Synergistic Zn-Cd Bimetallic Engineering in ZIFs for High-Chloride 2e− ORR to H2O2 in Simulated Neutral Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZnCd-ZIF Catalysts with Different Doping Proportions
2.3. Characterization Equipment
2.4. Electrochemical Measurements
2.5. Detection of H2O2 Production
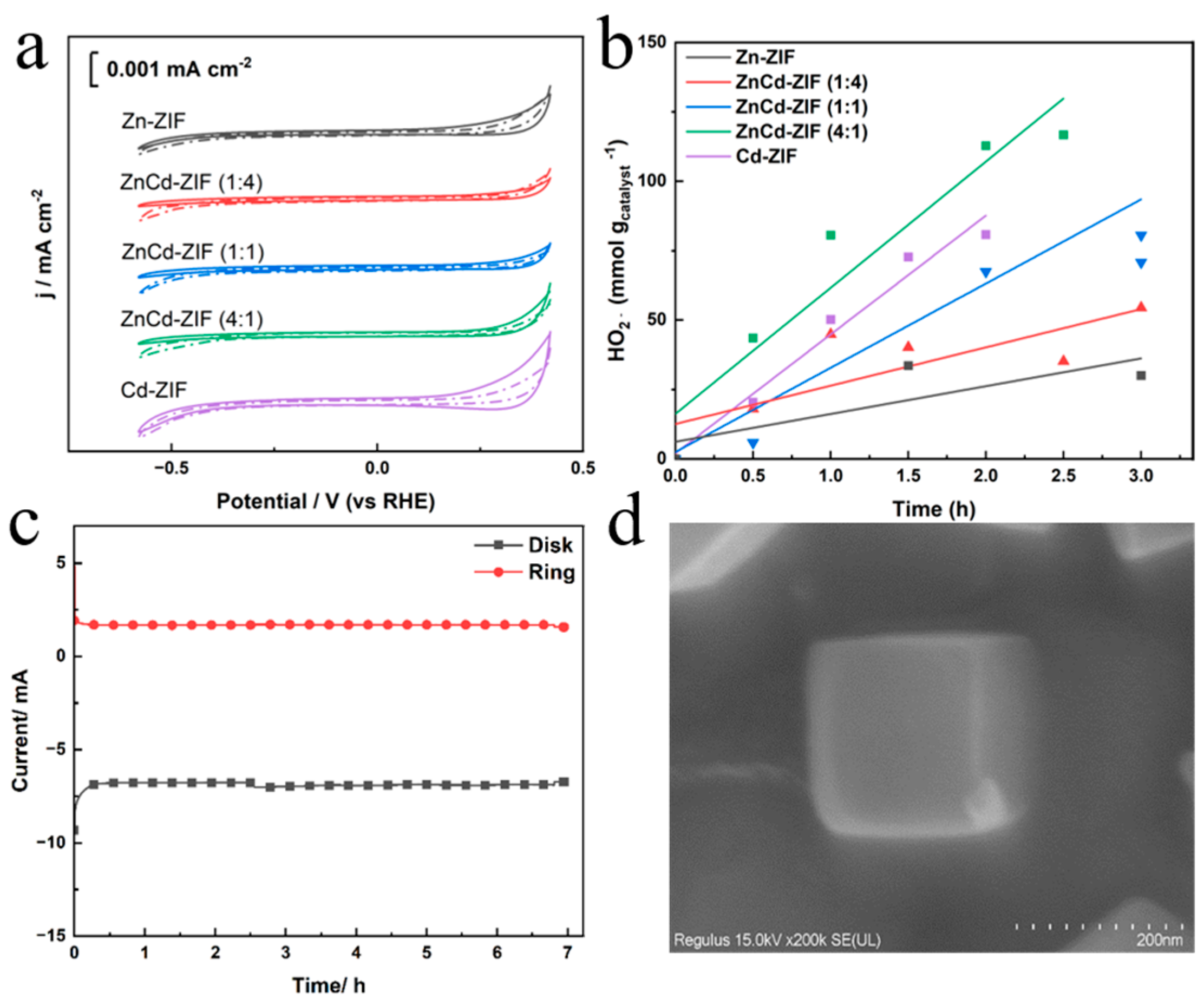
3. Results and Discussion
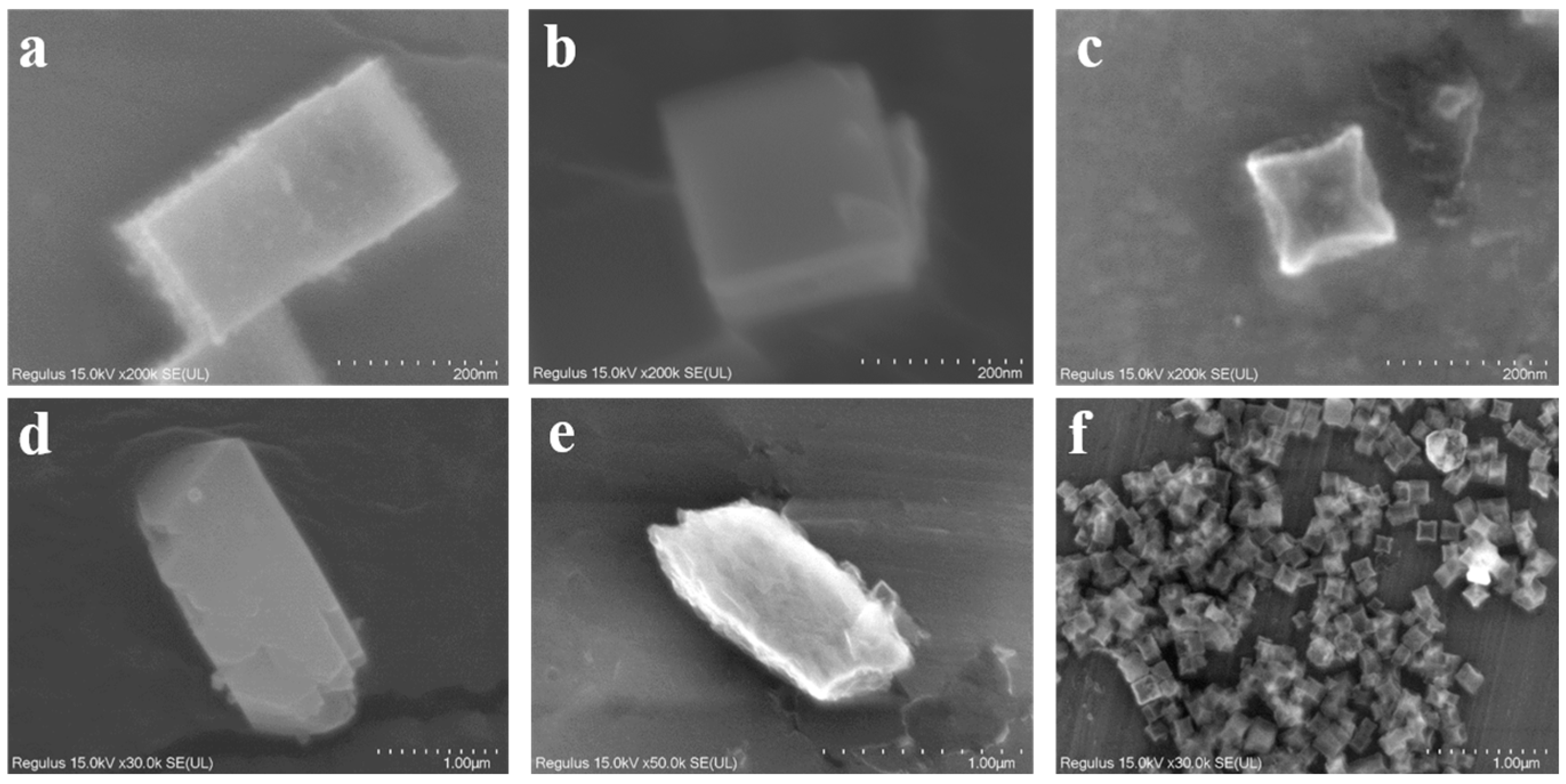
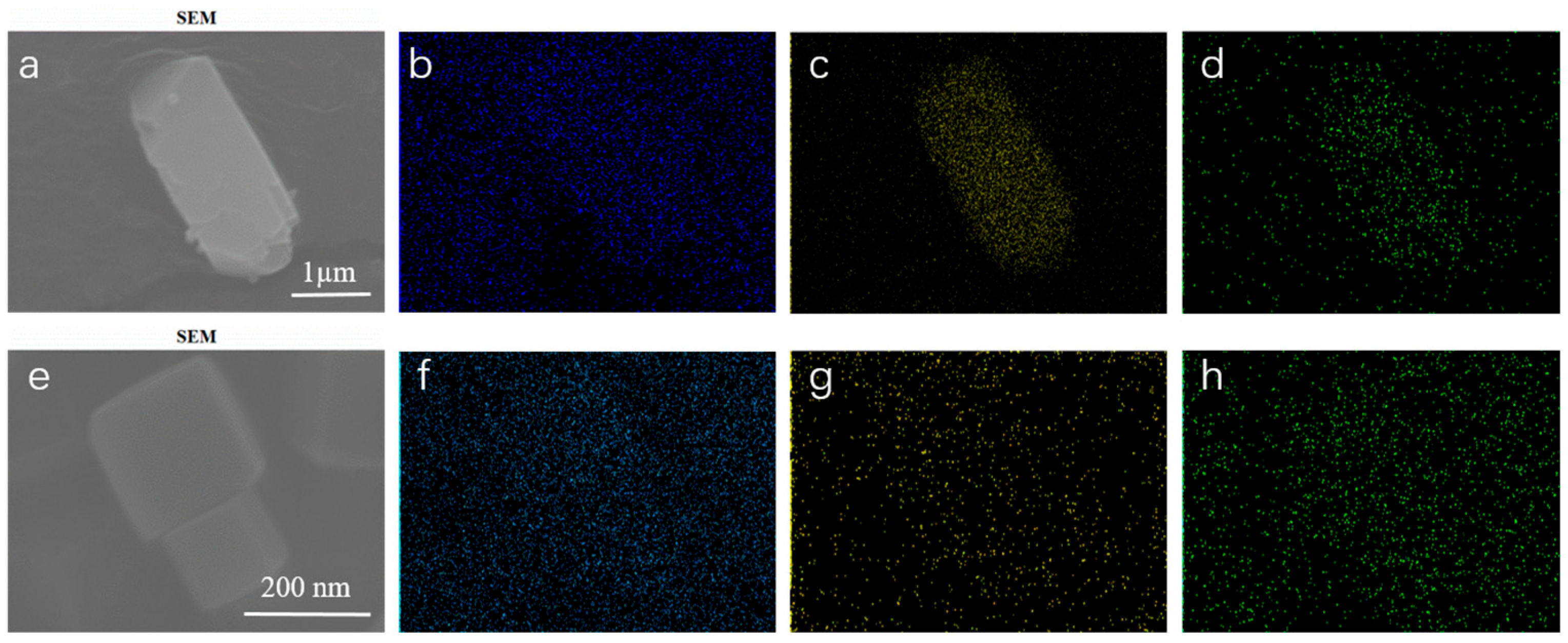
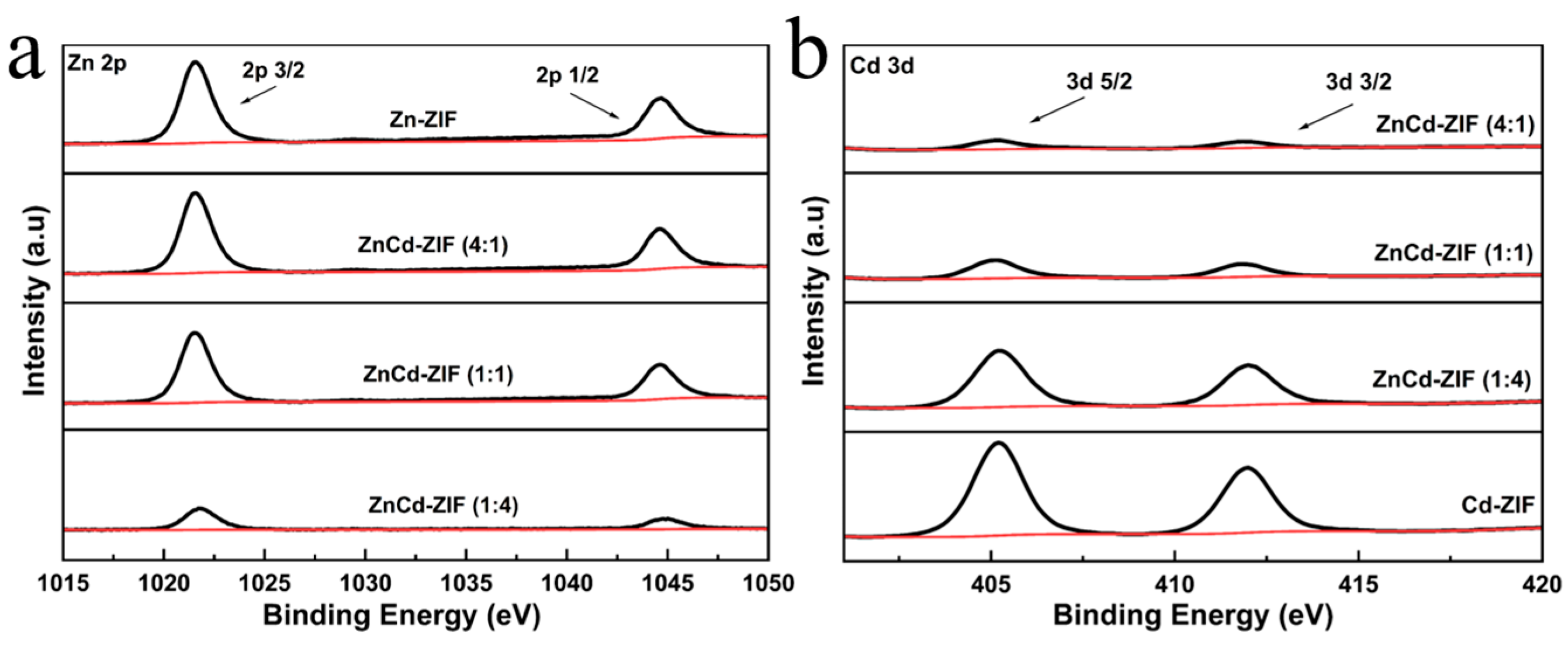
Characterizations of Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, H.; Wu, Q.; Yu, C.; Zhao, T.; Liu, M. Recent Progress of Biomimetic Antifouling Surfaces in Marine. Adv. Mater. Interfaces 2020, 7, 2000966. [Google Scholar] [CrossRef]
- Wu, S.; Wu, S.; Xing, S.; Wang, T.; Hou, J.; Zhao, Y.; Li, W. Research Progress of Marine Anti-Fouling Coatings. Coatings 2024, 14, 1227. [Google Scholar] [CrossRef]
- Gipperth, L. The legal design of the international and European Union ban on tributyltin antifouling paint: Direct and indirect effects. J. Environ. Manag. 2009, 90, S86–S95. [Google Scholar] [CrossRef]
- Li, J.; Wang, N.; Liu, K.; Duan, J.; Hou, B. Efficient electrocatalytic H2O2 production in simulated seawater on ZnO/reduced graphene oxide nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2023, 668, 131446. [Google Scholar] [CrossRef]
- Kosydar, R.; Drelinkiewicz, A.; Ganhy, J.P. Degradation Reactions in Anthraquinone Process of Hydrogen Peroxide Synthesis. Catal. Lett. 2010, 139, 105–113. [Google Scholar] [CrossRef]
