Molecular Ir-Based Coordination Compound Grafted onto Covalent Organic Framework for Efficient Photocatalytic H2 Evolution
Abstract
1. Introduction
2. Experimental
2.1. Photocatalytic Hydrogen Production
2.2. Characterization
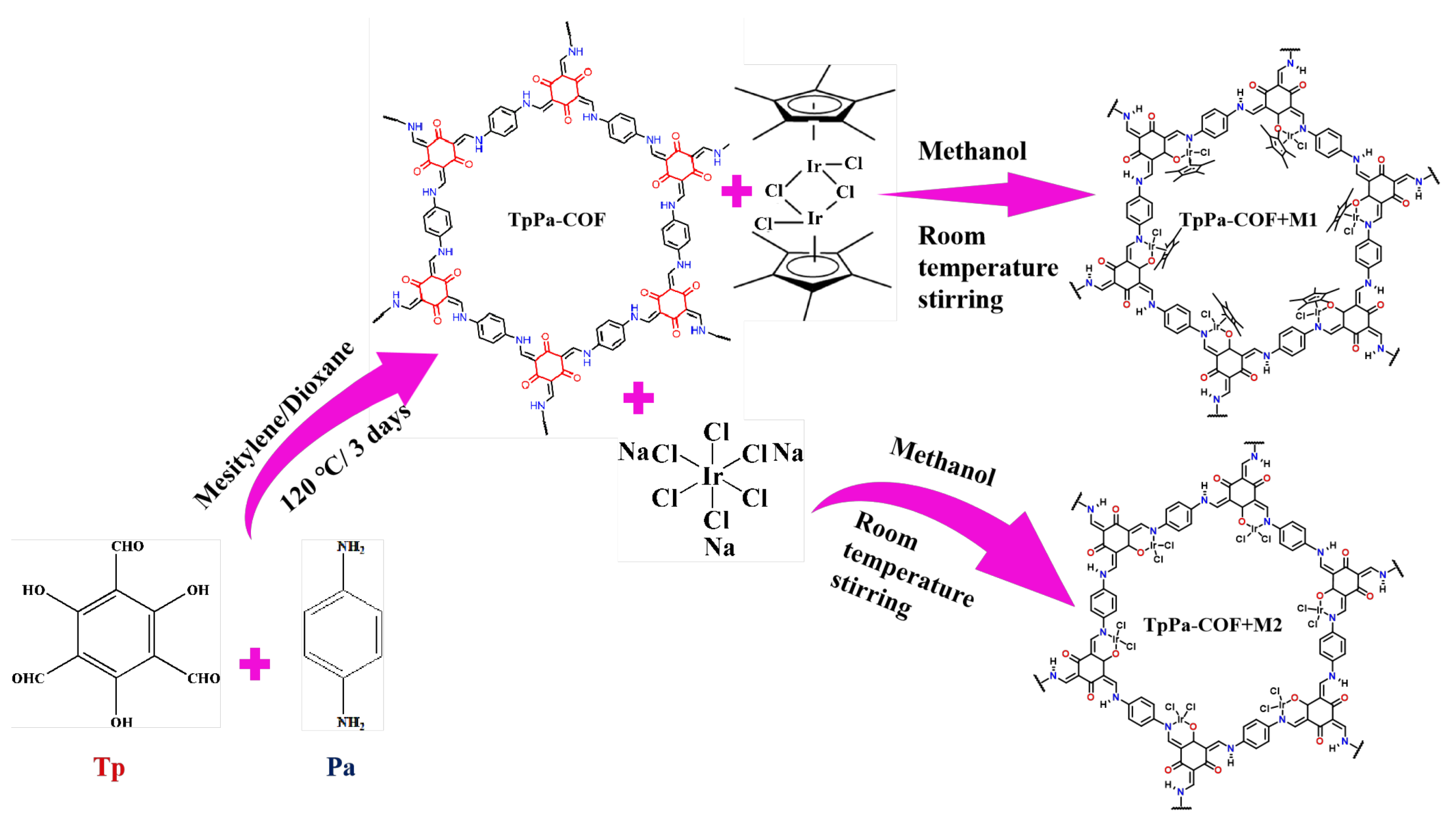
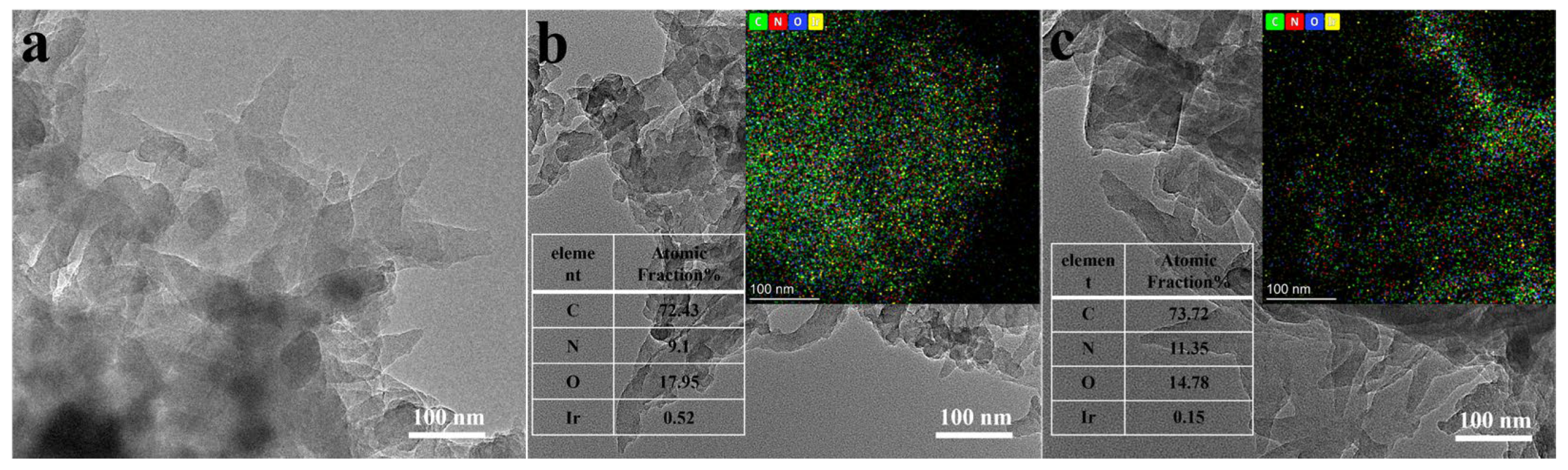
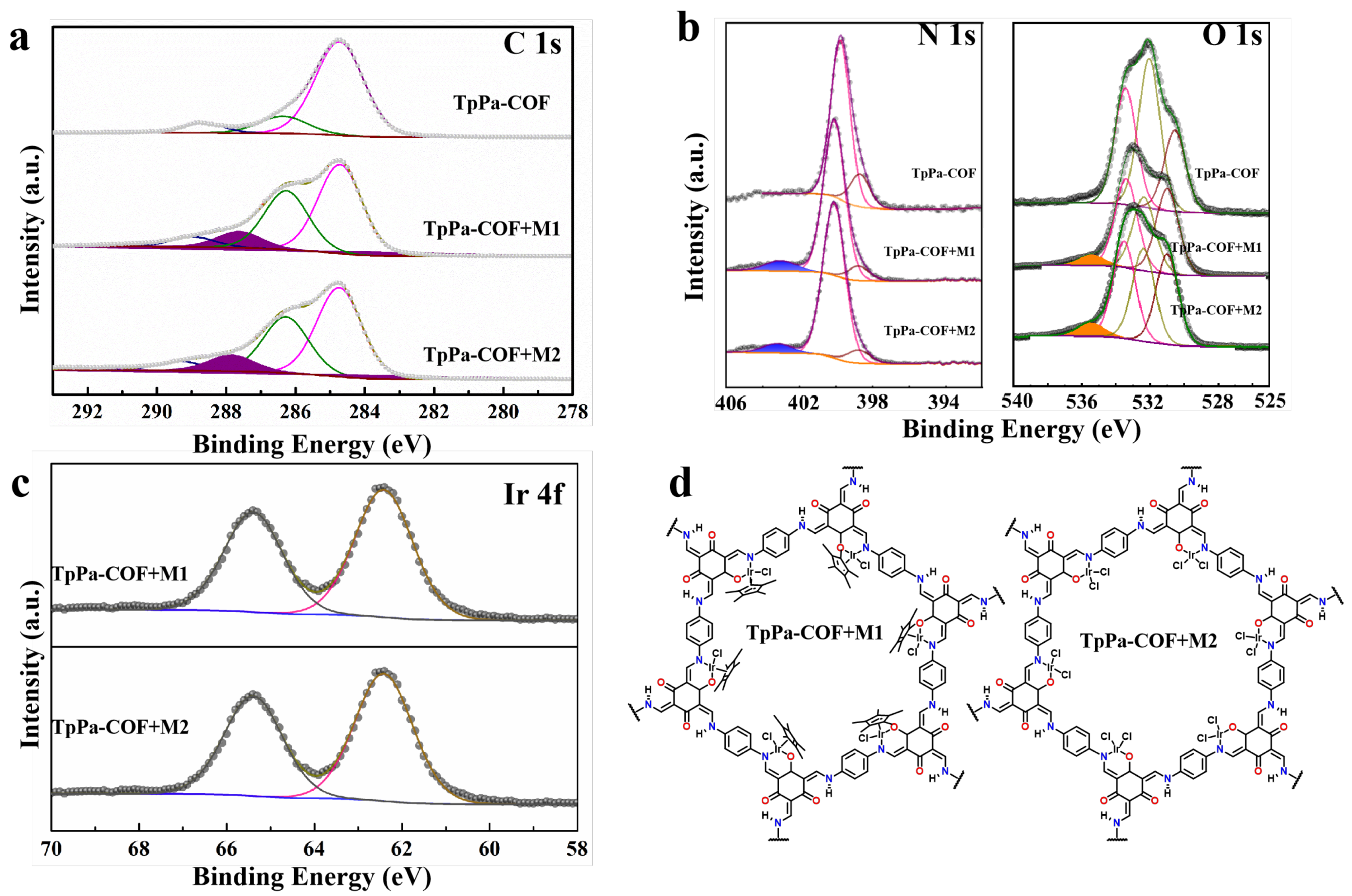
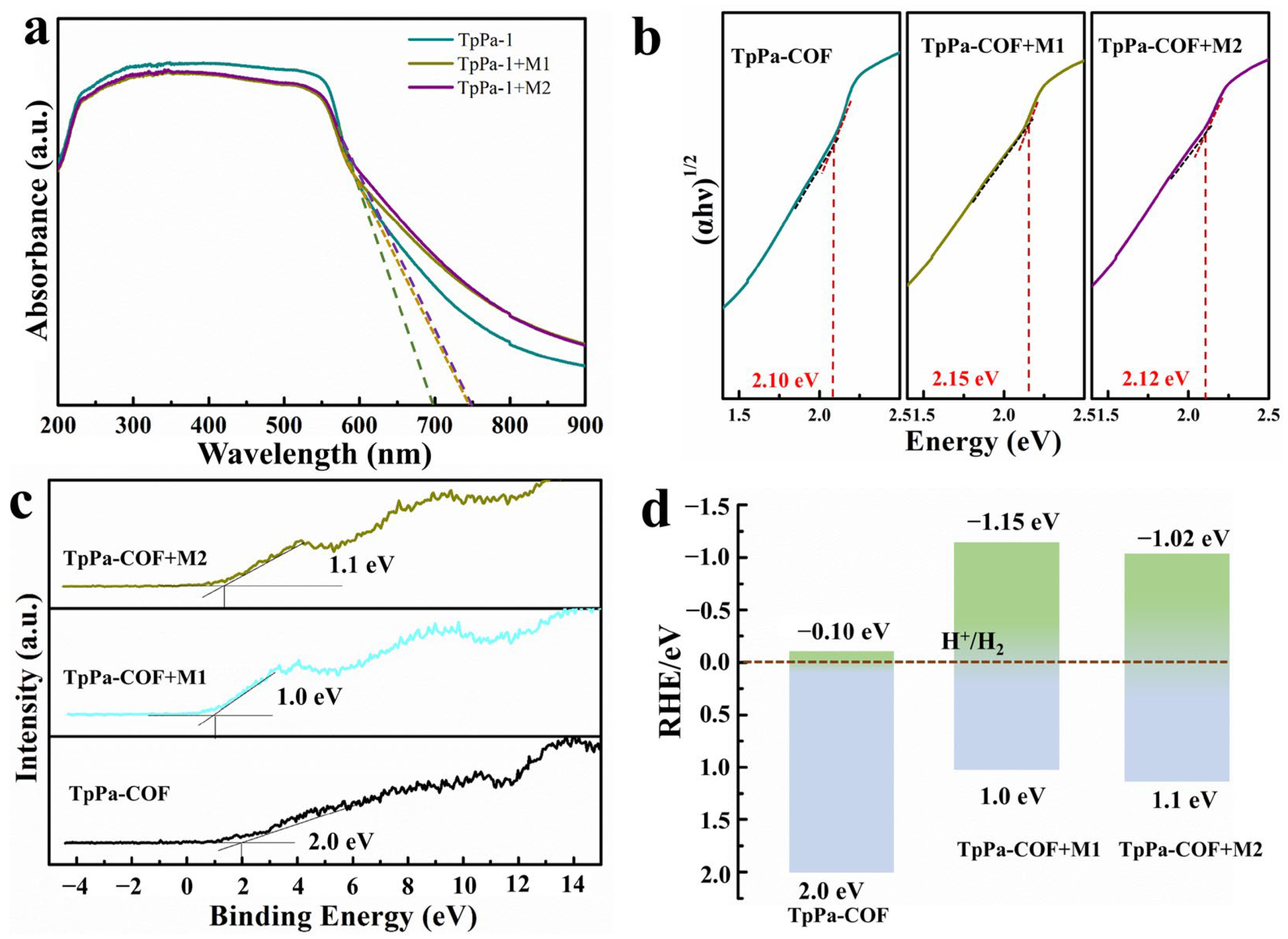
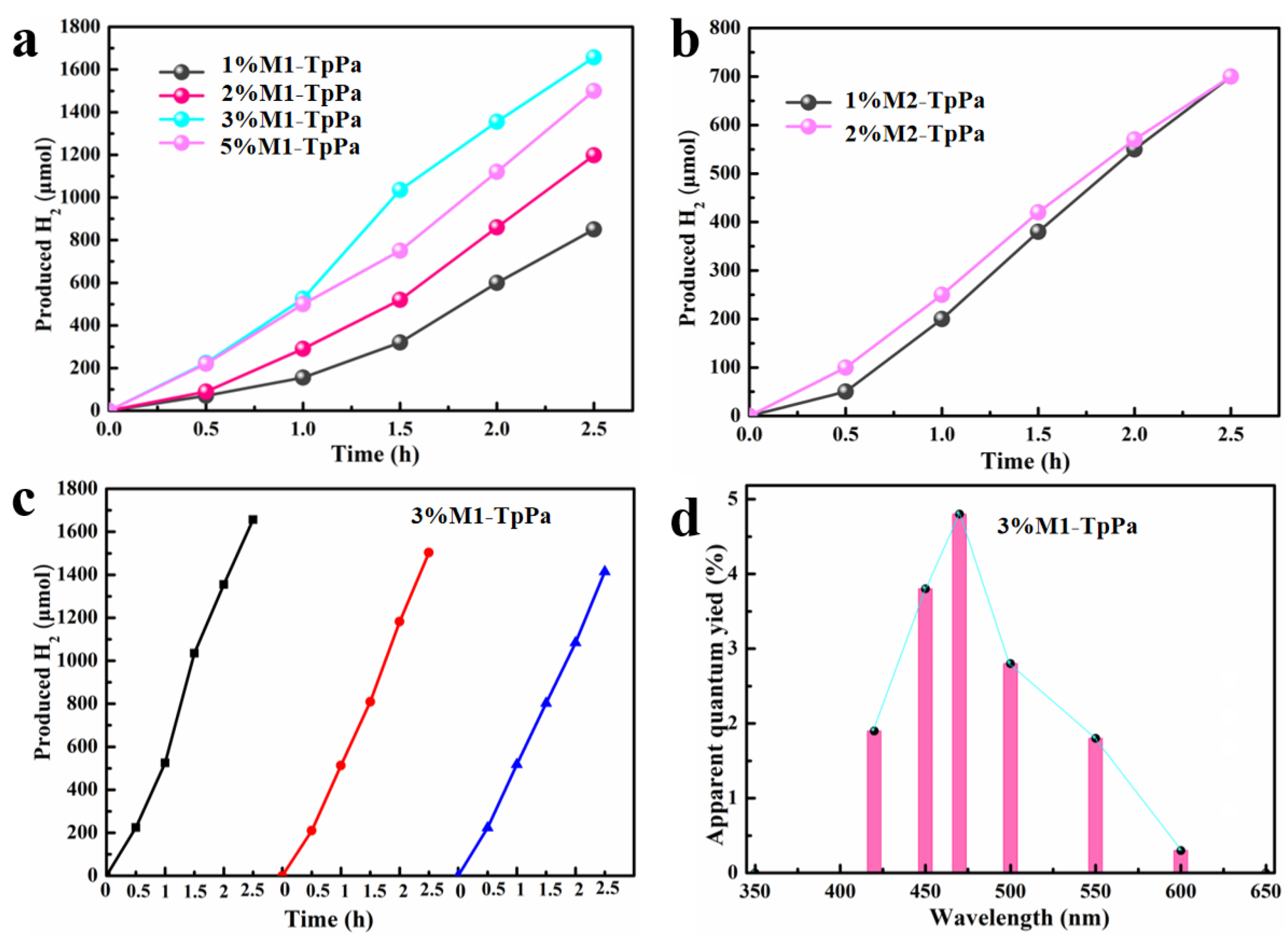
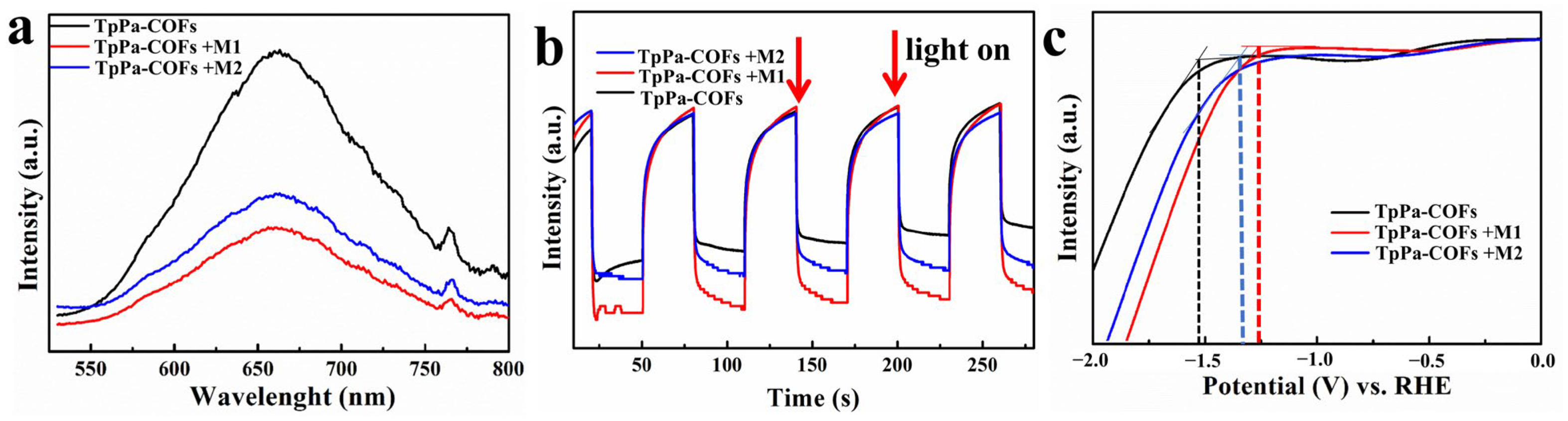
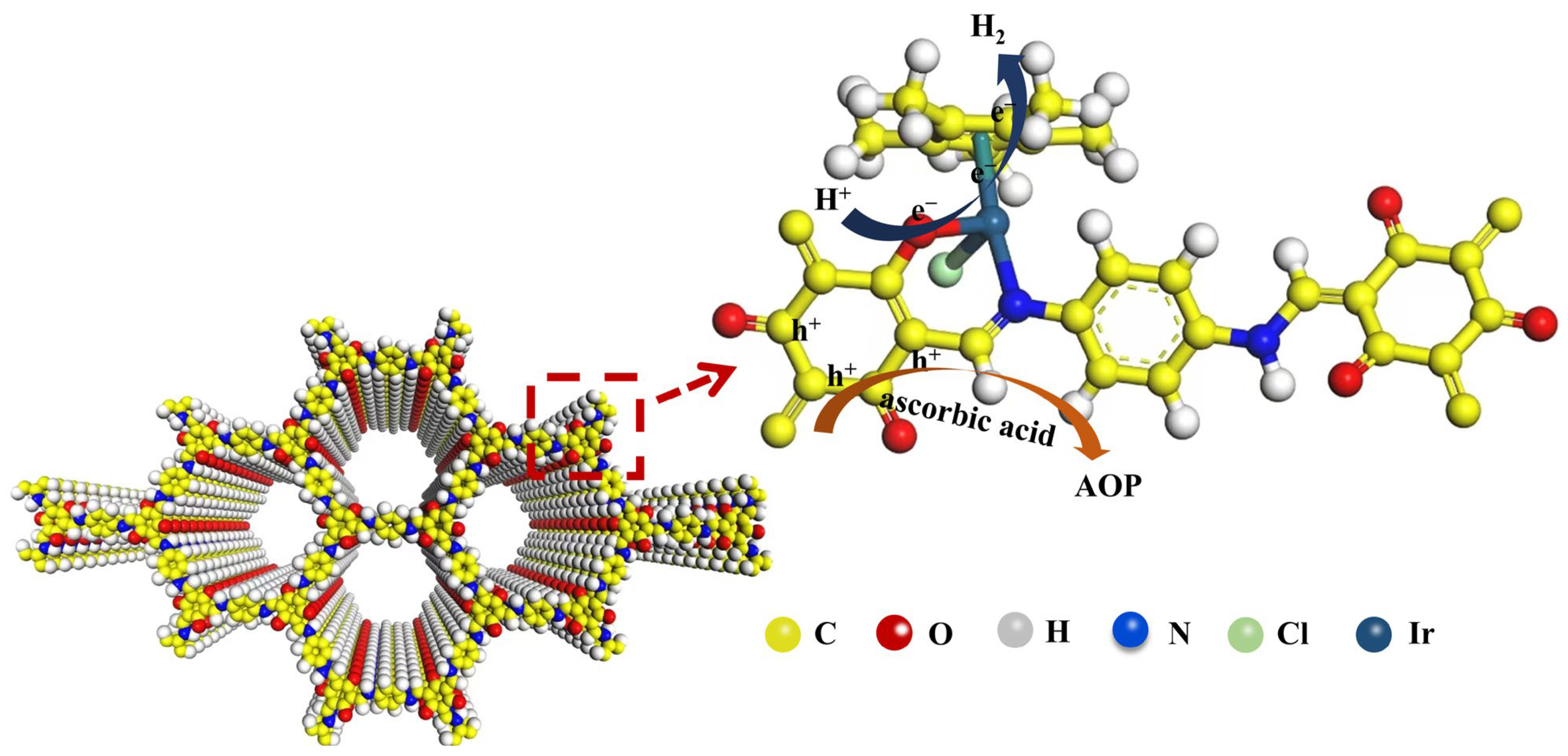
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klingler, S.; Bagemihl, B.; Mengele, A.K.; Kaufhold, S.; Myllyperkiö, P.; Ahokas, J.; Pettersson, M.; Rau, S.; Mizaikoff, B. Rationalizing In Situ Active Repair in Hydrogen Evolution Photocatalysis via Non-Invasive Raman Spectroscopy. Angew. Chem. Int. Ed. 2023, 62, e202306287. [Google Scholar] [CrossRef]
- Silver, S.C.; Niklas, J.; Du, P.; Poluektov, O.G.; Tiede, D.M.; Utschig, L.M. Protein Delivery of a Ni Catalyst to Photosystem I for Light-Driven Hydrogen Production. J. Am. Chem. Soc. 2013, 135, 13246–13249. [Google Scholar] [CrossRef]
- Sun, K.; Qian, Y.; Li, D.; Jiang, H.L. Reticular Materials for Photocatalysis. Adv. Mater. 2024, 2411118. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Qi, R.; Cao, C.; Liu, X.; Zheng, Y.; Song, W. Enhanced electron separation on in-plane benzene-ring doped g-C3N4 nanosheets for visible light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 244, 459–464. [Google Scholar] [CrossRef]
- Abhishek, B.; Jayarama, A.; Rao, A.S.; Nagarkar, S.S.; Dutta, A.; Duttagupta, S.P.; Prabhu, S.S.; Pinto, R. Challenges in photocatalytic hydrogen evolution: Importance of photocatalysts and photocatalytic reactors. Int. J. Hydrogen Energy 2024, 81, 1442–1466. [Google Scholar]
