Mechanism Study on the Influence of Clay-Type Lithium Slag on the Properties of Cement-Based Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methodology
2.2.1. Sample Preparation
2.2.2. Standard Consistency and Setting Time
2.2.3. Calorimetric Measurements
2.2.4. Compressive Strength
2.2.5. Thermogravimetry
2.2.6. X-Ray Diffraction (XRD)
2.2.7. Volcanic Ash Availability Coefficient (PEC)
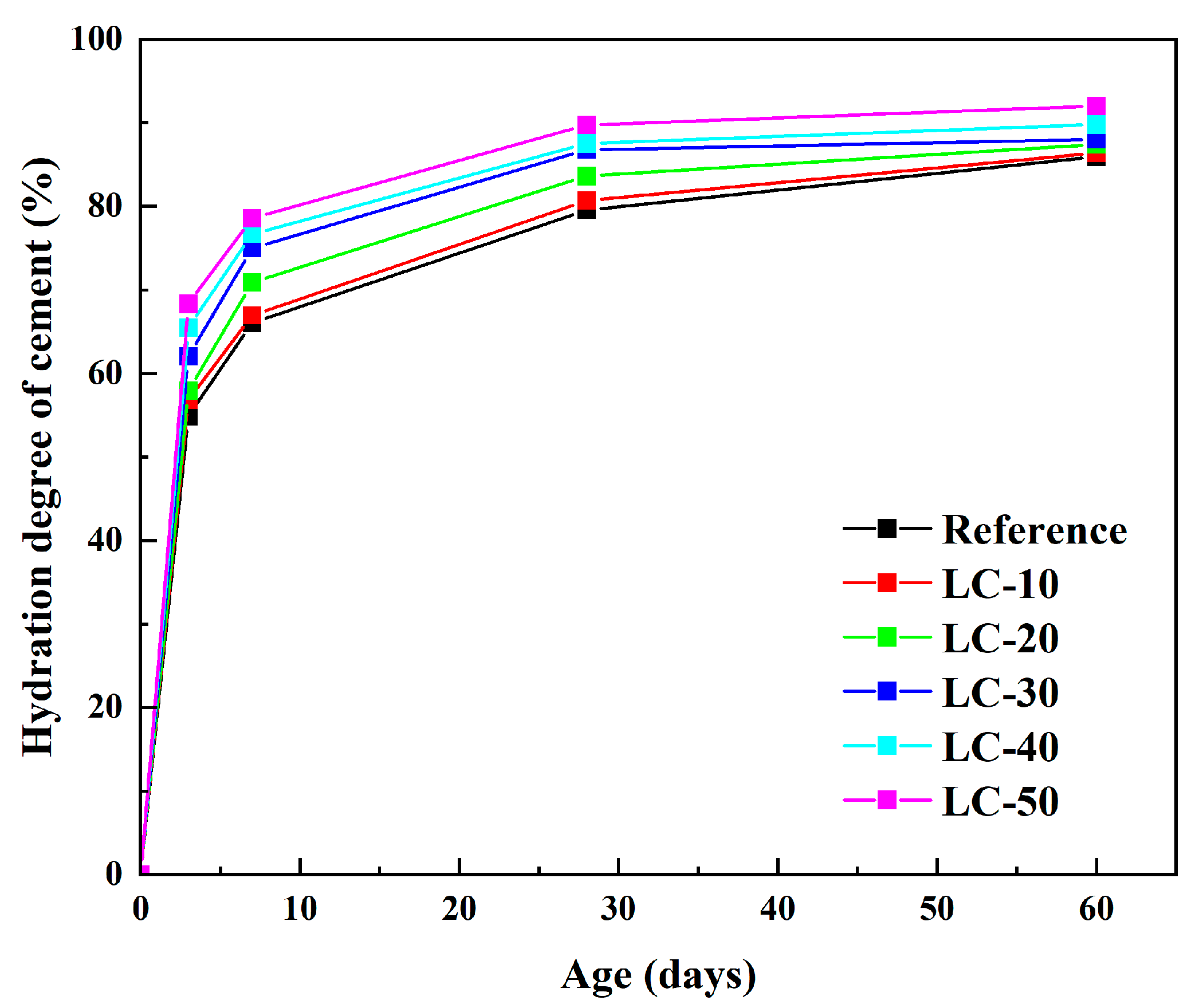
2.2.8. Hydration Degree
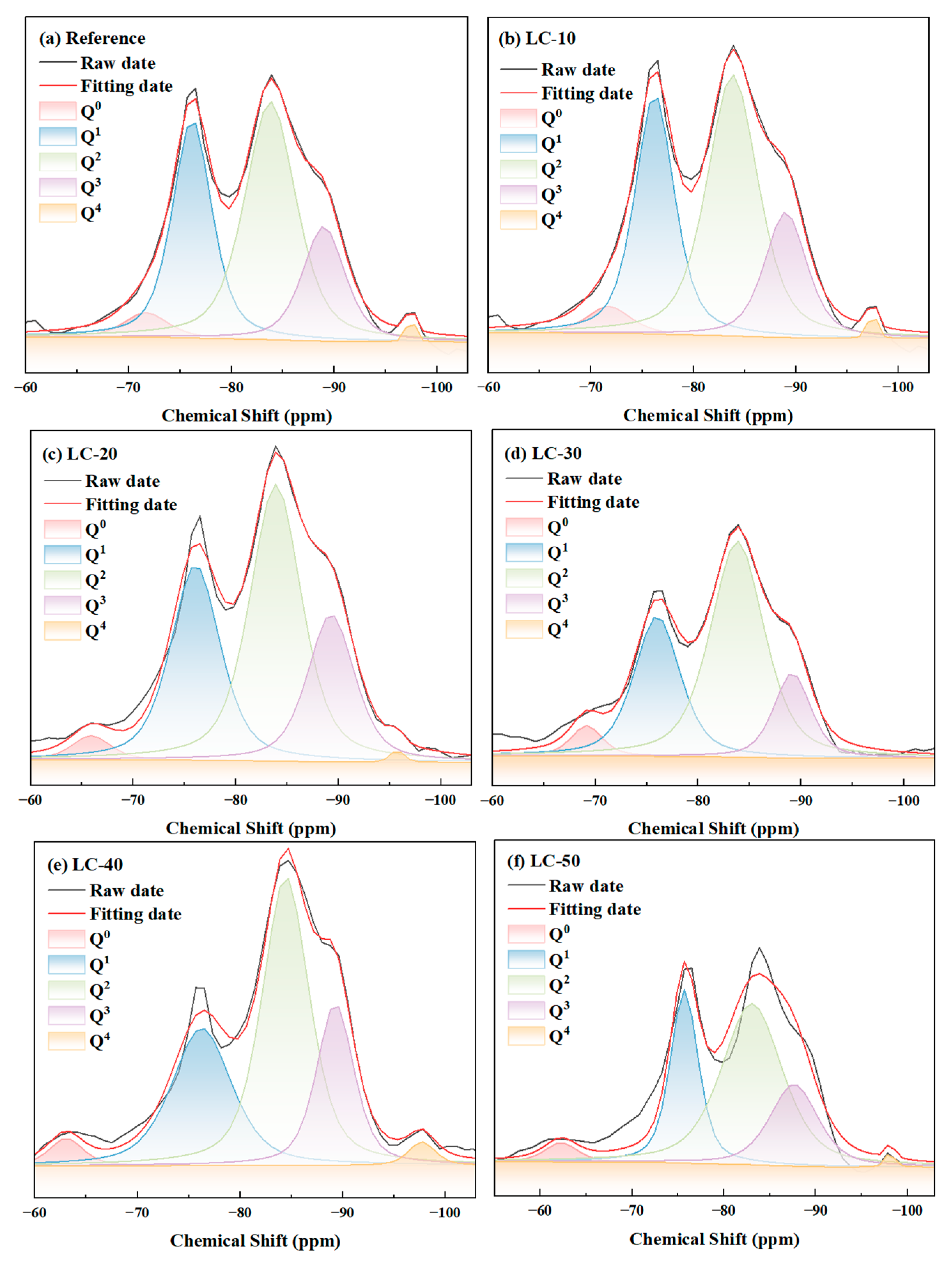
2.2.9. Nuclear Magnetic Resonance (NMR)
2.2.10. Scanning Electron Microscopy (SEM)
2.2.11. Mercury Intrusion Porosimetry (MIP)
3. Results and Discussion
3.1. Setting Time and Water for Standard Consistency
3.2. Heat of Hydration
3.3. Mechanical Properties
3.4. XRD and Thermogravimetric Analysis
3.5. Nuclear Magnetic Resonance
3.6. Pore Structure Analysis
3.7. Environmental Evaluation
4. Conclusions
- At the initial stage of hydration, with the increase in clay-type LS and LP content, the compressive strength showed a gradually decreasing trend. This was mainly due to the dilution effect of the clay-type LS. However, proper incorporation of clay-type LS and LP had a positive effect on the development of compressive strength in the later period. At 180 days, the compressive strength of LC-10 and LC-20 exceeded that of the reference by 3.7% and 1.1%, respectively. The volcanic ash availability coefficient of LC-10 and LC-20 were both greater than 1, indicating that there was synergy in the LC3 system.