- Chen, H.; Chen, R.; Liu, S.; Zhou, Y.; Chen, X.; Cai, J.; Lan, X.; Jiang, H.; Lin, L.; Sun, Z. Efficient H2O2 Synthesis Through a Two-Electron Oxygen Reduction Reaction by Electrocatalysts. ChemPlusChem 2024, 89, e202400422. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Qian, J.; Luan, J.; Mu, Y.; Xiao, C.; Zhang, Q.; Lam, S.S.; Li, W.; Zeng, L. Enhanced Electrocatalytic Hydrogen Peroxide Production via a CuWO4/WO3 Heterojunction with High Selectivity and Stability. ACS Appl. Mater. Interfaces 2025, 17, 5026–5037. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Choksi, T.S.; Zhou, D.; Han, S.; Liao, Y.-F.; Yang, H.B.; Liu, D.; Zeng, Z.; Liu, W.; et al. Strong Metal–Support Interaction Boosts Activity, Selectivity, and Stability in Electrosynthesis of H2O2. J. Am. Chem. Soc. 2022, 144, 2255–2263. [Google Scholar] [CrossRef]
- Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E.L. Toward the Decentralized Electrochemical Production of H2O2: A Focus on the Catalysis. ACS Catal. 2018, 8, 4064–4081. [Google Scholar] [CrossRef]
- He, H.; Liu, S.; Liu, Y.; Zhou, L.; Wen, H.; Shen, R.; Zhang, H.; Guo, X.; Jiang, J.; Li, B. Review and perspectives on carbon-based electrocatalysts for the production of H2O2via two-electron oxygen reduction. Green Chem. 2023, 25, 9501–9542. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, P.; Mostaghimi, A.H.B.; Zhao, X.; Denny, S.R.; Lee, J.H.; Gao, H.; Zhang, Y.; Xin, H.L.; Siahrostami, S.; et al. Promoting H2O2 production via 2-electron oxygen reduction by coordinating partially oxidized Pd with defect carbon. Nat. Commun. 2020, 11, 2178. