Low-Temperature Fabrication of Carbon Nanotube–Aluminum Composite Powders via Rotary Chemical Vapor Deposition: Process Optimization and Growth Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Al-CNTs Composite Powder
2.2. Characterization Techniques
3. Results
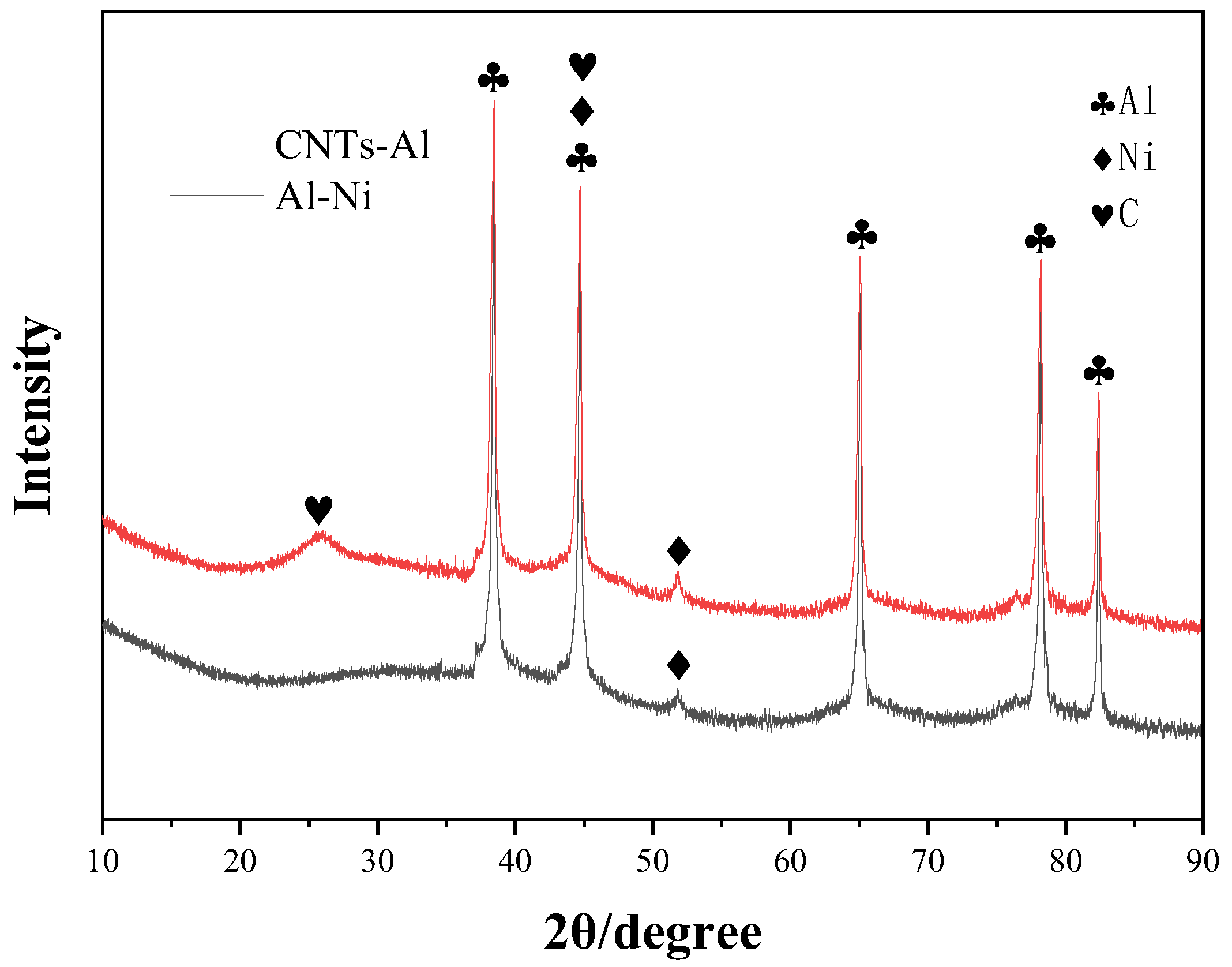
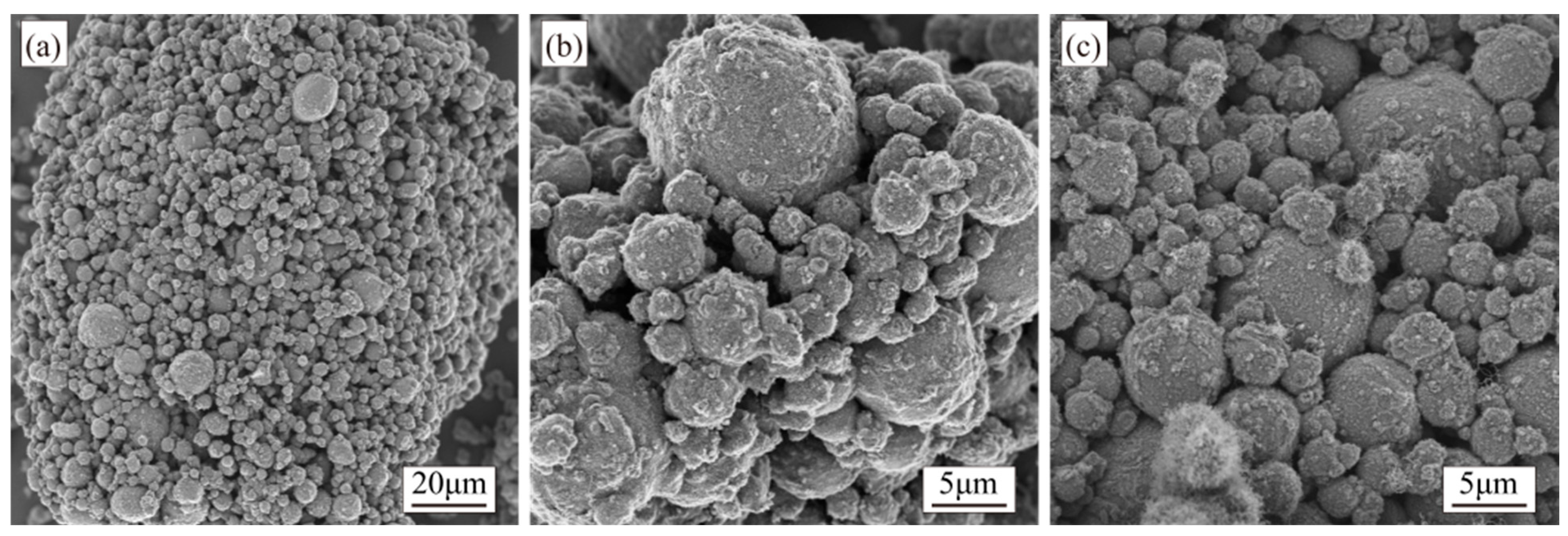
