Effect of Temperature and Ceramization Atmosphere on the Structure and Microstructure of Boron-Modified SiBOC Materials
Abstract
:1. Introduction
- (1)
- Synthesis of preceramic precursors
- (2)
- Initial thermal treatment in order to initiate the transformation from sol to gel.
- (3)
- Pyrolysis—high-temperature treatment in an inert atmosphere, obtaining the final material.
2. Materials and Methods
2.1. Synthesis of SiBOC Materials’ Precursors
2.2. Initial Thermal Treatment and Pyrolysis Process
2.3. Microstructural and Structural Research
2.3.1. Microstructure Analysis—Scanning Electron Microscopy (SEM)
2.3.2. Porosimetry Measurements—Mercury Intrusion Porosimetry (MIP)
2.3.3. Structural Studies—X-Ray Diffraction, FT-IR Spectroscopy, Raman Spectroscopy
3. Results and Discussion
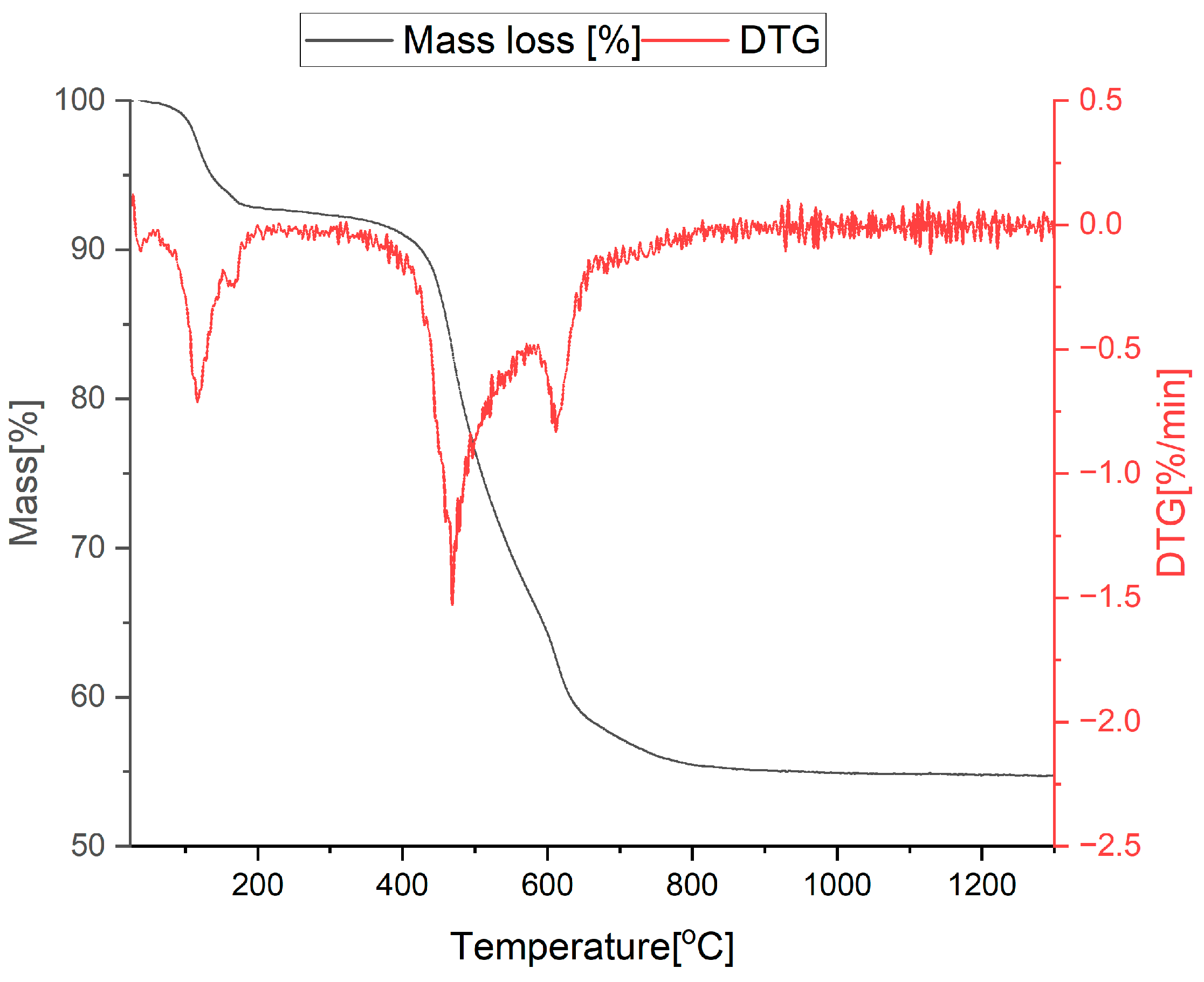
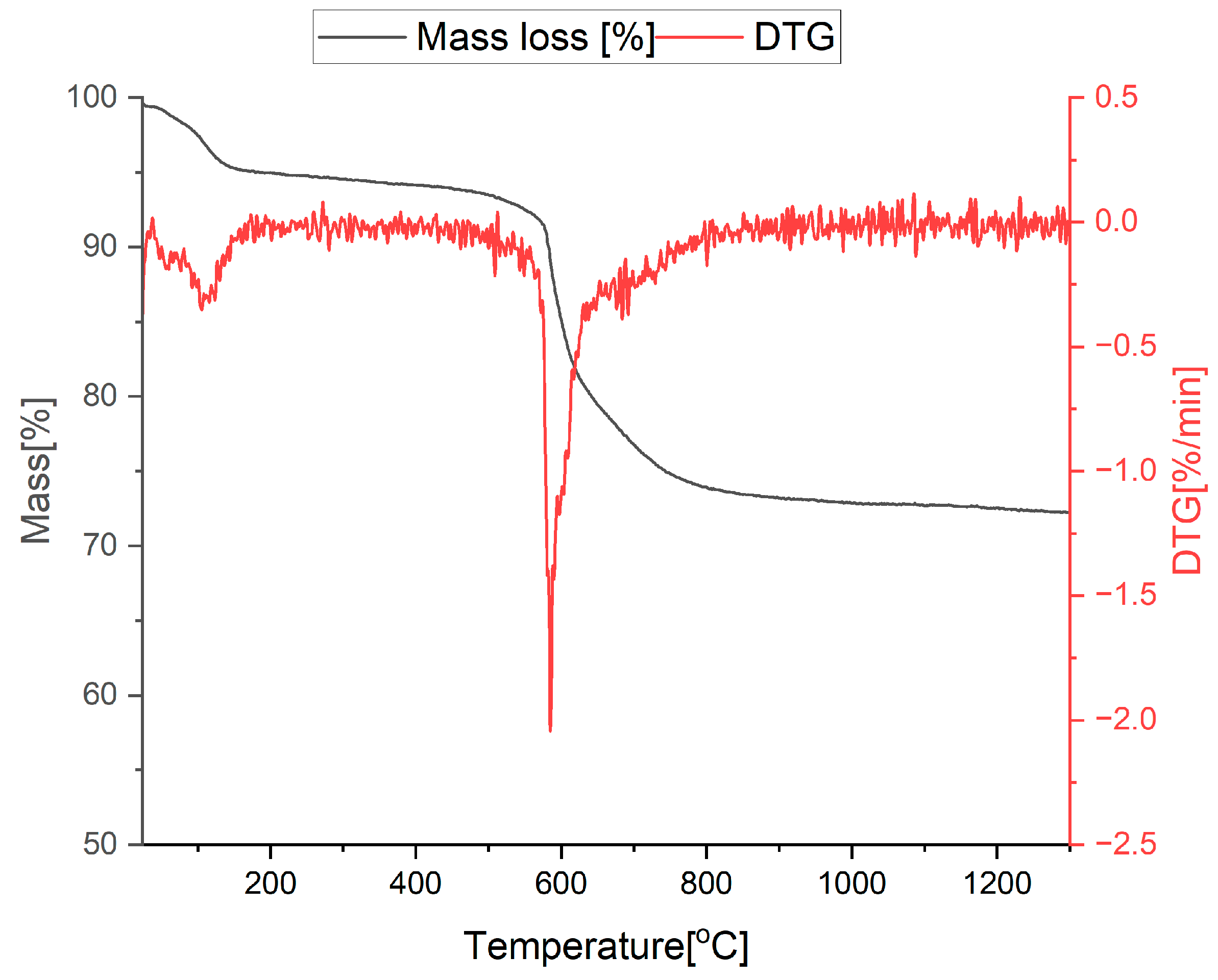
3.1. Thermal Analysis
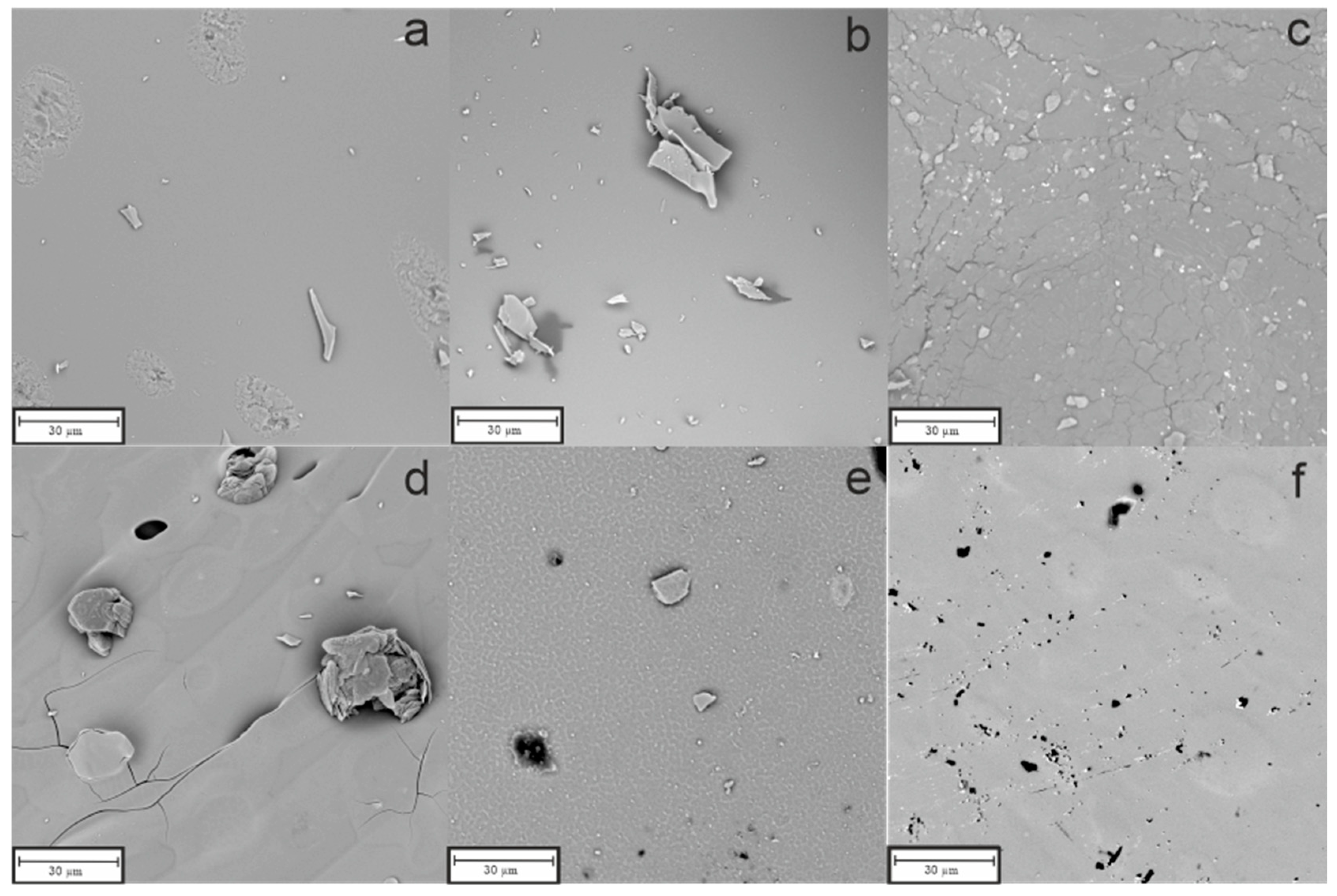
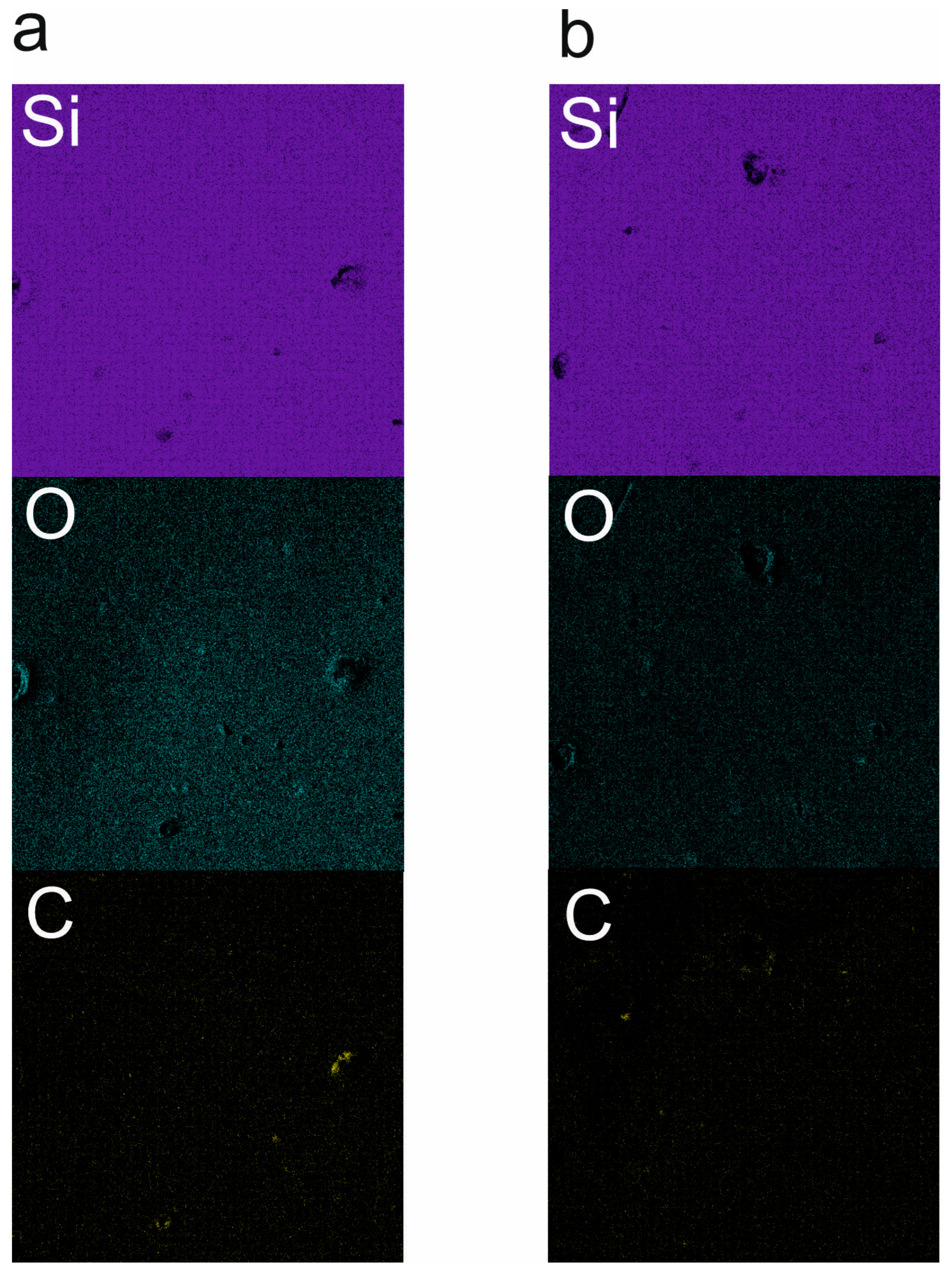
3.2. Scanning Electron Microscopy