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, S.; Zhang, R.; Wang, L.; Wang, Y.; Yang, L.; Li, J.; Guan, F.; Duan, J.; Hou, B. Regulating N Species in N-Doped Carbon Electro-Catalysts for High-Efficiency Synthesis of Hydrogen Peroxide in Simulated Seawater. Adv. Sci. 2023, 10, 2302446. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Zhang, Y.; Luo, W.; Wu, Y.; Wang, Y. Rational Design of Covalent Organic Frameworks for Photocatalytic Hydrogen Peroxide Production. Macromol. Rapid Commun. 2025, 2401149. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wang, K.; Xie, H.; Sun, Y.; Titirici, M.-M.; Chai, G.-L. Mesoporous Carbon Hollow Spheres as Efficient Electrocatalysts for Oxygen Reduction to Hydrogen Peroxide in Neutral Electrolytes. ACS Catal. 2020, 10, 7434–7442. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, L.; Liu, C.; Li, Z.; Zou, Y.; Zhang, X.; Zhang, Y.; Zhang, Z.; Wei, G.; Yu, C. Crystal Engineering Enables Cobalt-Based Metal–Organic Frameworks as High-Performance Electrocatalysts for H2O2 Production. J. Am. Chem. Soc. 2023, 145, 7791–7799. [Google Scholar] [CrossRef]
- Gao, L.; Xiao, M.; Jin, Z.; Liu, C.; Ge, J.; Xing, W. Hydrogen etching induced hierarchical meso/micro-pore structure with increased active density to boost ORR performance of Fe-N-C catalyst. J. Energy Chem. 2019, 35, 17–23. [Google Scholar] [CrossRef]
- Siahrostami, S.; Villegas, S.J.; Bagherzadeh Mostaghimi, A.H.; Back, S.; Farimani, A.B.; Wang, H.; Persson, K.A.; Montoya, J. A Review on Challenges and Successes in Atomic-Scale Design of Catalysts for Electrochemical Synthesis of Hydrogen Peroxide. ACS Catal. 2020, 10, 7495–7511. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Zhang, J. A review of advanced metal-free carbon catalysts for oxygen reduction reactions towards the selective generation of hydrogen peroxide. J. Mater. Chem. A 2020, 8, 20849–20869. [Google Scholar] [CrossRef]
