Recycling of Carbon Fiber Reinforced Plastic-Containing Waste and Iron Oxide-Containing Dusts as Aggregates in Metallurgical Processes
Abstract
1. Introduction
2. Requirements for CFRPs for Utilization in Metallurgical Processes
2.1. Material Requirements
2.2. Process Requirements for the Thermal Conversion of CFRP
3. Requirements for CFRP Iron Pellets for Use in Shaft Furnaces
4. Experimental Materials
4.1. CFRP
4.2. Magnetite Iron Ore
4.3. Bentonite
5. Stoichiometric Considerations
6. Agglomeration of Iron Ore Pellets by Pelletizing
6.1. Agglomeration by Pelletizing
6.2. Parameters Influencing the Pelletizing Process
6.3. Experimental Design
| approx. 2 wt.% bentonite added, |
| approx. 2 wt.% bentonite and 0.5 to 10 wt.% CFRP-O added, |
| approx. 2 wt.% bentonite and 0.5 to 1 wt.% CFRP-A added. |
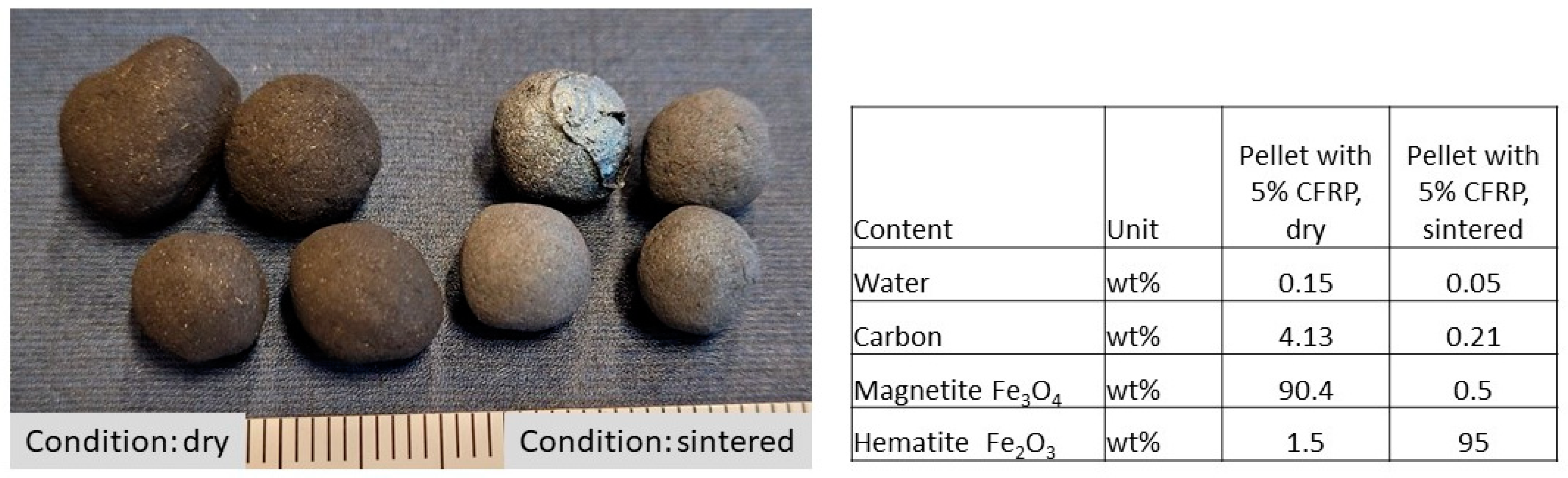
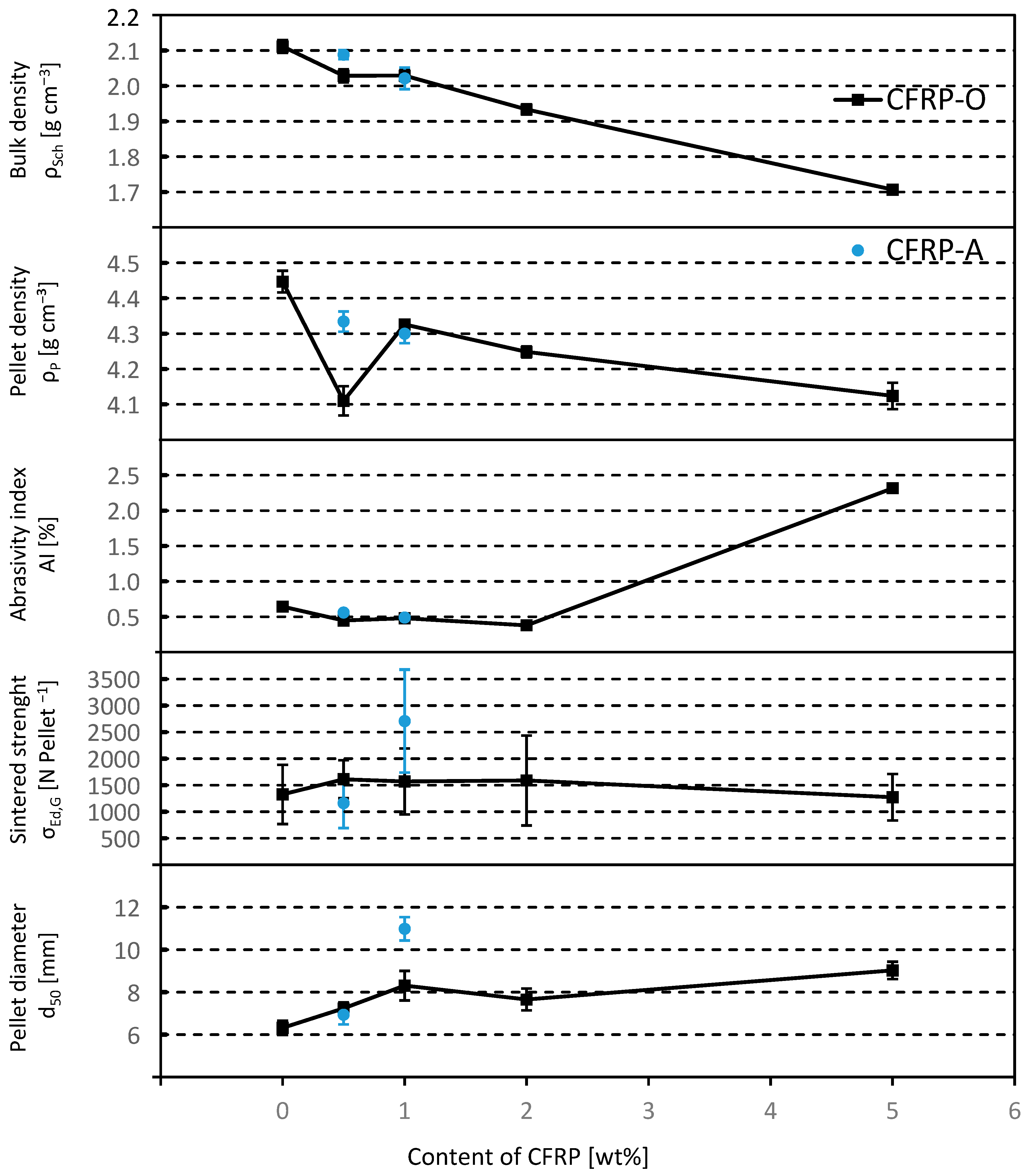
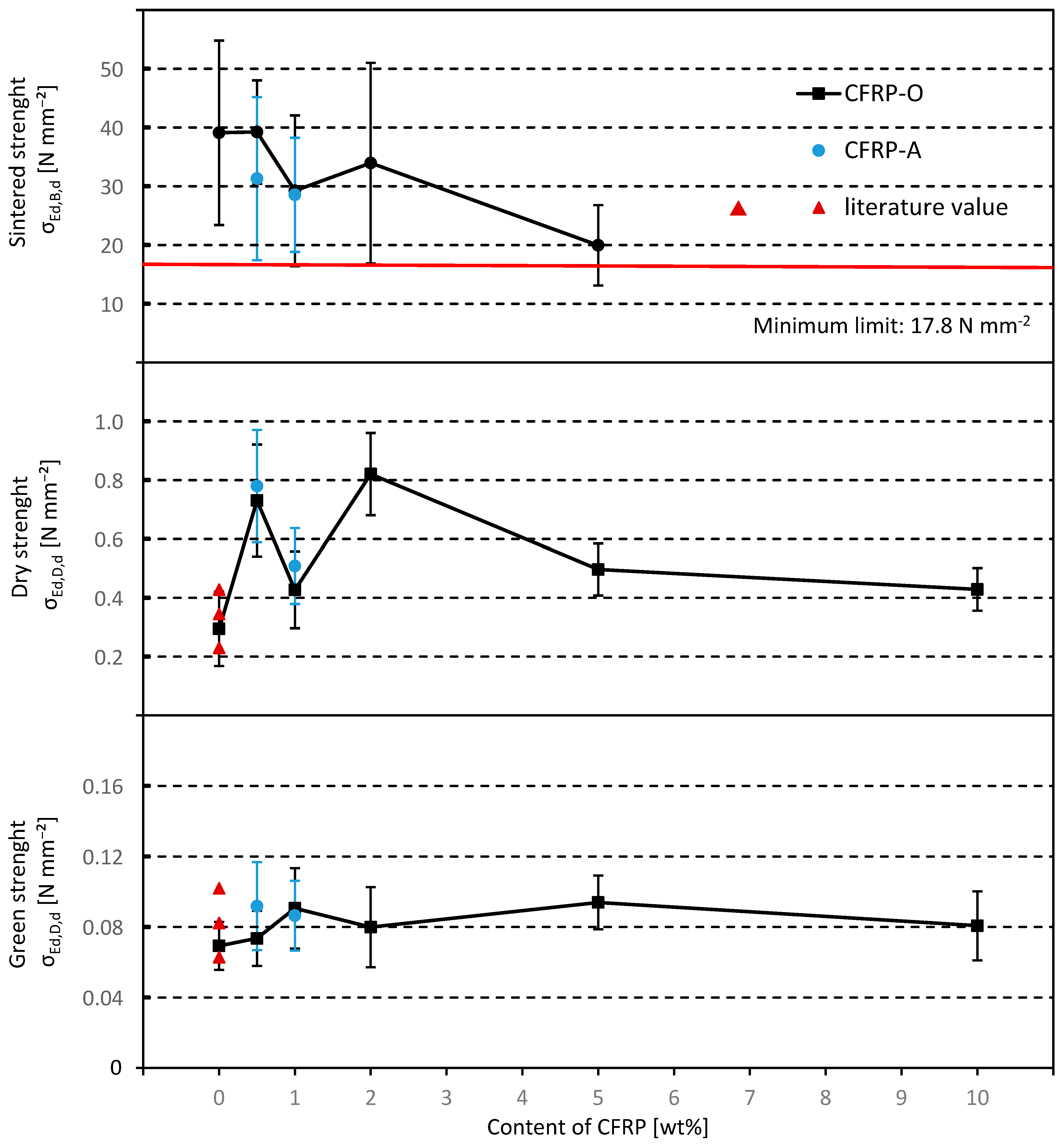
7. Comparison of the Characteristic Properties of the Sintered Pellets
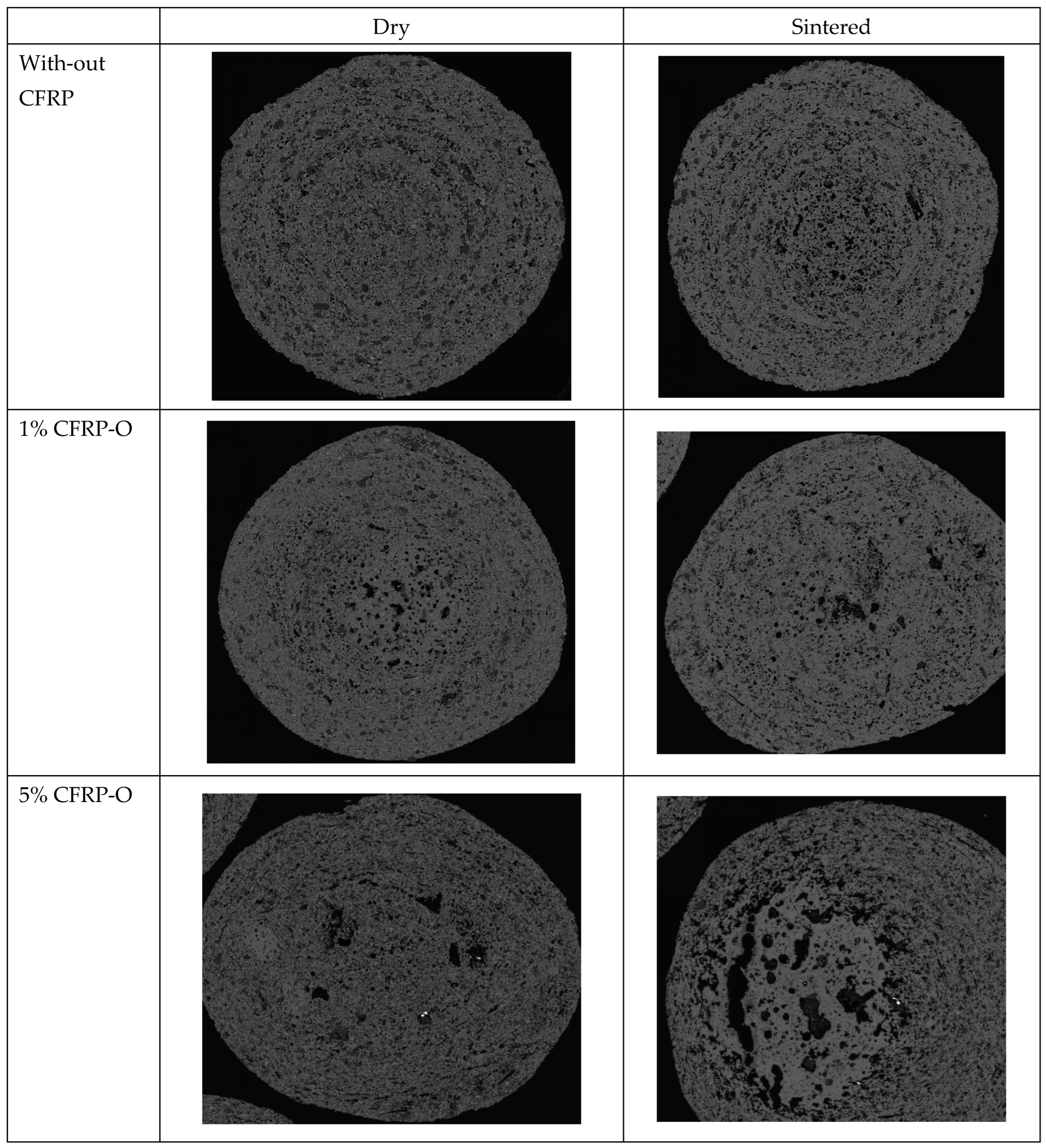
8. Structure of the Pellets
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sauer, M.; Kühnel, M.; Witten, E. Composites-Marktbericht 2018; Industrieverein Verstärkte Kunststoffe AVK: Frankfurt am Main, Germany, 2018. [Google Scholar]
- Schürmann, H. Konstruieren mit Faser-Kunststoff-Verbunden. 2; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-72189-5. [Google Scholar] [CrossRef]