3.1. Al-Ni Catalyst Powder
3.2. Synthesis of CNTs with Different Catalyst Contents
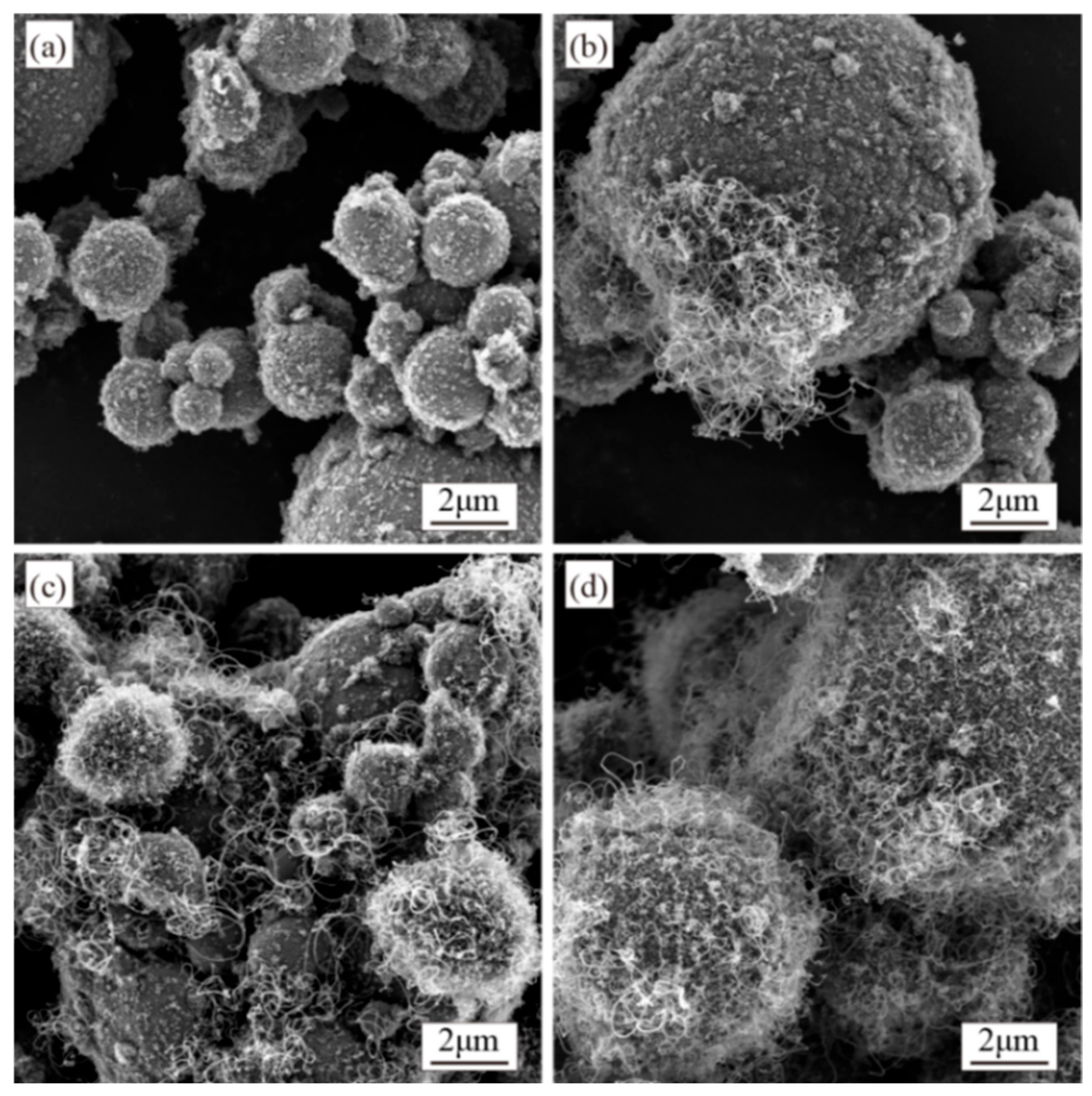
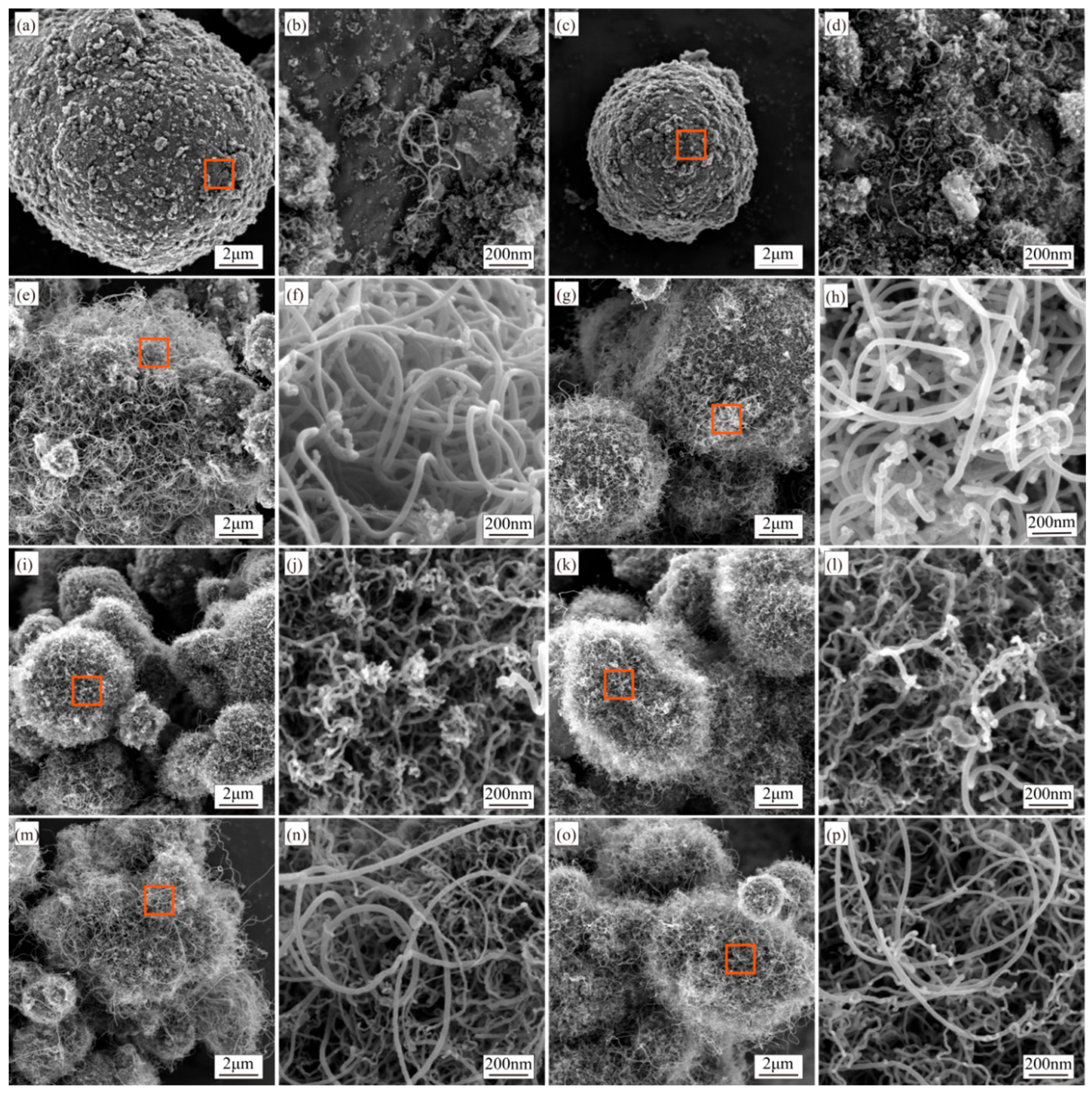
3.3. Synthesis of CNTs with Different Synthesis Temperatures
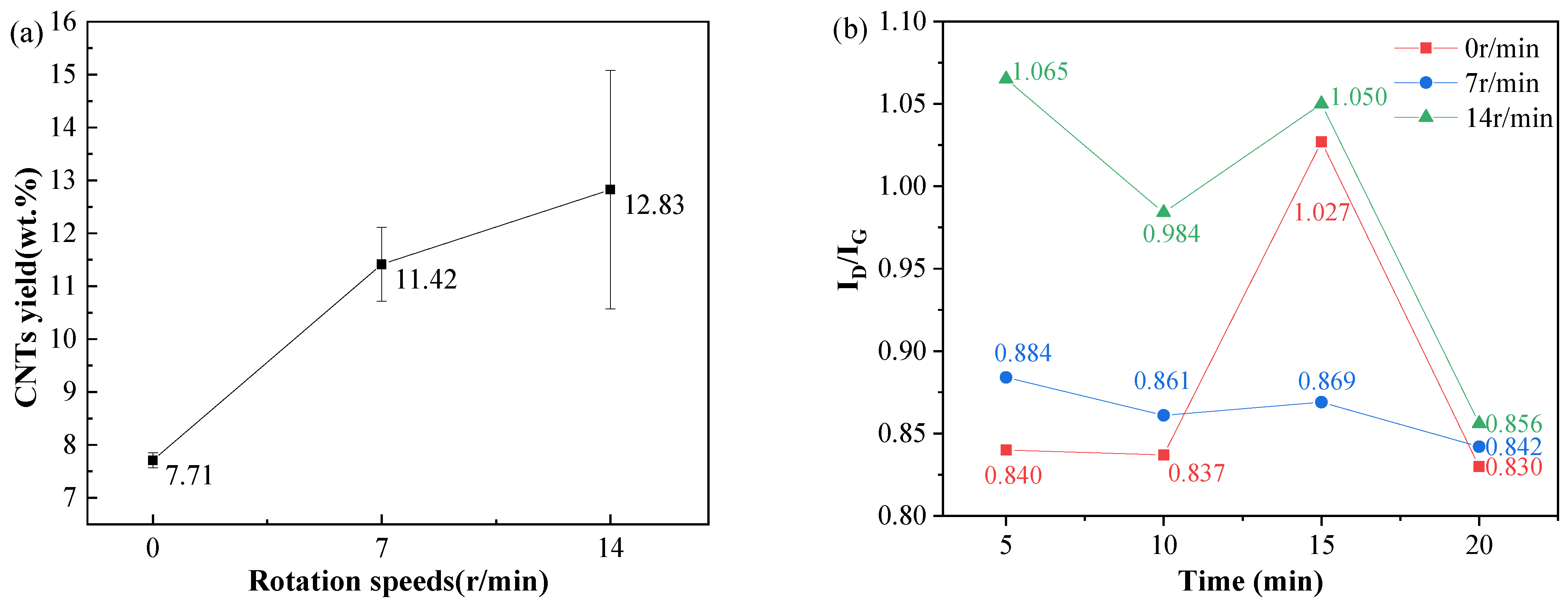
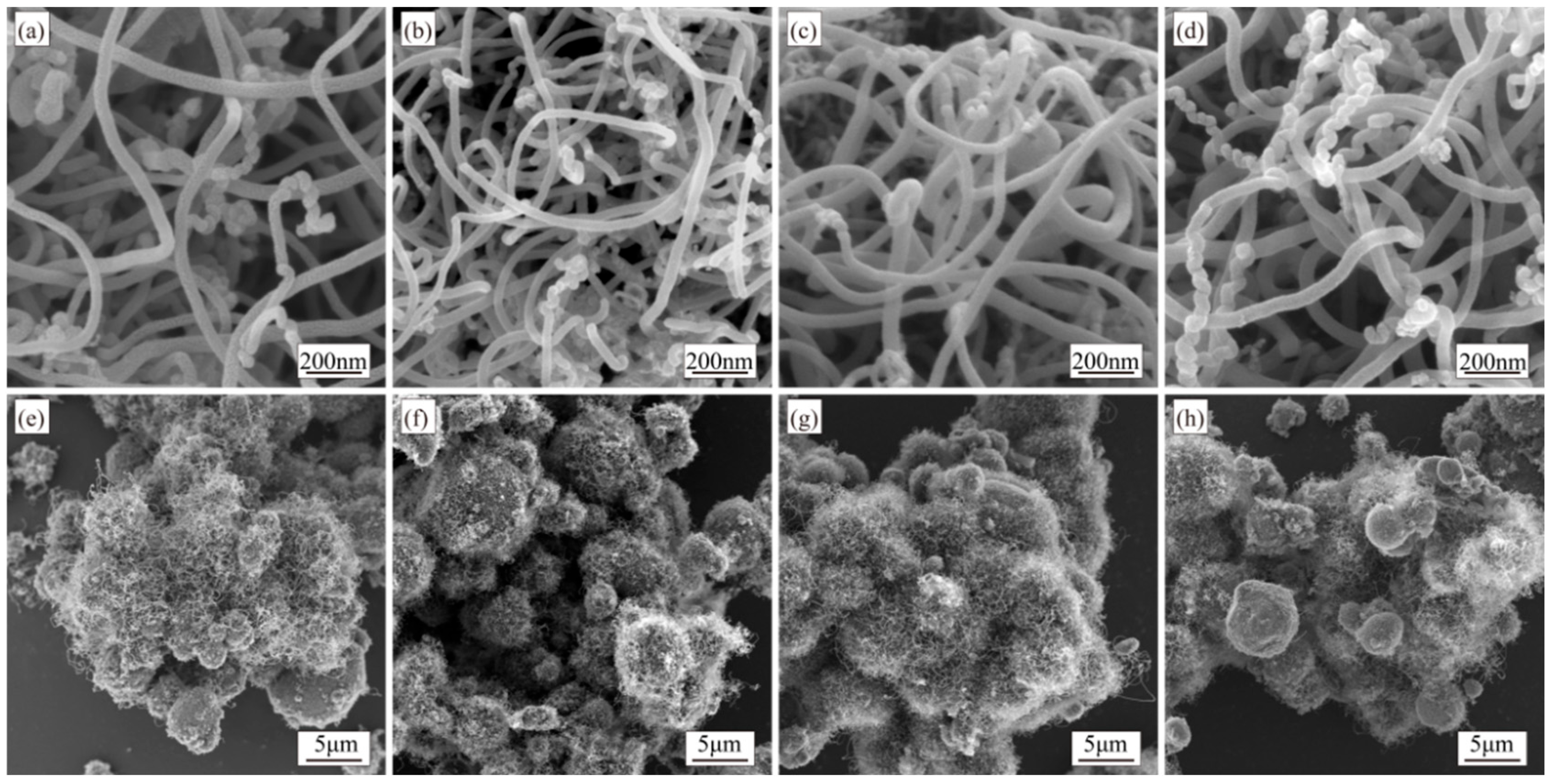
3.4. Synthesis of CNTs with Different Rotation Speeds and Growth Times During Synthesis
3.5. Synthesis of CNTs with Different C2H2 to N2 Gas Flow Ratios
4. Discussion
4.1. Growth Mechanism of CNTs
4.2. Effect of Catalyst Contents on CNT Synthesis
4.3. Effect of Synthesis Temperature on CNTs’ Synthesis
4.4. Effects of Rotation Speed and Growth Time During Synthesis on CNT Synthesis
4.5. Effects of C2H2/N2 Gas Flow Ratio on CNT Synthesis
5. Conclusions
- Tip-growth and base-growth mechanisms coexist during CNT synthesis on the Al substrate.
- Excessive catalyst loading hinders subsequent processing, while insufficient loading impedes CNT growth. A Ni content of 2.0 wt.% achieves optimal CNT morphology and purity.