- Proper incorporation of clay-type LS and LP could add additional hydration products, thus helping to fill large pores. In particular, the calcined clay-type LS reacted with calcium hydroxide to form C-A-S-H gel and amorphous aluminosilicates of aluminate hydrate.
- From the point of view of low-carbon development, the optimum dosage of LC3 cement was studied by considering many factors such as mechanical properties, setting time, heat of hydration, and nuclear magnetic analysis. The studies showed that the optimal content of clay-type LS and LP should not exceed 30%.
- LC3 cement partially replaced cement clinker with calcined clay-type LS and limestone, which significantly reduced CO2 emissions and energy consumption during the cement production. Its promotion and application strongly promoted the green and low-carbon transformation of the cement industry and provided important support for achieving the sustainable development goals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Liu, S.; Du, Y.; Huang, Q.; Qu, W.; Zheng, W. Effect of Mineral-Generated Lithium Slag on the Properties of Magnesium Oxychloride Cement. Crystals 2023, 13, 513. [Google Scholar] [CrossRef]
- Gu, T.; Zhang, G.; Wang, Z.; Liu, L.; Zhang, L.; Wang, W.; Huang, Y.; Dan, Y.; Zhao, P.; He, Y.; et al. The Formation, Characteristics, and Resource Utilization of Lithium Slag. Constr. Build. Mater. 2024, 432, 136648. [Google Scholar] [CrossRef]
- Survey, U.S.G. Mineral Commodity Summaries 2023; U.S. Geological Survey: Reston, VA, USA, 2023.