- Yu, W.; Hu, C.; Bai, L.; Tian, N.; Zhang, Y.; Huang, H. Photocatalytic hydrogen peroxide evolution: What is the most effective strategy? Nano Energy 2022, 104, 107906. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Bi, J.-N.; Pan, K.-Y.; Chiao, Y.-C. Microwave-Assisted Synthesis of SnO2@ZnIn2S4 Composites for Highly Efficient Photocatalytic Hydrogen Evolution. Materials 2024, 17, 2367. [Google Scholar] [CrossRef]
- Dai, C.; Liu, B. Conjugated polymers for visible-light-driven photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Fang, Y.; Hou, Y.; Fu, X.; Wang, X. Semiconducting Polymers for Oxygen Evolution Reaction under Light Illumination. Chem. Rev. 2022, 122, 4204–4256. [Google Scholar] [CrossRef]
- Sun, L.; Han, L.; Huang, J.; Luo, X.; Li, X. Single-atom catalysts for photocatalytic hydrogen evolution: A review. Int. J. Hydrogen Energy 2022, 47, 17583–17599. [Google Scholar] [CrossRef]
- Chu, X.; Sathish, C.I.; Yang, J.H.; Guan, X.; Zhang, X.; Qiao, L.; Domen, K.; Wang, S.; Vinu, A.; Yi, J. Strategies for Improving the Photocatalytic Hydrogen Evolution Reaction of Carbon Nitride-Based Catalysts. Small 2023, 19, 2302875. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X. Sub-nanometric materials: Electron transfer, delocalization, and beyond. Chem Catal. 2022, 2, 1257–1266. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, S.; Wang, X. Cluster–Nuclei Coassembled One-Dimensional Subnanometer Heteronanostructures. Nano Lett. 2021, 21, 9845–9852. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y. Impact of Interfaces on the Performance of Covalent Organic Frameworks for Photocatalytic Hydrogen Production. Small 2024, 21, 2408395. [Google Scholar] [CrossRef]
- Yang, F.; Qu, J.; Zheng, Y.; Cai, Y.; Yang, X.; Li, C.M.; Hu, J. Recent advances in high-crystalline conjugated organic polymeric materials for photocatalytic CO2 conversion. Nanoscale 2022, 14, 15217–15241. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, R.; Kim, Y. Single-Atom Catalysts on Covalent Organic Frameworks for Energy Applications. ACS Appl. Mater. Interfaces 2024, 16, 66874–66899. [Google Scholar] [CrossRef]
