Molecular Simulation Study on the Impact of a Cross-Linked Network Structure on the Tensile Mechanical Properties of PBT Substrates
Abstract
1. Introduction
2. Model Construction and Simulation Details
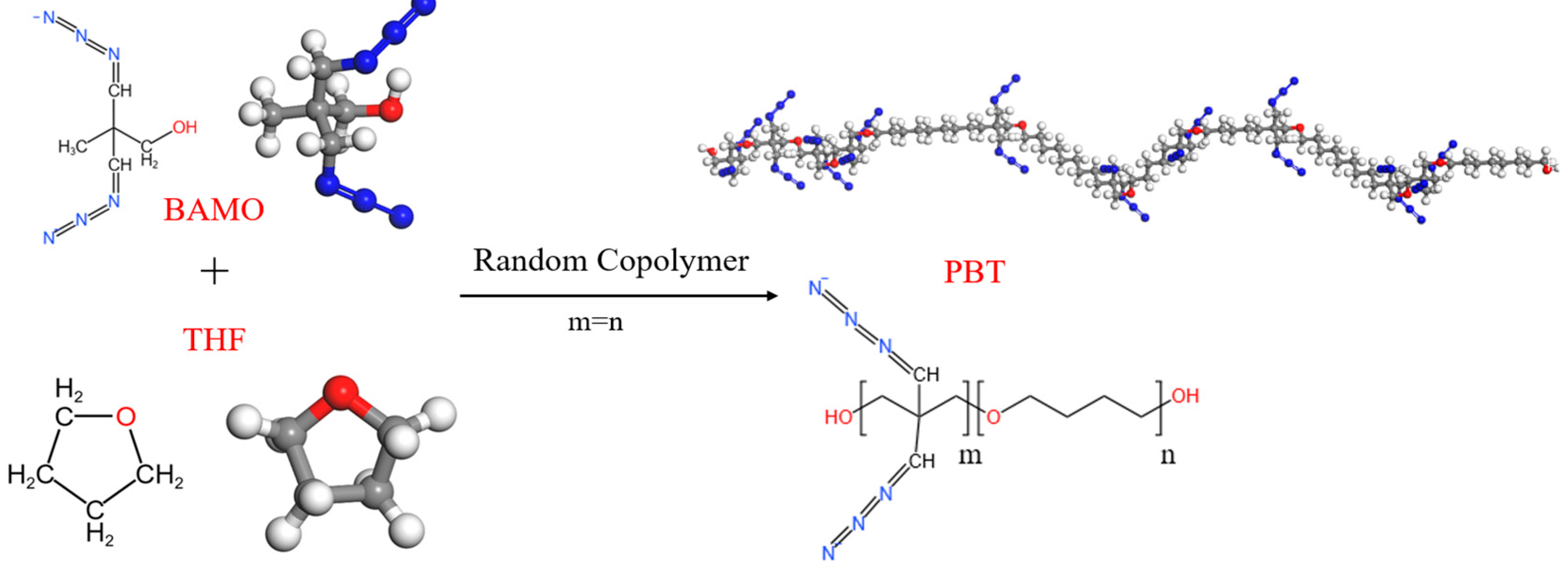
2.1. Cross-Linking Modeling
2.2. Uniaxial Tensile Simulation
3. Results and Discussion
3.1. Cross-Linking Analysis
3.1.1. Generated Cross-Linking Model
3.1.2. Cross-Linking Process Analysis
3.1.3. Radial Distribution Function Analysis
3.1.4. Diffusion Coefficient Analysis
3.1.5. Glass Transition Temperature Analysis
3.2. Analysis of Tensile Results
3.2.1. Effect of Cross-Link Density on Stress–Strain
3.2.2. Effect of Strain Rate on Cross-Linked Meshes
4. Conclusions
- (1)
- Reduced Motility and Increased Rigidity: Increasing the cross-linking density leads to a gradual reduction in the motility of the molecular chain segments, resulting in enhanced rigidity. This is reflected in a denser system with a reduced free volume and stronger interactions between the molecular chains, making the structure more compact.
- (2)
- Increased Glass Transition Temperature: The glass transition temperature (Tg) increases with the cross-linking density.
- (3)
- Energy Reduction: The cross-linking process reduces the system’s energy by approximately 40%, with the non-bonding energy being the primary contributor to this decrease.