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of Lithium from Primary and Secondary Sources by Pre-Treatment, Leaching and Separation: A Comprehensive Review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Sun, S.; Cai, L.; Nie, X.; Song, X.; Yu, J. Separation of magnesium and lithium from brine using a Desal nanofiltration membrane. J. Water. Process. Eng. 2015, 7, 210–217. [Google Scholar] [CrossRef]
- Li, Y.; Miao, W.; He, M.; Li, C.; Gu, H.; Zhang, X. Origin of lithium-rich salt lakes on the western Kunlun Mountains of the Tibetan Plateau: Evidence from hydrogeochem-istry and lithium isotopes. Ore. Geol. Rev. 2023, 155, 105356. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Cheng, H. Recent Advances in Lithium Extraction from Lithium-Bearing Clay Minerals. Hydrometallurgy 2023, 217, 106025. [Google Scholar] [CrossRef]
- Xie, R.; Zhao, Z.; Tong, X.; Xie, X.; Song, Q.; Fan, P. Review of the research on the development and utilization of clay-type lithium resources. Particuology 2024, 87, 46–53. [Google Scholar] [CrossRef]
- Gu, H.; Guo, T.; Wen, H.; Luo, C.; Cui, Y.; Du, S.; Wang, N. Leaching Efficiency of Sulfuric Acid on Selective Lithium Leachability from Bauxitic Claystone. Miner. Eng. 2020, 145, 106076. [Google Scholar] [CrossRef]
- An, J.W.; Kang, D.J.; Tran, K.T.; Kim, M.J.; Lim, T.; Tran, T. Recovery of Lithium from Uyuni Salar Brine. Hydrometallurgy 2012, 117–118, 64–70. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Lu, Y.; Zhu, D. The Influence of Industrial Solid Waste in Conjunction with Lepidolite Tailings on the Mechanical Properties and Microstructure of Cemented Backfill Materials. Constr. Build. Mater. 2024, 419, 135422. [Google Scholar] [CrossRef]
- Khair, S.; Rahman, S.M.A.; Shaikh, F.U.A.; Sarker, P.K. Evaluating Lithium Slag for Geopolymer Concrete: A Review of Its Properties and Sustainable Construction Applications. Case. Stud. Constr. Mater. 2024, 20, e02822. [Google Scholar] [CrossRef]
- Guan, B.; Cao, X.; Yu, J.; Zhao, H.; Dai, L. Influence of Silica Fume on the Properties of Cement Binders with Lithium Tailings Mud. Constr. Build. Mater. 2024, 431, 136614. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Zhou, F.-S.; Evelina, L.M.A.; Liu, J.-L.; Zhou, Y. A Review on the Industrial Solid Waste Application in Pelletizing Additives: Composition, Mechanism and Process Characteristics. J. Hazard. Mater. 2022, 423, 127056. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yan, J.; Zhou, M.; Xu, Y.; Lu, Y.; Duan, P.; Zhu, Y.; Zhang, Z.; Li, W.; Wang, A.; et al. Heavy Metals Immobilization of Ternary Geopolymer Based on Nickel Slag, Lithium Slag and Metakaolin. J. Hazard. Mater. 2023, 453, 131380. [Google Scholar] [CrossRef]