- Low temperatures reduce carbon diffusion rates, limiting yield, while excessive temperatures promote amorphous carbon formation. A synthesis temperature of 500 °C balances yield and CNT purity.
- Near the melting point of Al, CNTs exhibit smaller diameters and narrower size distributions but significantly reduced crystallinity.
- Prolonged synthesis times enhance CNT length and density, while higher rotation speeds increase yield but degrade crystallinity.
- Short synthesis results in non-uniform CNT distributions, elevated defect densities, and amorphous carbon impurities. To achieve high-quality, uniformly dispersed CNTs, sufficient synthesis duration is required.
- Reducing the C2H2/N2 flow ratio initially increases CNT yield and crystallinity, peaking at 1:7. Further reduction causes carbon starvation, sharply decreasing both parameters.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tu, S.; Wang, Q.; Ramachandran, C.S. Parametric Investigation of In-Situ Synthesis of Carbon Nanotubes on Al2O3 Powder by the Rotary Chemical Vapor Deposition Method. Ceram. Int. 2022, 48, 28258–28267. [Google Scholar] [CrossRef]
- Lou, G.; Zhao, Z.; Wang, Y.; Wang, Y.; Yue, Z.; Gao, J.; Liu, S. Preparation and Performance Study of Carbon Nanotube/Polyphenylene Sulfidecomposite Materials Based on Floating Catalytic Chemical Vapor Deposition and Continuous Dusting Powder. Polym. Compos. 2024, 46, 3239–3250. [Google Scholar] [CrossRef]
- Guo, M.; Du, J.; Zhang, Y. Effect of CNT Content and Size on the High-Temperature Particle-Erosion Resistance of Ablative Materials for Thermal Protection Systems. Compos. Sci. Technol. 2023, 235, 109969. [Google Scholar] [CrossRef]
- Nyanor, P.; El-Kady, O.; Yehia, H.M.; Hamada, A.S.; Hassan, M.A. Effect of Bimodal-Sized Hybrid TiC–CNT Reinforcement on the Mechanical Properties and Coefficient of Thermal Expansion of Aluminium Matrix Composites. Met. Mater. Int. 2021, 27, 753–766. [Google Scholar] [CrossRef]
- Liu, B.; Sun, J.; Zhao, J.; Yun, X. Hybrid Graphene and Carbon Nanotube–Reinforced Composites: Polymer, Metal, and Ceramic Matrices. Adv. Compos. Hybrid Mater. 2024, 8, 1. [Google Scholar] [CrossRef]
- Wang, M.; Yong, J.; Cai, L.; Li, Z.; Ou, Y.; Zhu, L.; Yi, X.; Mao, D. Significantly Improved Interfacial and Overall Mechanical Properties of Aramid Fiber/Phenolic Resin Matrix Composite Reinforced with Short CNT. J. Mater. Res. Technol. 2023, 26, 5225–5235. [Google Scholar] [CrossRef]
- Krishna, A.; Aravinda, L.S.; Murugan, A.; Kumar, N.S.; Sankar, M.R.; Nagahanumaiah; Reddy, K.N.; Balashanmugam, N. A Study on Wafer Scalable, Industrially Applicable CNT Based Nanocomposites of al-CNT, Cu-CNT, Ti-CNT, and Ni-CNT as Thermal Interface Materials Synthesised by Thin Film Techniques. Surf. Coat. Technol. 2022, 429, 127926. [Google Scholar] [CrossRef]
- Fattahi, M.; Nabhani, N.; Rashidkhani, E.; Fattahi, Y.; Akhavan, S.; Arabian, N. A New Technique for the Strengthening of Aluminum Tungsten Inert Gas Weld Metals: Using Carbon Nanotube/Aluminum Composite as a Filler Metal. Micron 2013, 54–55, 28–35. [Google Scholar] [CrossRef]
- Mohammed, S.M.A.K.; Chen, D.L. Carbon Nanotube-Reinforced Aluminum Matrix Composites. Adv. Eng. Mater. 2020, 22, 1901176. [Google Scholar] [CrossRef]
- Tjong, S.C. Recent Progress in the Development and Properties of Novel Metal Matrix Nanocomposites Reinforced with Carbon Nanotubes and Graphene Nanosheets. Mater. Sci. Eng. R Rep. 2013, 74, 281–350. [Google Scholar] [CrossRef]