- Li, Y.; Song, X.; Zhang, G.; Wang, L.; Liu, Y.; Chen, W.; Chen, L. 2D Covalent Organic Frameworks Toward Efficient Photocatalytic Hydrogen Evolution. ChemSusChem 2022, 15, e202200901. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Niu, H.; Sun, Q.; Zhao, R.; Li, N.; Cai, Y. Significant improvement of photocatalytic hydrogen evolution performance in covalent organic frameworks: Substituent fine-tuning. J. Mater. Chem. A 2024, 12, 11416–11423. [Google Scholar] [CrossRef]
- Wang, S.-D.; Huang, L.-Y.; Xue, L.-J.; Kang, Q.; Wen, L.-L.; Lv, K.-L. Sulfur-vacancy-modified ZnIn2S4/TpPa-1 S-scheme heterojunction with enhanced internal electric field for boosted photocatalytic hydrogen production. Appl. Catal. B Environ. Energy 2024, 358, 124366. [Google Scholar] [CrossRef]
- Yang, D.; Li, Z.-G.; Zhang, X.; Ren, Z.; Lu, W.; Liu, H.; Guo, X.; Zhang, J.; Bu, X.-H. Rational design of ZnCdS/TpPa-1-COF heterostructure photocatalyst by strengthening the interface connection in solar hydrogen production reactions. Nano Res. 2023, 17, 1027–1034. [Google Scholar] [CrossRef]
- Yin, L.; Zhao, Y.; Xing, Y.; Tan, H.; Lang, Z.; Ho, W.; Wang, Y.; Li, Y. Structure-Property relationship in β-keto-enamine-based covalent organic frameworks for highly efficient photocatalytic hydrogen production. Chem. Eng. J. 2021, 419, 129984. [Google Scholar] [CrossRef]
- Yan, G.; Sun, X.; Zhang, K.; Zhang, Y.; Li, H.; Dou, Y.; Yuan, D.; Huang, H.; Jia, B.; Li, H.; et al. Integrating Covalent Organic Framework with Transition Metal Phosphide for Noble-Metal-Free Visible-Light-Driven Photocatalytic H2 Evolution. Small 2022, 18, 2201340. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Y.; Zhang, A.; Cheng, T.; Xi, X.; Zhang, J. Platinum Single Atoms Anchored on a Covalent Organic Framework: Boosting Active Sites for Photocatalytic Hydrogen Evolution. ACS Catal. 2021, 11, 13266–13279. [Google Scholar] [CrossRef]