- Meng, F.; Olivetti, A.; Zhao, Y.; Chang, J.; Pickering, S.; Stephen, J.; McKechie, J. Comparing Life Cycle Energy and Global Warming Potential of Carbon Fiber Composite Recycling Technologies and Waste Management Options. Am. Chem. Soc. 2018, 6, 9854–9865. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.; Leeke, G. Current status of recycling of fibre reinforced polymers. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef]
- Zöllner, M.; Hamann, D.; Krampitz, T.; Thuem, S.; Lieberwirth, H. Carbon Fibers. In Handbook of Recycling; Elsevier Inc.: Amsterdam, The Netherlands, 2024; Volume 2, Chapter 34. [Google Scholar] [CrossRef]
- Achternbosch, A.; Brauetigam, K.; Kupsch, C.; Ressler, B. Analyse der Umweltauswirkung bei der Herstellung, dem Einsatz und der Entsorgung von CFK-bzw. Aluminiumrumpfkomponenten; FZKA, 6879; Forschungszentrum Karlsruhe: Karlsruhe, Germany; Institut für Technikfolgeabschätzung und Systemanalyse: Karlsruhe, Germany, 2003; ISSN 0947-8620. [Google Scholar]
- Weißhaupt, P. Faserverbundwerkstoffe und ihr Potential für Klima-und Ressourcenschonung; Umweltbundesamt: Dessau-Roßlau, Germany, 2020. [Google Scholar]
- Stapf, D.; Baumann, W.; Hauser, M.; Muellhopt, S.; Weiss, C. Stand der Untersuchungen zum Freisetzungsverhalten von Carbonfasern in thermischen Prozessen; Umweltbundesamt; LAGA Ad-hoc-Arbeitskreis; Aufbereitung und Verwertung carbonfaserhaltige Abfälle; Karlsruher Institut für Technologie (KIT): Dessau-Roßlau, Germany, 2019. [Google Scholar]
- Quicker, P.; Stockschläder, J. Möglichkeiten und Grenzen der Entsorgung Carbonfaserverstärkter Kunststoffabfälle in thermischen Prozessen; Texte 00/2019, Abschlussbericht; Umweltbundesamt (Hrsg.): Dessau-Roßlau, Germany, 2021; ISSN 1862-4804. [Google Scholar]
