Semiconductor-Based Photoelectrocatalysts in Water Splitting: From the Basics to Mechanistic Insights—A Brief Review
Abstract
1. Introduction
2. Fundamentals of PEC Technology and Characterization
2.1. Fundamentals of PEC Water Splitting
2.2. Electrochemical Characterization
3. Photoelectrocatalysis
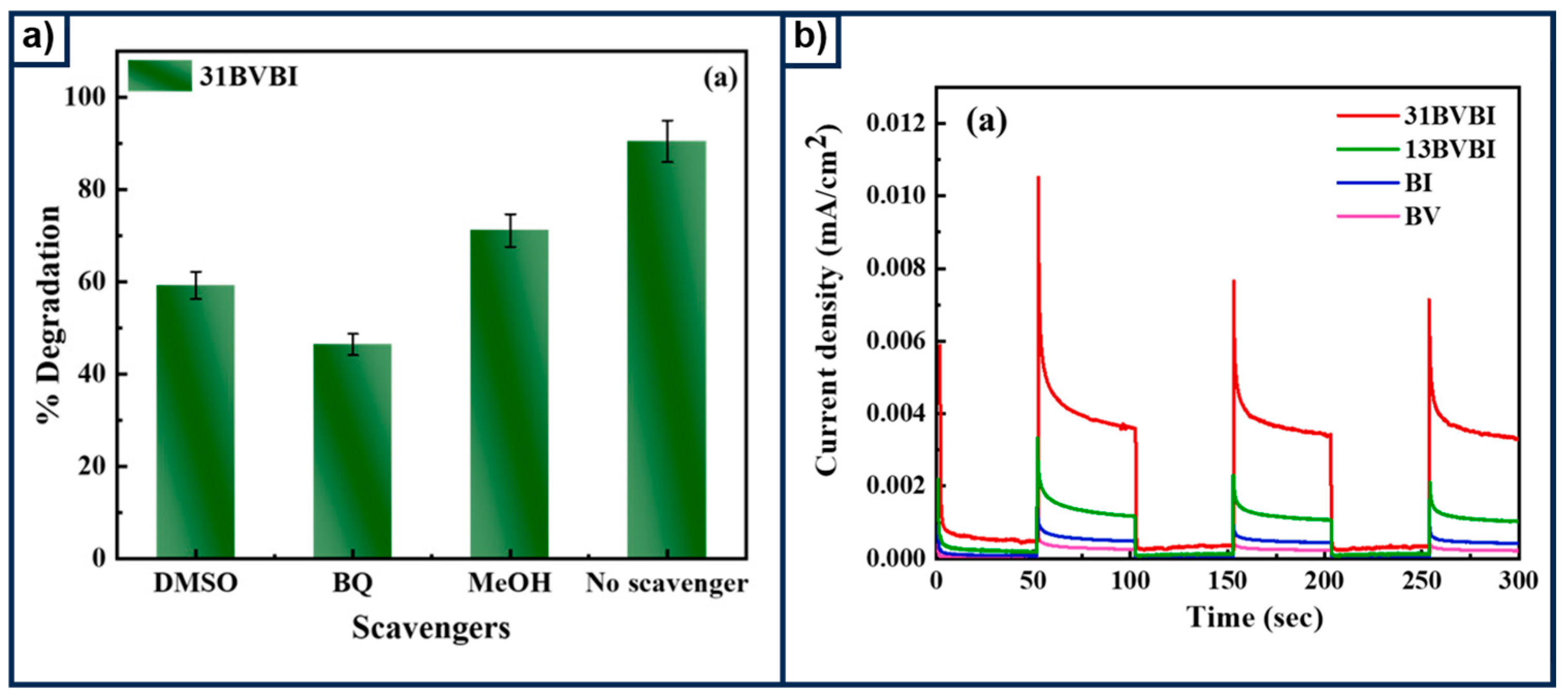
3.1. Traditional Semiconductive Photoelectrocatalysts
3.2. CdSe-Based Photoelectrocatalysts
3.3. NiWO4-Based Photoelectrocatalysts
3.4. Graphitic Carbon Nitride-Based Photoelectrocatalysts
3.5. Fe2O3-Based Photoelectrocatalysts
3.6. Other Promising Photoanode Candidates
4. Outlooks, Perspectives, and Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Li, F.; Xiang, Q. Advances and challenges in the modification of photoelectrode materials for photoelectrocatalytic water splitting. Mater. Horiz. 2024, 11, 1638–1657. [Google Scholar] [CrossRef]
- Khalili, S.; Rantanen, E.; Bogdanov, D.; Breyer, C. Global Transportation Demand Development with Impacts on the Energy Demand and Greenhouse Gas Emissions in a Climate-Constrained World. Energies 2019, 12, 3870. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, C.; Chen, X.; Cao, J.; Chen, A.; Shao, S.; Lian, Y. The design and optimization of energy level alignment in dual-photoelectrode solar PEC cells for efficient dye degradation at low bias. Surf. Interfaces 2025, 56, 105646. [Google Scholar] [CrossRef]
- Méndez-Ramos, J.; Borges, M.E.; Torres-García, S.; Medina-Alayón, M.; Acosta-Mora, P.; del-Castillo, J.; Menéndez-Velázquez, A.; García-Delgado, A.B.; Mullins, C.B.; Esparza, P. “There is plenty of energy at the bottom”: A spectral conversion approach for upconversion-powered water-splitting PEC cell. J. Power Sources 2025, 625, 235668. [Google Scholar] [CrossRef]
- Baviskar, V.S.; Jadhav, C.D.; Salunkhe, D.B.; Narkhede, N.M.; Patil, G.P. Enhancing 3G ETA solar cells with novel Bi2Se3 nanoparticles synthesized on TiO2: Impact of immersion cycles on PEC performance. Next Energy 2025, 6, 100190. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Hsiao, Y.-L.; Wu, C.-S.; Pu, Y.-C.; Liu, C.-P. Enhancing the photoelectrochemical water splitting efficiency of ZnO P–N homojunction nanorod arrays under the piezocatalyst effect. Catal. Sci. Technol. 2025, 15, 165–172. [Google Scholar] [CrossRef]
- El Ouardi, M.; El Idrissi, A.; Arab, M.; Zbair, M.; Haspel, H.; Saadi, M.; Ait Ahsaine, H. Review of photoelectrochemical water splitting: From quantitative approaches to effect of sacrificial agents, oxygen vacancies, thermal and magnetic field on (photo)electrolysis. Int. J. Hydrogen Energy 2024, 51, 1044–1067. [Google Scholar] [CrossRef]
- Clarizia, L.; Nadagouda, M.N.; Dionysiou, D.D. Recent advances and challenges of photoelectrochemical cells for hydrogen production. Curr. Opin. Green Sustain. Chem. 2023, 41, 100825. [Google Scholar] [CrossRef]
- Vilanova, A.; Dias, P.; Lopes, T.; Mendes, A. The route for commercial photoelectrochemical water splitting: A review of large-area devices and key upscaling challenges. Chem. Soc. Rev. 2024, 53, 2388–2434. [Google Scholar] [CrossRef]
- Liu, D.; Kuang, Y. Particle-Based Photoelectrodes for PEC Water Splitting: Concepts and Perspectives. Adv. Mater. 2024, 36, 2311692. [Google Scholar] [CrossRef]
- Ngo, V.T.; Lim, S.Y.; Law, C.S.; Wang, J.; Hamza, M.A.; Abell, A.D.; Zhang, H.; Santos, A. Semiconductor Nanoporous Anodic Alumina Photonic Crystals as a Model Photoelectrocatalytic Platform for Solar Light-Driven Reactions. Adv. Energy Sustain. Res. 2024, 5, 2400125. [Google Scholar] [CrossRef]
- Wang, B.; Yang, S.; Zhang, T.; Liu, Y.; Yang, S.; Li, L.; Wang, W.; Su, J. Performance analysis of a novel unassisted photoelectrochemical water splitting hybrid system based on spectral beam splitting. Front. Energy 2025. [Google Scholar] [CrossRef]
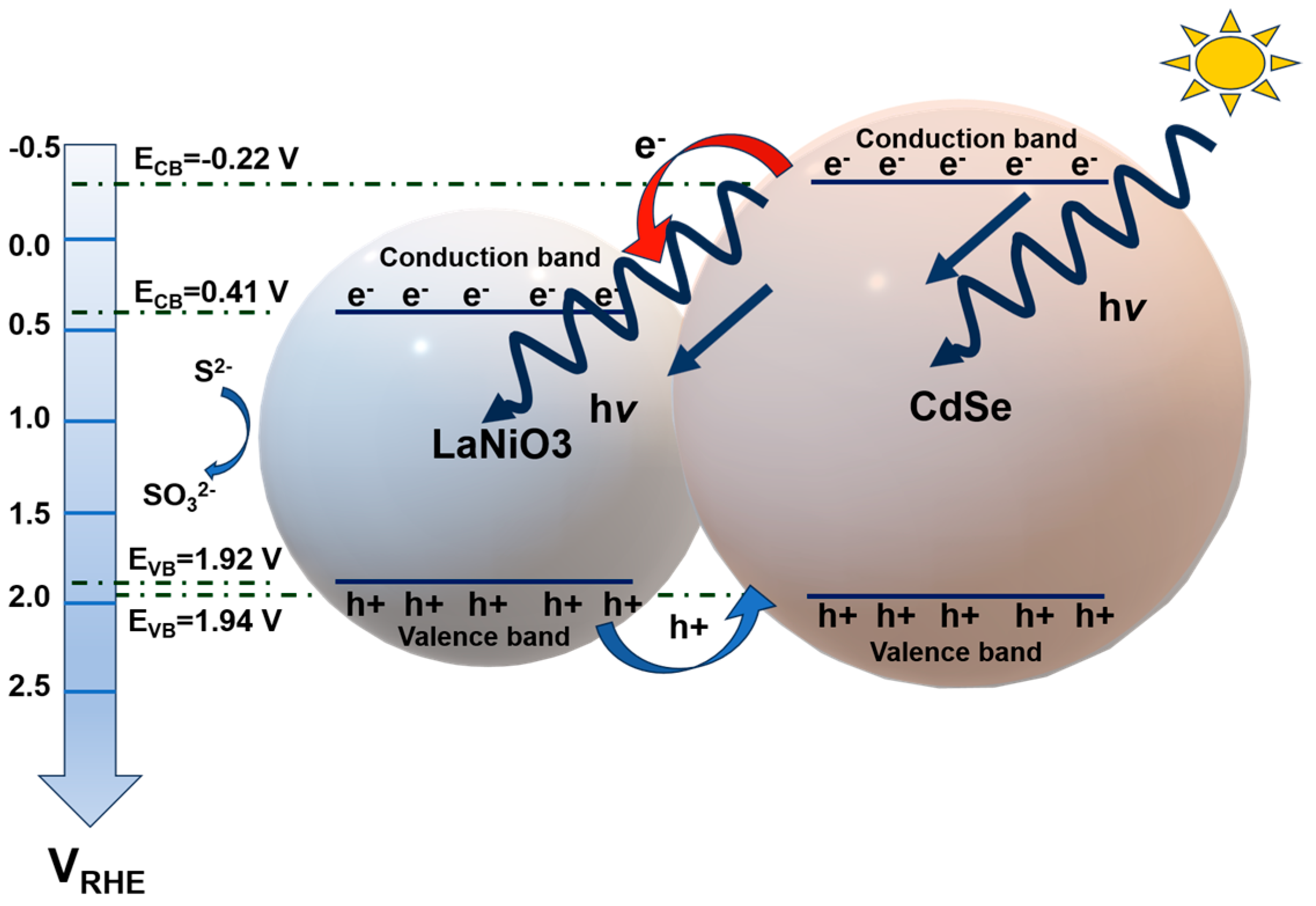
- Kuchipudi, A.; Nandigana, P.; Prasanna, M.; Anupriya; Panda, S.K.; Sreedhar, G. Enhanced photoelectrocatalytic performance of LaNiO3 sensitized with CdSe quantum dots for photoelectrochemical water splitting. Mater. Chem. Phys. 2023, 307, 128123. [Google Scholar] [CrossRef]
- Shi, Q.; Duan, H. Recent progress in photoelectrocatalysis beyond water oxidation. Chem Catal. 2022, 2, 3471–3496. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Wang, Y.; Tian, R.; Wang, J. Innovations in Photocatalytic and Photoelectrocatalytic Water Splitting: Pathways to Efficiently Convert Biomass into Renewable Energy and Chemicals. ChemCatChem 2024, 16, e202400413. [Google Scholar] [CrossRef]