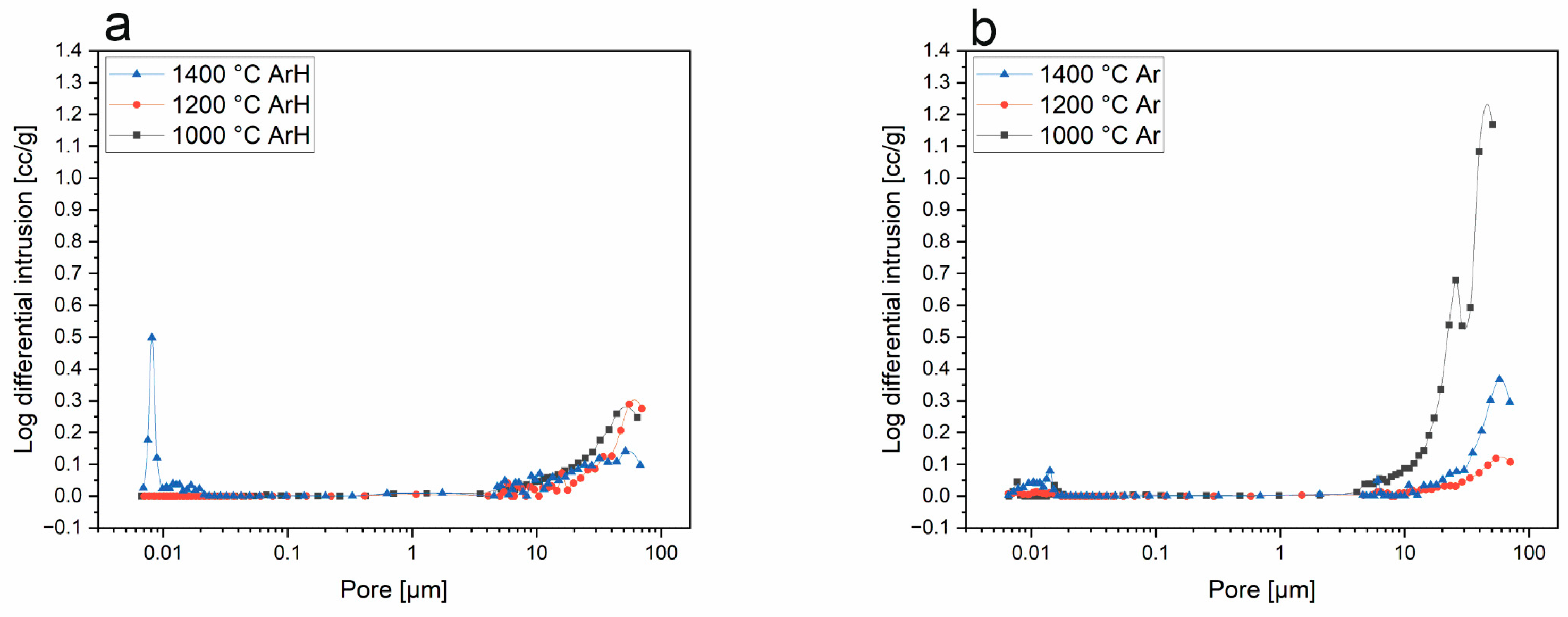
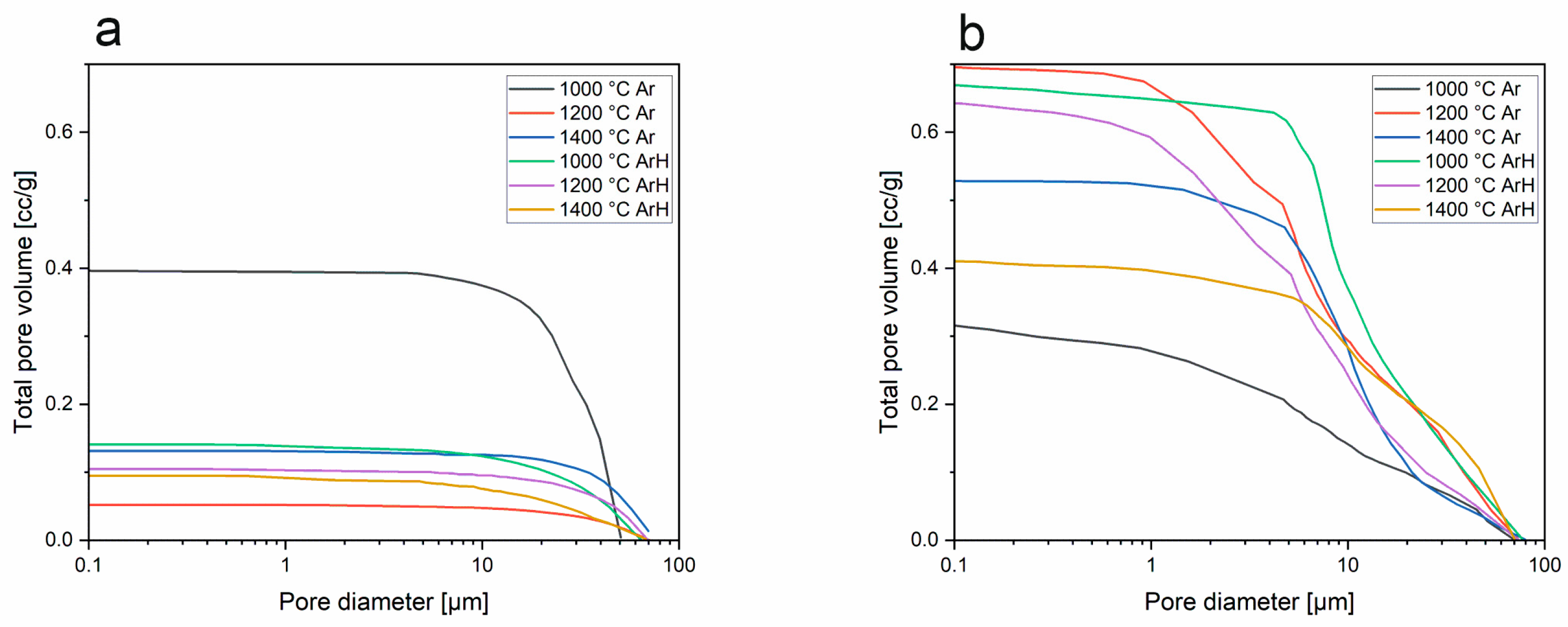
3.3. Mercury Intrusion Porosimetry
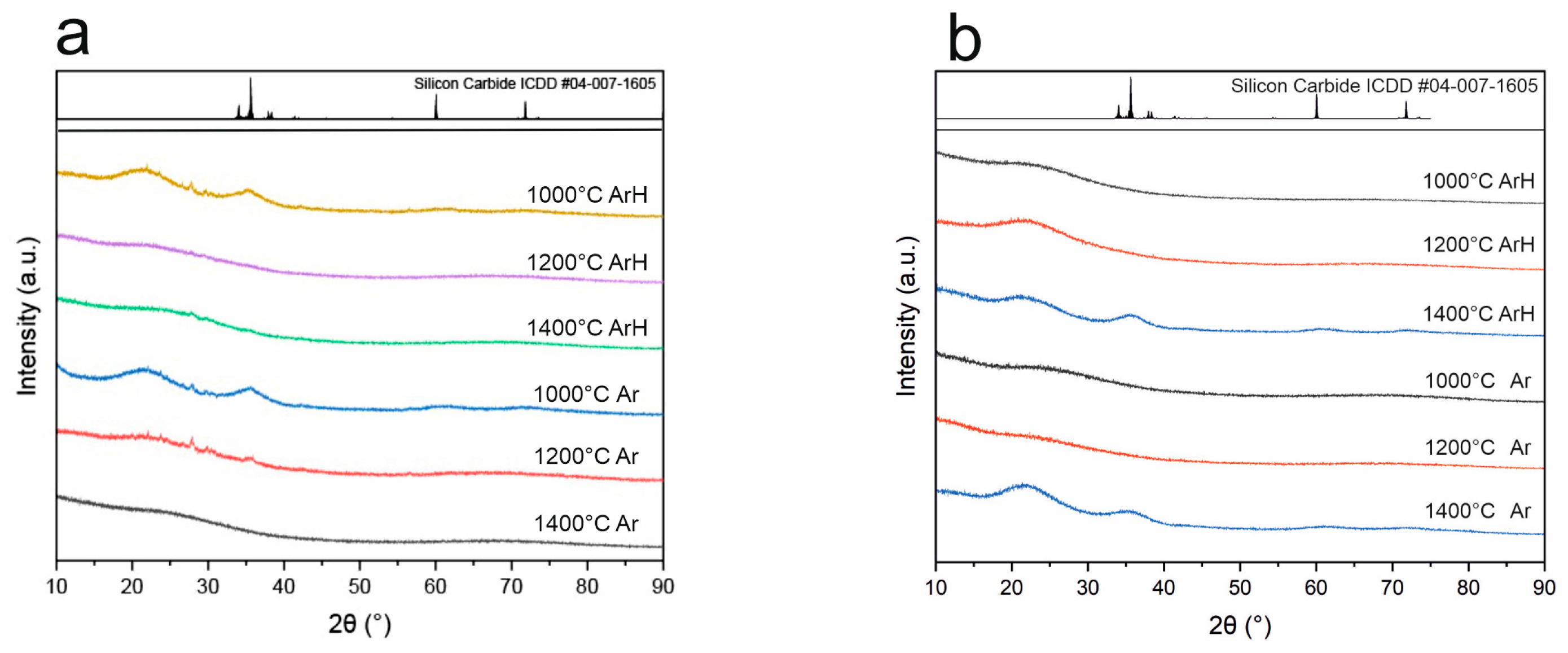
3.4. X-Ray Diffraction
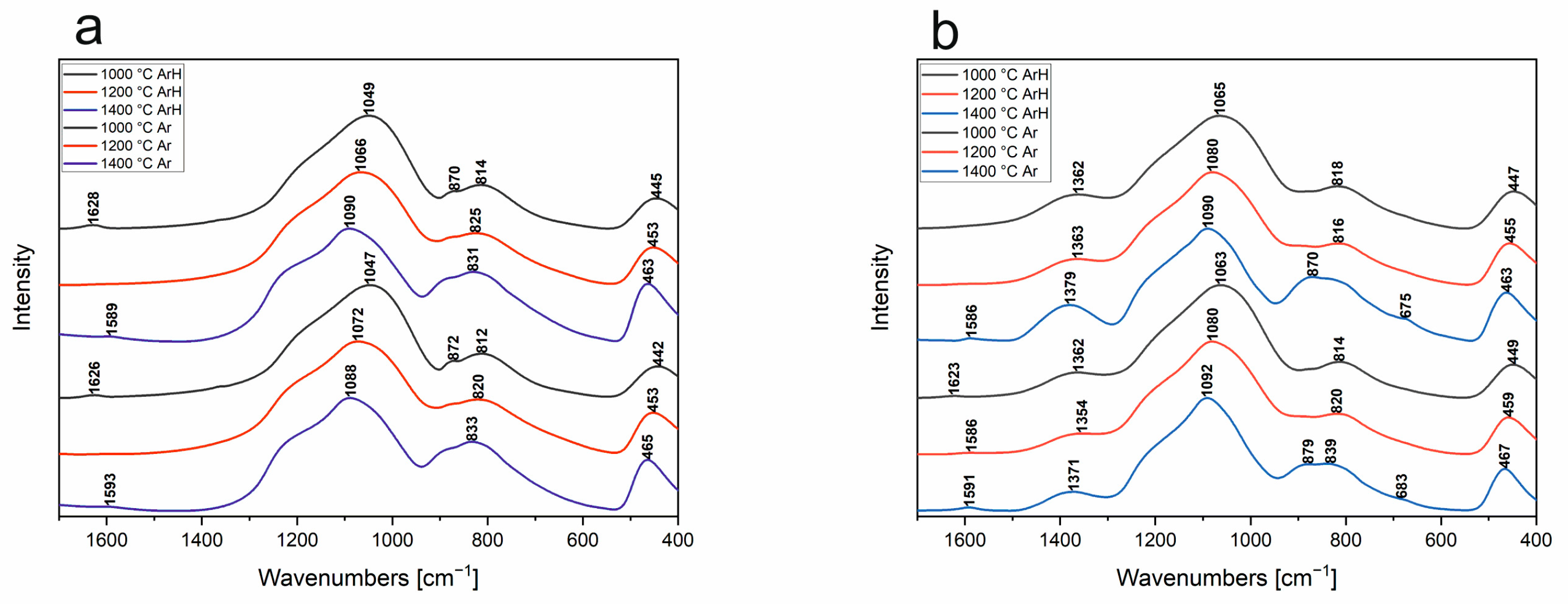
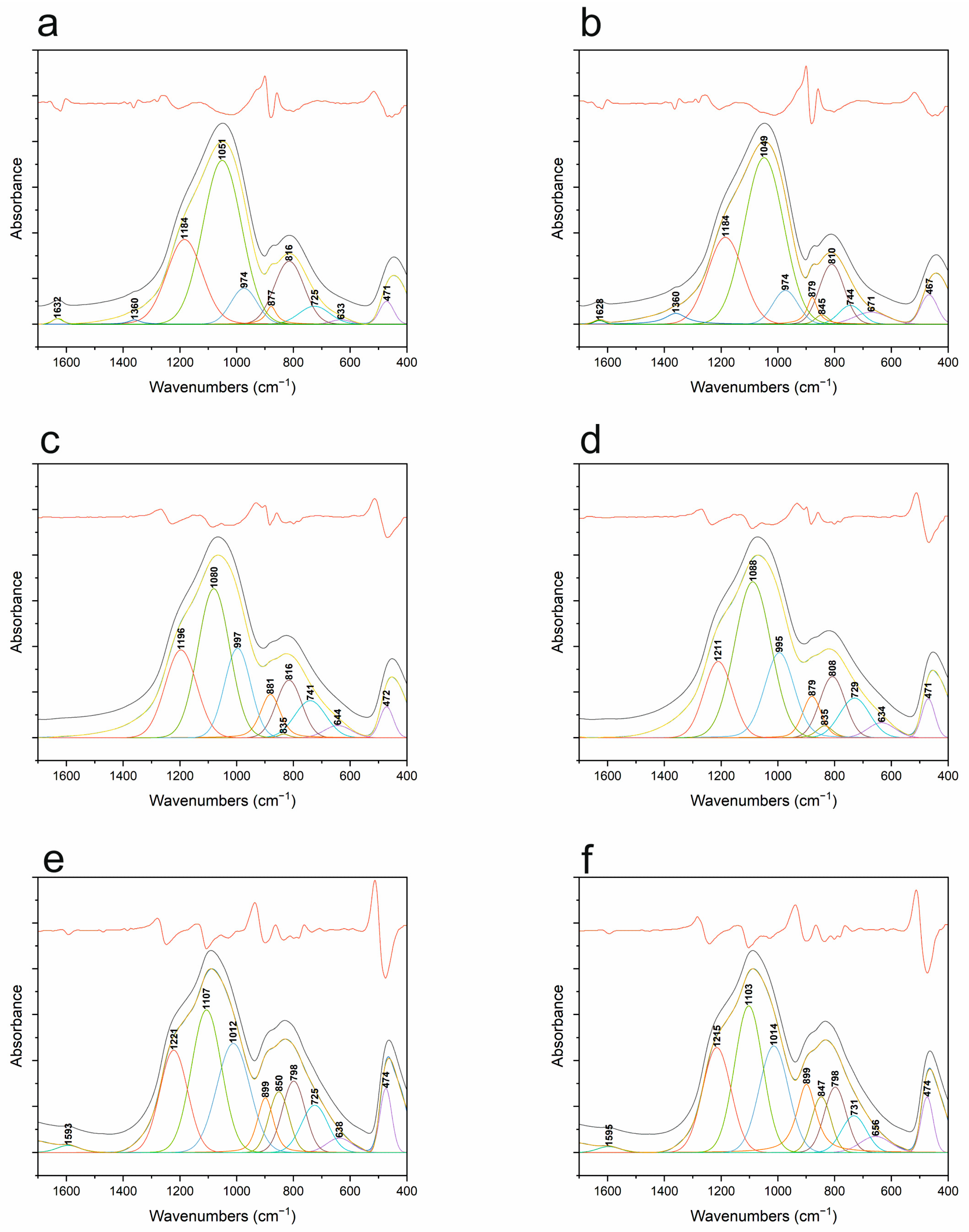
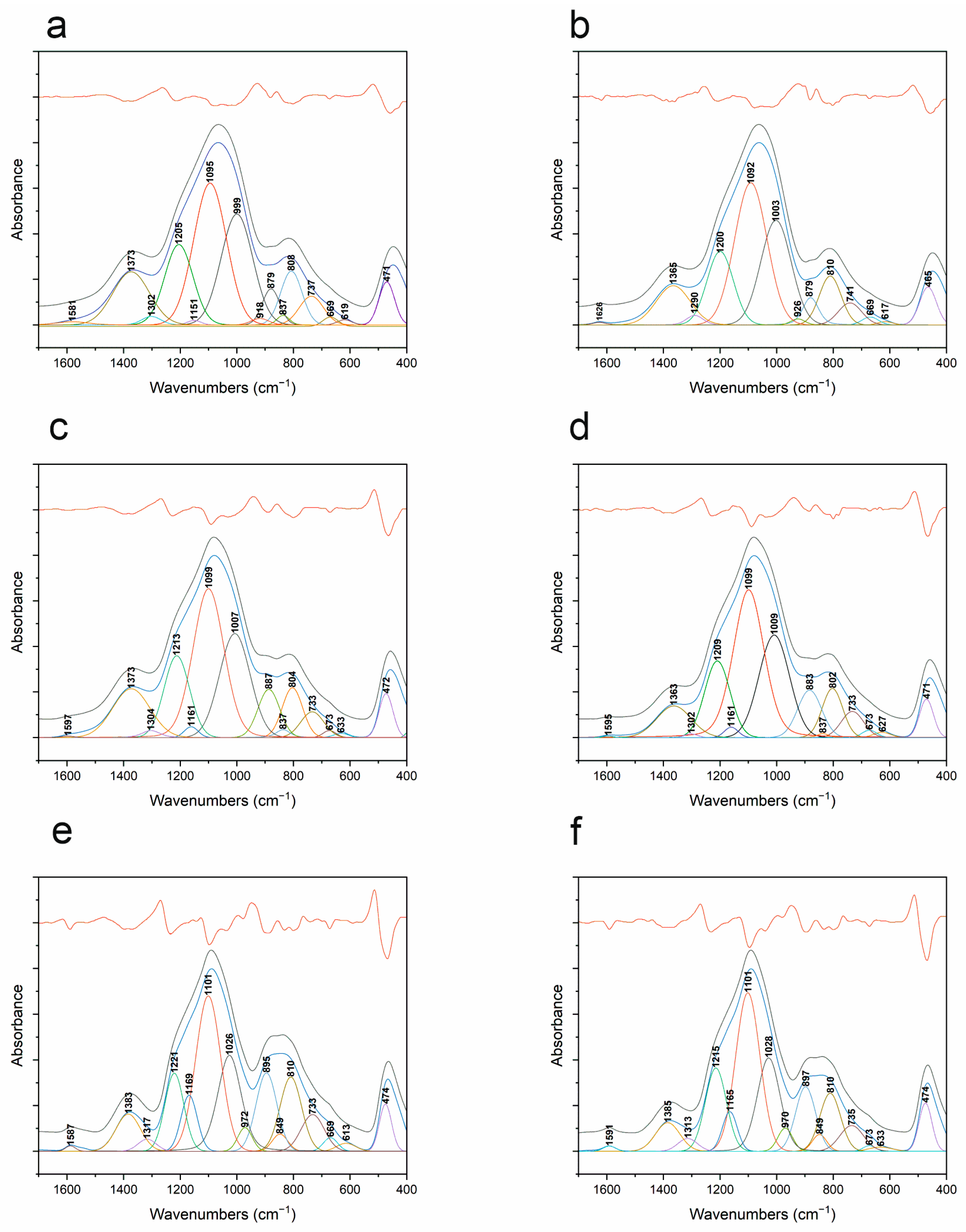
3.5. FT-IR
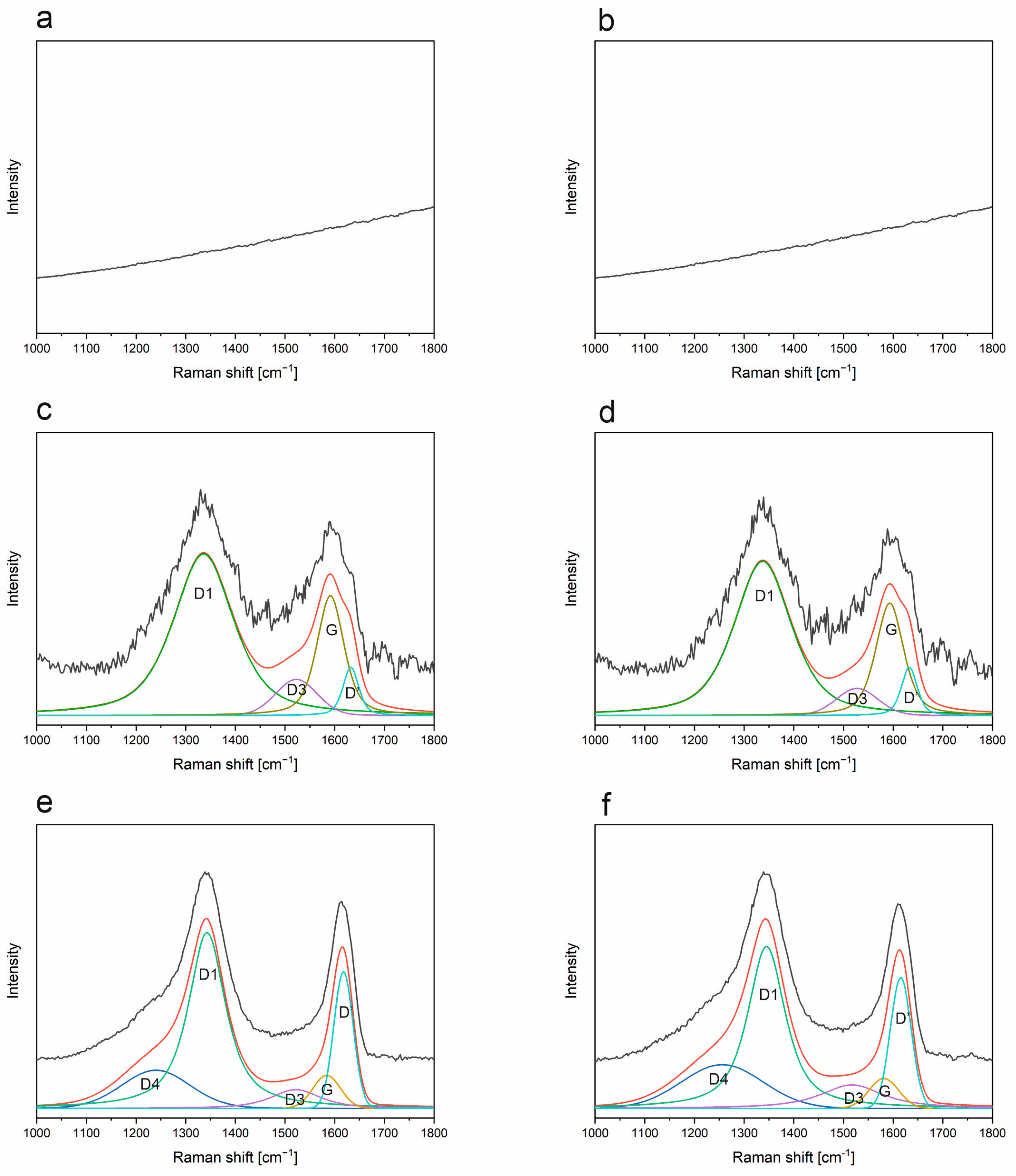
3.6. Raman Spectroscopy
4. Conclusions
- (a)
- SEM with EDS studies revealed homogenous material surfaces in all samples.
- (b)
- Porosity studies indicated that SiBOC materials regarding temperature and atmosphere were porous, with the highest porosity in samples treated at 1200 °C under Ar. However, SiOC samples do not possess pores in the structure. MIP studies suggest that TMB, which was the reagent introducing boron to the system, can be a porosity initiator.
- (c)
- XRD studies revealed that both SiOC and SiBOC materials remain fully amorphous up to 1200 °C, and at 1400 °C, samples pyrolyzed in both ArH and Ar exhibited amorphous structures, probably with a nanocrystalline SiC phase. These results suggest that the crystalline structure of the material depends on the pyrolysis temperature and that the atmosphere does not have an impact as long as it is inert.
- (d)
- FT-IR detailed studies showed the temperature dependence of chemical structure changes in SiBOC materials. With its increase, boron moved to a coordination number of 4, forming an oxyborosilicate matrix. FT-IR measurements of SiOC materials partially confirmed the nanocrystalline phase of Si-C in samples pyrolyzed in 1400 °C.