- Li, J.; Lian, P.; Huang, S.; Huang, L. Recycling of Lithium Slag Extracted from Lithium Mica by Preparing White Portland Cement. J. Environ. Manage. 2020, 265, 110551. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Liu, M.; Li, Y.; Tang, Y.; Zhuang, L.; Tian, B. Comprehensive Utilization of Waste Residue from Lithium Extraction Process of Spodumene. Miner. Eng. 2021, 170, 106986. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.; Liu, D.; Wang, M. Preparation of Lightweight Ceramsite from Solid Waste Lithium Slag and Fly Ash. Constr. Build. Mater. 2023, 398, 132419. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Y.; Zhang, W.; Sun, H.; Wang, Q.; Han, D. Recycling Lithium Slag into Eco-Friendly Ultra-High Performance Concrete: Hydration Process, Microstructure Development, and Environmental Benefits. J. Build. Eng. 2024, 91, 109563. [Google Scholar] [CrossRef]
- Liang, G.; Yao, W.; She, A. Rheology and Microstructure of Lithium Slag/Metakaolin Geopolymer Pastes: Insights from Particle Packing and Water Dynamic Evolution. J. Build. Eng. 2024, 95, 110261. [Google Scholar] [CrossRef]
- He, Z.; Li, L.; Du, S. Mechanical Properties, Drying Shrinkage, and Creep of Concrete Containing Lithium Slag. Constr. Build. Mater. 2017, 147, 296–304. [Google Scholar] [CrossRef]
- Dong, S.; Tu, S.; Chen, L.; Wu, F.; Xie, L.; Zhuo, Q.; Yu, S. Investigation of the Performance of Cement Mortar Incorporating Lithium Slag as a Super-Fine Aggregate. Front. Mater. 2023, 10, 1134622. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Q.; Chen, Q.; Bian, J.; Qi, C.; Kang, Q.; Feng, Y. Mechanical and Environmental Characteristics of Cemented Paste Backfill Containing Lithium Slag-Blended Binder. Constr. Build. Mater. 2021, 271, 121567. [Google Scholar] [CrossRef]
- Pan, H.; Xiao, Q.; Huang, N.; Xiao, A.; Zhu, D. Effect of Alkaline Rice Straw Fibers Content and Curing Ages on Static Mechanical Properties of Cemented Lithium Mica Tailings Backfill. Case. Stud. Constr. Mater. 2024, 21, e03450. [Google Scholar] [CrossRef]
- He, Y.; Chen, Q.; Qi, C.; Zhang, Q.; Xiao, C. Lithium Slag and Fly Ash-Based Binder for Cemented Fine Tailings Backfill. J. Environ. Manag. 2019, 248, 109282. [Google Scholar] [CrossRef]
- Golewski, G.L. Determination of fracture mechanic parameters of concretes based on cement matrix enhanced by fly ash and nano-silica. Materials 2024, 17, 4230. [Google Scholar] [CrossRef]
- Huang, L.; Lan, Z.; Chen, Y.; Li, T. Preparation and Mechanism Analysis of Stainless Steel AOD Slag Mixture Base Materials. Materials 2024, 17, 970. [Google Scholar] [CrossRef]
- Park, S.; Wu, S.; Liu, Z.; Pyo, S. The Role of Supplementary Cementitious Materials (SCMs) in Ultra High Performance Concrete (UHPC): A Review. Materials 2021, 14, 1472. [Google Scholar] [CrossRef]
- Navarrete, I.; Valdes, J.; Lopez, M.; Vargas, F. Replacement of pozzolanic blended cement by supplementary cementitious materials: Mechanical and environmental approach. Constr. Build. Mater. 2023, 394, 132263. [Google Scholar] [CrossRef]