- Santos, G.O.S.; Goulart, L.A.; Cordeiro-Junior, P.J.M.; Sánchez-Montes, I.; Lanza, M.R.V. Pharmaceutical contaminants: Ecotoxicological aspects and recent advances in oxidation technologies for their removal in aqueous matrices. J. Environ. Chem. Eng. 2022, 10, 108932. [Google Scholar] [CrossRef]
- Singh, S.; Patidar, R.; Srivastava, V.C.; Lo, S.-L.; Nidheesh, P.V. A critical review on the degradation mechanism of textile effluent during electrocatalytic oxidation: Removal optimization and degradation pathways. J. Environ. Chem. Eng. 2023, 11, 111277. [Google Scholar] [CrossRef]
- Ramgopal, N.C.; Sreedevi, G.; Alhammadi, S.; El-marghany, A.; reddy Gutturu, R.; Arla, S.K.; Kim, J.S.; Joo, S.W. Cavity-engineered light utilization in NiCoMnO4 2D sheets for superior PEC water splitting performance. J. Alloys Compd. 2025, 1017, 179007. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Lu, B.; Wang, D.; Guo, X.; Zhou, X.; Lei, Z. Nickel tungstate-based electrocatalyst, photocatalyst, and photoelectrocatalyst in water splitting applications. Int. J. Hydrogen Energy 2024, 53, 859–874. [Google Scholar] [CrossRef]
- Yu, S.; Su, C.; Xiao, Z.; Kuang, Y.; Gong, X.; He, X.; Liu, J.; Jin, Q.; Sun, Z. Tuning surface hydrophilicity of a BiVO4 photoanode through interface engineering for efficient PEC water splitting. RSC Adv. 2025, 15, 815–823. [Google Scholar] [CrossRef]
- Ladhane, S.; Shah, S.; Doiphode, V.; Shinde, P.; Punde, A.; Kale, D.; Rahane, S.; Thombare, J.; Hase, Y.; Waghmare, A.; et al. Direct growth of WS2 nanosheets using RF-magnetron sputtering on hydrothermally grown TiO2 nanorods for enhancing photoelectrochemical water splitting. Mater. Chem. Phys. 2025, 334, 130440. [Google Scholar] [CrossRef]
- Yusuf, T.L.; Orimolade, B.O.; Masekela, D.; Mamba, B.; Mabuba, N. The application of photoelectrocatalysis in the degradation of rhodamine B in aqueous solutions: A review. RSC Adv. 2022, 12, 26176–26191. [Google Scholar] [CrossRef]
- Villanueva Martinez, B.; Odier, H.; Coetsier, C.; Groenen Serrano, K. Recent advances in sub-stoichiometric TiO2 as reactive electrochemical membranes (REM) for bio-refractory pollutants removal: A critical review. J. Environ. Chem. Eng. 2023, 11, 110203. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Recent progress of applied TiO2 photoelectrocatalysis for the degradation of organic pollutants in wastewaters. J. Environ. Chem. Eng. 2023, 11, 109635. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Geng, J.; Zong, S.; Wang, P. Photo(electro)catalytic Water Splitting for Hydrogen Production: Mechanism, Design, Optimization, and Economy. Molecules 2025, 30, 630. [Google Scholar] [CrossRef]
- Qiao, F. Photoelectrocatalytic hydrogen production: Hydrogen production principle, performance optimization strategy, application and prospect. Nano Res. Energy 2024, 4, e9120132. [Google Scholar] [CrossRef]
- Harris-Lee, T.R.; Marken, F.; Bentley, C.L.; Zhang, J.; Johnson, A.L. A chemist’s guide to photoelectrode development for water splitting—The importance of molecular precursor design. EES Catal. 2023, 1, 832–873. [Google Scholar] [CrossRef]
- Cui, J.; Ding, D.; Yue, S.; Chen, Z. Photoelectrochemical water splitting with In2O3−x nanofilm/black Ti–Si–O composite photoanode. RSC Adv. 2025, 15, 4987–4996. [Google Scholar] [CrossRef]
- Orton, J. (Ed.) CHAPTER 1—What Exactly is a Semiconductor: And what can it do? In Semiconductors and the Information Revolution; Academic Press: Amsterdam, The Netherlands, 2009; pp. 1–30. [Google Scholar]
- Iwase, A. Band Engineering of Semiconductors Toward Visible-Light-Responsive Photocatalysts. In Heterogeneous Catalysts; Wiley-VCH GmbH: Weinheim, Germany, 2021; pp. 203–213. [Google Scholar]
- Kusmierek, E. Semiconductor Electrode Materials Applied in Photoelectrocatalytic Wastewater Treatment—An Overview. Catalysts 2020, 10, 439. [Google Scholar] [CrossRef]
- Khamgaonkar, S.S.; Leudjo Taka, A.; Maheshwari, V. Engineering and Design of Halide Perovskite Photoelectrochemical Cells for Solar-Driven Water Splitting. Adv. Funct. Mater. 2024, 34, 2405414. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, Y.; Zhang, X.; Yang, H.; Xie, R.; Zhang, S.; Lv, Y.; Xiong, L. Progress in Promising Semiconductor Materials for Efficient Photoelectrocatalytic Hydrogen Production. Molecules 2024, 29, 289. [Google Scholar] [CrossRef] [PubMed]