- (4)
- Improved Mechanical Properties: As the cross-linking density increases, the mechanical properties of the PBT-based solid propellant improve significantly. Higher cross-linking densities result in greater structural rigidity and tensile strength. Materials with higher cross-linking densities exhibit greater resistance to deformation and higher rigidity under the same strain, with significantly improved stress responses at high tensile rates.
- (5)
- Enhanced Tensile Resistance: At higher cross-linking densities (e.g., 90%), the tensile resistance of the material is notably enhanced, characterized by higher stress values and reduced stress fluctuations compared to those of the lower-density models. In contrast, low-cross-linking-density materials show greater stress fluctuations, while high-density materials maintain a more stable stress response.
- (6)
- Stress Stabilization During Stretching: During stretching, cross-linked mesh materials exhibit a plateau in the stress curve after an initial increase, where stress stabilizes due to the inability of the cross-linking points to stretch further. This stabilization results from microcracks and fractures within the network, leading to stress redistribution. Increasing the cross-linking density reduces the likelihood of such microcracks and fractures, thereby enabling the material to withstand greater stress.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, S.; Fan, H.; Zhang, N.; Li, W.; Li, Y.; Li, Y.; Huang, D.; Zeng, L.; Shi, X.; Ran, X.; et al. Investigation of a Low-Toxicity Energetic Binder for a Solid Propellant: Curing, Microstructures, and Performance. ACS Omega 2020, 5, 30538–30548. [Google Scholar] [PubMed]
- Hafner, S. Internal Plasticized Glycidyl Azide Copolymers for Energetic Solid Propellant Binders. Ph.D. Dissertation, LMU, Los Angeles, CA, USA, 2019. [Google Scholar]
- Jarosz, T.; Stolarczyk, A.; Wawrzkiewicz-Jalowiecka, A.; Pawlus, K.; Miszczyszyn, K. Glycidyl Azide Polymer and Its Derivatives—Versatile Binders for Explosives and Pyrotechnics: Tutorial Review of Recent Progress. Molecules 2019, 24, 4475. [Google Scholar] [CrossRef]
- Balcer, K.; Boroński, D. Mechanical Properties of Twill Carbon Fiber Fabric-Reinforced Single-Layer Thermoplastic Polyamide and Polybutylene Terephthalate-Based Composite Materials Manufactured by Hot Pressing. Materials 2025, 18, 343. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Wu, Y.; Shentu, B.; Weng, Z. Toughening of PBT by POE/POE-g-GMA Elastomer through Regulating Interfacial Adhesion and Toughening Mechanism. Ind. Eng. Chem. Res. 2019, 58, 12650–12663. [Google Scholar]
- Qiu, C.; Chen, J.; Huan, F.; Deng, S.; Yao, Z.; Wang, S.; Wang, J. Curing and Cross-Linking Processes in the Poly(3,3-Bis-Azidomethyl Oxetane)-Tetrahydrofuran/Toluene Diisocyanate/Trimethylolpropane System: A Density Functional Theory and Accelerated ReaxFF Molecular Dynamics Investigation. ACS Omega 2024, 9, 33153–33161. [Google Scholar]
- Ahn, Y.H.; Kim, J.S.; Kim, S.H. Reaction Monitoring of Toluenediisocyanate (TDI) Polymerization on a Non-Mixable Aqueous Surface by MALDI Mass Spectrometry. Anal. Sci. 2013, 29, 703–708. [Google Scholar]
- Jia, X.; Tang, L.; Liu, R.; Liao, H.; Cao, L.; Tang, X.; Cao, P. Analysis of Interfacial Adhesion Properties Between PBT Azide Propellant Matrix and Defective AP Fillers Using Molecular Dynamics Simulations. Polymers 2024, 16, 3497. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, X.; Zhang, X.; Huang, H.; Chang, J.; Chen, H. Structural Investigations of Toluene Diisocyanate (TDI) and Trimethylolpropane (TMP)-Based Polyurethane Prepolymer. J. Ind. Eng. Chem. 2012, 18, 1620–1627. [Google Scholar]