- Rahman, S.A.; Shaikh, F.U.A.; Sarker, P.K. A Comprehensive Review of Properties of Concrete Containing Lithium Refinery Residue as Partial Replacement of Cement. Constr. Build. Mater. 2022, 328, 127053. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Dodd, A.; Khair, S.; Shaikh, F.U.A.; Sarker, P.K.; Hosan, A. Assessment of Lithium Slag as a Supplementary Cementitious Material: Pozzolanic Activity and Microstructure Development. Cem. Concr. Compos. 2023, 143, 105262. [Google Scholar] [CrossRef]
- Shah, S.F.A.; Chen, B.; Ahmad, M.R.; Haque, M.A. Development of Cleaner One-Part Geopolymer from Lithium Slag. J. Clean. Prod. 2021, 291, 125241. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, B.; Tan, H.; Liu, X.; Chen, P.; Luo, Z. Effect of TIPA on Mechanical Properties and Hydration Properties of Cement-Lithium Slag System. J. Environ. Manag. 2020, 276, 111274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.; Zheng, Y.; Hua, K.; Liu, J. Thermal Activation Mechanism and Activity Evaluation of Lithium Slag: Insights from Simulated Hydration. Constr. Build. Mater. 2024, 411, 134615. [Google Scholar] [CrossRef]
- Liu, N.; Huang, B.; Dong, Z. Effect of Lithium Mica Slag on the Internal Sulfate Attack of Cement Mortar. Appl. Sci. 2024, 14, 2723. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.; Yang, C.; Gu, X.; Zhao, J. Study on the Activation and Pozzolanic Reaction Mechanism of Lithium Slag under the Effect of Composite Activation. Constr. Build. Mater. 2024, 448, 138223. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, S.; Tang, M.; Ding, J.; Buekens, A.; Yang, J.; Qiu, Q.; Yan, J. Mechanical Activation of Fly Ash from MSWI for Utilization in Cementitious Materials. Waste Manage. 2019, 88, 182–190. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, Y.; Bao, S. Preparation of Geopolymers from Vanadium Tailings by Mechanical Activation. Constr. Build. Mater. 2017, 145, 236–242. [Google Scholar] [CrossRef]
- Hu, Z.; Gu, X.; Cheng, B.; Wang, Q.; Liu, J.; Ge, X.; Yin, S. The Role of Chemical Activation in Strengthening Iron Ore Tailings Supplementary Cementitious Materials. Buildings 2024, 14, 963. [Google Scholar] [CrossRef]
- Tchadjie, L.N.; Ekolu, S.O. Enhancing the Reactivity of Aluminosilicate Materials toward Geopolymer Synthesis. J. Mater. Sci. 2018, 53, 4709–4733. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Jiang, Q.; Cheng, G.; Li, L.; Kang, Y.; Wang, D. A Green Route to Sustainable Alkali-Activated Materials by Heat and Chemical Activation of Lithium Slag. J. Clean. Prod. 2019, 225, 1184–1193. [Google Scholar] [CrossRef]
- He, Y.; You, C.; Liu, S.; Jiang, M.; Shi, P. Effects of Thermal Activation on the Hydration Performance of Lithium Slag–Cement Composite Binder. Adv. Cem. Res. 2024, 36, 240–248. [Google Scholar] [CrossRef]
- Liang, J.; Zou, W.; Tian, Y.; Wang, C.; Li, W. Effect of High Temperature on Mechanical Properties of Lithium Slag Concrete. Sci. Rep. 2024, 14, 11872. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.T.E.; Sarker, P.K.; Shaikh, F.U.A. Transport Properties of Concrete Containing Lithium Slag. Constr. Build. Mater. 2024, 416, 135073. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Zheng, X.; Hao, E.; Zhang, D.; Wang, T. Study on Chloride Attack Resistance of Concrete with Lithium Slag Content. J. Build. Eng. 2024, 97, 110723. [Google Scholar] [CrossRef]