- (e)
- The presence of the free carbon phase was detected using Raman spectroscopy for samples obtained above 1000 °C. The free carbon phase was identified in different forms—sp2 and sp3. The amount of defects and fluorescence depends on the addition of boron.
- (f)
- Both ArH and Ar atmospheres do not affect the chemical structure of the SiBOC matrix, but their effect on the microstructure is significant. On the other hand, the effect on base SiOC materials is negligible.
- (g)
- The free carbon phase is dependent on both the temperature of pyrolysis and the atmosphere. With increasing temperature, the intensity of the G band decreases (ordered sp2 domains decrease), the D’ band intensity increases (defects increase), and the FWHM of both D1 and D’ decreases (defects become more localized).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, V.L.; Proust, V.; Quievryn, C.; Bernard, S.; Miele, P.; Soraru, G.D. Processing, Mechanical Characterization, and Alkali Resistance of SiliconBoronOxycarbide (SIBOC) Glass Fibers. J. Am. Ceram. Soc. 2014, 97, 3143–3149. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Y.; Lei, Y.; Wang, B.; Wu, N.; Gou, Y.; Fang, D. A Simply Prepared Flexible SiBOC Ultrafine Fiber Mat with Enhanced High-Temperature Stability and Chemical Resistance. RSC Adv. 2015, 5, 64911–64917. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Preparation and Structure of Silicon Oxycarbide Glasses Derived from Polysiloxane Precursors. J. Solgel Sci. Technol. 1997, 8, 327–330. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared Spectroscopy of Sol–Gel Derived Silica-Based Films: A Spectra-Microstructure Overview. J. Non Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Yu, S.; Tu, R.; Goto, T. Preparation of SiOC Nanocomposite Films by Laser Chemical Vapor Deposition. J. Eur. Ceram. Soc. 2016, 36, 403–409. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ohshita, J.; Mizumo, T.; Tsuru, T. Efficient Synthesis of SiOC Glasses from Ethane, Ethylene, and Acetylene-Bridged Polysilsesquioxanes. J. Non Cryst. Solids 2015, 408, 137–141. [Google Scholar] [CrossRef]
- Strachota, A.; Černý, M.; Chlup, Z.; Šlouf, M.; Hromádková, J.; Pleštil, J.; Šandová, H.; Glogar, P.; Sucharda, Z.; Havelcová, M.; et al. Optimization of Sol–Gel/Pyrolysis Routes to Silicon Oxycarbide Glasses. J. Non Cryst. Solids 2012, 358, 2771–2782. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Chen, X.; Yu, S. Influence of Ti Addition on the Structure, Thermal Stability of SiTiOC Ceramics and Pyrolysis Behavior of SiTiOC Xerogel. Ceram. Int. 2024, 51, 908–916. [Google Scholar] [CrossRef]
- Garrigues Mateo, S. PAINTS | Organic Solvent-Based. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 9–18. [Google Scholar]
- Zarei-Jelyani, F.; Salahi, F.; Zarei-Jelyani, M.; Rahimpour, M.R. Various Industrial Wastes to Energy Technologies. In Encyclopedia of Renewable Energy, Sustainability and the Environment; Elsevier: Amsterdam, The Netherlands, 2024; pp. 17–28. [Google Scholar]
- Erb, D.; Lu, K. Additive and Pyrolysis Atmosphere Effects on Polysiloxane-Derived Porous SiOC Ceramics. J. Eur. Ceram. Soc. 2017, 37, 4547–4557. [Google Scholar] [CrossRef]
- Vallero, D.A. Control of Hazardous Air Pollutants. In Fundamentals of Air Pollution; Elsevier: Amsterdam, The Netherlands, 2008; pp. 825–851. [Google Scholar]
- Rezapoor, P.; Rahimpour, M.R. Animal Waste to Energy, Technologies, Economics, and Challenges. In Encyclopedia of Renewable Energy, Sustainability and the Environment; Elsevier: Amsterdam, The Netherlands, 2024; pp. 61–70. [Google Scholar]
- Okoroanyanwu, U.; Bhardwaj, A.; Einck, V.; Ribbe, A.; Hu, W.; Rodriguez, J.M.; Schmidt, W.R.; Watkins, J.J. Rapid Preparation and Electrochemical Energy Storage Applications of Silicon Carbide and Silicon Oxycarbide Ceramic/Carbon Nanocomposites Derived Via Flash Photothermal Pyrolysis of Organosilicon Preceramic Polymers. Chem. Mater. 2021, 33, 678–694. [Google Scholar] [CrossRef]
- Pogodin, V.A.; Rabinskiy, L.N.; Sitnikov, S.A. Obtaining a Composite Material Based on Quartz Woven Filler and Pyrolysis Matrix of Organosilicon Resin. Orig. Res. 2021, 9, 347–356. [Google Scholar] [CrossRef]
- Brus, J.; Kolář, F.; Machovič, V.; Svítilová, J. Structure of Silicon Oxycarbide Glasses Derived from Poly(Methylsiloxane) and Poly[Methyl(Phenyl)Siloxane] Precursors. J. Non Cryst. Solids 2001, 289, 62–74. [Google Scholar] [CrossRef]
- Bai, H.W.; Wen, G.; Huang, X.X.; Han, Z.X.; Zhong, B.; Hu, Z.X.; Zhang, X.D. Synthesis and Structural Characterization of SiBOC Ceramic Fibers Derived from Single-Source Polyborosiloxane. J. Eur. Ceram. Soc. 2011, 31, 931–940. [Google Scholar] [CrossRef]
- Schuhladen, K.; Pantulap, U.; Engel, K.; Jeleń, P.; Olejniczak, Z.; Hupa, L.; Sitarz, M.; Boccaccini, A.R. Influence of the Replacement of Silica by Boron Trioxide on the Properties of Bioactive Glass Scaffolds. Int. J. Appl. Glass Sci. 2021, 12, 293–312. [Google Scholar] [CrossRef]
- Park, C.; Kim, B.; Balaji, N.; Lee, Y.-J.; Ju, M.; Lee, H.; Yi, J. Boron Oxygen Pair Effect in p+ Emitter and Nanosized Boron Rich Layer by Fold Coordination Analysis for Crystalline Silicon Solar Cell Applications. J. Nanosci. Nanotechnol. 2016, 16, 4846–4850. [Google Scholar] [CrossRef]
- Peña-Alonso, R.; Mariotto, G.; Gervais, C.; Babonneau, F.; Soraru, G.D. New Insights on the High-Temperature Nanostructure Evolution of SiOC and B-Doped SiBOC Polymer-Derived Glasses. Chem. Mater. 2007, 19, 5694–5705. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, H.; Yuan, F.; Zhou, S.; Zhou, H.; Han, W. Effect of B Incorporation on the SiOC Precursor Fabrication and High-Temperature Oxidation Behavior of SiBOC Ceramics. Ann. Mater. Sci. Eng. 2021, 5, 1043. [Google Scholar]
- Yang, M.; Li, J.; Zhang, J.; Cao, J.; Luo, P.; Liu, J.; Gao, G.; Dong, J.; Jiang, Z. DLP-Printed SiBOC Ceramic Components from Preceramic Polymers Based on Boric Acid. J. Eur. Ceram. Soc. 2024, 44, 116747. [Google Scholar] [CrossRef]