- Abdelaziz, A.; Trache, D.; Tarchoun, A.F.; Boukeciat, H.; Kadri, D.E.; Hassam, H.; Ouahioune, S. Application of Co-Crystallization Method for the Production of Ammonium Perchlorate/Ammonium Nitrate Oxidizer for Solid Rocket Propellants. Chem. Eng. J. 2024, 487, 150654. [Google Scholar]
- Jeon, Y.J.; Yun, J.H.; Kang, M.S. Analysis of Elastic Properties of Al/PET Isotropic Composite Materials Using Finite Element Method. Materials 2022, 15, 8007. [Google Scholar] [CrossRef]
- Ibrahim, T.K.; Yawas, D.S.; Thaddaeus, J.; Danasabe, B.; Iliyasu, I.; Adebisi, A.A.; Ahmadu, T.O. Development, Modelling and Optimization of Process Parameters on the Tensile Strength of Aluminum, Reinforced with Pumice and Carbonated Coal Hybrid Composites for Brake Disc Application. Sci. Rep. 2024, 14, 16999. [Google Scholar] [CrossRef]
- Hu, Y.; Song, X.; Yang, W.; Kang, J. Stress Relaxation Behavior of Azido Propellant Based on BAMO-THF at High Temperatures. Materials 2024, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, F.; Zhu, L.; Geng, Z.; Yang, R.; Zhai, J. Synergistic Effect of Covalent Cross-Linking and Physical Hydrogen Bonding Associations on the Mechanical Properties of Polyurethane Thermoset Elastomers. J. Appl. Polym. Sci. 2024, 141, e56114. [Google Scholar]
- Wolińska-Grabczyk, A.; Kaczmarczyk, B.; Jankowski, A. Investigations of Hydrogen Bonding in the Poly(Urethane-Urea)-Based Membrane Materials by Using FTIR Spectroscopy. Pol. J. Chem. Technol. 2008, 10, 53–56. [Google Scholar]
- Wang, H.; Cao, L.; Wang, X.; Lang, X.; Cong, W.; Han, L.; Zhang, H.; Zhou, H.; Sun, J.; Zong, C. Effects of Isocyanate Structure on the Properties of Polyurethane: Synthesis, Performance, and Self-Healing Characteristics. Polymers 2024, 16, 3045. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.E.; Piacenti, A.R.; Waters, S.L.; Contera, S. Enhancing Nanoscale Viscoelasticity Characterization in Bimodal Atomic Force Microscopy. Soft Matter 2024, 20, 7457–7470. [Google Scholar] [CrossRef]
- Wittenberg, E.; Meyer, A.; Eggers, S.; Abetz, V. Hydrogen Bonding and Thermoplastic Elastomers—A Nice Couple with Temperature-Adjustable Mechanical Properties. Soft Matter 2018, 14, 2701–2711. [Google Scholar] [CrossRef]
- Solares, S.D. Nanoscale Effects in the Characterization of Viscoelastic Materials with Atomic Force Microscopy: Coupling of a Quasi-Three-Dimensional Standard Linear Solid Model with In-Plane Surface Interactions. Beilstein J. Nanotechnol. 2016, 7, 554–571. [Google Scholar]
- Li, C.; Voth, G.A. Accurate and Transferable Reactive Molecular Dynamics Models from Constrained Density Functional Theory. J. Phys. Chem. B 2021, 125, 10471–10480. [Google Scholar] [CrossRef]
- Patil, S.U.; Shah, S.P.; Olaya, M.; Deshpande, P.P.; Maiaru, M.; Odegard, G.M. Reactive Molecular Dynamics Simulation of Epoxy for the Full Cross-Linking Process. ACS Appl. Polym. Mater. 2021, 3, 5788–5797. [Google Scholar] [CrossRef]
- Shundo, A.; Yamamoto, S.; Tanaka, K. Network Formation and Physical Properties of Epoxy Resins for Future Practical Applications. JACS Au 2022, 2, 1522–1542. [Google Scholar] [CrossRef] [PubMed]
- Bugrov, A.N.; Gorshkova, Y.E.; Ivan’kova, E.M.; Kopitsa, G.P.; Pavlova, A.A.; Popova, E.N. Domain Structure, Thermal and Mechanical Properties of Polycaprolactone-Based Multiblock Polyurethane-Ureas under Control of Hard and Soft Segment Lengths. Polymers 2022, 14, 4145. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, W. Optimization and Design for the Curing Process of Solid Azide Propellant: Influence of Typical Components on the Curing Reactions of PBT Binders with TDI. J. Chin. Chem. Soc. 2022, 69, 419–439. [Google Scholar]