- Son, M.-K. Key Strategies on Cu2O Photocathodes toward Practical Photoelectrochemical Water Splitting. Nanomaterials 2023, 13, 3142. [Google Scholar] [CrossRef]
- Lianos, P. Review of recent trends in photoelectrocatalytic conversion of solar energy to electricity and hydrogen. Appl. Catal. B Environ. 2017, 210, 235–254. [Google Scholar] [CrossRef]
- Kumar, M.; Meena, B.; Subramanyam, P.; Suryakala, D.; Subrahmanyam, C. Recent trends in photoelectrochemical water splitting: The role of cocatalysts. NPG Asia Mater. 2022, 14, 88. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Huang, J.; Yao, S.; Jiang, R.; Hou, Y.; Tang, W.; Sun, P.; Huang, H.; Wang, M. Redox-active ligands enhance oxygen evolution reaction activity: Regulating the spin state of ferric ions and accelerating electron transfer. J. Colloid Interface Sci. 2023, 650, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.F.; Rejeb, O.; Ghenai, C. Green hydrogen production and solar to hydrogen ratio using innovative and integrated bifacial solar photovoltaics and cool roof technologies. Int. J. Hydrogen Energy 2025. [Google Scholar] [CrossRef]
- El Idrissi, M.; Mei, B.; Abd-Lefdil, M.; Atourki, L. Making Solar Hydrogen: A Review of the Challenges and Strategies of Synthesizing CuFeO2 Photocathodes for Photoelectrochemical Water Splitting. Molecules 2025, 30, 1152. [Google Scholar] [CrossRef]
- Li, W.; Duan, W.; Liao, G.; Gao, F.; Wang, Y.; Cui, R.; Zhao, J.; Wang, C. 0.68% of solar-to-hydrogen efficiency and high photostability of organic-inorganic membrane catalyst. Nat. Commun. 2024, 15, 6763. [Google Scholar] [CrossRef]
- Bora, L.V.; Bora, N.V. Photoelectrocatalytic water splitting for efficient hydrogen production: A strategic review. Fuel 2025, 381, 133642. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Santosh, M.S. The integration of flexible dye-sensitized solar cells and storage devices towards wearable self-charging power systems: A review. Renew. Sustain. Energy Rev. 2022, 159, 112252. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Alex, K.V.; Latha, M.B.; Kamakshi, K.; Sathish, S.; Silva, J.P.B.; Sekhar, K.C. Effect of the thickness on the photocatalytic and the photocurrent properties of ZnO films deposited by spray pyrolysis. Discov. Mater. 2022, 2, 10. [Google Scholar] [CrossRef]
- Penkova, Y.; Betova, I.; Karastoyanov, V.; Bojinov, M. Electrochemical synthesis and characterization of tungsten oxide catalysts for photoelectrochemical water splitting. Electrochim. Acta 2024, 480, 143908. [Google Scholar] [CrossRef]
- Crawford, S.; Thimsen, E.; Biswas, P. Impact of Different Electrolytes on Photocatalytic Water Splitting. J. Electrochem. Soc. 2009, 156, H346. [Google Scholar] [CrossRef]
- Arzaee, N.A.; Mohamad Noh, M.F.; Halim, A.A.; Faizal Abdul Rahim, M.A.; Haziqah Mohd Ita, N.S.; Mohamed, N.A.; Farhana Mohd Nasir, S.N.; Ismail, A.F.; Mat Teridi, M.A. Cyclic voltammetry—A promising approach towards improving photoelectrochemical activity of hematite. J. Alloys Compd. 2021, 852, 156757. [Google Scholar] [CrossRef]
- Bui, T.K.; Le, H.V.; Nguyen, H.T.; Van Pham, V. Non-binder synthesis of g-C3N4 decorated on TiO2 nanotube arrays toward photo/electrochemical water splitting. Mol. Catal. 2025, 577, 114960. [Google Scholar] [CrossRef]
- Li, Q.; Cui, X.; Liu, X.; Wang, W. Ti3C2 quantum dots decorated BiVO4 photoelectrode for both photoelectrochemical water splitting and H2O2 determination. J. Alloys Compd. 2025, 1011, 178399. [Google Scholar] [CrossRef]
- Ravishankar, S.; Bisquert, J.; Kirchartz, T. Interpretation of Mott–Schottky plots of photoanodes for water splitting. Chem. Sci. 2022, 13, 4828–4837. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-González, P.C.; Pech-Rodríguez, W.J.; Luévano-Hipólito, E.; Hernández-Ramírez, A.; Hernández-López, J.M. Innovative syntheses of immobilized CuxO semiconductors grown on 3D prints and their photoelectrochemical activity for sulfamethoxazole degradation. J. Environ. Chem. Eng. 2024, 12, 112551. [Google Scholar] [CrossRef]
- Darowicki, K.; Krakowiak, S.; Ślepski, P. Selection of measurement frequency in Mott–Schottky analysis of passive layer on nickel. Electrochim. Acta 2006, 51, 2204–2208. [Google Scholar] [CrossRef]
- Mittal, S.; Khosya, M.; Singh, M.; Khare, N. 2D/2D heterojunction of SnS2/g-C3N4 nanocomposite photoelectrode for improved photoelectrochemical water splitting performance. Appl. Surf. Sci. 2024, 677, 161016. [Google Scholar] [CrossRef]