- Ding, J.; Li, W.; Thiem, M.; Fan, W.; Zhang, S.; Teppala, D.T.; Lu, K.; Ionescu, E.; Riedel, R.; Weidenkaff, A.; et al. In-Situ Polymer-Derived SiC/Si(B)OC Ceramic Nanocomposites: A Sustainable Potential Candidate for High-Temperature Thermoelectric Applications. Chem. Eng. J. 2025, 503, 158420. [Google Scholar] [CrossRef]
- Gencer, A.; Oksal, B.S. Synthesis and Characterization of Novel SiBOC Ceramics: Comparison of Microwave and Ultrasonic Application on Gelation Time. J. Solgel Sci. Technol. 2015, 73, 171–180. [Google Scholar] [CrossRef]
- Wen, Q.; Yu, Z.; Riedel, R. The Fate and Role of in Situ Formed Carbon in Polymer-Derived Ceramics. Prog. Mater. Sci. 2020, 109, 100623. [Google Scholar] [CrossRef]
- Parchovianský, M.; Parchovianská, I.; Švančárek, P.; Medveď, D.; Lenz-Leite, M.; Motz, G.; Galusek, D. High-Temperature Oxidation Resistance of PDC Coatings in Synthetic Air and Water Vapor Atmospheres. Molecules 2021, 26, 2388. [Google Scholar] [CrossRef] [PubMed]
- Stabler, C.; Ionescu, E.; Graczyk-Zajac, M.; Gonzalo-Juan, I.; Riedel, R. Silicon Oxycarbide Glasses and Glass-ceramics: “All-Rounder” Materials for Advanced Structural and Functional Applications. J. Am. Ceram. Soc. 2018, 101, 4817–4856. [Google Scholar] [CrossRef]
- Barrios, E.; Zhai, L. A Review of the Evolution of the Nanostructure of SiCN and SiOC Polymer Derived Ceramics and the Impact on Mechanical Properties. Mol. Syst. Des. Eng. 2020, 5, 1606–1641. [Google Scholar] [CrossRef]
- Zhang, H.; Pantano, C.G. Synthesis and Characterization of Silicon Oxycarbide Glasses. J. Am. Ceram. Soc. 1990, 73, 958–963. [Google Scholar] [CrossRef]
- Jeleń, P.; Bik, M.; Nocuń, M.; Gawęda, M.; Długoń, E.; Sitarz, M. Free Carbon Phase in SiOC Glasses Derived from Ladder-like Silsesquioxanes. J. Mol. Struct. 2016, 1126, 172–176. [Google Scholar] [CrossRef]
- Kim, K.J.; Eom, J.-H.; Kim, Y.-W.; Seo, W.-S. Electrical Conductivity of Dense, Bulk Silicon-Oxycarbide Ceramics. J. Eur. Ceram. Soc. 2015, 35, 1355–1360. [Google Scholar] [CrossRef]
- Kleebe, H.-J.; Blum, Y.D. SiOC Ceramic with High Excess Free Carbon. J. Eur. Ceram. Soc. 2008, 28, 1037–1042. [Google Scholar] [CrossRef]
- Stabler, C.; Reitz, A.; Stein, P.; Albert, B.; Riedel, R.; Ionescu, E. Thermal Properties of SiOC Glasses and Glass Ceramics at Elevated Temperatures. Materials 2018, 11, 279. [Google Scholar] [CrossRef]
- Momma, T.; Aoki, S.; Nara, H.; Yokoshima, T.; Osaka, T. Electrodeposited Novel Highly Durable SiOC Composite Anode for Li Battery above Several Thousands of Cycles. Electrochem. Commun. 2011, 13, 969–972. [Google Scholar] [CrossRef]
- Shen, J.; Ahn, D.; Raj, R. C-Rate Performance of Silicon Oxycarbide Anodes for Li+ Batteries Enhanced by Carbon Nanotubes. J. Power Sources 2011, 196, 2875–2878. [Google Scholar] [CrossRef]
- Jeleń, P.; Szumera, M.; Gawęda, M.; Długoń, E.; Sitarz, M. Thermal Evolution of Ladder-like Silsesquioxanes during Formation of Black Glasses. J. Therm. Anal. Calorim. 2017, 130, 103–111. [Google Scholar] [CrossRef]
- Gervais, C.; Babonneau, F.; Dallabonna, N.; Sorarù, G. Sol-Gel-Derived Silicon-Boron Oxycarbide Glasses Containing Mixed Silicon Oxycarbide (SiCxO4−x) and Boron Oxycarbide (BCyO3−y) Units. J. Am. Ceram. Soc. 2001, 84, 2160–2164. [Google Scholar] [CrossRef]
- Marchewka, J.; Jeleń, P.; Rutkowska, I.; Bezkosty, P.; Sitarz, M. Chemical Structure and Microstructure Characterization of Ladder-Like Silsesquioxanes Derived Porous Silicon Oxycarbide Materials. Materials 2021, 14, 1340. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, E.; Gozzi, M.F.; Gonc, M.C.; Yoshida, I.V.P. Silicon Oxycarbide Glasses from Silicone Networks. J. Non-Crystalline Solids 1999, 248, 37–48. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Tang, K.; Ostrovski, O.; Tronstad, R. Synthesis of Silicon Carbide by Carbothermal Reduction of Quartz in H2-Ar Gas Mixtures. In Proceedings of the Fourteenth International Ferroalloys Congress 2015, Kyiv, Ukraine, 31 May–4 June 2015. [Google Scholar]
- Ricohermoso, E.I.; Heripre, E.; Solano-Arana, S.; Riedel, R.; Ionescu, E. Hierarchical Microstructure Growth in a Precursor-derived SiOC Thin Film Prepared on Silicon Substrate. Int. J. Appl. Ceram. Technol. 2023, 20, 735–746. [Google Scholar] [CrossRef]
- George Socrates. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Łagowska, B.; Wacławska, I.; Sitarz, M.; Szumera, M. Spectroscopic Studies of Structural Interactions in Silicate-Borate-Phosphate Glass. J. Mol. Struct. 2018, 1171, 110–116. [Google Scholar] [CrossRef]
- Gautam, C.; Yadav, A.K.; Singh, A.K. A Review on Infrared Spectroscopy of Borate Glasses with Effects of Different Additives. ISRN Ceram. 2012, 2012, 428497. [Google Scholar] [CrossRef]
- Sitarz, M.; Czosnek, C.; Jeleń, P.; Odziomek, M.; Olejniczak, Z.; Kozanecki, M.; Janik, J.F. SiOC Glasses Produced from Silsesquioxanes by the Aerosol-Assisted Vapor Synthesis Method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 112, 440–445. [Google Scholar] [CrossRef]
- Sitarz, M.; Jastrzębski, W.; Jeleń, P.; Długoń, E.; Gawęda, M. Preparation and Structural Studies of Black Glasses Based on Ladder-like Silsesquioxanes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 884–888. [Google Scholar] [CrossRef]
- Bik, M.; Galetz, M.; Dąbrowa, J.; Mroczka, K.; Zając, P.; Gil, A.; Jeleń, P.; Gawęda, M.; Owińska, M.; Stygar, M.; et al. Polymer Derived Ceramics Based on SiAlOC Glasses as Novel Protective Coatings for Ferritic Steel. Appl. Surf. Sci. 2022, 576, 151826. [Google Scholar] [CrossRef]
