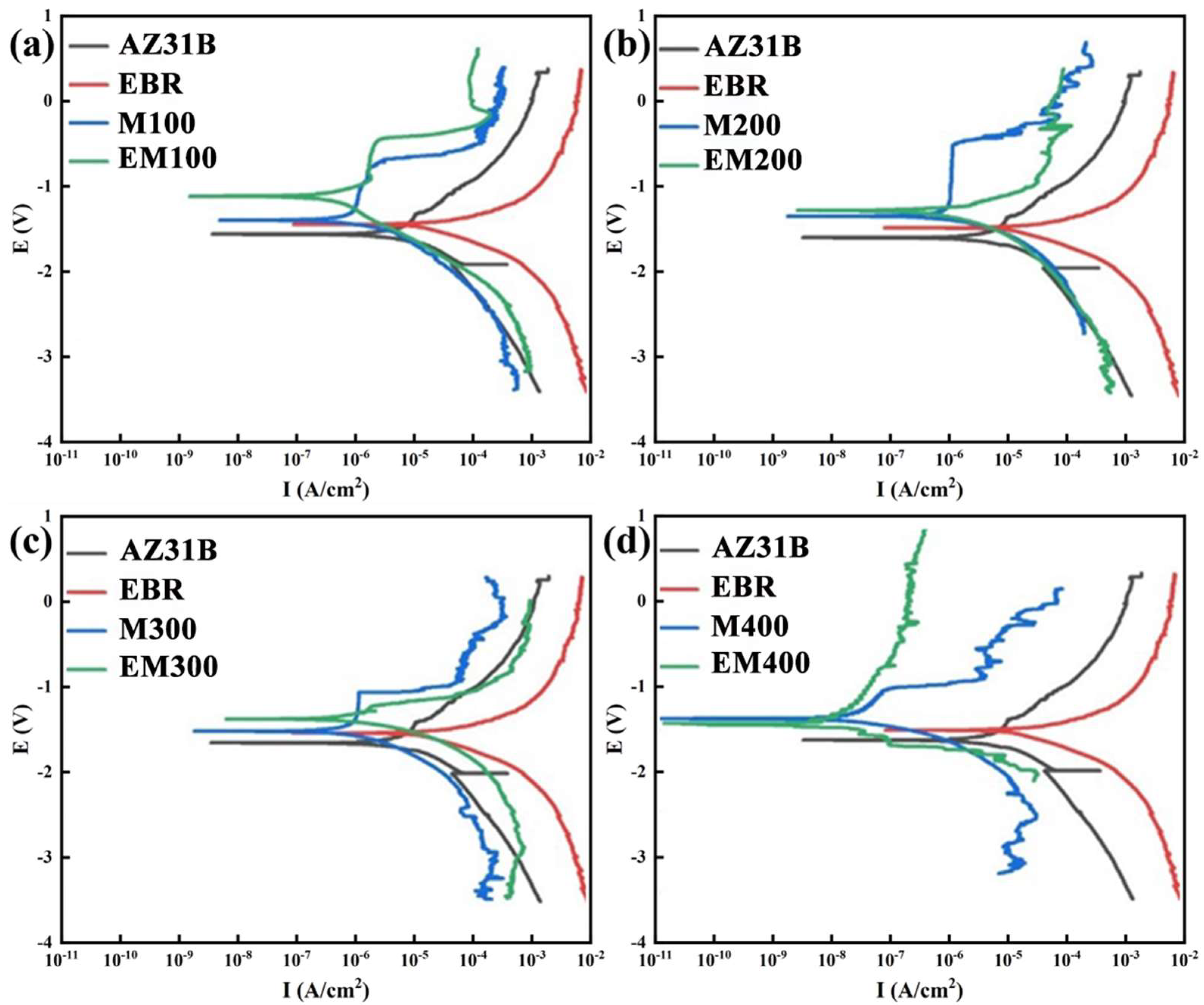
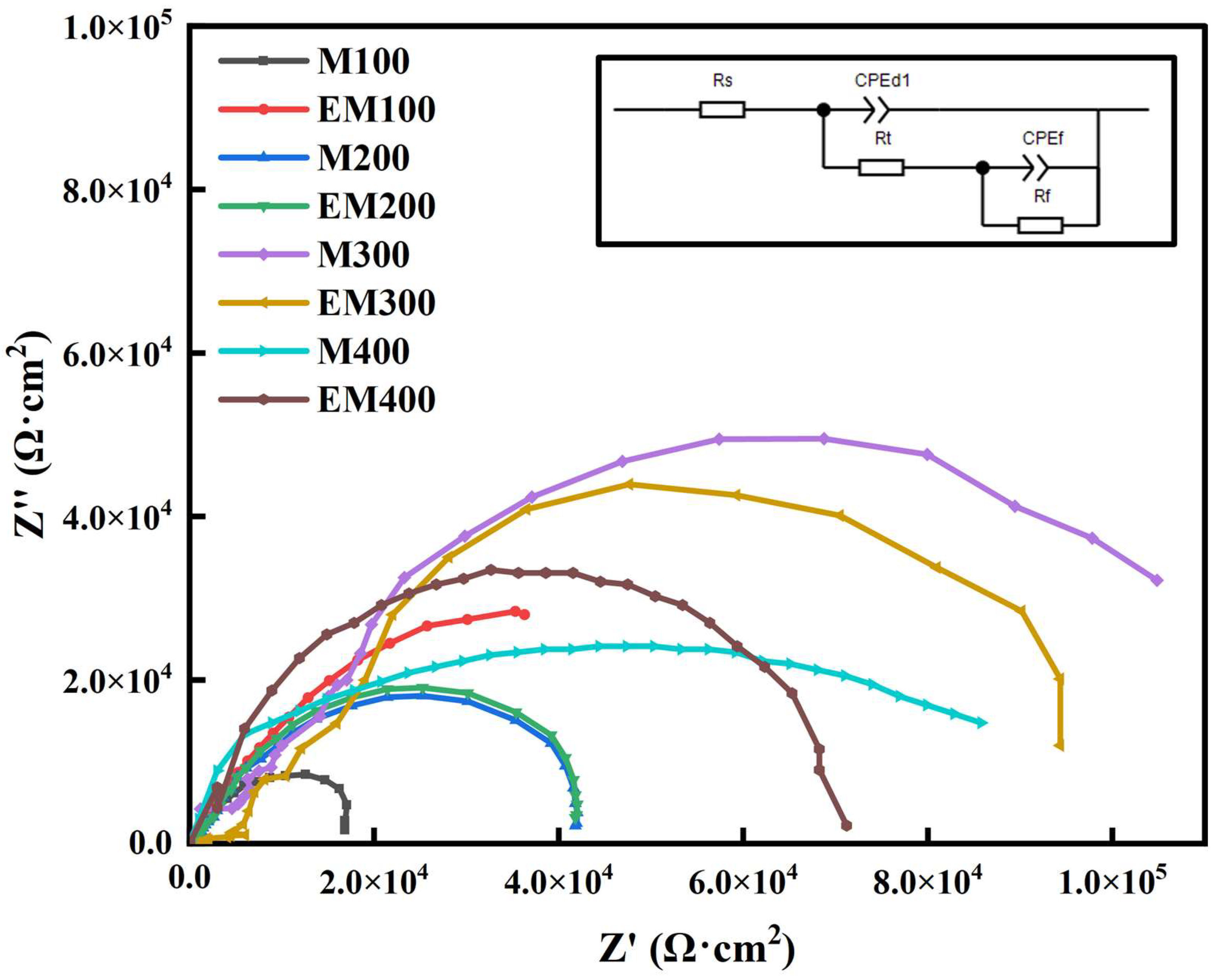
| Ecorr/V | Icorr/(A·cm−2) | |
|---|---|---|
| AZ31B | −1.672 | 1.607 × 10−5 |
| EBR | −1.521 | 1.144 × 10−5 |
| M100 | −1.460 | 2.012 × 10−6 |
| EM100 | −1.162 | 6.423 × 10−7 |
| M200 | −1.367 | 1.929 × 10−6 |
| EM200 | −1.295 | 5.681 × 10−7 |
| M300 | −1.487 | 1.802 × 10−6 |
| EM300 | −1.337 | 4.238 × 10−7 |
| M400 | −1.364 | 1.261 × 10−7 |
| EM400 | −1.438 | 2.897 × 10−8 |
| Rs (Ω·cm2) | CPEd1 (Ω−1sncm−2) | N1 | Rt (Ω·cm2) | CPEf (Ω−1sncm−2) | N2 | Rf (Ω·cm2) | |
|---|---|---|---|---|---|---|---|
| AZ31B | 33.15 | 1.33 × 10−6 | 0.93 | 4.20 × 102 | 1.25 × 10−6 | 0.91 | 1.64 × 103 |
| EBR | 39.56 | 8.77 × 10−6 | 0.95 | 6.40 × 102 | 4.99 × 10−7 | 1.06 | 1.69 × 103 |
| M100 | 48.30 | 1.38 × 10−7 | 0.72 | 4.60 × 104 | 4.44 × 10−8 | 0.84 | 2.22 × 104 |
| EM100 | 35.26 | 3.24 × 10−7 | 0.78 | 6.08 × 104 | 5.99 × 10−7 | 0.69 | 1.14 × 104 |