- Shao, M.; Chen, H.; Hao, S.; Liu, H.; Cao, Y.; Zhao, Y.; Jin, J.; Dang, H.; Meng, Y.; Huo, Y.; et al. N-doped vanadium carbide combined with Pt as a multifunctional cocatalyst to boost photocatalytic hydrogen production. Appl. Surf. Sci. 2022, 577, 151857. [Google Scholar] [CrossRef]
- Yao, Y.-H.; Yang, Y.; Wang, Y.; Zhang, H.; Tang, H.-L.; Zhang, H.-Y.; Zhang, G.; Wang, Y.; Zhang, F.-M.; Yan, H. Photo-induced synthesis of ternary Pt/rGO/COF photocatalyst with Pt nanoparticles precisely anchored on rGO for efficient visible-light-driven H2 evolution. J. Colloid Interface Sci. 2022, 608, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, Y.; Qu, B.; Bai, L.; Liu, Y.; Yang, Z.D.; Zhang, W.; Jing, L.; Fu, H. Construction of Six-Oxygen-Coordinated Single Ni Sites on g-C3N4 with Boron-Oxo Species for Photocatalytic Water-Activation-Induced CO2 Reduction. Adv. Mater. 2021, 33, 2105482. [Google Scholar] [CrossRef]
- Lazaar, N.; Wu, S.; Qin, S.; Hamrouni, A.; Bikash Sarma, B.; Doronkin, D.E.; Denisov, N.; Lachheb, H.; Schmuki, P. Single-Atom Catalysts on C3N4: Minimizing Single Atom Pt Loading for Maximized Photocatalytic Hydrogen Production Efficiency. Angew. Chem. Int. Ed. 2025, 64, e202416453. [Google Scholar] [CrossRef]
- Xiao, X.; Gao, Y.; Zhang, L.; Zhang, J.; Zhang, Q.; Li, Q.; Bao, H.; Zhou, J.; Miao, S.; Chen, N.; et al. A Promoted Charge Separation/Transfer System from Cu Single Atoms and C3N4 Layers for Efficient Photocatalysis. Adv. Mater. 2020, 32, 2003082. [Google Scholar] [CrossRef]
- Qiu, Z.; Luo, Z.; Zhou, T.; Zi, B.; Chen, M.; Hu, R.; Lv, T.; He, T.; Ma, Y.; Zhang, J.; et al. Oxygen vacancy enriched and Cu single-atom contained covalent organic frameworks: A competitive photocatalyst to promote hydrogen evolution under visible light. Mater. Today Energy 2025, 47, 101750. [Google Scholar] [CrossRef]