- Quicker, P.; Limburg, M. Entsorgung von Carbonfasern—Probleme des Recyclings und Auswirkungen auf die Abfallverbrennung. Neuruppin; Energie aus Abfall-Band 13, K.J. Thomè-Kozmiensky und M.Beckmann; TK-Verlag: Aachen, Germany, 2016; pp. 135–144. ISBN 978-3-944310-24-4. [Google Scholar]
- EUWID. CFK-haltige Abfälle dürfen nicht im Restmüll landen. Recycl. Und Entsorgung 2016, 23, 7–8. [Google Scholar]
- Reckter, B. Der Verbund ist das Problem. Vdi Nachrichten 2016, 4, 24. [Google Scholar]
- Stapf, D.; Beckmann, M. Entwicklung von Rückbau-und Recyclingstandards für Rotorblätter; Texte 92/2022; Umwelt-Bundesamt (UBA): Dessau-Roßlau, Germany, 2022; pp. 504–514. [Google Scholar]
- Walter, T. CFK-Einsatz in der Calciumcarbidproduktion; LAGA Ad-hoc-Arbeitskreis; Aufbereitung und Verwertung carbonfaserhaltige Abfälle; AlzChem; Umweltbundesamt: Dessau-Roßlau, Germany, 2019. [Google Scholar]
- Adam, C.; Weimann, K. Verwertung Carbonhaltiger Reststoffe in der Pyrometallurgie; LAGA Ad-hoc-Arbeitskreis; Aufbereitung und Verwertung carbonfaserhaltige Abfälle; BAM; Umweltbundesamt: Dessau-Roßlau, Germany, 2019. [Google Scholar]
- Rammler, E.; Heide, K. Versuche zur Brikettierung von Eisenfeinerz mit Braunkohle im Hinblick auf die Schwefelverhüttung; A3; Freiberger Forschungshefte: Freiberg, Germany, 1952; pp. 127–134. ISSN 0071-9390. [Google Scholar]