- Li, J.; Huang, S. Recycling of Lithium Slag as a Green Admixture for White Reactive Powder Concrete. J. Mater. Cycles. Waste. Manag. 2020, 22, 1818–1827. [Google Scholar] [CrossRef]
- He, Z.; Du, S.; Chen, D. Microstructure of Ultra High Performance Concrete Containing Lithium Slag. J. Hazard. Mater. 2018, 353, 35–43. [Google Scholar] [CrossRef]
- Consoli, N.C.; Saldanha, R.B.; Mallmann, J.E.C.; de Paula, T.M.; Hoch, B.Z. Enhancement of Strength of Coal Fly Ash–Carbide Lime Blends through Chemical and Mechanical Activation. Constr. Build. Mater. 2017, 157, 65–74. [Google Scholar] [CrossRef]
- Luo, X.; Huang, L.; Yan, L.; Li, Y.; Wei, L.; Chen, Z.; Qu, Y. Preparation of Geopolymers from Thermally Activated Lithium Slag: Activity Enhancement and Microstructure. J. Build. Eng. 2024, 88, 109256. [Google Scholar] [CrossRef]
- Zhai, M.; Zhao, J.; Wang, D.; Wang, Y.; Wang, Q. Hydration Properties and Kinetic Characteristics of Blended Cement Containing Lithium Slag Powder. J. Build. Eng. 2021, 39, 102287. [Google Scholar] [CrossRef]
- Lu, J.; Yu, Z.; Zhu, Y.; Huang, S.; Luo, Q.; Zhang, S. Effect of Lithium-Slag in the Performance of Slag Cement Mortar Based on Least-Squares Support Vector Machine Prediction. Materials 2019, 12, 1652. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, S.; Liu, L.; Zhou, Y. Effects of Lithium Slag on the Frost Resistance of Cement-Soil. Materials 2022, 15, 5531. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Rupasinghe, M.; Sofi, M.; Sharma, R.; Ranzi, G.; Mendis, P.; Zhang, Z. A Review on Cementitious and Geopolymer Composites with Lithium Slag Incorporation. Materials 2024, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, L.; Li, L.; Zhou, P.; Shi, Z.; Cai, J.; Zhang, T. Investigation into the Alkali-Activation of Lithium Slag: A Sustainable Alternative to Conventional Cement with Optimized Mechanical Properties. Constr. Build. Mater. 2024, 416, 135022. [Google Scholar] [CrossRef]
- Junior, E.S.A.; Braga, N.T.D.S.; Barata, M.S. Life cycle assessment to produce LC3 cements with kaolinitic waste from the Amazon region, Brazil. Case Stud. Constr. Mater. 2023, 18, e01729. [Google Scholar]
- Avet, F.; Snellings, R.; Diaz, A.A.; Haha, M.N.; Scrivener, K. Development of a new rapid, relevant and reliable (R3) test method to evaluate the pozzolanic reactivity of calcined kaolinitic clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Scrivener, K.; Avet, F.; Maraghechi, H.; Zunino, F.; Ston, J.; Hanpongpun, W.; Favier, A. Impacting factors and properties of limestone calcined clay cements (LC3). Green Mater. 2019, 7, 3–14. [Google Scholar] [CrossRef]