- Zhang, W.; You, X.; Fang, D.; Yang, P.; Yi, J.; Yu, X.; Bao, R.; Li, C.; Liu, Y.; Tao, J.; et al. Influence of Acid-Treated Time of Carbon Nanotubes on Mechanical Property in Carbon Nanotubes Reinforced Copper Matrix Composites. Diam. Relat. Mater. 2020, 109, 108069. [Google Scholar] [CrossRef]
- Sadeghi, B.; Fan, G.; Tan, Z.; Li, Z.; Kondo, A.; Naito, M. Smart Mechanical Powder Processing for Producing Carbon Nanotube Reinforced Aluminum Matrix Composites. KONA Powder Part. J. 2022, 39, 219–229. [Google Scholar] [CrossRef]
- Pan, J.; Zou, L.; Liao, Z.; Lin, Z.; Chen, J. Study on the Properties of Carbon Nanotube (CNTs) Reinforced AlSi10Mg Composites Fabricated by Powder Metallurgy. Materials 2023, 16, 3905. [Google Scholar] [CrossRef] [PubMed]
- Azarniya, A.; Safavi, M.S.; Sovizi, S.; Azarniya, A.; Chen, B.; Madaah Hosseini, H.R.; Ramakrishna, S. Metallurgical Challenges in Carbon Nanotube-Reinforced Metal Matrix Nanocomposites. Metals 2017, 7, 384. [Google Scholar] [CrossRef]
- Kumar, N.; Soren, S.; Nirala, A.; Almakayeel, N.; Yunus Khan, T.M.; Khan, M.A. Distribution of Carbon Nanotubes in an Aluminum Matrix by a Solution-Mixing Process. ACS Omega 2023, 8, 33845–33856. [Google Scholar] [CrossRef]
- Raju, T.N.; Shivappa, H.A. Development and Characterization of Carbon Nanotube Reinforced Aluminium-6061 Metal Matrix Composites. J. Miner. Mater. Charact. Eng. 2021, 9, 290–300. [Google Scholar] [CrossRef]
- José-Yacamán, M.; Miki-Yoshida, M.; Rendón, L.; Santiesteban, J.G. Catalytic Growth of Carbon Microtubules with Fullerene Structure. Appl. Phys. Lett. 1993, 62, 202–204. [Google Scholar] [CrossRef]
- Heydari Gharahcheshmeh, M. Fabrication of Conjugated Conducting Polymers by Chemical Vapor Deposition (CVD) Method. Nanomaterials 2025, 15, 452. [Google Scholar] [CrossRef]
- Faggio, G.; Politano, G.G.; Lisi, N.; Capasso, A.; Messina, G. The Structure of Chemical Vapor Deposited Graphene Substrates for Graphene-Enhanced Raman Spectroscopy. J. Phys. Condens. Matter. 2024, 36, 195303. [Google Scholar] [CrossRef]
- Vahlas, C.; Caussat, B.; Serp, P.; Angelopoulos, G.N. Principles and Applications of CVD Powder Technology. Mater. Sci. Eng. R Rep. 2006, 53, 1–72. [Google Scholar] [CrossRef]
- See, C.H.; Harris, A.T. A Review of Carbon Nanotube Synthesis via Fluidized-Bed Chemical Vapor Deposition. Ind. Eng. Chem. Res. 2007, 46, 997–1012. [Google Scholar] [CrossRef]
- Danafar, F.; Fakhru’l-Razi, A.; Salleh, M.A.M.; Biak, D.R.A. Fluidized Bed Catalytic Chemical Vapor Deposition Synthesis of Carbon Nanotubes—A Review. Chem. Eng. J. 2009, 155, 37–48. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, R.; Goto, T. Preparation of Ni-Precipitated hBN Powder by Rotary Chemical Vapor Deposition and Its Consolidation by Spark Plasma Sintering. J. Alloys Compd. 2010, 502, 371–375. [Google Scholar] [CrossRef]
- Itoh, H.; Watanabe, N.; Naka, S. Preparation of Titanium Nitride Coated Powders by Rotary Powder Bed Chemical Vapour Deposition. J. Mater. Sci. 1988, 23, 43–47. [Google Scholar] [CrossRef]
- Itoh, H.; Hattori, K.; Naka, S. Rotary Powder Bed Chemical Vapour Deposition of Titanium Nitride on Spherical Iron Powder. J. Mater. Sci. 1989, 24, 3643–3646. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Wang, Q.; Wang, T.; Zhong, L.; Pan, W.-P. Insights into the Removal of Volatile Organic Compounds by Three-Dimensional Ordered Microporous Zeolite-Templated Carbon: A Combined Experimental Study and Density Functional Theory Calculation. Chem. Eng. J. 2024, 494, 152965. [Google Scholar] [CrossRef]
- Wang, J.; Obrovac, M.N. Lab-Scale Chemical Vapor Deposition onto Powders. AIP Adv. 2022, 12, 075209. [Google Scholar] [CrossRef]