- Konstantinidis, P.; Charitos, A. Ausgediente Karbonfasern in Metallurgische Schlacke; Stahleisen.de: Germany, 2023; p. 50. [Google Scholar]
- Krampitz, T.; Zöllner, M.; Lieberwirth, H.; Kamptner, A.; Friedrich, J. Feinstzerkleinerung von CFK-Abfällen und Chrakterisierung der Zerkleinerungsprodukte; Chemie Ingenieur Technik (CIT); 92; No.4; Wiley-VCH Verlag GmbH & Co.KGaA: Weinheim, Germany, 2020; pp. 476–484. [Google Scholar] [CrossRef]
- Niebel, P.; Krampitz, T.; Lieberwirth, H.; Zeisberg, M. Grundlagenuntersuchungen zum Einfluss verschiedener Zerkleinerungsparameter auf die spezifische Zerkleinerungsarbeit von kohlenstofffaserverstärkten Kunststoffen. Chem. Ing. Tech. 2024, 96, 913–923. [Google Scholar] [CrossRef]
- Buschmann, W. Koks, Gas, Kohlechemie—Geschichte und gegenständliche Überlieferung der Kohleveredelung; Klartext-Verlag: Essen, Germany, 1993; ISBN 978-3-88474-028-6. [Google Scholar]
- Pelc, H. Flammschutzmittel Reduzieren Gefahren Durch Freigesetzte CFK-Fasern; Springer Prefessional: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Krampitz, T. Rohstoffliche Verwertung von CFK-Haltigen Abfällen in Metallurgischen Prozessen und deren Anforderungen an die Vorbehandlung; Heft 24; Berichtsheft zum 73. BHT; FK5: Recyclingstrategien in der Mobilität; TU Bergakademie Freiberg: Freiberg, Germany, 2024; ISBN 978-3-86012-715-5. [Google Scholar]
- Hausmann, M. RECOSIC: Technologie für Nachhaltige Siliciumcarbid-Rohstoffe; UVR-FIA-Tagung Aufbereitung und Recycling; ESK SiC GmbH: Frechen, Germany, 2022. [Google Scholar]
