Distinguishing the Contribution of Extracellular Electron Transfer in the Desulfovibrio caledoniensis-Induced Total Corrosion of Q235 Carbon Steel
Highlights
- Corrosion current induced by EMIC was distinguished from the total MIC.
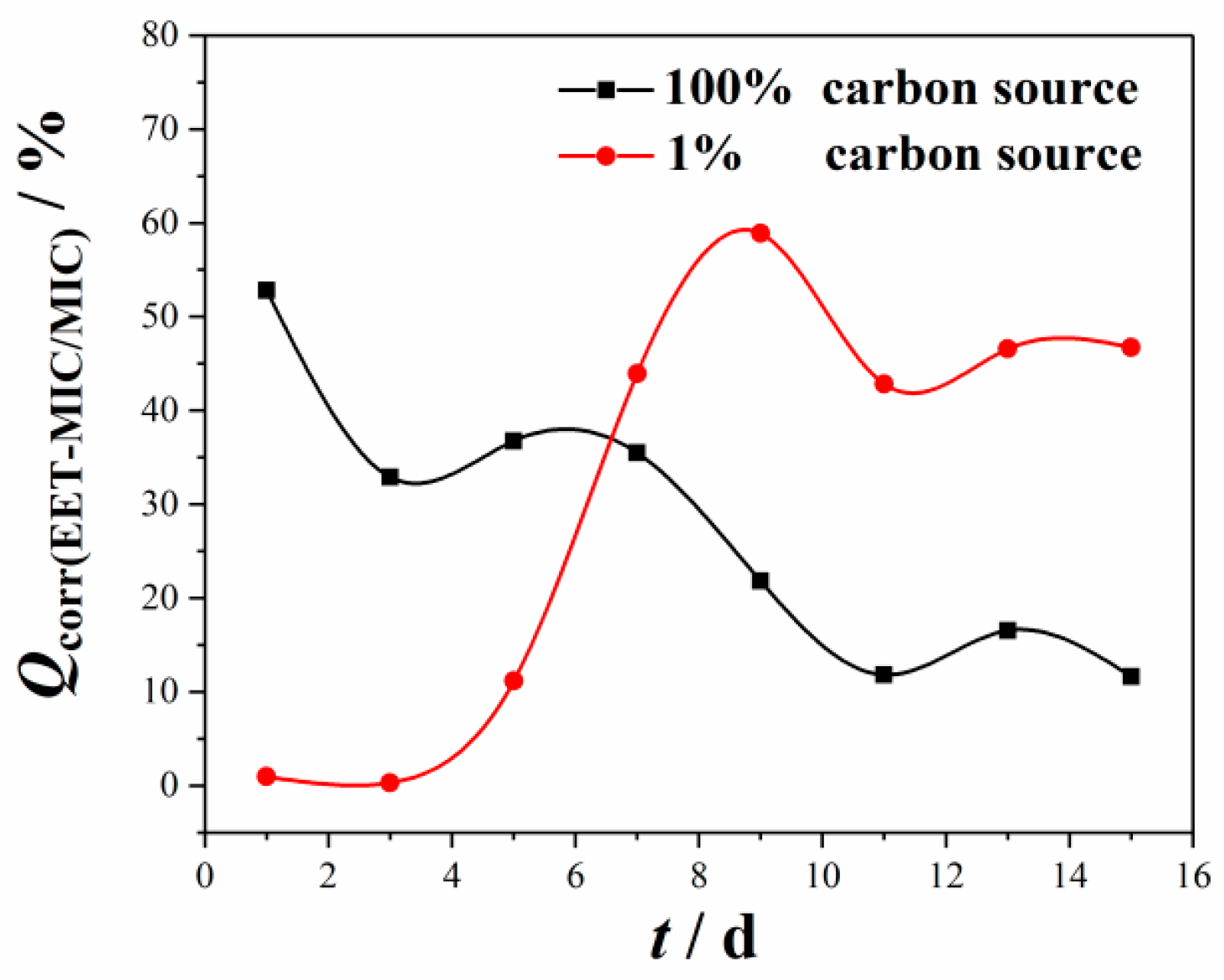
- The quotient of EMIC to total MIC is 27.69 % and 37.68 % in 100% and 1% CS media, respectively.