- Wang, N.; Ma, S.; Zuo, P.; Duan, J.; Hou, B. Recent Progress of Electrochemical Production of Hydrogen Peroxide by Two-Electron Oxygen Reduction Reaction. Adv. Sci. 2021, 8, 2100076. [Google Scholar] [CrossRef]
- Jiao, D.X.; Ding, C.S.; Xu, M.H.; Ruan, X.W.; Ravi, S.K.; Cui, X.Q. Modulating Yeager Adsorption Configuration of O2 Through Cd Doping in Zn3In2S6 for Photosynthesis of H2O2. Adv. Funct. Mater. 2025, 35, 2416753. [Google Scholar] [CrossRef]
- Ye, Y.; Cai, F.; Yan, C.; Li, Y.; Wang, G.; Bao, X. Two-step pyrolysis of ZIF-8 functionalized with ammonium ferric citrate for efficient oxygen reduction reaction. J. Energy Chem. 2017, 26, 1174–1180. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, G.; Siahrostami, S.; Chen, Z.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D.; Liu, Y.; et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162. [Google Scholar] [CrossRef]
- Liu, K.; Wang, N.; Li, J.; Du, F.; Hou, B.; Zhang, R. Phtotoelectrochemical water oxidation to H2O2 based on N-TiO2 derived from NH2-MIL-125 and in-situ application on degradation dye. J. Ind. Eng. Chem. 2023, 128, 586–596. [Google Scholar] [CrossRef]
- Sankar ganesh, R.; Navaneethan, M.; Mani, G.K.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Kawasaki, S.; Hayakawa, Y. Influence of Al doping on the structural, morphological, optical, and gas sensing properties of ZnO nanorods. J. Alloys Compd. 2017, 698, 555–564. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Wang, Z.; Wang, R.; Chen, C.; Dong, C. Nanoparticles Assembled CdIn2O4 Spheres with High Sensing Properties towards n-Butanol. Nanomaterials 2019, 9, 1714. [Google Scholar] [CrossRef]