- Dong, G.; Liu, H.; Deng, L.; Yu, H.; Zhou, X.; Tang, X.; Li, W. Study on the Interfacial Interaction between Ammonium Perchlorate and Hydroxyl-Terminated Polybutadiene in Solid Propellants by Molecular Dynamics Simulation. e-Polymers 2022, 22, 264–275. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended Coverage for Polymer and Drug-Like Molecule Databases. J. Mol. Model. 2016, 22, 47. [Google Scholar]
- Sun, H. COMPASS: An Ab Initio Force-Field Optimized for Condensed-Phase Applications Overview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Chang, K.S.; Yoshioka, T.; Kanezashi, M.; Tsuru, T.; Tung, K.L. A Molecular Dynamics Simulation of a Homogeneous Organic–Inorganic Hybrid Silica Membrane. Chem. Commun. 2010, 46, 9140–9142. [Google Scholar] [CrossRef]
- Geng, Z.; Guo, X.Y.; Ding, T.F.; Zhai, J.X.; Zhao, H.P. Effect of Hard Segment Composition of 3,3-Diazidomethyloxetane-Tetrahydrofuran Copolyethers Elastomer on Its Mechanical Properties. Polym. Mater. Sci. Eng. 2020, 36, 1–6. [Google Scholar]
- Santos, J.M.; Guthrie, J.T. Lewis Acid/Base Character and Crystallisation Properties of Poly(Butylene Terephthalate). J. Chromatogr. A 2015, 1379, 92–99. [Google Scholar]
- Xie, Q.; Fu, K.; Liang, S.; Liu, B.; Lu, L.; Yang, X.; Huang, Z.; Lü, F. Micro-Structure and Thermomechanical Properties of Crosslinked Epoxy Composite Modified by Nano-SiO2: A Molecular Dynamics Simulation. Polymers 2018, 10, 801. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, X.; Chen, L.; Li, Y.; Sun, M.; Duan, X.; Liang, W. Structures and Impact Strength Variation of Chemically Crosslinked High-Density Polyethylene: Effect of Crosslinking Density. RSC Adv. 2021, 11, 6791–6797. [Google Scholar] [PubMed]
- Gurina, D.; Budkov, Y.; Kiselev, M. Molecular Dynamics Study of the Swelling of Poly(Methyl Methacrylate) in Supercritical Carbon Dioxide. Materials 2019, 12, 3315. [Google Scholar] [CrossRef] [PubMed]
- Lawrimore, C.J.; Lawrimore, J.; He, Y.; Chave, S.; Bloom, K. Polymer Perspective of Genome Mobilization. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2020, 821, 111706. [Google Scholar]
- Rajakumar, P.R.; Nanthini, R. Thermal and Morphological Behaviours of Polybutylene Terephthalate/Polyethylene Terephthalate Blend Nanocomposites. Rasayan J. Chem. 2011, 4, 567–579. [Google Scholar]











| Cross-Link Density (%) | Density (g/cm3) | Free Volume (Å3) | FFV (%) |
|---|---|---|---|
| 0 | 1.00 | 12,437.14 | 19.43 |
| 50 | 1.05 | 11,234.74 | 17.55 |
| 60 | 1.11 | 10,424.41 | 16.29 |
| 70 | 1.13 | 9863.15 | 15.41 |
| 80 | 1.14 | 9641.23 | 15.06 |
| 90 | 1.18 | 8537.21 | 13.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Zhao, K.; Cao, P.; Cao, L.; Liao, H.; Tang, X. Molecular Simulation Study on the Impact of a Cross-Linked Network Structure on the Tensile Mechanical Properties of PBT Substrates. Materials 2025, 18, 1675. https://doi.org/10.3390/ma18071675
Huang R, Zhao K, Cao P, Cao L, Liao H, Tang X. Molecular Simulation Study on the Impact of a Cross-Linked Network Structure on the Tensile Mechanical Properties of PBT Substrates. Materials. 2025; 18(7):1675. https://doi.org/10.3390/ma18071675
Chicago/Turabian StyleHuang, Renlong, Kang Zhao, Peng Cao, Liang Cao, Hongjun Liao, and Xianqiong Tang. 2025. "Molecular Simulation Study on the Impact of a Cross-Linked Network Structure on the Tensile Mechanical Properties of PBT Substrates" Materials 18, no. 7: 1675. https://doi.org/10.3390/ma18071675
APA StyleHuang, R., Zhao, K., Cao, P., Cao, L., Liao, H., & Tang, X. (2025). Molecular Simulation Study on the Impact of a Cross-Linked Network Structure on the Tensile Mechanical Properties of PBT Substrates. Materials, 18(7), 1675. https://doi.org/10.3390/ma18071675