- Syrek, K.; Zych, M.; Pisarek, M.; Gondek, Ł.; Gurgul, M.; Palowska, R.; Liu, L.; Sulka, G.D. Novel anodic WO3-SeO2-CuO photoelectrode operating under solar illumination for water-splitting applications. J. Power Sources 2025, 640, 236656. [Google Scholar] [CrossRef]
- Bedoya-Lora, F.E.; Holmes-Gentle, I.; Hankin, A. Electrochemical techniques for photoelectrode characterisation. Curr. Opin. Green Sustain. Chem. 2021, 29, 100463. [Google Scholar] [CrossRef]
- Talasila, G.; Sachdev, S.; Bera, T.; Badhe, R.M.; Srivastva, U.; Sharma, A. Experimental study on critical role of heterostructures for efficient water splitting activity. Chem. Eng. J. 2025, 508, 160868. [Google Scholar] [CrossRef]
- Seferlis, A.K.; Neophytides, S.G. On the kinetics of photoelectrocatalytic water splitting on nanocrystalline TiO2 films. Appl. Catal. B Environ. 2013, 132–133, 543–552. [Google Scholar] [CrossRef]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Abanades, S.; Charvin, P.; Lemont, F.; Flamant, G. Novel two-step SnO2/SnO water-splitting cycle for solar thermochemical production of hydrogen. Int. J. Hydrogen Energy 2008, 33, 6021–6030. [Google Scholar] [CrossRef]
- Lamm, B.; Zhou, L.; Rao, P.; Stefik, M. Atomic Layer Deposition of Space-Efficient SnO2 Underlayers for BiVO4 Host–Guest Architectures for Photoassisted Water Splitting. ChemSusChem 2019, 12, 1916–1924. [Google Scholar] [CrossRef]
- Gong, J.; Lai, Y.; Lin, C. Electrochemically multi-anodized TiO2 nanotube arrays for enhancing hydrogen generation by photoelectrocatalytic water splitting. Electrochim. Acta 2010, 55, 4776–4782. [Google Scholar] [CrossRef]
- Moridon, S.N.F.; Arifin, K.; Yunus, R.M.; Minggu, L.J.; Kassim, M.B. Photocatalytic water splitting performance of TiO2 sensitized by metal chalcogenides: A review. Ceram. Int. 2022, 48, 5892–5907. [Google Scholar] [CrossRef]
- Yang, P.; Li, W.; Lian, Y.; Yu, F.; Dai, B.; Guo, X.; Liu, Z.; Peng, B. A facile approach to synthesize CoO-Co3O4/TiO2 NAs for reinforced photoelectrocatalytic water oxidation. J. Solid State Electrochem. 2020, 24, 941–950. [Google Scholar] [CrossRef]
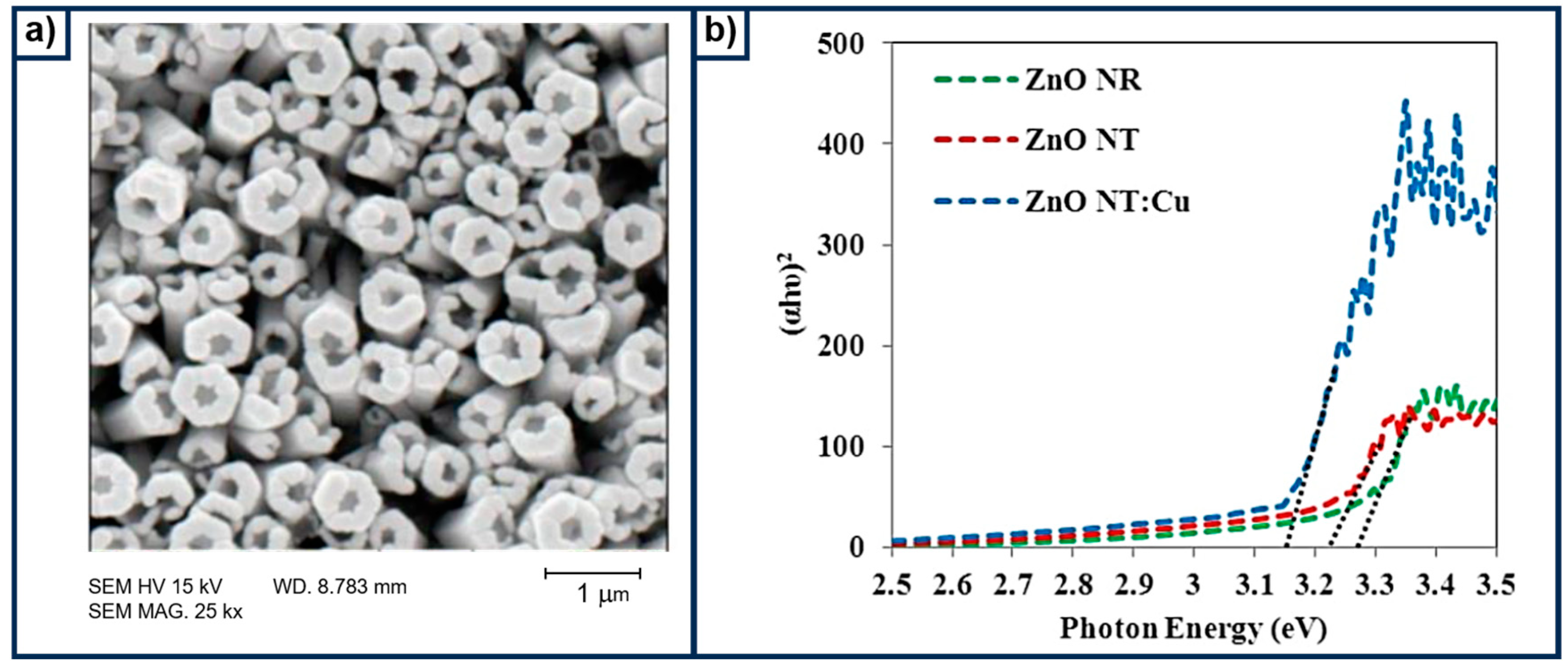
- Mollaei, T.; Rouhollahi, A.; Hadi, M.; Rasouli, F. Electrodeposition of Copper Doped ZnO Nanotubes and Its Impact on Photoelectrocatalytic Property of ZnO Nanostructure in Improving Photoelectrochemical Water Splitting. Anal. Bioanal. Electrochem. 2024, 16, 423–437. [Google Scholar] [CrossRef]
- Araujo, M.d.S.; dos Santos, H.L.S.; Medina, M.; Salomao, A.C.; Mascaro, L.H.; Andrade, M.A.S. Vanquishing CZTSSe deep defects to enhance photoelectrocatalytic water splitting. Electrochim. Acta 2023, 464, 142935. [Google Scholar] [CrossRef]
- Pan, A.; Zhu, X. 12—Optoelectronic properties of semiconductor nanowires. In Semiconductor Nanowires; Arbiol, J., Xiong, Q., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 327–363. [Google Scholar]
- Chopade, P.; Jagtap, S.; Gosavi, S. 5—Material properties and potential applications of CdSe semiconductor nanocrystals. In Nanoscale Compound Semiconductors and their Optoelectronics Applications; Pawade, V.B., Dhoble, S.J., Swart, H.C., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 105–153. [Google Scholar]
- Cao, K.; Chen, M.-M.; Chang, F.-Y.; Cheng, Y.-Y.; Tian, L.-J.; Li, F.; Deng, G.-Z.; Wu, C. The biosynthesis of cadmium selenide quantum dots by Rhodotorula mucilaginosa PA-1 for photocatalysis. Biochem. Eng. J. 2020, 156, 107497. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Li, H.; Jiang, H.; Tian, G. Achieving cadmium selenide-decorated zinc ferrite@titanium dioxide hollow core/shell nanospheres with improved light trapping and charge generation for photocatalytic hydrogen generation. J. Colloid Interface Sci. 2020, 575, 158–167. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Li, Y.; Zhang, M.; Zheng, Y. Designing a 0D/1D S-Scheme Heterojunction of Cadmium Selenide and Polymeric Carbon Nitride for Photocatalytic Water Splitting and Carbon Dioxide Reduction. Molecules 2022, 27, 6286. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ali, S.; Zulfiqar, S.; Kang, K.T.; Khan, T.; Khattak, S.; Khan, G.; Rahman, M.U.; Shaik, M.R. Investigation of ZnO@CdS nanocomposite with amplified photocatalytic H2 production under visible light irradiation. Surf. Interfaces 2024, 52, 104836. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, D.; Wang, Y.; Liu, E.; Miao, H. Fabricating S-scheme Sb2S3@CdSexS1–x quasi-one-dimensional heterojunction photoanodes by in-situ growth strategy towards photoelectrochemical water splitting. J. Mater. Sci. Technol. 2024, 201, 250–260. [Google Scholar] [CrossRef]