- Woidasky, J.; Seile, E.; Henning, F.; Wolf, M.; Harsch, M. 2. Umwelt und Recycling, Polymer Engineering 3 Werkstoff-und Bauteilprüfung, Recycling, Entwicklung; Springer: Berlin, Germany, 2020; ISBN 978-3-662-59839-9. [Google Scholar]
- Schliephake, H. „Können CFK-Abfällen als Primärkohleersatz im Elektrolichtbogenofen bei der Stahlerzeugung Eingesetzt Werden? Werkstoffe der Zukunft—Faserverbundwerkstoffe“; Vortrag; NoWASTE Georgsmarienhütte GmbH: Georgsmarienhütte, Germany, 2019. [Google Scholar]
- Warzelhahn, V. Vorstellung des Arbeitskreises Recycling; CCe.V.: Stuttgart, Germany, 2019. [Google Scholar]
- Kramer, B.; Jäckel, H.-G. Verfahren zur Brikettierung Pulverförmiger Legierungszuschläge der Stahl-und Gießerei-und Ne-Metallurgie mit Hilfe Faserhaltiger Strukturbilder und ein Brikett. DE 10 2015 011 067 A1, 20 April 2017. [Google Scholar]
- Jäckel, H.-G.; Hamann, D. Konzeption und Pilotierung Eines Verfahrens zur Vollständigen Stofflichen Verwertung von Kohlefaserhaltigen Abfällen; Umweltbundesamt; TU Bergakademie Freiberg; Institut für Aufbereitungsmaschinen; Freiberg: Dessau-Roßlau, Germany, 2019. [Google Scholar]
- Krampitz, T.; Lieberwirth, H. Pellet als Zuschlagstoff für Metallurgische Prozesse und Verfahren zu Dessen Herstellung und Verwendung. DE 10 2017 212 583 A1, 24 January 2019. [Google Scholar]
- Taube, K. Stahlerzeugung Kompakt, Grundlagen der Eisen-und Stahlmetallurgie; Vieweg Technik Werkstofftechnik: Braunschweig, Germany; Wiesbaden, Germany, 1998; ISBN 3528038632. [Google Scholar]