- Yang, F.F.; Fang, L.; Zhang, S.F.; Sun, J.S.; Xu, Q.T.; Wu, S.Y.; Dong, J.X.; Kong, C.Y. Structure and electrical properties of CdIn2O4 thin films prepared by DC reactive magnetron sputtering. Appl. Surf. Sci. 2008, 254, 5481–5486. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Z.; Fan, S.; Han, X.; Li, L.; Gao, Z.; Wang, C. From structure to function: MOF-based and COF-based catalysts for efficient electrocatalytic H2O2 production via 2e− ORR. J. Mater. Chem. A 2024, 12, 27180–27205. [Google Scholar] [CrossRef]
- Wang, N.; Ma, S.; Duan, J.; Zhai, X.; Guan, F.; Wang, X.; Hou, B. Electrocatalytic oxygen reduction to hydrogen peroxide by oxidized graphene aerogel supported cubic MnCO3 for antibacteria in neutral media. Electrochim. Acta 2020, 340, 135880. [Google Scholar] [CrossRef]
- Carneiro, J.F.; Paulo, M.J.; Siaj, M.; Tavares, A.C.; Lanza, M.R.V. Nb2O5 nanoparticles supported on reduced graphene oxide sheets as electrocatalyst for the H2O2 electrogeneration. J. Catal. 2015, 332, 51–61. [Google Scholar] [CrossRef]
- Sun, Y.; Sinev, I.; Ju, W.; Bergmann, A.; Dresp, S.; Kühl, S.; Spöri, C.; Schmies, H.; Wang, H.; Bernsmeier, D.; et al. Efficient Electrochemical Hydrogen Peroxide Production from Molecular Oxygen on Nitrogen-Doped Mesoporous Carbon Catalysts. ACS Catal. 2018, 8, 2844–2856. [Google Scholar] [CrossRef]