- Attached biofilm mainly accounted for MIC and the cathodic current stimulation.
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain, Growth Medium, and Culturing Conditions
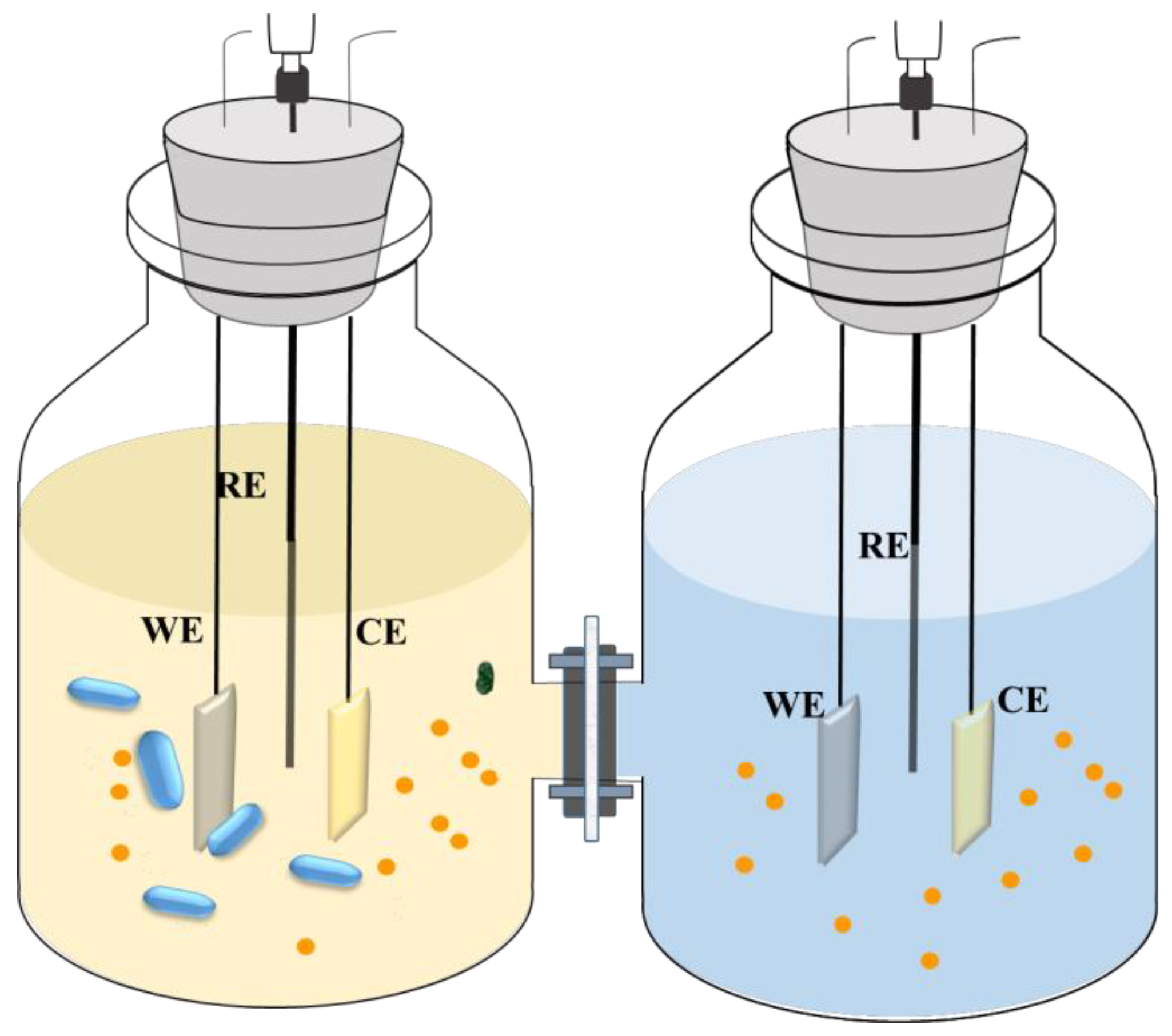
2.2. Electrochemical Cell and Bacterial Inoculation
2.3. Electrochemical Measurements
2.4. Weight Loss Determination
3. Results
3.1. Corrosion of the Q235 Steel in Different Culture Media
3.2. Quotient of EMIC in the MIC of the Q235 Steel
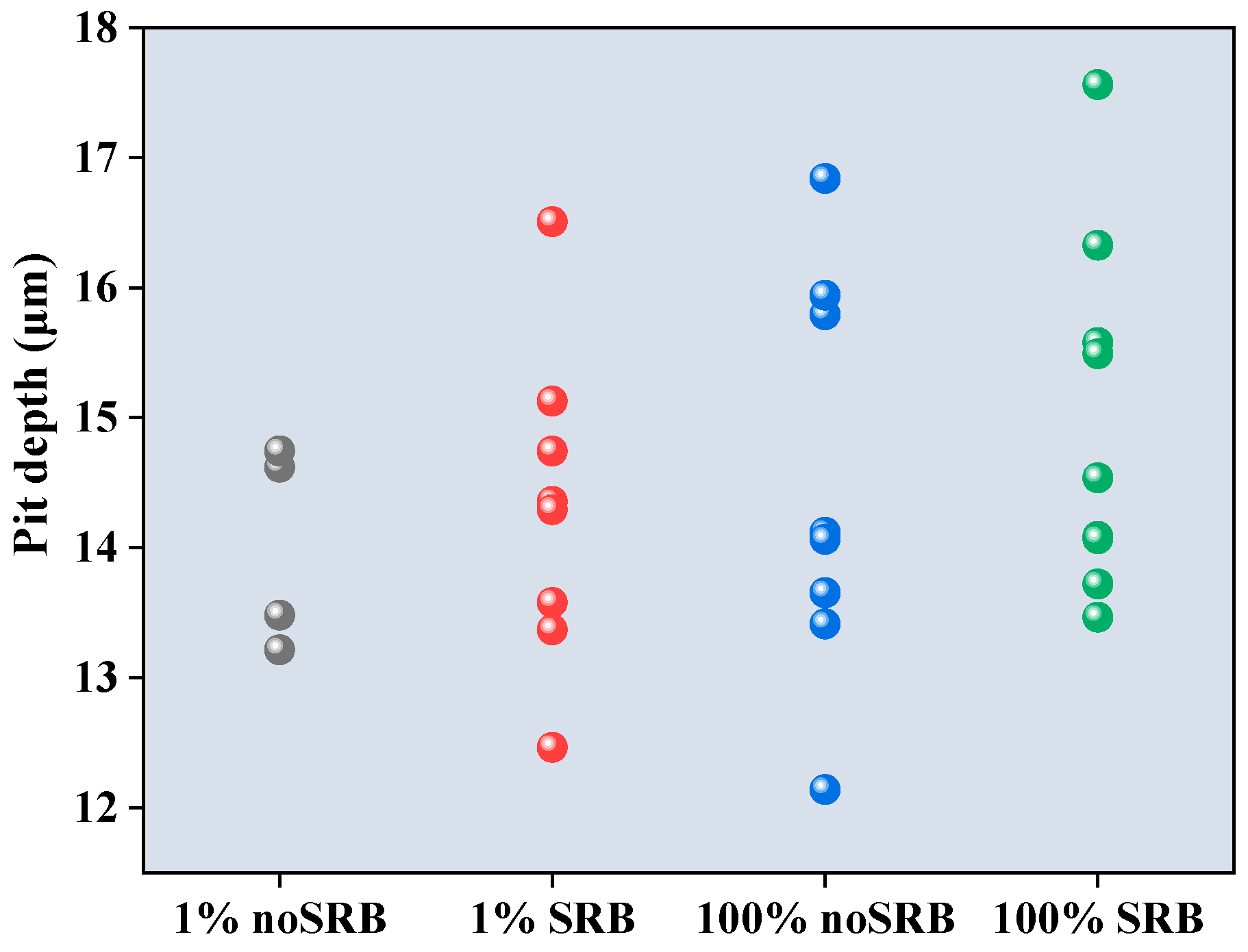
3.3. Weight Loss
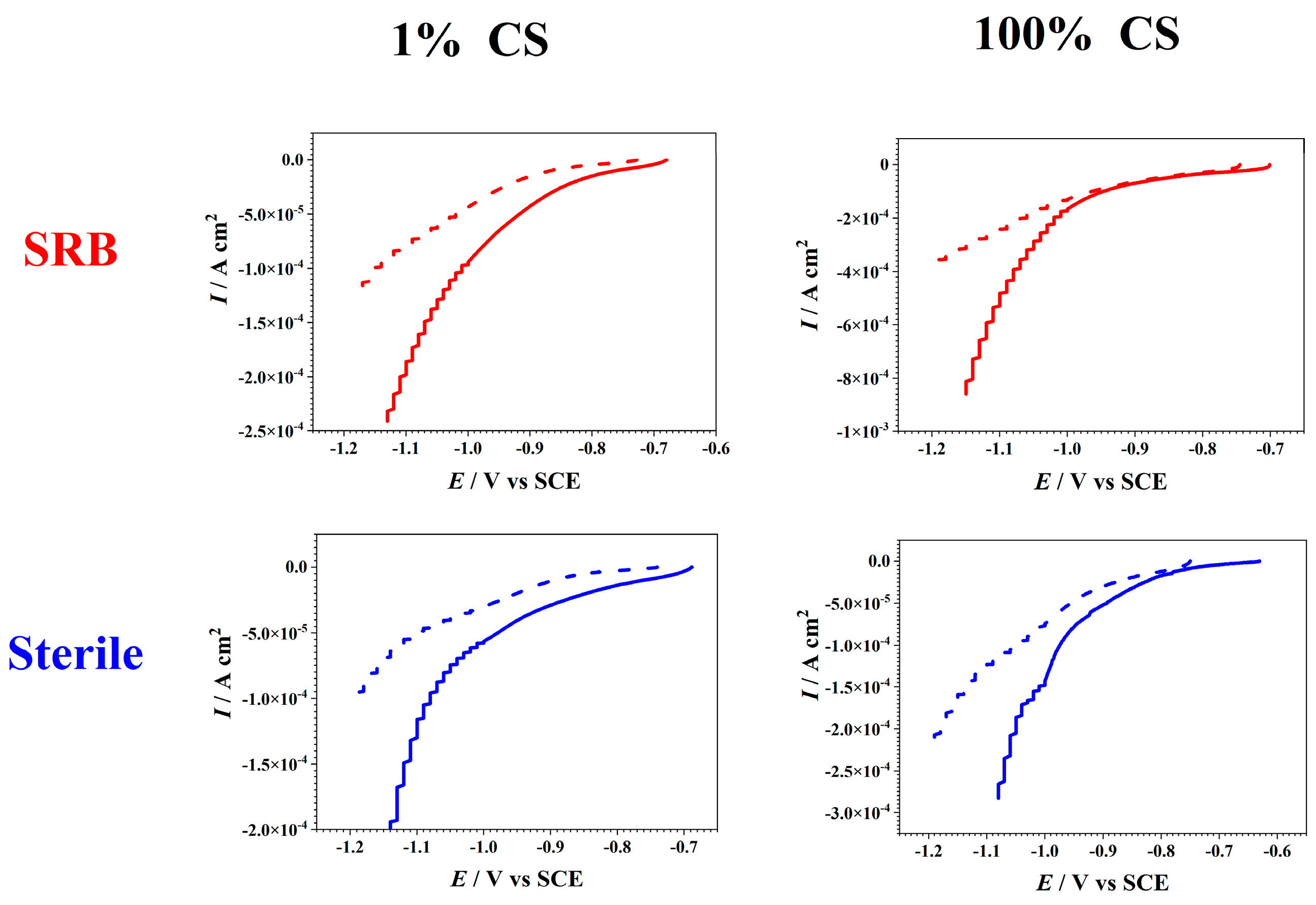
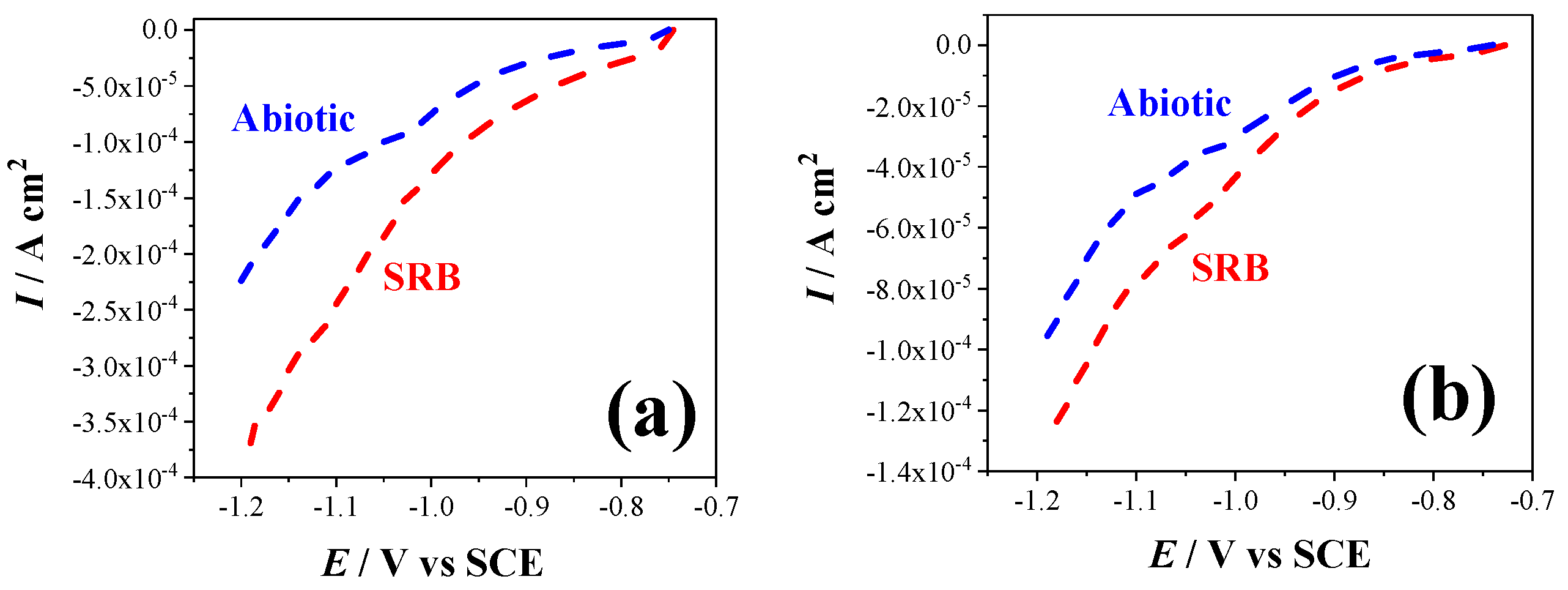