- Fang, K.; Chen, Z.; Wei, Y.; Fang, S.; Dong, Z.; Zhang, Y.; Li, W.; Wang, L. Single site Co-S anchored on carbon nitride as a highly active cocatalyst for photocatalytic hydrogen evolution. J. Alloys Compd. 2022, 925, 166257. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.; Kailath, A.J.; Sahu, R.K. Mechanistic investigation of hydrogen generation from water and magnesium catalyst reaction: Advanced reactive molecular dynamics simulation. Int. J. Hydrogen Energy 2024, 52, 1440–1445. [Google Scholar] [CrossRef]
- Messori, A.; Martelli, G.; Piazzi, A.; Basile, F.; De Maron, J.; Fasolini, A.; Mazzoni, R. Molecular Ruthenium Cyclopentadienone Bifunctional Catalysts for the Conversion of Sugar Platforms to Hydrogen. ChemPlusChem 2023, 88, e202300357. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-B.; Chu, Y.; Xie, P.; Xiong, L.; Wang, N.; Wang, H.; Tang, J. Molecular Cobalt Catalysts Grafted onto Polymers for Efficient Hydrogen Generation Cathodes. Sol. RRL 2020, 5, 2000281. [Google Scholar] [CrossRef]
- Bodedla, G.B.; Tritton, D.N.; Chen, X.; Zhao, J.; Guo, Z.; Cham-Fai Leung, K.; Wong, W.-Y.; Zhu, X. Correction to Cocatalyst-free Photocatalytic Hydrogen Evolution with Simple Heteroleptic Iridium(III) Complexes. ACS Appl. Energy Mater. 2021, 4, 6374. [Google Scholar] [CrossRef]
- Yao, X.; Fan, L.; Zhang, Q.; Zheng, C.; Yang, X.; Lu, Y.; Jiang, Y. Impact of Anchoring Groups on the Photocatalytic Performance of Iridium(III) Complexes and Their Toxicological Analysis. Molecules 2024, 29, 2564. [Google Scholar] [CrossRef]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.L.; Dong, H.; Meng, X.B.; Tang, H.L.; Yao, Y.H.; Liu, D.Q.; Bai, L.L.; Zhang, F.M.; Wei, J.Z.; Sun, X.J. Effect of Different Functional Groups on Photocatalytic Hydrogen Evolution in Covalent-Organic Frameworks. ChemCatChem 2019, 11, 2313–2319. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Li, H.; Wu, K.; Wang, J.; Yang, Q. Covalent organic frameworks with high quantum efficiency in sacrificial photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 2357. [Google Scholar] [CrossRef]
- Ming, J.; Liu, A.; Zhao, J.; Zhang, P.; Huang, H.; Lin, H.; Xu, Z.; Zhang, X.; Wang, X.; Hofkens, J.; et al. Hot pi-Electron Tunneling of Metal-Insulator-COF Nanostructures for Efficient Hydrogen Production. Angew. Chem. Int. Ed. Engl. 2019, 58, 18290–18294. [Google Scholar] [CrossRef]
- He, W.; Kong, K.; Wang, M.; Dong, B.; Yuan, D.; Bryliakov, K.P.; Wang, R. Photoelectron migration monitored by 3D orbital electron configuration of spinel cocatalysts for covalent organic framework-based photocatalytic hydrogen evolution. Appl. Catal. B Environ. Energy 2024, 350, 123916. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, W.; Zhang, G.; Chen, Y. Interface molecular wires induce electron transfer from COFs to Pt for enhanced photocatalytic H2 evolution. J. Mater. Chem. A 2023, 11, 26052–26062. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, D.; Guo, M.; Yu, J.; Zhang, S.; Zhang, Z.; Chen, Y. Engineering Olefin-Linked Covalent Organic Frameworks for Photoenzymatic Reduction of CO2. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200261. [Google Scholar] [CrossRef]
- Ding, J.; Li, Z.; Wang, Y.; Liu, Y.; Li, F.; Yu, X.; Huang, P.; Wang, Y. Ir doping improved oxygen activation of WO3 for boosting acetone sensing performance at low working temperature. Appl. Surf. Sci. 2025, 679, 161239. [Google Scholar] [CrossRef]
- Yan, M.; Jiang, F.; Wu, Y. Metal-free 2D-2D black phosphorus/covalent organic framework p-n heterojunction for efficient visible-light-driven hydrogen evolution without cocatalysts. Int. J. Hydrogen Energy 2023, 48, 8867–8876. [Google Scholar] [CrossRef]