- Pirard, S.L.; Douven, S.; Pirard, J.-P. Large-Scale Industrial Manufacturing of Carbon Nanotubes in a Continuous Inclined Mobile-Bed Rotating Reactor via the Catalytic Chemical Vapor Deposition Process. Front. Chem. Sci. Eng. 2017, 11, 280–289. [Google Scholar] [CrossRef]
- Dorbani, T.; Bouleklab, M.C.; Settar, A.; Chetehouna, K.; Naoui, Y.; Revo, S.; Hamamda, S. Synthesis and Characterization of Al+0.25%MWCNTs Nanocomposite with Reduced Thermal Expansion Coefficient. J. Mater. Res. Technol. 2022, 19, 1484–1492. [Google Scholar] [CrossRef]
- Dal Santo, V.; Gallo, A.; Gatti, M.M.; De Grandi, V.; Psaro, R.; Sordelli, L.; Recchia, S. Tailored Supported Metal Nanoparticles by CVD: An Easy and Efficient Scale-up by a Rotary Bed OMCVD Device. J. Mater. Chem. 2009, 19, 9030. [Google Scholar] [CrossRef]
- Ahmad, M.; Silva, S.R.P. Low Temperature Growth of Carbon Nanotubes—A Review. Carbon 2020, 158, 24–44. [Google Scholar] [CrossRef]
- Lin, J.; Jin, H.; Ge, X.; Yang, Y.; Huang, G.; Wang, J.; Li, F.; Li, H.; Wang, S. Investigation of the Parameters of Carbon Nanotube Growth on Zirconium Diboride Supported Ni Catalyst via CVD. Diam. Relat. Mater. 2021, 115, 108347. [Google Scholar] [CrossRef]
- Gergeroglu, H.; Ebeoglugil, M.F. Investigation of the Effect of Catalyst Type, Concentration, and Growth Time on Carbon Nanotube Morphology and Structure. Carbon Lett. 2022, 32, 1729–1743. [Google Scholar] [CrossRef]
- Dikonimos Makris, T.; Giorgi, L.; Giorgi, R.; Lisi, N.; Salernitano, E. CNT Growth on Alumina Supported Nickel Catalyst by Thermal CVD. Diam. Relat. Mater. 2005, 14, 815–819. [Google Scholar] [CrossRef]
- Baker, R.T.K.; Barber, M.A.; Harris, P.S.; Feates, F.S.; Waite, R.J. Nucleation and Growth of Carbon Deposits from the Nickel Catalyzed Decomposition of Acetylene. J. Catal. 1972, 26, 51–62. [Google Scholar] [CrossRef]
- Alstrup, I. A New Model Explaining Carbon Filament Growth on Nickel, Iron, and Ni-Cu Alloy Catalysts. J. Catal. 1988, 109, 241–251. [Google Scholar] [CrossRef]
- Yang, R.T.; Chen, J.P. Mechanism of Carbon Filament Growth on Metal Catalysts. J. Catal. 1989, 115, 52–64. [Google Scholar] [CrossRef]
- Baker, R.T.K.; Waite, R.J. Formation of Carbonaceous Deposits from the Platinum-Iron Catalyzed Decomposition of Acetylene. J. Catal. 1975, 37, 101–105. [Google Scholar] [CrossRef]
- Li, H.; Shi, C.; Du, X.; He, C.; Li, J.; Zhao, N. The Influences of Synthesis Temperature and Ni Catalyst on the Growth of Carbon Nanotubes by Chemical Vapor Deposition. Mater. Lett. 2008, 62, 1472–1475. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Yan, Y. Synthesis of CNTs on Stainless Steel Microfibrous Composite by CVD: Effect of Synthesis Condition on Carbon Nanotube Growth and Structure. Compos. Part B Eng. 2019, 160, 369–383. [Google Scholar] [CrossRef]
- Duan, P.; Xu, K.; Chen, X.; Liu, P.; Zhou, H.; Fu, S.; Bi, L.; Guo, X. Simple and Efficient Preparation of Uniformly Dispersed Carbon Nanotubes Reinforced Copper Matrix Composite Powders by in Situ Chemical Vapor Deposition without Additional Catalyst. Mater. Res. Express 2021, 8, 016513. [Google Scholar] [CrossRef]
- Noda, L.K.; Gonçalves, N.S.; Valentini, A.; Probst, L.F.D.; De Almeida, R.M. Effect of Ni Loading and Reaction Temperature on the Formation of Carbon Nanotubes from Methane Catalytic Decomposition over Ni/SiO2. J. Mater. Sci. 2007, 42, 914–922. [Google Scholar] [CrossRef]
- He, C.; Zhao, N.; Shi, C.; Du, X.; Li, J.; Li, H.; Cui, Q. An Approach to Obtaining Homogeneously Dispersed Carbon Nanotubes in Al Powders for Preparing Reinforced Al-Matrix Composites. Adv. Mater. 2007, 19, 1128–1132. [Google Scholar] [CrossRef]