3.4. Revealing the Cathodic Microbial Activity via LSV
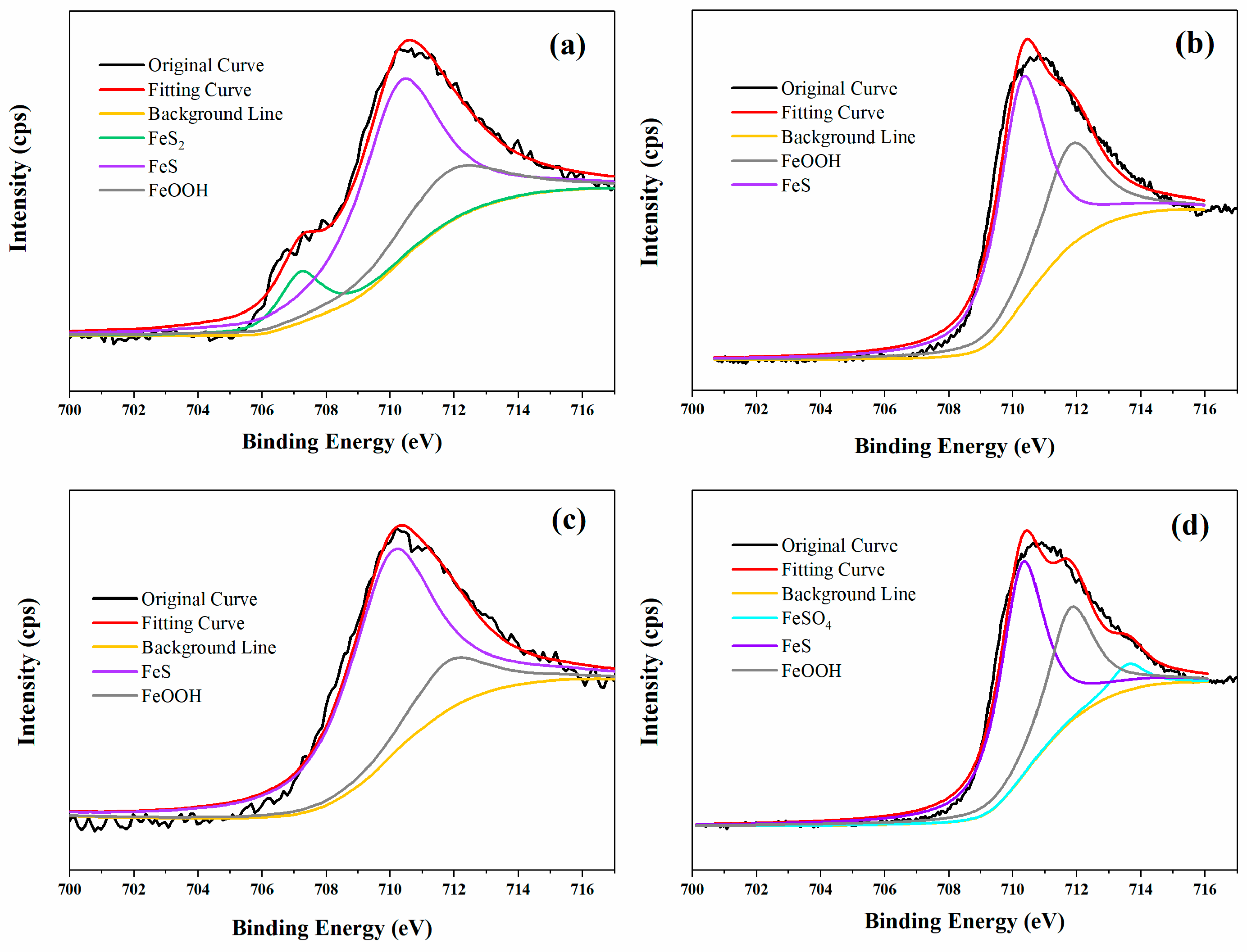
3.5. Corrosion Product Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaines, R.H. Bacterial Activity as a Corrosive Influence in the Soil. J. Ind. Eng. Chem. 1910, 2, 128–130. [Google Scholar] [CrossRef]
- AlAbbas, F.M.; Williamson, C.; Bhola, S.M.; Spear, J.R.; Olson, D.L.; Mishra, B.; Kakpovbia, A.E. Influence of sulfate reducing bacterial biofilm on corrosion behavior of low-alloy, high-strength steel (API-5L X80). Int. Biodeterior. Biodegrad. 2013, 78, 34–42. [Google Scholar] [CrossRef]