- Patrycja, M.; Michał, P.; Wojciech, M. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV−Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar]
- Wang, Y.; Huang, Y.; Liu, S.; Cui, S.; Zhang, Y.; Deng, P. Iridium complex modified MOFs for enhancing photocatalytic hydrogen evolution. Energy Adv. 2024, 3, 1414–1421. [Google Scholar] [CrossRef]
- Matt, B.; Fize, J.; Moussa, J.; Amouri, H.; Pereira, A.; Artero, V.; Izzet, G.; Proust, A. Charge Photo-Accumulation and Photocatalytic Hydrogen Evolution Under Visible Light at an Iridium(III)-Photosensitized Polyoxotungstate. Energy Environ. Sci. 2013, 6, 1504–1508. [Google Scholar] [CrossRef]
- Tritton, D.N.; Tang, F.-K.; Bodedla, G.B.; Lee, F.-W.; Kwan, C.-S.; Leung, K.C.-F.; Zhu, X.; Wong, W.-Y. Development and advancement of iridium(III)-based complexes for photocatalytic hydrogen evolution. Coord. Chem. Rev. 2022, 459, 214390. [Google Scholar] [CrossRef]
- Li, C.-C.; Gao, M.-Y.; Sun, X.-J.; Tang, H.-L.; Dong, H.; Zhang, F.-M. Rational combination of covalent-organic framework and nano TiO2 by covalent bonds to realize dramatically enhanced photocatalytic activity. Appl. Catal. B Environ. 2020, 266, 118586. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Gao, Y.; Li, R.; An, K.; Wang, W.; Zhao, Z.; Xin, X.; Ren, H.; Jiang, Z. On-Surface Bottom-Up Construction of COF Nanoshells towards Photocatalytic H2 Production. Research 2021, 12, 9798564. [Google Scholar]
- Zhang, L.; Lu, X.; Sun, J.; Wang, C.; Dong, P. Insights into the plasmonic “hot spots” and efficient hot electron injection induced by Ag nanoparticles in a covalent organic framework for photocatalytic H2 evolution. J. Mater. Chem. A 2024, 12, 5392–5405. [Google Scholar] [CrossRef]