- Papacek, H.-G. Einfluss des Durchmessers von Eisenerz-Pellets auf Deren Herstellung und auf ihr Verhalten im Hochofen. Ph.D. Dissertation, RWTH, Aachen, Germany, 1984. HBZ: HT002729116. [Google Scholar]
- Furnas, C. Bulletin 307—Flow of Gases Through Beds of Broken Solids; United States Government Printing Office: Washington, DC, USA, 1929. [Google Scholar]
- Pietsch, W. Agglomeration Processes; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002; ISBN 3-527-30369-3. [Google Scholar]
- Capes, C. Particle Size Enlargement; Elsevier: New York, NY, USA, 1980. [Google Scholar]
- ISO 4700:2015; Iron Ore Pellets for Blast Furnace and Direct Reduction Feedstocks. Determination of the Crushing Strength. s.l. International Organization for Standardization (ISO): Geneva, Switzerland, 2015; AC-Code: GB30265140.
- Meyer, K. Pelletizing of Iron Ores; Springer: Berlin/Heidelberg, Germany, 1980; ISBN 9780387102153. [Google Scholar]
- Geerdes, M. Modern Blast Furnace Ironmaking; IOS PressBV: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Hütter, B. Die Herstellung Gebrannter Eisenerzpelltes aus Grünpellets mit Geringer Brennstoffbeimischung. Ph.D. Dissertation, RWTH, Aachen, Germany, 1977. [Google Scholar]
- ISO 3271:2015; Iron Ores for Blast Furnace and Direct Reduction Feedstocks. Determination of the Tumble and Abrasion Indices. International Organization for Standardization (ISO): Geneva, Switzerland, 2015; AC-Code: GB30265107.
- DIN 51733; Prüfung Fester Brennstoffe—Bestimmung der Elementarzusammensetzung und Berechnung des Sauerstoffgehalts. Deutsches Institut für Normung: Berlin, Germany, 2016.