- Anand, A.; Unnikrishnan, B.; Wang, C.Y.; Lai, J.Y.; Lin, H.J.; Huang, C.C. Phosphate Ester-Linked Carbonized Polymer Nanosheets to Limit Microbiological Contamination in Aquaculture Water. npj Clean. Water 2024, 7, 84. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Pach, A.; Kutyła, D.; Kula, A.; Małecki, S.; Jeleń, P.; Hessel, V. Waste for Product—Synthesis and Electrocatalytic Properties of Palladium Nanopyramid Layer Enriched with PtNPs. Materials 2024, 17, 4165. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. Carbon 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Krasovskii, P.V.; Sigalaev, S.K.; Grigoriev, Y.V. Characterization of Free Carbon Forms in β-SiC Nanopowders by Temperature-Programmed Oxidation and Raman Spectroscopy. Ceram. Int. 2021, 47, 7957–7965. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Hong, C.; Han, J.; Han, W.; Du, S. Sol-Gel-Derived SiBOC Ceramics with Highly Graphitized Free Carbon. Ceram. Int. 2015, 41, 15292–15296. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman Spectroscopy in Graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Meyer, G.; Arneodo Larochette, P.; Baruj, A.; Castro, F.J.; Lacharmoise, P.; Zacur, E.; Talagañis, B.A. Equipment for Hydrogen Absorption-Desorption Cycling Characterization of Hydride Forming Materials. Rev. Sci. Instrum. 2007, 78, 023903. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman Spectroscopy of Carbon Materials and Their Composites: Graphene, Nanotubes and Fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Roy, D.; Kanojia, S.; Mukhopadhyay, K.; Eswara Prasad, N. Analysis of Carbon-Based Nanomaterials Using Raman Spectroscopy: Principles and Case Studies. Bull. Mater. Sci. 2021, 44, 31. [Google Scholar] [CrossRef]



| Atomic Concentration [%] | |||
|---|---|---|---|
| Si | O | C | |
| 1000 °C ArH SiOC | 43.26 | 50.50 | 6.25 |
| 1000 °C Ar SiOC | 45.17 | 51.82 | 3.01 |
| 1200 °C ArH SiOC | 47.57 | 49.30 | 3.13 |
| 1200 °C Ar SiOC | 43.68 | 53.61 | 2.71 |
| 1400 °C ArH SiOC | 40.85 | 51.50 | 7.64 |
| 1400 °C Ar SiOC | 41.23 | 53.56 | 5.22 |
| 1000 °C ArH SiBOC | 39.72 | 42.53 | 17.75 |
| 1000 °C Ar SiBOC | 31.62 | 59.34 | 9.05 |
| 1200 °C ArH SiBOC | 29.46 | 39.90 | 30.64 |
| 1200 °C Ar SiBOC | 32.34 | 45.84 | 21.83 |
| 1400 °C ArH SiBOC | 33.06 | 43.57 | 23.37 |
| 1400 °C ArH SiBOC | 29.79 | 57.19 | 13.02 |
| Band Position [cm−1] | Vibration Origin | |||||
|---|---|---|---|---|---|---|
| 1000 °C Ar | 1000 °C ArH | 1200 °C Ar | 1200 °C ArH | 1400 °C Ar | 1400 °C ArH | |
| 467 | 471 | 471 | 472 | 474 | 474 | δ O-Si-O |
| 671 | 633 | 634 | 644 | 656 | 638 | S4R + S6R 4 and 6 membered rings |
| 744 | 725 | 729 | 741 | 731 | 725 | S3R 3 membered rings |
| 810 | 816 | 808 | 816 | 798 | 798 | vs Si-O-Si |
| 845 | - | 835 | 835 | 847 | 850 | v Si-C |
| 879 | 877 | 879 | 881 | 899 | 899 | δ Si-H |
| 974 | 974 | 995 | 997 | 1014 | 1012 | Si-O− |
| 1049 | 1051 | 1088 | 180 | 1103 | 1107 | vas Si-O-Si (C) |
| 1184 | 1184 | 1211 | 1196 | 1215 | 1221 | vas Si-O-Si (Si=O) |
| 1360 | 1360 | - | - | - | - | v Si-CHx-Si |
| 1628 | 1632 | - | - | - | - | δ H-O-H |
| - | - | - | - | 1595 | 1593 | v C-C |
| Band Position [cm−1] | Vibration Origin | |||||
|---|---|---|---|---|---|---|
| 1000 °C Ar | 1000 °C ArH | 1200 °C Ar | 1200 °C ArH | 1400 °C Ar | 1400 °C ArH | |
| 465 | 468 | 469 | 469 | 474 | 474 | δ O-Si-O |
| 616 | 617 | 628 | 632 | 632 | 614 | δ B-O-Si |
| 670 | 668 | 672 | 669 | 673 | 669 | δ B-O in BO3 |
| 741 | 735 | 732 | 733 | 735 | 733 | δ BO3-O-BO4 |
| 811 | 805 | 803 | 804 | 810 | 810 | vs Si-O-Si |
| 838 | 834 | 837 | 837 | 849 | 849 | v Si-C |
| 880 | 877 | 884 | 887 | 897 | 895 | δ Si-H/v BO4 |
| 925 | 916 | - | - | - | - | δ Si-OH |
| - | - | - | - | 970 | 972 | B-O-B or B-O-Si |
| 1002 | 998 | 1009 | 1007 | 1028 | 1026 | Si-O− or B-O-Si/pentaborate/Si-O− |
| 1091 | 1093 | 1099 | 1100 | 1101 | 1101 | vas Si-O-Si |
| 1148 | 1149 | 1160 | 1161 | 1165 | 1169 | v B-O in BO4 |
| 1200 | 1203 | 1209 | 1212 | 1215 | 1221 | Si-O-Si |
| 1289 | 1300 | 1303 | 1303 | 1313 | 1317 | BO4 |
| 1365 | 1371 | 1363 | 1373 | 1385 | 1383 | v B-O in BO3 |
| - | - | 1595 | - | 1591 | 1587 | v C-C |
| 1626 | - | - | - | - | - | δ H-O-H in H2O |
| Sample | Band | Raman Shift [cm−1] | Intensity [a.u.] | FWHM [cm−1] | Integral Intensity [a.u.] |
|---|---|---|---|---|---|
| 1000 °C ArH SiBOC | D1 | 1341 | 1.69 | 146 | 320.62 |
| D4 | 1493 | 0.52 | 128 | 87.40 | |
| G | 1563 | 0.84 | 47 | 51.87 | |
| D′ | 1608 | 1.20 | 41 | 63.99 | |
| 1200 °C ArH SiBOC | D4 | 1287 | 0.29 | 236 | 73.69 |
| D1 | 1336 | 1.70 | 127 | 293.95 | |
| D3 | 1521 | 0.39 | 145 | 60.63 | |
| G | 1577 | 0.91 | 65 | 77.98 | |
| D′ | 1610 | 0.93 | 42 | 42.49 | |
| 1200 °C ArH SiOC | D1 | 1335 | 1.7 | 137 | 309 |
| G | 1554 | 0.6 | 181 | 134 | |
| D′ | 1603 | 1.0 | 74 | 90 | |
| 1200 °C Ar SiBOC | D4 | 1251 | 0.28 | 226 | 66.49 |
| D1 | 1339 | 1.78 | 128 | 310.64 | |
| D3 | 1504 | 0.37 | 139 | 53.97 | |
| G | 1573 | 0.71 | 65 | 64.05 | |
| D′ | 1611 | 1.10 | 46 | 54.57 | |
| 1200 °C Ar SiOC | D1 | 1335 | 1.6 | 123 | 262 |
| G | 1554 | 0.6 | 181 | 134 | |
| D′ | 1605 | 0.9 | 67 | 83 | |
| 1400 °C ArH SiBOC | D4 | 1293 | 0.39 | 170 | 98.14 |
| D1 | 1352 | 1.73 | 73 | 183.95 | |
| D3 | 1513 | 0.17 | 115 | 22.63 | |
| G | 1569 | 0.28 | 59 | 17.69 | |
| D′ | 1610 | 1.38 | 51 | 74.12 | |
| 1400 °C ArH SiOC | D4 | 1235 | 0.4 | 156 | 59 |
| D1 | 1343 | 1.9 | 91 | 268 | |
| G | 1562 | 0.3 | 108 | 42 | |
| D′ | 1616 | 1.5 | 48 | 76 | |
| 1400 °C Ar SiBOC | D4 | 1291 | 0.31 | 221 | 85.23 |
| D1 | 1350 | 1.75 | 93 | 240.15 | |
| D3 | 1516 | 0.27 | 109 | 31.37 | |
| G | 1571 | 0.40 | 58 | 24.67 | |
| D′ | 1609 | 1.46 | 52 | 80.25 | |
| 1400 °C Ar SiOC | D4 | 1242 | 0.4 | 175 | 68 |
| D1 | 1345 | 1.9 | 95 | 273 | |
| G | 1553 | 0.3 | 118 | 50 | |
| D′ | 1613 | 1.4 | 52 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyszczarz, K.; Jeleń, P.; Szymczak, P.; Sitarz, M. Effect of Temperature and Ceramization Atmosphere on the Structure and Microstructure of Boron-Modified SiBOC Materials. Materials 2025, 18, 1794. https://doi.org/10.3390/ma18081794
Łyszczarz K, Jeleń P, Szymczak P, Sitarz M. Effect of Temperature and Ceramization Atmosphere on the Structure and Microstructure of Boron-Modified SiBOC Materials. Materials. 2025; 18(8):1794. https://doi.org/10.3390/ma18081794
Chicago/Turabian StyleŁyszczarz, Klaudia, Piotr Jeleń, Patryk Szymczak, and Maciej Sitarz. 2025. "Effect of Temperature and Ceramization Atmosphere on the Structure and Microstructure of Boron-Modified SiBOC Materials" Materials 18, no. 8: 1794. https://doi.org/10.3390/ma18081794
APA StyleŁyszczarz, K., Jeleń, P., Szymczak, P., & Sitarz, M. (2025). Effect of Temperature and Ceramization Atmosphere on the Structure and Microstructure of Boron-Modified SiBOC Materials. Materials, 18(8), 1794. https://doi.org/10.3390/ma18081794