- Yao, Y.H.; Li, J.; Zhang, H.; Tang, H.L.; Fang, L.; Niu, G.D.; Sun, X.J.; Zhang, F.M. Facile Synthesis of Covalently Connected rGO-COF Hybrid Material by In-Situ Reaction for Enhanced Visible-light Induced Photocatalytic H2 Evolution. J. Mater. Chem. A 2020, 8, 8949–8956. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Tang, H.L.; Dong, H.; Gao, M.Y.; Li, C.C.; Sun, X.J.; Wei, J.Z.; Qu, Y.; Li, Z.J.; Zhang, F.M. Covalent-Organic Framework Based Z-Scheme Heterostructured Noble-Metal-Free Photocatalysts for Visible-Light-Driven Hydrogen Evolution. J. Mater. Chem. A 2020, 8, 4334–4340. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Xu, S.; Li, M.; Chen, F. The preparation of 2D TpPa-COF/2D g-C3N4 heterojunction via in-situ growth for enhanced visible-light photocatalysis. Int. J. Hydrogen Energy 2024, 60, 1433–1441. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zhang, H.; Zheng, X.; Ding, J.; Li, Y.; Chen, F.; Zhao, Z. Molecular Ir-Based Coordination Compound Grafted onto Covalent Organic Framework for Efficient Photocatalytic H2 Evolution. Materials 2025, 18, 1874. https://doi.org/10.3390/ma18081874
Wu C, Zhang H, Zheng X, Ding J, Li Y, Chen F, Zhao Z. Molecular Ir-Based Coordination Compound Grafted onto Covalent Organic Framework for Efficient Photocatalytic H2 Evolution. Materials. 2025; 18(8):1874. https://doi.org/10.3390/ma18081874
Chicago/Turabian StyleWu, Chao, Haoyan Zhang, Xuan Zheng, Jing Ding, Yuanyuan Li, Feiyong Chen, and Zhengfeng Zhao. 2025. "Molecular Ir-Based Coordination Compound Grafted onto Covalent Organic Framework for Efficient Photocatalytic H2 Evolution" Materials 18, no. 8: 1874. https://doi.org/10.3390/ma18081874
APA StyleWu, C., Zhang, H., Zheng, X., Ding, J., Li, Y., Chen, F., & Zhao, Z. (2025). Molecular Ir-Based Coordination Compound Grafted onto Covalent Organic Framework for Efficient Photocatalytic H2 Evolution. Materials, 18(8), 1874. https://doi.org/10.3390/ma18081874