- Javaherdashti, R. MIcrobiologically Influenced Corrosion—An Engineering Insight; Springer: London, UK, 2008. [Google Scholar]
- Xu, D.; Gu, T.; Lovley, D.R. Microbially mediated metal corrosion. Nat. Rev. Microbiol. 2023, 21, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Booth, G.H. Sulphur Bacteria in Relation to Corrosion. J. Appl. Bacteriol. 1964, 27, 174–181. [Google Scholar] [CrossRef]
- Graves, J.W.; Sullivan, E.H. Internal corrosion in gas gathering systems and transmission lines. Mater. Prot. 1966, 5, 33–37. [Google Scholar]
- Jacobson, G.A. Corrosion at Prudhoe Bay: A lesson on the line. Mater. Perform. 2007, 46, 26–34. [Google Scholar]
- Zhang, L.J.; Zhang, Z.; Cao, F.H.; Zhang, J.Q.; Cao, C.N. Study of the X70 pipeline steel corroding in 3.0wt% NaCl solution using electrochemical impedance spectroscopy technique. Acta Metall. Sin. (Engl. Lett.) 2004, 17, 907–911. [Google Scholar]
- Enning, D.; Venzlaff, H.; Garrelfs, J.; Dinh, H.T.; Meyer, V.; Mayrhofer, K.; Hassel, A.W.; Stratmann, M.; Widdel, F. Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ. Microbiol. 2012, 14, 1772–1787. [Google Scholar] [CrossRef]