- Hanpongpun, W.; Scrivener, K. The Effect of Curing Temperature on the Properties of Limestone Calcined Clay Cement (LC3); Springer: Singapore, 2020; Volume 14, pp. 293–298. [Google Scholar]
- Mañosa, J.; Calderón, A.; Salgado-Pizarro, R.; Maldonado-Alameda, A.; Chimenos, J.M. Research evolution of limestone calcined clay cement (LC3), a promising low-carbon binder—A comprehensive overview. Heliyon 2024, 10, e25117. [Google Scholar] [CrossRef]
- Avram, S.E.; Tudoran, L.B.; Cuc, S.; Borodi, G.; Birle, B.V.; Petean, I. Conditioning Influence of Kaolinite Matrices on Flexural Strength of Raw Pressed Slurry Collected from Ceramic Tile Production Wastewater. J. Compos. Sci. 2024, 8, 219. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). State Administration for Market Regulation and Standardization Administration of China: Beijing, China, 2021.
- ISO679-2009; Cement—Test Methods—Determination of Strength. International organization for Standardization: Geneva, Switzerland, 2009.
- GSB 08-1337-2017; China ISO Standard Sand. State Administration for Market Regulation and Standardization Administration of China: Beijing, China, 2021.
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. The General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China and the Standardization Administration of China: Beijing, China, 2011.
- GB/T 12959-2008; Test Methods for Heat of Hydration of Cement. The General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China and the Standardization Administration of China: Beijing, China, 2008.
- EN 197-1:2000; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. CEN, European Committee for Standardization: Brussels, Belgium, 2000.
- Záleská, M.; Pavlíková, M.; Pavlík, Z.; Jankovský, O.; Pokorný, J.; Tydlitát, V.; Svora, P.; Černý, R. Physical and chemical characterization of technogenic pozzolans for the application in blended cements. Constr. Build. Mater. 2018, 160, 106–116. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, G.; Yang, R.; Yu, R.; Xiao, R.; Wang, Z.; Xie, G.; Cheng, J. Hydration Kinetics and Microstructure Development of Ultra-High Performance Concrete (UHPC) by High Volume of Phosphorus Slag Powder. Cem. Concr. Compos. 2023, 138, 104978. [Google Scholar] [CrossRef]
- Kanagaraj, B.; Anand, N.; Johnson Alengaram, U.; Samuvel Raj, R.; Karthick, S. Limestone Calcined Clay Cement (LC3): A Sustainable Solution for Mitigating Environmental Impact in the Construction sector. Res. Cons. Recy. Advan. 2024, 21, 200197. [Google Scholar] [CrossRef]
- Wang, J.; Shi, D.; Xia, Y.; Liu, M.; Ma, X.; Yu, K.; Zhao, Y.; Zhang, J. Stabilization/Solidification of Radioactive Borate Waste via Low-Carbon Limestone Calcined Clay Cement (LC3). J. Environ. Chem. Eng. 2024, 12, 113129. [Google Scholar] [CrossRef]
- Wu, H.; Song, H.; Sun, X.; Bi, Y.; Fu, S.; Yang, N. Geo-Environmental Properties and Microstructural Characteristics of Sustainable Limestone Calcined Clay Cement (LC3) Binder Treated Zn-Contaminated Soils. J. Zhejiang Univ. Sci. 2023, 24, 898–911. [Google Scholar] [CrossRef]