- Zhu, J.; Li, W.; Li, J.; Li, Y.; Hu, H.; Yang, Y. Photoelectrochemical activity of NiWO4/WO3 heterojunction photoanode under visible light irradiation. Electrochim. Acta 2013, 112, 191–198. [Google Scholar] [CrossRef]
- Do, T.H.; Nguyen Van, C.; Tsai, K.-A.; Quynh, L.T.; Chen, J.-W.; Lin, Y.-C.; Chen, Y.-C.; Chou, W.-C.; Wu, C.-L.; Hsu, Y.-J.; et al. Superior photoelectrochemical activity of self-assembled NiWO4–WO3 heteroepitaxy. Nano Energy 2016, 23, 153–160. [Google Scholar] [CrossRef]
- Hosseini, S.; Farsi, H.; Moghiminia, S.; Zubkov, T.; Lightcap, I.V.; Riley, A.; Peters, D.G.; Li, Z. Nickel tungstate (NiWO4) nanoparticles/graphene composites: Preparation and photoelectrochemical applications. Semicond. Sci. Technol. 2018, 33, 055008. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Arunachalam, P.; Hezam, M.; Al-Mayouf, A.M. Cooperative Catalytic Behavior of SnO2 and NiWO4 over BiVO4 Photoanodes for Enhanced Photoelectrochemical Water Splitting Performance. Catalysts 2019, 9, 879. [Google Scholar] [CrossRef]
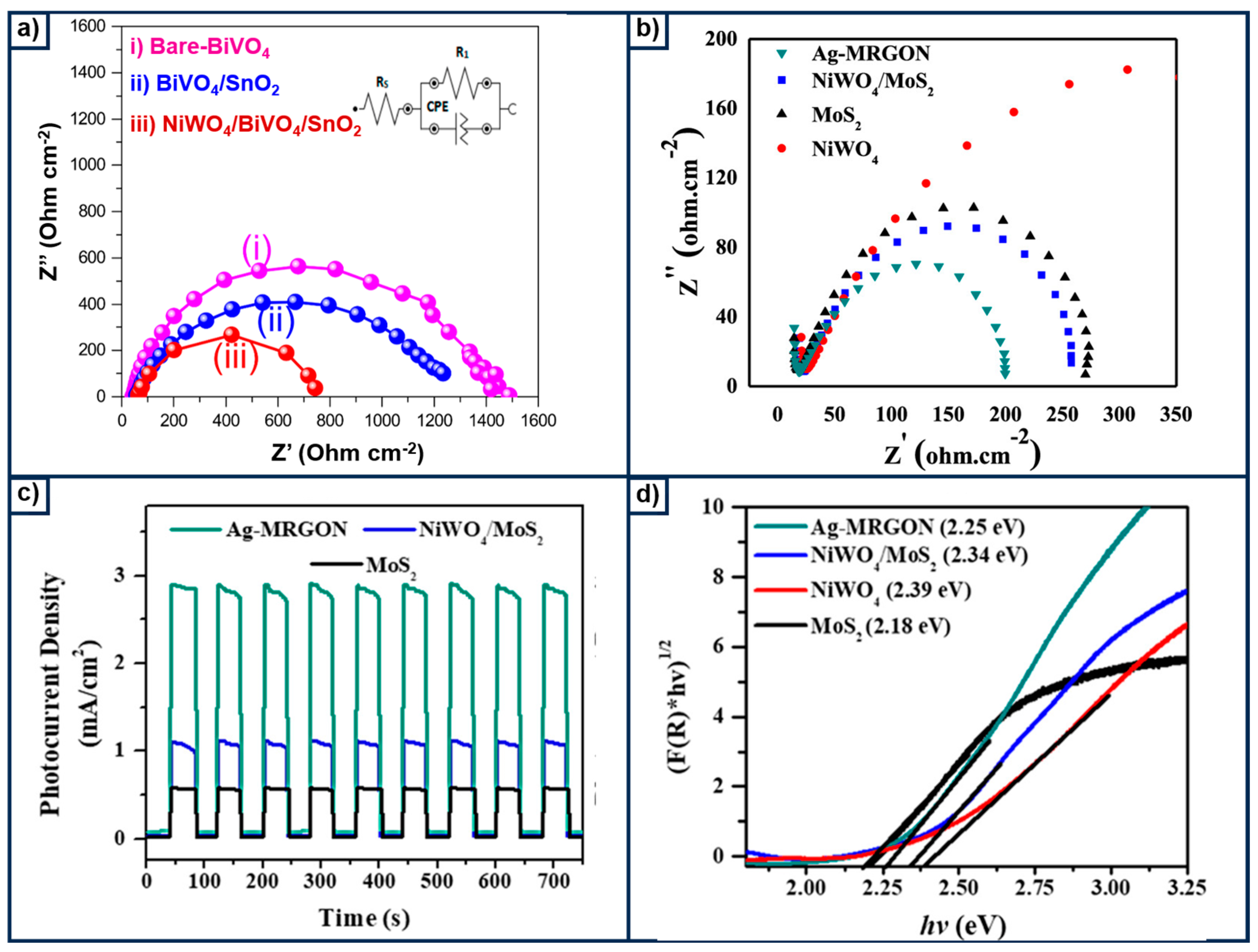
- Hendi, A.H.; Osman, A.M.; Khan, I.; Saleh, T.A.; Kandiel, T.A.; Qahtan, T.F.; Hossain, M.K. Visible Light-Driven Photoelectrocatalytic Water Splitting Using Z-Scheme Ag-Decorated MoS2/RGO/NiWO4 Heterostructure. ACS Omega 2020, 5, 31644–31656. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, X.; Pan, D.; Li, G. Nanotube array-like WO3/W photoanode fabricated by electrochemical anodization for photoelectrocatalytic overall water splitting. Chin. J. Catal. 2017, 38, 2132–2140. [Google Scholar] [CrossRef]
- Shembade, U.V.; Mane, J.V.; Mali, S.S.; Magadum, M.G.; Patil, S.S.; Wategaonkar, S.B.; Padalkar, N.S.; Patil, P.S.; Park, J.P.; Moholkar, A.V. Hydrothermal synthesized nickel tungstate (NiWO4) microflowers for supercapacitor and water-splitting. Colloids Surf. A Physicochem. Eng. Asp. 2024, 697, 134403. [Google Scholar] [CrossRef]
- Li, J.; Du, X.; Zhang, X. Controlled synthesis of NiWO4 combined with NiSe2 with heterostructure on nickel foam for efficient overall water splitting. J. Alloys Compd. 2023, 951, 169941. [Google Scholar] [CrossRef]
- Kong, L.-X.; Fang, Y.; Wang, M.-M.; Li, H.-H.; Huang, S.; Li, J.-S.; Xiao, Q. Self-supported Ni-NiWO4@NC heterojunction as high-efficient electrocatalyst for overall water splitting. J. Alloys Compd. 2024, 972, 172836. [Google Scholar] [CrossRef]
- Jing, L.; Ong, W.-J.; Zhang, R.; Pickwell-MacPherson, E.; Yu, J.C. Graphitic carbon nitride nanosheet wrapped mesoporous titanium dioxide for enhanced photoelectrocatalytic water splitting. Catal. Today 2018, 315, 103–109. [Google Scholar] [CrossRef]
- Mary Rajaitha, P.; Shamsa, K.; Murugan, C.; Bhojanaa, K.B.; Ravichandran, S.; Jothivenkatachalam, K.; Pandikumar, A. Graphitic carbon nitride nanoplatelets incorporated titania based type-II heterostructure and its enhanced performance in photoelectrocatalytic water splitting. SN Appl. Sci. 2020, 2, 572. [Google Scholar] [CrossRef]
- Ashfaq, T.; Khan, M.; Arshad, I.; Ahmad, A.; Ali, S.; Aftab, K.; Al-Kahtani, A.A.; Mohamed Tighezza, A. Electro-Oxidation of Metal Oxide-Fabricated Graphitic Carbon Nitride for Hydrogen Production via Water Splitting. Coatings 2022, 12, 548. [Google Scholar] [CrossRef]
- Murugan, C.; Bhojanaa, K.B.; Ong, W.-J.; Jothivenkatachalam, K.; Pandikumar, A. Improving hole mobility with the heterojunction of graphitic carbon nitride and titanium dioxide via soft template process in photoelectrocatalytic water splitting. Int. J. Hydrogen Energy 2019, 44, 30885–30898. [Google Scholar] [CrossRef]
- Yang, P.; Liu, Q.; Yu, F.; Wu, J.; Liu, Z.; Peng, B. Cobalt substituted polyoxophosphomolybdate modified TiO2 for boosted photoelectrocatalytic water oxidation. J. Alloys Compd. 2021, 854, 157232. [Google Scholar] [CrossRef]
- Yang, M.; Oldham, L.I.; Daboczi, M.; Baghdadi, Y.; Cui, J.; Benetti, D.; Zhang, W.; Durrant, J.R.; Hankin, A.; Eslava, S. Advancing Hematite Photoanodes for Photoelectrochemical Water Splitting: The Impact of g-C3N4 Supported Ni-CoP on Photogenerated Hole Dynamics. Adv. Energy Mater. 2024, 14, 2401298. [Google Scholar] [CrossRef]