| M200 | 34.6 | 3.69 × 10−8 | 0.80 | 4.24 × 104 | 2.22 × 10−7 | 0.79 | 3.05 × 104 |
| EM200 | 30.6 | 5.67 × 10−8 | 0.78 | 5.14 × 104 | 2.80 × 10−8 | 0.86 | 4.81 × 104 |
| M300 | 44.6 | 4.62 × 10−8 | 0.84 | 7.54 × 104 | 1.59 × 10−8 | 0.87 | 2.35 × 104 |
| EM300 | 34.2 | 3.54 × 10−8 | 0.80 | 8.34 × 104 | 1.09 × 10−8 | 0.78 | 8.41 × 104 |
| M400 | 33.2 | 1.18 × 10−7 | 0.95 | 5.36 × 104 | 3.97 × 10−7 | 0.89 | 4.15 × 104 |
| EM400 | 32.7 | 3.34 × 10−7 | 0.87 | 5.89 × 104 | 3.97 × 10−8 | 0.79 | 4.57 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Yu, Z.; Zhang, J.; Hu, Y.; Zhou, M.; Mei, J.; Cai, Z.; Zheng, W.; Yang, J. The Effect of Pulse Frequency on the Microstructure and Corrosion Resistance of an AZ31B Magnesium Alloy Composite Coating with Electron-Beam Remelting and Micro-Arc Oxidation. Materials 2025, 18, 1962. https://doi.org/10.3390/ma18091962