| Electrocatalysts | Electrolytes | H2O2 Yield (%) | Onset Potential | Stability (h) | Reference |
|---|---|---|---|---|---|
| Nb2O5-rGO | 0.1 M K2SO4 | 85.3% | 0.300 VRHE | 5 | [30] |
| NCMK3IL50-800T | 0.1 M K2SO4 | 55–85% | 0.450 VRHE | - | [31] |
| GOX/MnCO3 | 3.5% NaCl | 50% | 0.635 VRHE | - | [29] |
| ZnO/rGO | 0.5 M NaCl | 75–78% | 0.335 VRHE | 6 | [4] |
| rGO | 0.5 M NaCl | 56–60% | 0.409 VRHE | 6 | [4] |
| ZnCd-ZIF | 3.5% NaCl | 65–70% | 0.448 VRHE | 7 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, N.; Liu, K.; Yang, M.; Zhang, R.; Khan, S.; Pang, J.; Duan, J.; Hou, B.; Sand, W. Synergistic Zn-Cd Bimetallic Engineering in ZIFs for High-Chloride 2e− ORR to H2O2 in Simulated Neutral Seawater. Materials 2025, 18, 1786. https://doi.org/10.3390/ma18081786
Wang X, Wang N, Liu K, Yang M, Zhang R, Khan S, Pang J, Duan J, Hou B, Sand W. Synergistic Zn-Cd Bimetallic Engineering in ZIFs for High-Chloride 2e− ORR to H2O2 in Simulated Neutral Seawater. Materials. 2025; 18(8):1786. https://doi.org/10.3390/ma18081786
Chicago/Turabian StyleWang, Xu, Nan Wang, Kunpeng Liu, Meinan Yang, Ruiyong Zhang, Sikandar Khan, Jinhui Pang, Jizhou Duan, Baorong Hou, and Wolfgang Sand. 2025. "Synergistic Zn-Cd Bimetallic Engineering in ZIFs for High-Chloride 2e− ORR to H2O2 in Simulated Neutral Seawater" Materials 18, no. 8: 1786. https://doi.org/10.3390/ma18081786
APA StyleWang, X., Wang, N., Liu, K., Yang, M., Zhang, R., Khan, S., Pang, J., Duan, J., Hou, B., & Sand, W. (2025). Synergistic Zn-Cd Bimetallic Engineering in ZIFs for High-Chloride 2e− ORR to H2O2 in Simulated Neutral Seawater. Materials, 18(8), 1786. https://doi.org/10.3390/ma18081786