- Sundararaj, S.B.; Amir, H.; Chinnusamy, V.; Thangavelu, S. Interfacial charge separation of nickel tungstate anchored on g-C3N4 heterojunction stimulates visible-light driven direct Z-scheme photoelectrochemical hydrogen evolution. Int. J. Hydrogen Energy 2023, 48, 26221–26237. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Miao, X.; Zhao, L.; Zhu, C. Deposition of FeOOH Layer on Ultrathin Hematite Nanoflakes to Promote Photoelectrochemical Water Splitting. Micromachines 2024, 15, 387. [Google Scholar] [CrossRef]
- Berardi, S.; Cristino, V.; Bignozzi, C.A.; Grandi, S.; Caramori, S. Hematite-based photoelectrochemical interfaces for solar fuel production. Inorg. Chim. Acta 2022, 535, 120862. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.-Y.; Zou, Y.; Li, T.-T.; Liu, H.; Wang, J.-J. Enhanced charge separation and conductivity of hematite enabled by versatile NiSe2 nanoparticles for improved photoelectrochemical water oxidation. Appl. Mater. Today 2022, 28, 101552. [Google Scholar] [CrossRef]
- Li, L.; Liang, P.; Liu, C.; Zhang, H.; Mitsuzaki, N.; Chen, Z. New method for improving the bulk charge separation of hematite with enhanced water splitting. Int. J. Hydrogen Energy 2019, 44, 4208–4217. [Google Scholar] [CrossRef]
- Xi, L.; Lange, K.M. Surface Modification of Hematite Photoanodes for Improvement of Photoelectrochemical Performance. Catalysts 2018, 8, 497. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Kawashima, K.; Yang, X.; Yin, X.; Zhan, F.; Liu, M.; Qiu, X.; Li, W.; Mullins, C.B.; et al. Modulating Charge Transfer Efficiency of Hematite Photoanode with Hybrid Dual-Metal–Organic Frameworks for Boosting Photoelectrochemical Water Oxidation. Adv. Sci. 2020, 7, 2002563. [Google Scholar] [CrossRef]
- Nasejje, S.; Mukhokosi, E.P.; Diale, M.; Velauthapillai, D. Device architectures for photoelectrochemical water splitting based on hematite: A review. Discov. Mater. 2024, 4, 44. [Google Scholar] [CrossRef]
- Peng, Y.; Ruan, Q.; Lam, C.H.; Meng, F.; Guan, C.-Y.; Santoso, S.P.; Zou, X.; Yu, E.T.; Chu, P.K.; Hsu, H.-Y. Plasma-implanted Ti-doped hematite photoanodes with enhanced photoelectrochemical water oxidation performance. J. Alloys Compd. 2021, 870, 159376. [Google Scholar] [CrossRef]
- Fu, Y.; Dong, C.-L.; Zhou, W.; Lu, Y.-R.; Huang, Y.-C.; Liu, Y.; Guo, P.; Zhao, L.; Chou, W.-C.; Shen, S. A ternary nanostructured α-Fe2O3/Au/TiO2 photoanode with reconstructed interfaces for efficient photoelectrocatalytic water splitting. Appl. Catal. B Environ. 2020, 260, 118206. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Li, Y.; Xue, S.; Wang, Y. Hematite Photoanodes Decorated with a Zn-doped Fe2O3 Catalyst for Efficient Photoelectrochemical Water Oxidation. Int. J. Electrochem. Sci. 2022, 17, 22106. [Google Scholar] [CrossRef]
- Li, L.; Tang, H.; Chen, Y.; Yang, R.; Tian, D.; Chen, Z. Effect of Lactic Acid on the Photoelectrocatalytic Water Splitting of Hematite Prepared by Hydrothermal Method. Electron. Mater. Lett. 2020, 16, 481–490. [Google Scholar] [CrossRef]
- Chong, R.; Wang, Z.; Fan, M.; Wang, L.; Chang, Z.; Zhang, L. Hematite decorated with nanodot-like cobalt (oxy)hydroxides for boosted photoelectrochemical water oxidation. J. Colloid Interface Sci. 2023, 629, 217–226. [Google Scholar] [CrossRef]
- Xie, H.; Song, Y.; Jiao, Y.; Gao, L.; Shi, S.; Wang, C.; Hou, J. Engineering Surface Passivation and Hole Transport Layer on Hematite Photoanodes Enabling Robust Photoelectrocatalytic Water Oxidation. ACS Nano 2024, 18, 5712–5722. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Li, D.; Wang, X.; Li, C. Surface Passivation Effect of Ferrihydrite with Hole-Storage Ability in Water Oxidation on BiVO4 Photoanode. J. Phys. Chem. C 2021, 125, 8369–8375. [Google Scholar] [CrossRef]
- Jeon, T.H.; Park, C.; Kang, U.; Moon, G.-h.; Kim, W.; Park, H.; Choi, W. Photoelectrochemical water oxidation using hematite modified with metal-incorporated graphitic carbon nitride film as a surface passivation and hole transfer overlayer. Appl. Catal. B Environ. 2024, 340, 123167. [Google Scholar] [CrossRef]
- Cai, J.; Tang, X.; Zhong, S.; Li, Y.; Wang, Y.; Liao, Z.; Wang, J.; Mao, K.; Xie, Q. Elucidation the role of Co-MOF on hematite for boosting the photoelectrochemical performance toward water oxidation. Int. J. Hydrogen Energy 2023, 48, 12342–12353. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, Y.; Li, Y. Improved photoelectrochemical water splitting performance of Sn-doped hematite photoanode with an amorphous cobalt oxide layer. Int. J. Hydrogen Energy 2024, 51, 1176–1183. [Google Scholar] [CrossRef]