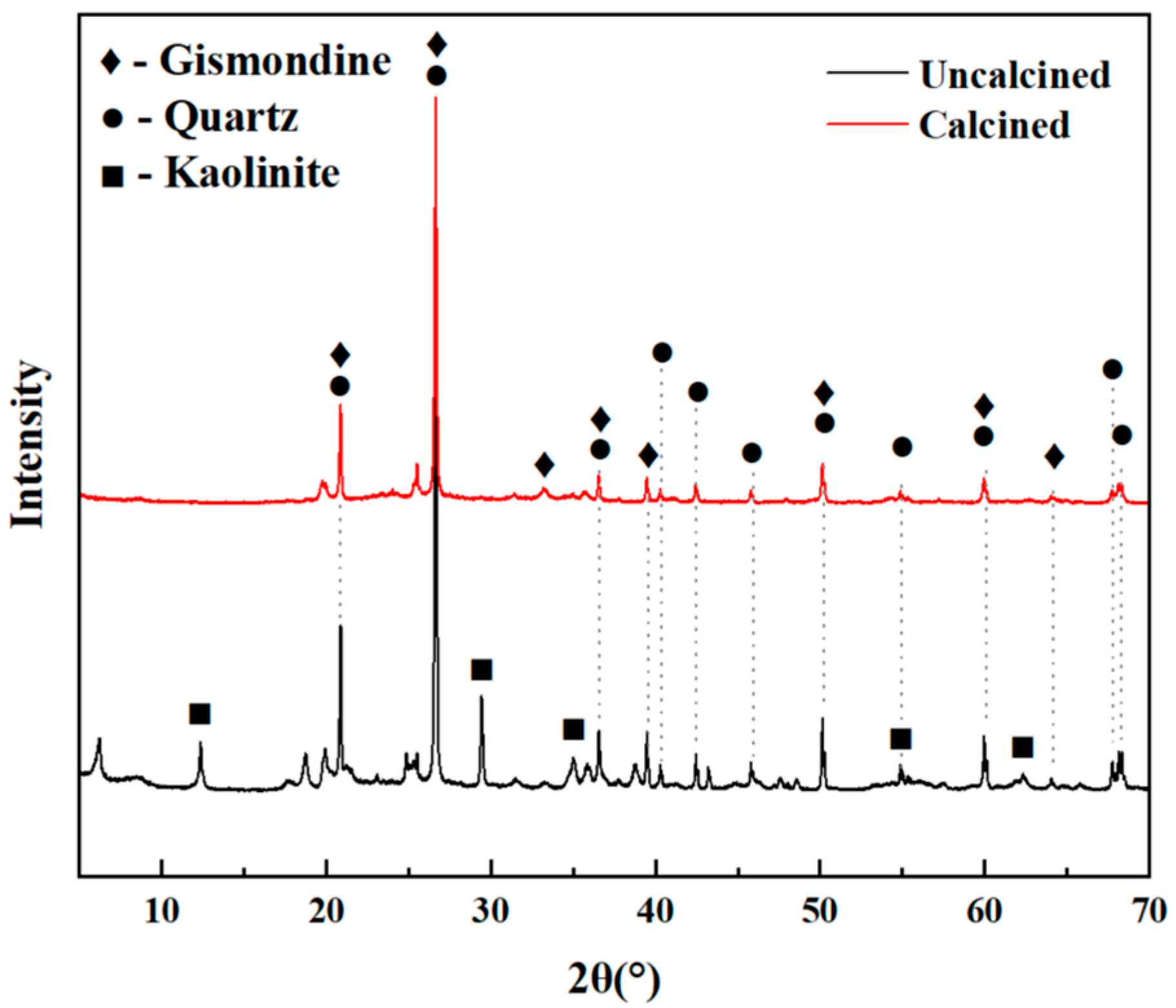
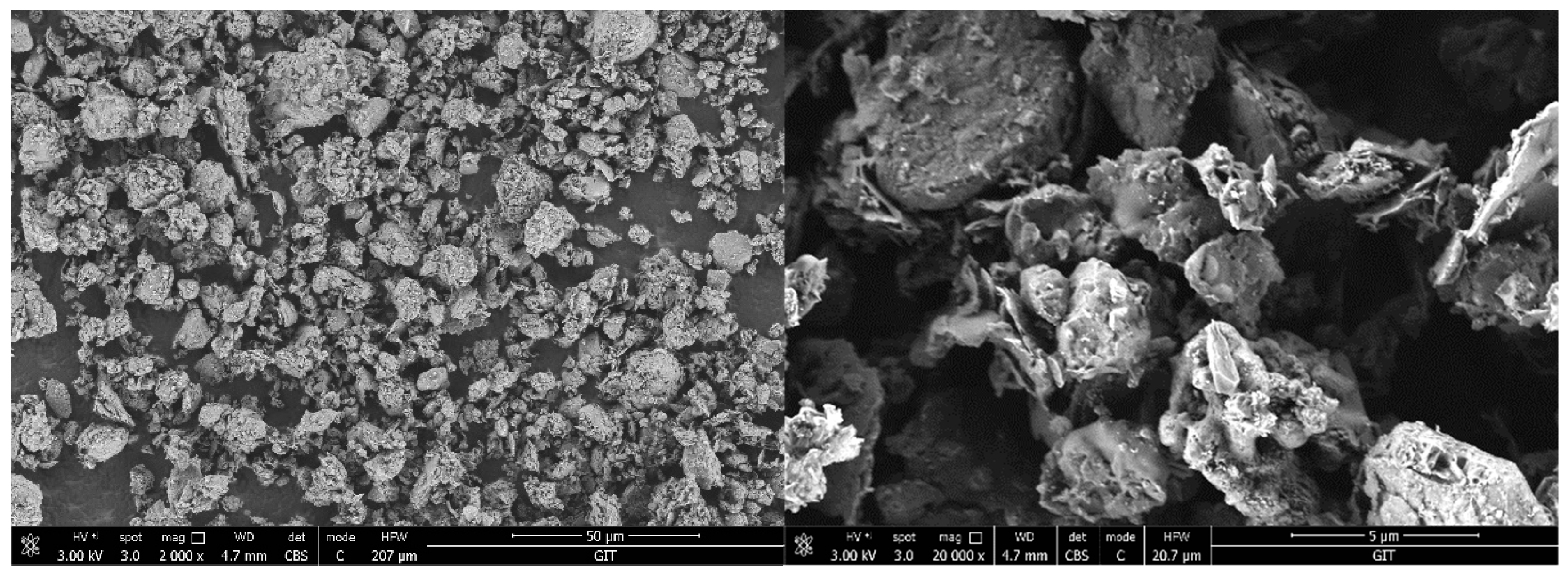
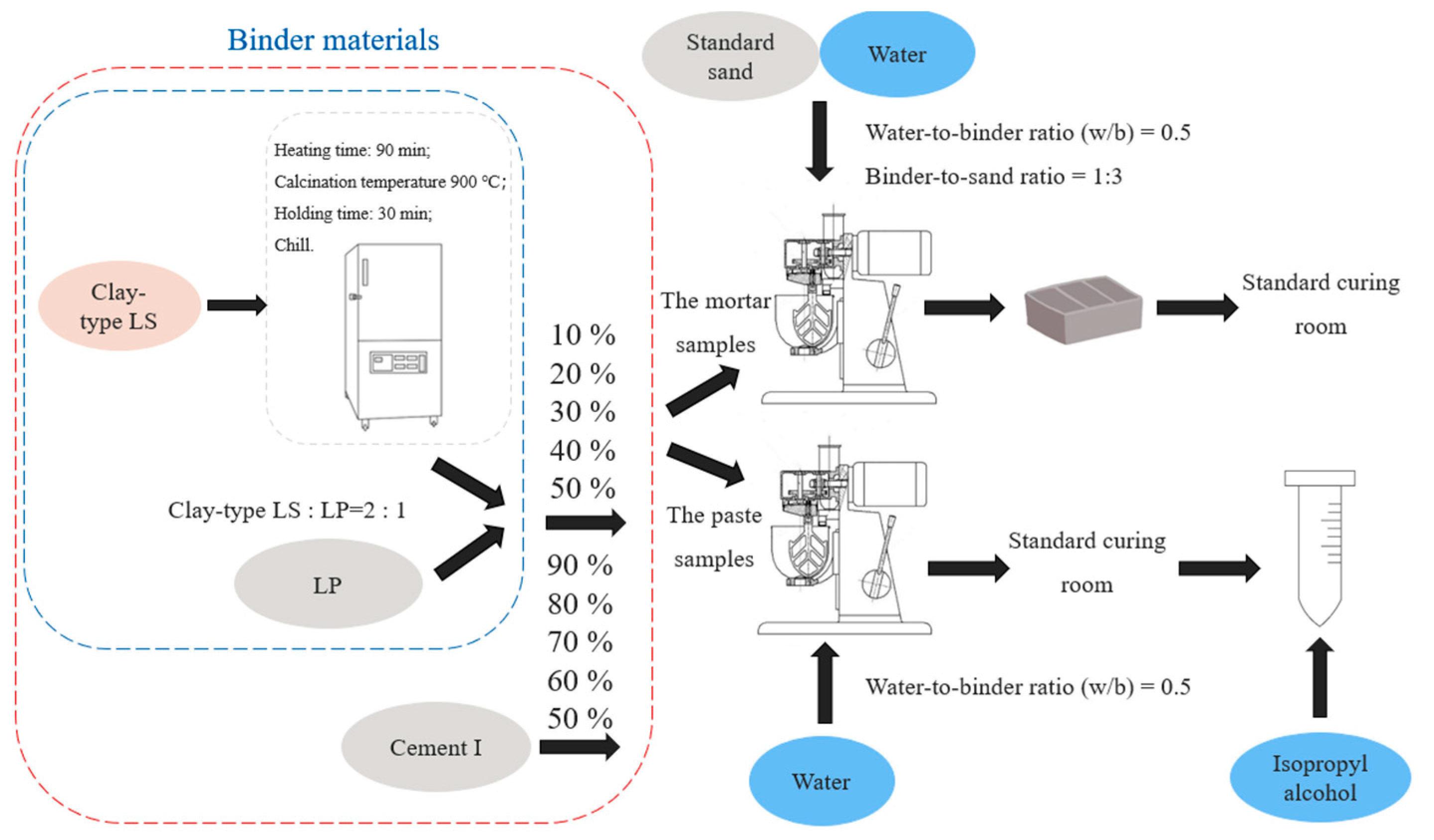



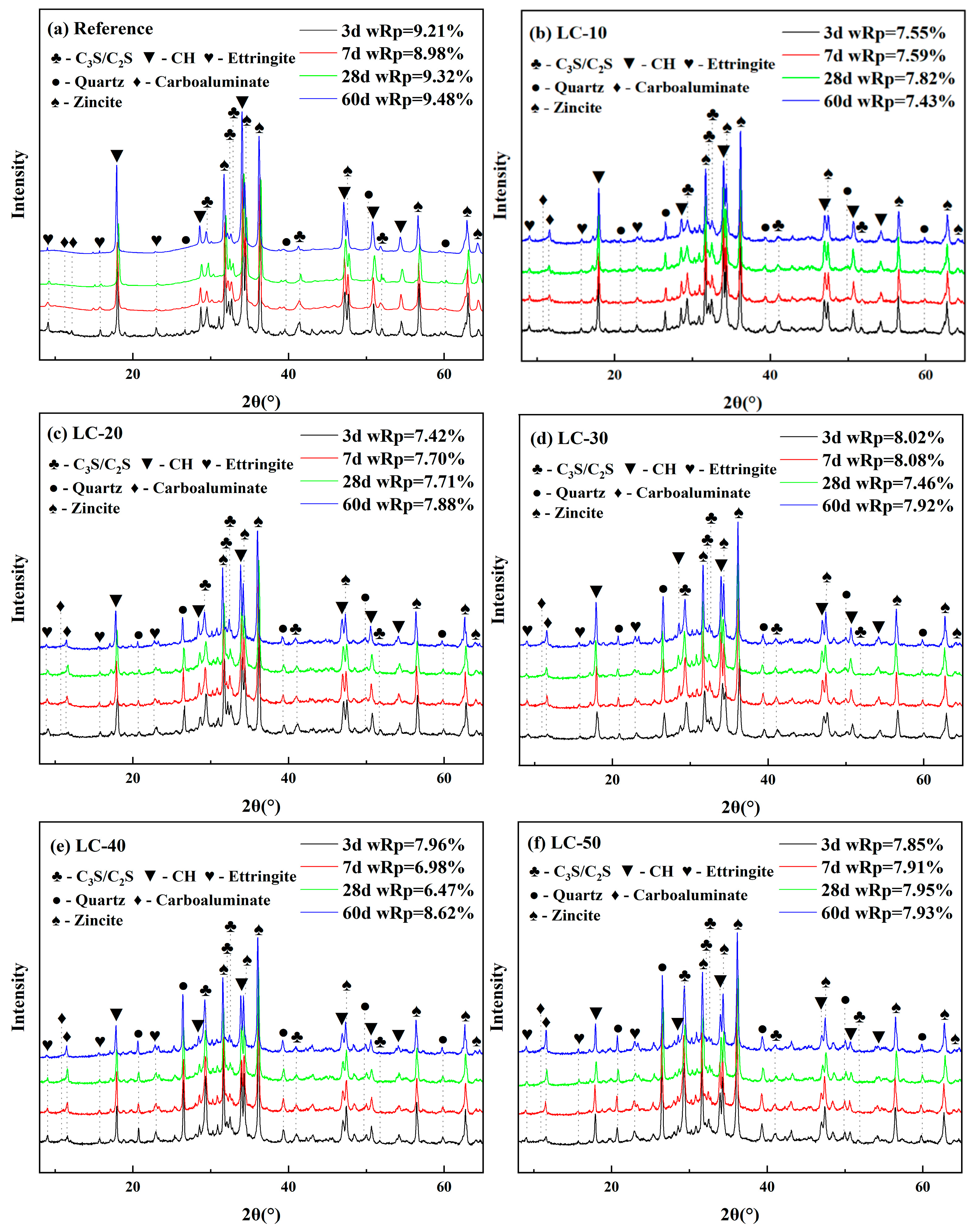


| Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | TiO2 | Cr2O3 | Fe2O3 | LOL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement I | 0.58 | 0.86 | 4.78 | 19.19 | 0.11 | 3.31 | 0.55 | 65.8 | 0.51 | - | 3.99 | 0.32 |
| LS | 0.07 | 0.23 | 19.60 | 73.53 | 0.09 | 0.03 | 1.30 | 2.17 | 1.12 | 0.01 | 1.83 | 0.02 |
| LP | - | 2.17 | 0.11 | 0.23 | - | 0.05 | - | 53.40 | - | - | 0.03 | 44.01 |
| Cement I | LS | LP | Sand | Water | |
|---|---|---|---|---|---|
| Reference | 450 | - | - | 1350 | 225 |
| LC-10 | 405 | 30 | 15 | 1350 | 225 |
| LC-20 | 360 | 60 | 30 | 1350 | 225 |
| LC-30 | 315 | 90 | 45 | 1350 | 225 |
| LC-40 | 270 | 120 | 60 | 1350 | 225 |
| LC-50 | 225 | 150 | 75 | 1350 | 225 |
| Mixture | Reference | LC-10 | LC-20 | LC-30 | LC-40 | LC-50 |
|---|---|---|---|---|---|---|
| 3 days | 19.32 | 15.99 | 14.68 | 12.70 | 11.10 | 8.63 |
| 7 days | 20.19 | 17.06 | 15.54 | 12.74 | 10.89 | 8.55 |
| 28 days | 21.01 | 18.01 | 15.91 | 13.85 | 11.59 | 8.72 |
| 60 days | 21.34 | 17.68 | 15.42 | 13.44 | 10.65 | 8.30 |
| Mixture | Reference | LC-10 | LC-20 | LC-30 | LC-40 | LC-50 |
|---|---|---|---|---|---|---|
| Q0 | 26.78 | 25.04 | 22.35 | 22.09 | 21.90 | 16.65 |
| Q1 | 211.22 | 201.65 | 227.47 | 150.65 | 171.93 | 127.54 |
| Q2 | 190.39 | 287.13 | 335.88 | 272.22 | 319.16 | 258.38 |
| Q3 | 121.84 | 114.29 | 164.30 | 74.22 | 141.83 | 104.23 |
| Q4 | 5.11 | 6.25 | 3.90 | 0.10 | 14.97 | 3.52 |
| φ | 3.80 | 4.85 | 4.95 | 5.61 | 5.71 | 6.05 |
| PD | 0.90 | 1.42 | 1.48 | 1.83 | 1.86 | 2.03 |
| Mixture | Ref. | LC-10 | LC-20 | LC-30 | LC-40 | LC-50 |
|---|---|---|---|---|---|---|
| Total Porosity (%) | 25.62 | 34.47 | 32.93 | 31.58 | 33.33 | 34.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, K.; Yang, G.; Zhou, W.; Ran, Q.; Yao, X.; Xiao, R.; Zhou, S. Mechanism Study on the Influence of Clay-Type Lithium Slag on the Properties of Cement-Based Materials. Materials 2025, 18, 1788. https://doi.org/10.3390/ma18081788
Xiao K, Yang G, Zhou W, Ran Q, Yao X, Xiao R, Zhou S. Mechanism Study on the Influence of Clay-Type Lithium Slag on the Properties of Cement-Based Materials. Materials. 2025; 18(8):1788. https://doi.org/10.3390/ma18081788
Chicago/Turabian StyleXiao, Kejia, Guangshao Yang, Wei Zhou, Qihao Ran, Xin Yao, Rengui Xiao, and Shaoqi Zhou. 2025. "Mechanism Study on the Influence of Clay-Type Lithium Slag on the Properties of Cement-Based Materials" Materials 18, no. 8: 1788. https://doi.org/10.3390/ma18081788
APA StyleXiao, K., Yang, G., Zhou, W., Ran, Q., Yao, X., Xiao, R., & Zhou, S. (2025). Mechanism Study on the Influence of Clay-Type Lithium Slag on the Properties of Cement-Based Materials. Materials, 18(8), 1788. https://doi.org/10.3390/ma18081788