- Schubert, H. Handbuch der Mechanischen Verfahrenstechnik; Wiley-VCH Verlag GmbH: Freiberg, Germany; Weinheim, Germany, 2003; ISBN 3-527-30577-7. [Google Scholar]
- Stieß, M. Mechanische Verfahrenstechnik-Partikeltechnologie 1; Kennzeichnung von Partikeln und dispersen Stoffsystemen; Springer-Lehrbuch: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-32551-2. [Google Scholar]
- Heinze, G. Handbuch der Agglomerationstechnik; Wiley-VCH: Weinheim, Germany, 2005; ISBN 3527603573. [Google Scholar]
- Pal, J.; Ghoari, S.; Ayyandurai, A. Improving reducibility of iron ore pellets by optimization of physical parameters. J. Min. Metall. Sect. B Metall. 2016, 53, 37–46. [Google Scholar] [CrossRef]
- Srb, J.; Ruzickova, Z. Pelletization of Fines; SNTL: Prag, Czech Republic, 1988. [Google Scholar]
- Gründer, W.; Hildenbrand, H. Untersuchung der Arbeitsweise eines Labor-Pelletiertellers. Chem. Ing. Tech. (CIT) 1961, 33, 749–753. [Google Scholar] [CrossRef]
- Sommer, K.; Herrmann, W. Auslegung von Granulierteller und Granuliertrommel. Chem. Ing. Tech. (CIT) 1978, 50, 518–524. [Google Scholar] [CrossRef]
- Bhrany, U. Entwurf und Betrieb von Pelletiertellern. At Aufbereit. 1977, 12, 641–647. [Google Scholar]
- Ries, H. Granulaterzeugung in Mischgranulatoren und Granuliertellern. Aufbereitungstechnik 1975, 12, 639. [Google Scholar]
- Bombled, P.M. The granulation of cement raw materials. Mag. Concr. Res. 1961, 549, 289–299. [Google Scholar] [CrossRef]
- Forsmo, S.; Samskog, P.; Björkman, B. A study on plasticity and compression strenght in wet iron ore green pellets related to real process variations in raw material fineness. Powder Technol. 2008, 181, 321–330. [Google Scholar] [CrossRef]
- Forsmo, S.; Apelqvist, A.; Björkman, B.; Samskog, P. Binding mechanisms in wet iron ore green pellets with a bentonite binder. Powder Technol. 2006, 169, 147–158. [Google Scholar] [CrossRef]
- Dietz, D. Untersuchungen zur Pelletierbarkeit von CFK-Eisen-Pellets. In Masterthesis; Institut für Aufbereitungsmaschinen, TU Bergakademie Freiberg: Freiberg, Germany, 2019. [Google Scholar]
- Schmidtke, K. Rohstahlproduktion in Deutschland; Wirtschaftsvereinigung Stahl (WV Stahl): Berlin, Germany, 2023; Pressemitteilung. [Google Scholar]

| Type | CFRP-O | CF-O | CFRP-A | CFRP-B | CF-T | CF-K | CFRP/GFRP | GFRP | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Description | 0/90/0 prepreg | Vehicle manufacturer A | Vehicle manufacturer B | Combinated textile | Combinated prepreg | Wind turbine blade | ||||
| Resin type | Epoxy | - | Epoxy | Epoxy | Thermo-plast PA6 | Epoxy | Epoxy | Epoxy | ||
| Parameters | Unit | Method | Value | Value | Value | Value | Value | Value | Value | Value |
| Carbon | wt.-% | DIN 51732 | 86.6 | 94.8 | 87.0 | 91.7 | 73.5 | 68.8 | 64.5 | 29.7 |
| Calorific Value Hu | kJ kg−1 | DIN 51900 | 30,600 | 31,900 | 31,400 | 31,300 | 29,300 | 24,500 | 25,900 | 11,600 |
| Chlorine | wt.-% | DIN 51727 | 0.18 | 0.01 | 0.04 | 0.47 | 0.01 | 0.02 | 0.03 | 0.15 |
| Requirements | Process Management |
| Complete combustion | Longer dwell time and higher ambient temperatures (>800 °C) compared to conventional waste incineration plants |
| Reducing atmosphere | High carbon content and calorific value for the chemical reduction system |
| Minimal exposure of fibers in the process | Largely closed process chamber with the greatest possible avoidance of fiber discharge into the exhaust air Favoring the use of liquid phases at high temperatures and high residence time |
| Tolerance to material mixtures | CFRP-containing waste is mostly combined with other materials or contains residual adhesions; therefore, a high tolerance to GFRP, other fiber reinforcements and low quantities of metallic materials or halogens is required |