- Bai, P.; Xie, J.; Wang, H.; Kang, X.; Jiang, K.; Yang, C.; Wang, X. Enhancing photoelectrocatalytic water splitting performance through PdCoP co-modification of Ti:Fe2O3. Int. J. Hydrogen Energy 2024, 79, 130–138. [Google Scholar] [CrossRef]
- Niu, H.; Gao, L.; Liu, M.; Zou, Y.; Wang, J.; Hu, G.; Jin, J. Rapid charge extraction via hole transfer layer and interfacial coordination bonds on hematite photoanode for efficient photoelectrochemical water oxidation. Appl. Catal. B Environ. Energy 2024, 358, 124369. [Google Scholar] [CrossRef]
- Chen, H.; Wang, P.; Ye, H.; Yin, H.; Rao, L.; Luo, D.; Hou, X.; Zhou, G.; Nötzel, R. Vertically aligned InGaN nanowire arrays on pyramid textured Si (1 00): A 3D arrayed light trapping structure for photoelectrocatalytic water splitting. Chem. Eng. J. 2021, 406, 126757. [Google Scholar] [CrossRef]
- Singla, S.; Devi, P.; Basu, S. Revolutionizing the Role of Solar Light Responsive BiVO4/BiOBr Heterojunction Photocatalyst for the Photocatalytic Deterioration of Tetracycline and Photoelectrocatalytic Water Splitting. Materials 2023, 16, 5661. [Google Scholar] [CrossRef]
- Ramgopal, N.C.; Roy, N.; El-marghany, A.; Alhammadi, S.; Sreedevi, G.; Arla, S.K.; Merum, D.; Joo, S.W. Enhancing photo electrocatalytic water splitting efficiency using Bi2O2CO3@Ni(OH)2 composite with flower-like morphology. Ceram. Int. 2025, 51, 4388–4399. [Google Scholar] [CrossRef]
- Du, H.; Yang, C.; Pu, W.; Zhao, H.; Gong, J. Highly Active Sb2S3-Attached Mo–WO3 Composite Film for Enhanced Photoelectrocatalytic Water Splitting at Extremely Low Input Light Energy. ACS Sustain. Chem. Eng. 2019, 7, 9172–9181. [Google Scholar] [CrossRef]
- Zhu, Z.; Daboczi, M.; Chen, M.; Xuan, Y.; Liu, X.; Eslava, S. Ultrastable halide perovskite CsPbBr3 photoanodes achieved with electrocatalytic glassy-carbon and boron-doped diamond sheets. Nat. Commun. 2024, 15, 2791. [Google Scholar] [CrossRef]
- Mondal, S.; Banerjee, S.; Bera, S.; Mondal, S.; Midya, S.P.; Jana, R.; Behera, R.K.; Datta, A.; Pradhan, N.; Ghosh, P. CsPbBr3 Perovskite Polyhedral Nanocrystal Photocatalysts for Decarboxylative Alkylation via Csp3–H Bond Activation of Unactivated Ethers. ACS Catal. 2024, 14, 6633–6643. [Google Scholar] [CrossRef]
- Gong, W.; Li, Y.; Yang, Y.; Guo, H.; Niu, X. Enhanced charge transport from Pd-doping in CsPbBr3 quantum dots for efficient photoelectrocatalytic water splitting. J. Mater. Chem. C 2023, 11, 6963–6970. [Google Scholar] [CrossRef]
- Li, Y.; Feng, J.; Li, H.; Wei, X.; Wang, R.; Zhou, A. Photoelectrochemical splitting of natural seawater with α-Fe2O3/WO3 nanorod arrays. Int. J. Hydrogen Energy 2016, 41, 4096–4105. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Yao, B.; Liu, S.; Ren, J.; Wang, Y.; Fang, Z.; Wu, R.; Wei, S. Construction of WO3 quantum dots/TiO2 nanowire arrays type II heterojunction via electrostatic self-assembly for efficient solar-driven photoelectrochemical water splitting. Dalton Trans. 2023, 52, 6284–6289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Jiang, S.; Cai, M.; Zhang, F.; Yu, H. WO3/FeOOH heterojunction for improved charge carrier separation and efficient photoelectrochemical water splitting. J. Alloys Compd. 2024, 981, 173637. [Google Scholar] [CrossRef]
- Jia, S.; Fang, Y.; Liu, Z.; Tian, K.; Zhao, X.; Bai, S. Design and fabrication of double heterojunctions of WO3/BiVO4/Cu2O photoanode for photoelectrochemical water splitting. J. Photochem. Photobiol. A Chem. 2025, 461, 116156. [Google Scholar] [CrossRef]
- Bai, S.; Han, J.; Zhao, Y.; Chu, H.; Wei, S.; Sun, J.; Sun, L.; Luo, R.; Li, D.; Chen, A. rGO decorated BiVO4/Cu2O n-n heterojunction photoanode for photoelectrochemical water splitting. Renew. Energy 2020, 148, 380–387. [Google Scholar] [CrossRef]
- Wang, P.; Hao, Z.; Liu, Z. FeOOH interlayer with storing holes applied to construct WO3/FeOOH/Cu2O ternary heterojunction photoanode with dual built-in electric filed for efficient PEC cell. J. Alloys Compd. 2022, 917, 165496. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, M.; Jiao, H.; Jiang, H.-Y.; Tang, J. Conformal BiVO4/WO3 nanobowl array photoanode for efficient photoelectrochemical water splitting. Chin. J. Catal. 2022, 43, 2321–2331. [Google Scholar] [CrossRef]