Ma Y, Yu Z, Zhang J, Hu Y, Zhou M, Mei J, Cai Z, Zheng W, Yang J. The Effect of Pulse Frequency on the Microstructure and Corrosion Resistance of an AZ31B Magnesium Alloy Composite Coating with Electron-Beam Remelting and Micro-Arc Oxidation. Materials. 2025; 18(9):1962. https://doi.org/10.3390/ma18091962
Chicago/Turabian StyleMa, Yinghe, Zhen Yu, Jinpeng Zhang, Yonghui Hu, Mengliang Zhou, Jinhui Mei, Zhihui Cai, Wenjian Zheng, and Jianguo Yang. 2025. "The Effect of Pulse Frequency on the Microstructure and Corrosion Resistance of an AZ31B Magnesium Alloy Composite Coating with Electron-Beam Remelting and Micro-Arc Oxidation" Materials 18, no. 9: 1962. https://doi.org/10.3390/ma18091962
APA StyleMa, Y., Yu, Z., Zhang, J., Hu, Y., Zhou, M., Mei, J., Cai, Z., Zheng, W., & Yang, J. (2025). The Effect of Pulse Frequency on the Microstructure and Corrosion Resistance of an AZ31B Magnesium Alloy Composite Coating with Electron-Beam Remelting and Micro-Arc Oxidation. Materials, 18(9), 1962. https://doi.org/10.3390/ma18091962