| Electric Arc Furnace | Shaft Furnace | |||
|---|---|---|---|---|
| Product | Steel | Calcium carbide | Silicon carbide | Pig iron |
| Temperature | Ca. 1600 °C | >2000 °C | >2000 °C | Ca. 1500 °C |
| Residence time | Ca. 40 min | hours | no indication | >12 h |
| Requirement | Advantage |
|---|---|
| Narrow particle size distribution with high permeability High pressure and abrasion resistance at high temperature High reducibility (high carbon content) Low swelling capacity or reduction softening | No segregation in the furnace Good gas flow through the pellets Increase in indirect reduction |
| CFRP-O | CFRP-A | |
|---|---|---|
| Laminat PREDO PR-DU 600/1250 FT1091 33 | Roving SIGRAVIL C T50-4.0/240 E100 | |
| Mass of resin [%] 33 | Fiber sizing Epoxy | Mass of resin [%] ca. 39.3 +/− 1.1 (a) |
| 0°—Tensil strenght [MPa] 1900 | Tensile strength [MPa] 4000 | |
| 0°—Elongation-modulus [GPa] 130 | Elongation modulus [GPa] 240 | |
| 0°—Elongation at break [%] 1.2 | Filament diameter [µm] 7 | |
| Interlaminate shear strength [MPa] 64 | Amount of filament in Roving 50,000 | |
| Wall thickness [mm] 1.9 | Wall thickness [mm] 1.7 | |
| Property | Unit | Analytical Method | Magnetite Ore | Bentonite | CFRP-O | CFRP-A |
|---|---|---|---|---|---|---|
| Particle size < 56 µm | % | DIN EN ISO 8130-13 | 88.6 | 83.1 | - | - |
| Particle size x50 | µm | 23.3 | 30.6 | 24 | 19 | |
| Particle size x90 | µm | DIN EN ISO 8130-13 | 58.4 | 64.7 | 101 | 87 |
| Fiber length x90 | µm | - | - | 222 | 254 | |
| Moisture content | % | ISO 10251 | 8.2 | 7.3 | 0.4 | 1.9 |
| Specific surface Sm according to Blaine | cm2 g−1 | DIN 66126 | 2095 | 8586 | 6621 | 6681 |
| Bulk density dry | g cm−3 | DIN ISO 693 | 2.26 | 0.86 | 0.29 | 0.24 |
| Batch | Magnetite Ore | CFRP-O | CFRP-A | Bentonite | Water | Magnetite Ore | Total | CFRP-O | CFRP-A | Bentonite | Water | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | g | g | g | g | g | % | % | % | % | % | % | |
| I | 100 | 0 | - | 2 | 2 | 104 | 96.2 | 0.0 | - | 1.9 | 1.9 | 100 |
| II | 100 | 10 | - | 2 | 2 | 114 | 87.7 | 8.8 | - | 1.8 | 1.8 | 100 |
| III | 100 | 1 | - | 2 | 2 | 105 | 95.2 | 1.0 | - | 1.9 | 1.9 | 100 |
| IV | 100 | 5 | - | 2 | 2 | 109 | 91.7 | 4.6 | - | 1.8 | 1.8 | 100 |
| V | 100 | 2 | - | 2 | 2 | 106 | 94.3 | 1.9 | - | 1.9 | 1.9 | 100 |
| VI | 100 | 0.5 | - | 2 | 2 | 104.5 | 95.7 | 0.5 | - | 1.9 | 1.9 | 100 |
| VII | 100 | - | 1 | 2 | 2 | 105 | 95.2 | - | 1.0 | 1.9 | 1.9 | 100 |
| VIII | 100 | - | 0.5 | 2 | 2 | 104.5 | 95.7 | - | 0.5 | 1.9 | 1.9 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krampitz, T.; Lampke, J.; Dietz, D.; Lieberwirth, H. Recycling of Carbon Fiber Reinforced Plastic-Containing Waste and Iron Oxide-Containing Dusts as Aggregates in Metallurgical Processes. Materials 2025, 18, 1838. https://doi.org/10.3390/ma18081838
Krampitz T, Lampke J, Dietz D, Lieberwirth H. Recycling of Carbon Fiber Reinforced Plastic-Containing Waste and Iron Oxide-Containing Dusts as Aggregates in Metallurgical Processes. Materials. 2025; 18(8):1838. https://doi.org/10.3390/ma18081838
Chicago/Turabian StyleKrampitz, Thomas, Jan Lampke, Dirk Dietz, and Holger Lieberwirth. 2025. "Recycling of Carbon Fiber Reinforced Plastic-Containing Waste and Iron Oxide-Containing Dusts as Aggregates in Metallurgical Processes" Materials 18, no. 8: 1838. https://doi.org/10.3390/ma18081838
APA StyleKrampitz, T., Lampke, J., Dietz, D., & Lieberwirth, H. (2025). Recycling of Carbon Fiber Reinforced Plastic-Containing Waste and Iron Oxide-Containing Dusts as Aggregates in Metallurgical Processes. Materials, 18(8), 1838. https://doi.org/10.3390/ma18081838