- Lu, C.; Liu, J. Controlling the Diameter of Carbon Nanotubes in Chemical Vapor Deposition Method by Carbon Feeding. J. Phys. Chem. B 2006, 110, 20254–20257. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Y.; Zhang, H.; Li, F. Effect of Source Gases on CVD Synthesis of CNTs@TiB2 Composite Powders Using Ni/Y2O3 as the Catalyst. Ceram. Int. 2020, 46, 10704–10709. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, X.; Liu, P.; Wang, W.; Liu, W. Study on Mechanical Properties and Wear Behavior of In-Situ Synthesized CNTs/CuCrZrY Composites Prepared by Spark Plasma Sintering. Vacuum 2021, 188, 110180. [Google Scholar] [CrossRef]
- Lin, B.; Chen, X.; Chen, J.; Chen, S.; Lu, R.; Liang, S.; Cui, X.; Chi, H.; Zou, L. Facile Synthesis of Homogeneously Dispersed Carbon Nanotubes on TC4 Alloy Powder by In-Situ CVD and Its Growth Behavior. J. Mater. Res. Technol. 2023, 24, 9928–9938. [Google Scholar] [CrossRef]
- Ummethala, R.; Khavrus, V.O.; Leonhardt, A.; Büchner, B.; Eckert, J. A Comparative Study of Various Supported Catalysts on the Growth of Aligned Carbon Nanotube Forests on Aluminum Foils. Chem. Vap. Depos. 2012, 18, 326–335. [Google Scholar] [CrossRef]
- Li, H.; Sun, X.; Dai, X.; Zhao, L.; Li, B.; Liang, C.; Wang, H.; Fan, J. Enhanced Mechanical Properties of Novel Aluminum Foams Synergistically Reinforced by NiO Particles and in Situ Grown Carbon Nanotubes. ECS J. Solid State Sci. Technol. 2021, 10, 051009. [Google Scholar] [CrossRef]
- San, M.T.; Prachakittikul, P.; Chainarong, K.; Sripisarn, T.; Kerdnawee, K.; Suttiponparnit, K.; Charinpanitkul, T.; Koo-Amornpattana, W.; Srifa, A.; Ratchahat, S.; et al. Potential Production of Carbon Nanotubes from Liquid Aromatic Hydrocarbons over Fe and Ni on Alumina Powder via Catalytic Chemical Vapor Deposition. Diam. Relat. Mater. 2023, 137, 110130. [Google Scholar] [CrossRef]




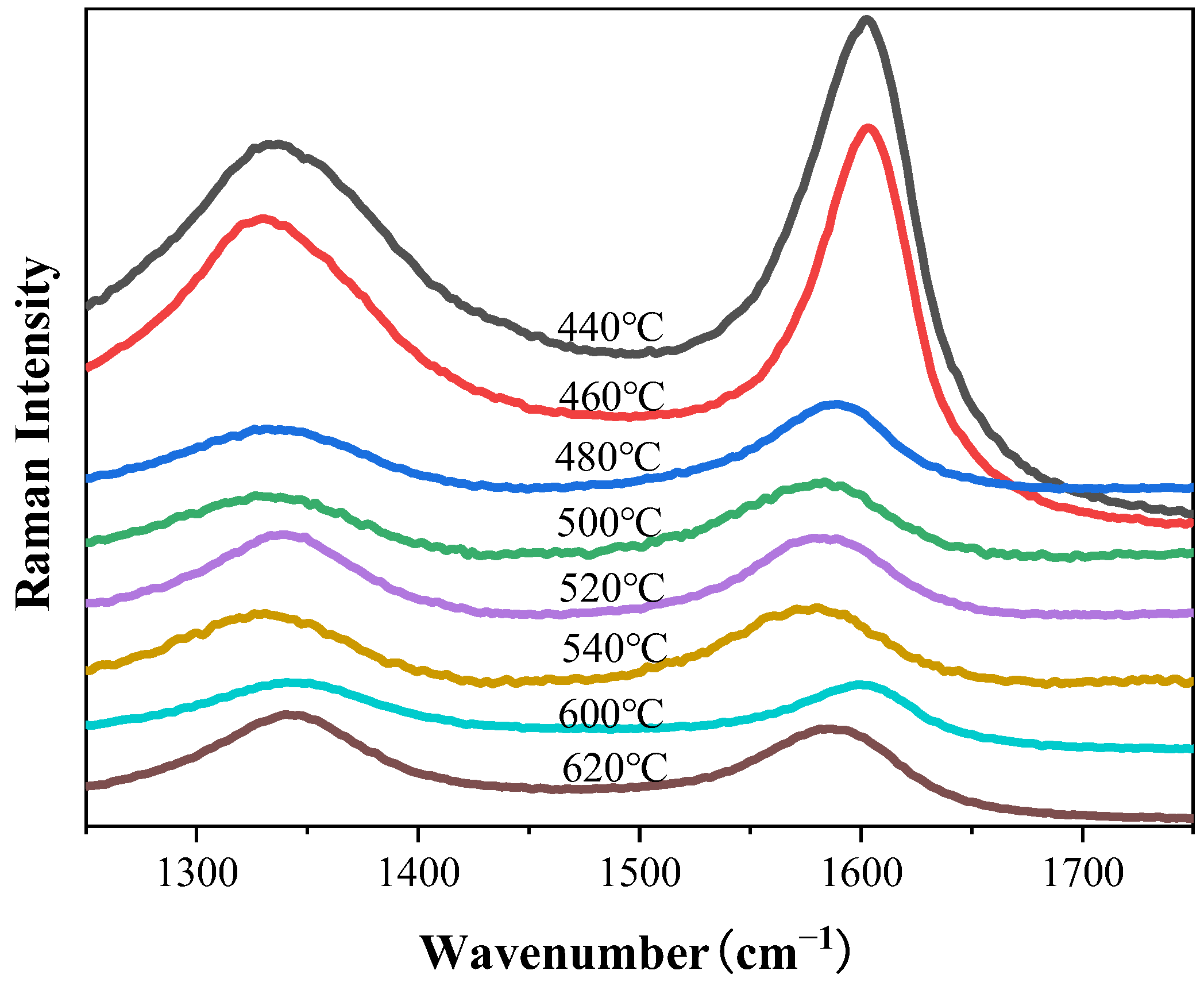






| Temperature (℃) | CNT Yield (wt.%) | ID/IG |
|---|---|---|
| 440 | 13.02 ± 0.47 | 0.750 |
| 460 | 14.02 ± 0.30 | 0.774 |
| 480 | 16.17 ± 1.77 | 0.708 |
| 500 | 23.56 ± 3.54 | 0.810 |
| 520 | 21.85 ± 1.48 | 1.047 |
| 540 | 19.22 ± 0.87 | 0.919 |
| 600 | 18.74 ± 0.54 | 1.027 |
| 620 | 23.99 ± 4.29 | 1.154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, R.; Li, H.; Liu, J.; Wu, Z.; Wang, Q.; Ramachandran, C.S. Low-Temperature Fabrication of Carbon Nanotube–Aluminum Composite Powders via Rotary Chemical Vapor Deposition: Process Optimization and Growth Mechanisms. Materials 2025, 18, 1654. https://doi.org/10.3390/ma18071654
Tan R, Li H, Liu J, Wu Z, Wang Q, Ramachandran CS. Low-Temperature Fabrication of Carbon Nanotube–Aluminum Composite Powders via Rotary Chemical Vapor Deposition: Process Optimization and Growth Mechanisms. Materials. 2025; 18(7):1654. https://doi.org/10.3390/ma18071654
Chicago/Turabian StyleTan, Ruodi, Haifeng Li, Jianwu Liu, Zizhao Wu, Qun Wang, and Chidambaram Seshadri Ramachandran. 2025. "Low-Temperature Fabrication of Carbon Nanotube–Aluminum Composite Powders via Rotary Chemical Vapor Deposition: Process Optimization and Growth Mechanisms" Materials 18, no. 7: 1654. https://doi.org/10.3390/ma18071654
APA StyleTan, R., Li, H., Liu, J., Wu, Z., Wang, Q., & Ramachandran, C. S. (2025). Low-Temperature Fabrication of Carbon Nanotube–Aluminum Composite Powders via Rotary Chemical Vapor Deposition: Process Optimization and Growth Mechanisms. Materials, 18(7), 1654. https://doi.org/10.3390/ma18071654