- Li, Y.; Mei, Q.; Liu, Z.; Hu, X.; Zhou, Z.; Huang, J.; Bai, B.; Liu, H.; Ding, F.; Wang, Q. Fluorine-doped iron oxyhydroxide cocatalyst: Promotion on the WO3 photoanode conducted photoelectrochemical water splitting. Appl. Catal. B Environ. 2022, 304, 120995. [Google Scholar] [CrossRef]
- Sang, P.; Kim, J.H. Role of g-C3N4 in Fabrication of BiVO4/WO3 Z-scheme Heterojunction for high Photoelectrochemical Performances with Enhanced Light Harvesting. Int. J. Precis. Eng. Manuf. -Green Technol. 2023, 10, 1015–1026. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, Z.; Liu, Y.; Liu, N.; Li, Y.; Cheng, Y.; Yu, J.; Yu, R.; Wang, D.; Li, H. Shear-Strained Pd Single-Atom Electrocatalysts for Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2024, 63, e202411396. [Google Scholar] [CrossRef]




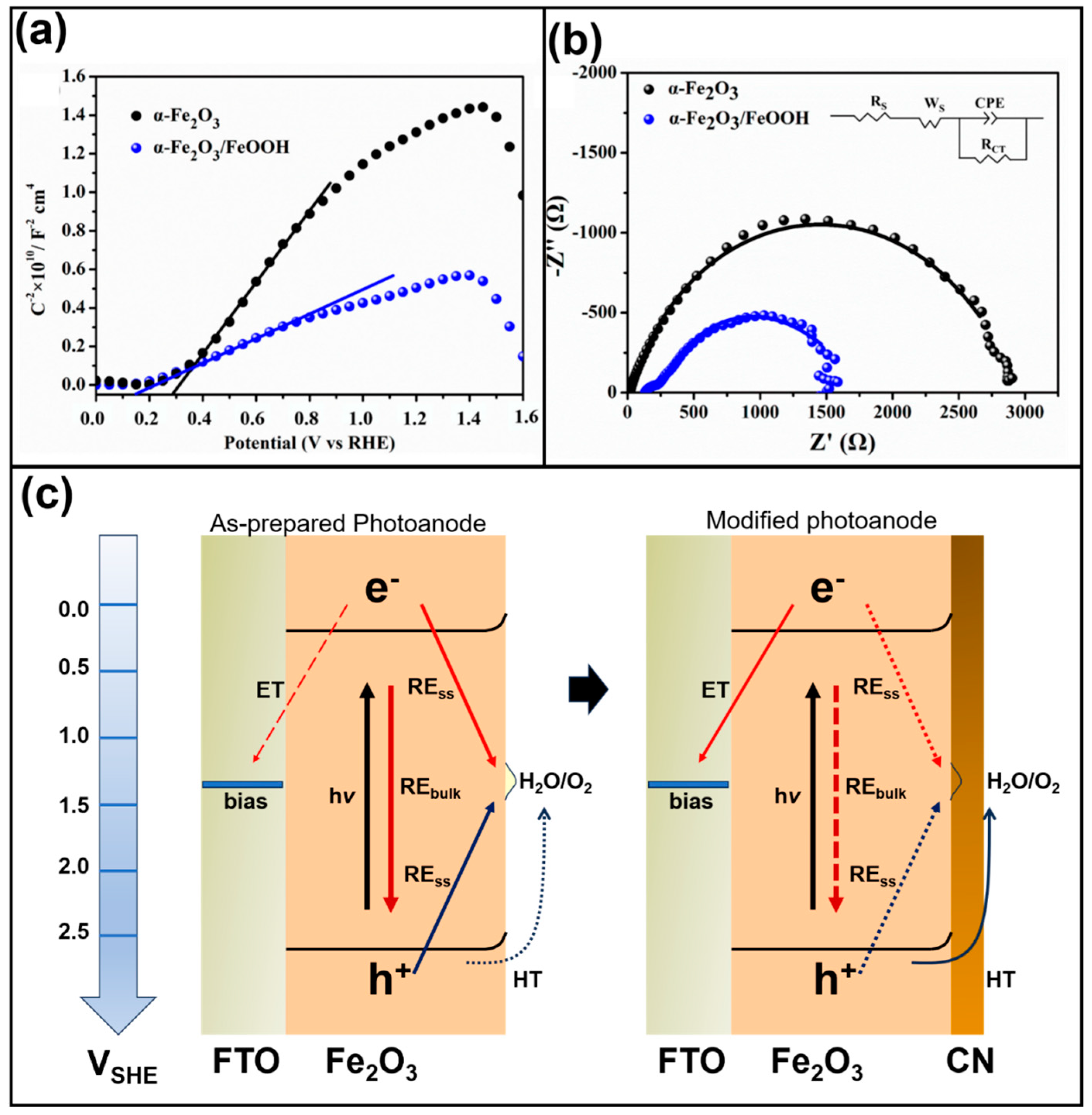
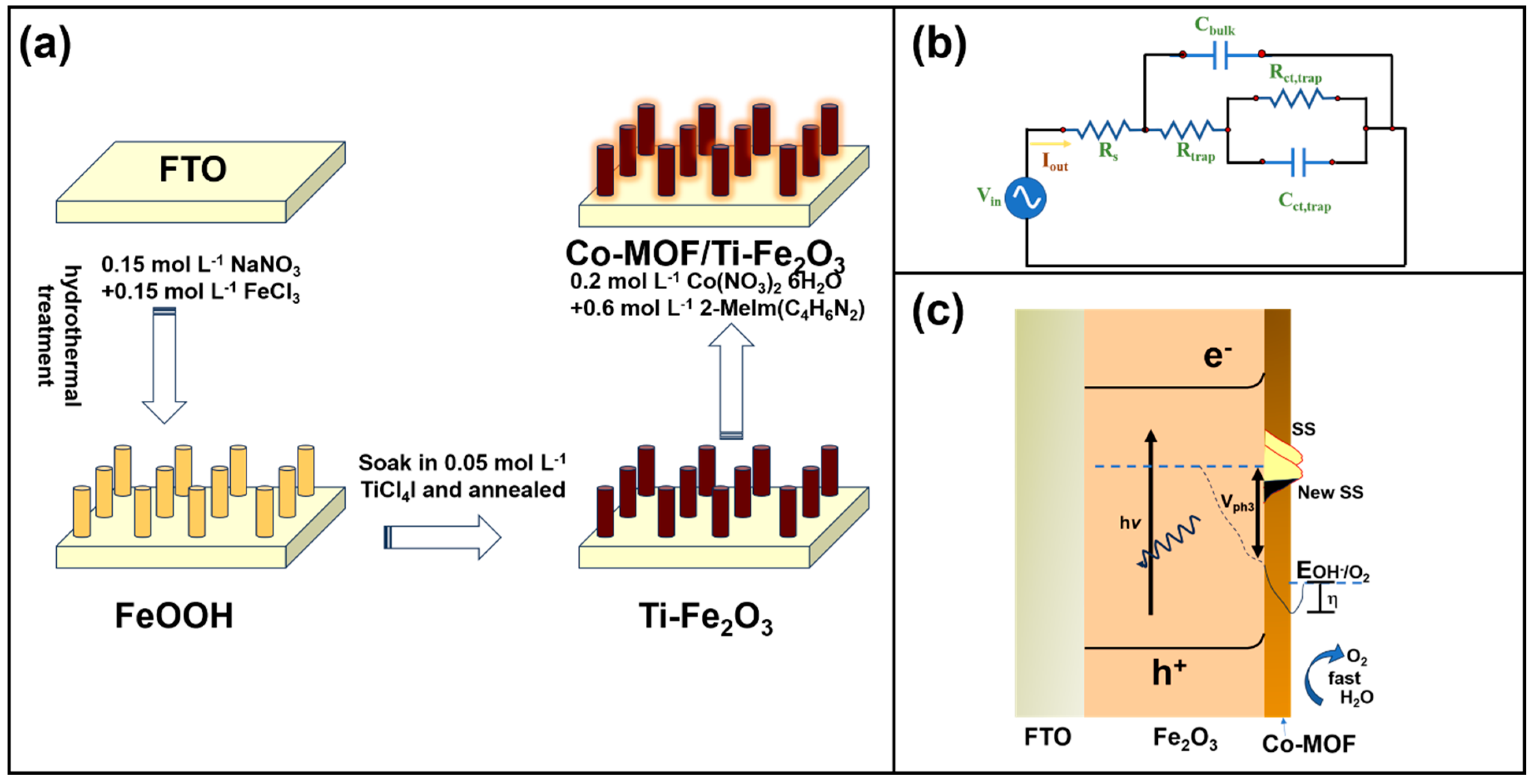
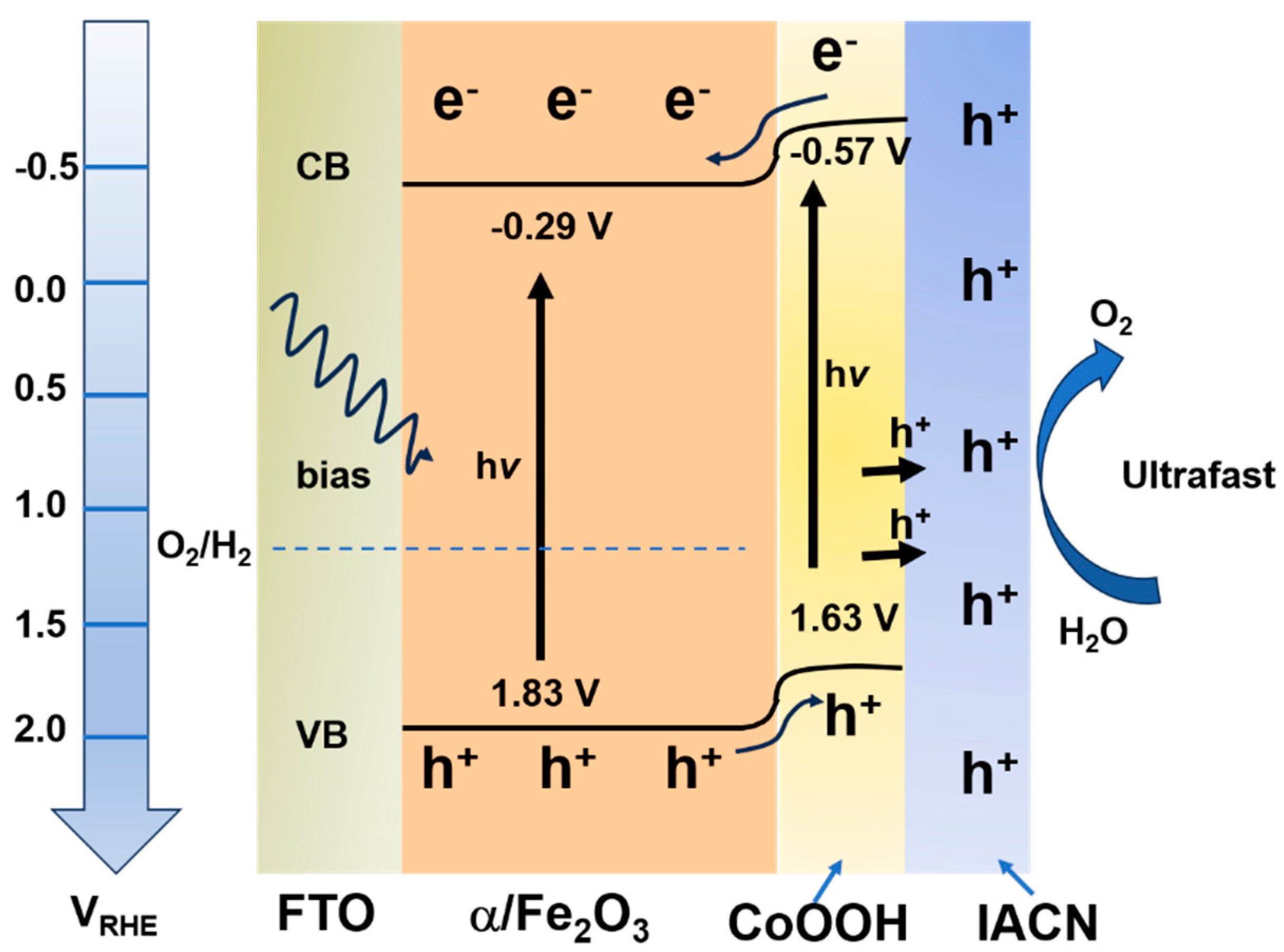
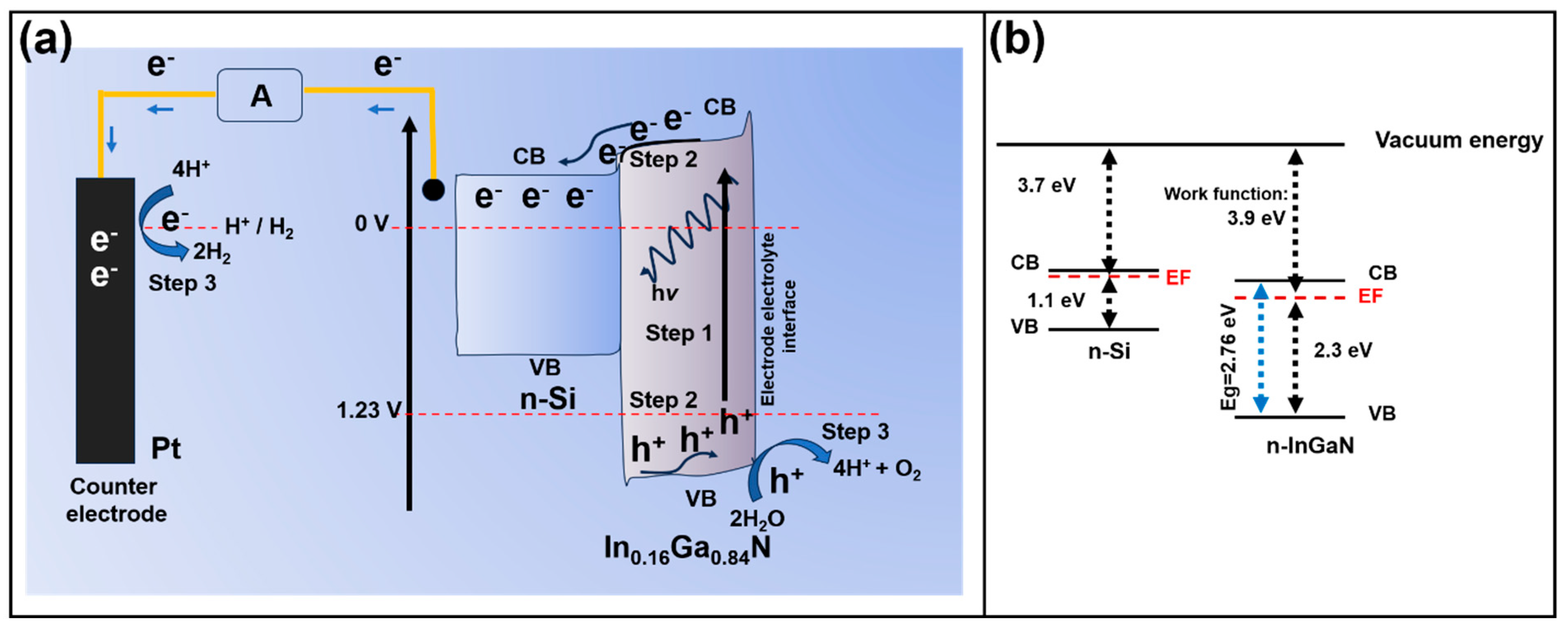
| Material | Structure | Synthesis Process | Electrolyte | Photocurrent Density (mA cm−2)@1.23 V | Light Source | Reference |
|---|---|---|---|---|---|---|
| Sn-Fe2O3/CoOx-ST | Nanowires | Hydrothermal and two-step solvothermal | 1 mol L−1 KOH | 1.40 | 300 W Xe lamp | [105] |
| PdCoP-Ti:Fe2O3 | Nanorods | Hydrothermal and thermal treatment | 1 mol L−1 NaOH | 2.82 | 300 W Xe lamp | [106] |
| α-Fe2O3/WO3 | Nanorods | Hydrothermal treatment and deposition-annealing. | 0.1 mol L−1 Na2SO4 | 1 | 300 W Xe lamp | [115] |
| WO3 quantum dots/TiO2 | Nanowire | Hydrothermal and photoreduction | 1 mol L−1 Na2SO4 | 1.5 | AM 1.5 G | [116] |
| BiVO4/BiOBr | Petal-like morphology | Hydrothermal | 0.5 M Na2SO4 | 0.2198 | 100 mWcm−2 | [109] |
| (Ti)-doped α-Fe2O3 | Nanoparticles | Hydrothermal, plasma ion implantation, and post-annealing | 1 mol L−1 NaOH | 0.55 | 300 W Xe lamp | [96] |
| WO3/FeOOH | Nanoplate | Hydrothermal spin-coated | 0.1 M Na2SO4 | 2.63 | 300 W Xe lamp | [117] |
| WO3/BiVO4/Cu2O | Nanoworm-like, nanoparticles | Dropcastin and electrodeposition | 0.5 M Na2SO4 | 5 | xenon lamp, 100 mWcm−2 | [118] |
| BiVO4/rGO/ Cu2O | Nanoparticle | Metal-organic decomposition and electrodeposition | 0.5 M Na2SO4 | 3 | 300 W Xe lamp | [119] |
| WO3/FeOOH/Cu2O | Nanoplates | Hydrothermal, precipitation, and electrodeposition | 0.2 M of Na2SO4 | 2.14 | 500 W Xe lamp | [120] |
| BiVO4/WO3 | Nanobowl array | Lithography and two-step electro-deposition | 0.2 mol L−1 Na2SO4 | 3.05 | 300 W Xe lamp | [121] |
| F:FeOOH/BiVO4/WO3 | Nanoplates | Spinning calcination and and hydrothermal | phosphate buffer + 1 mol−1 sodium sulfite | 3.1 | 300 W Xe lamp | [122] |
| CN-BiVO4/WO3 | Nanoparticles | Sol-gel spin-coating | 0.5 mol L−1 Na2SO4 | 0.538 | 150 W Xe lamp | [123] |
| Co-MOF/Ti-Fe2O3 | Nanoparticles | Hydrothermal, calcination, and impregnation | 1 mol L−1 NaOH | 1.01 | 300 W Xe lamp | [104] |
| CoOOH/Fe2O3 | Nanorods | Hydrothermal combined two-step calcination and solvothermal | 1 mol L−1 NaOH | 1.92 | 300 W Xe lamp | [100] |
| CN-FeNiOOH-CoOOH | Nanoparticle aggregates | One-step evaporation, spin-coating, photodeposition, and drop-casting. | 1 mol L−1 KOH | 3.5 | 300 W Xe arc lamp | [103] |
| α-Fe2O3/Au/TiO2 | Nanorods | Sputtering and pulsed laser deposition | 1 mol L−1 NaOH | 1.05 | 100 mW cm−2 | [97] |
| IACN/CoOOH/Fe2O3 | Nanorods | Hydrothermal | 1 mol L−1 KOH | 2.02 | 300 W Xe arc lamp | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pech-Rodríguez, W.J.; Şahin, N.E.; Suarez-Velázquez, G.G.; Meléndez-González, P.C. Semiconductor-Based Photoelectrocatalysts in Water Splitting: From the Basics to Mechanistic Insights—A Brief Review. Materials 2025, 18, 1952. https://doi.org/10.3390/ma18091952
Pech-Rodríguez WJ, Şahin NE, Suarez-Velázquez GG, Meléndez-González PC. Semiconductor-Based Photoelectrocatalysts in Water Splitting: From the Basics to Mechanistic Insights—A Brief Review. Materials. 2025; 18(9):1952. https://doi.org/10.3390/ma18091952
Chicago/Turabian StylePech-Rodríguez, W. J., Nihat Ege Şahin, G. G. Suarez-Velázquez, and P. C. Meléndez-González. 2025. "Semiconductor-Based Photoelectrocatalysts in Water Splitting: From the Basics to Mechanistic Insights—A Brief Review" Materials 18, no. 9: 1952. https://doi.org/10.3390/ma18091952
APA StylePech-Rodríguez, W. J., Şahin, N. E., Suarez-Velázquez, G. G., & Meléndez-González, P. C. (2025). Semiconductor-Based Photoelectrocatalysts in Water Splitting: From the Basics to Mechanistic Insights—A Brief Review. Materials, 18(9), 1952. https://doi.org/10.3390/ma18091952