- Dinh, H.T.; Kuever, J.; Muszmann, M.; Hassel, A.W.; Stratmann, M.; Widdel, F. Iron corrosion by novel anaerobic microorganisms. Nature 2004, 427, 829–832. [Google Scholar] [CrossRef]
- Gu, T.; Xu, D. Why Are Some Microbes Corrosive And Some Not? In Proceedings of the CORROSION/2013, Orlando, FL, USA, 17–21 March 2013. [Google Scholar]
- Li, Z.; Yang, J.; Guo, H.; Kumseranee, S.; Punpruk, S.; Mohamed, M.E.; Saleh, M.A.; Gu, T. Carbon Source Starvation of a Sulfate-Reducing Bacterium–Elevated MIC Deterioration of Tensile Strength and Strain of X80 Pipeline Steel. Front. Mater. 2021, 8, 794051. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Chen, C.; Li, X.; Jia, R.; Zhang, D.; Sand, W.; Wang, F.; Gu, T. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, E.; Ueki, T.; Zhang, D.; Fan, Y.; Xu, D.; Wang, F.; Lovley, D.R. Accelerated Microbial Corrosion by Magnetite and Electrically Conductive Pili through Direct Fe0-to-Microbe Electron Transfer. Angew. Chem. Int. Ed. 2023, 62, e202309005. [Google Scholar] [CrossRef]
- Guberman-Pfeffer, M.J.; Dorval Courchesne, N.-M.; Lovley, D.R. Microbial nanowires for sustainable electronics. Nat. Rev. Bioeng. 2024, 2, 869–886. [Google Scholar] [CrossRef]
- Schröder, U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys. Chem. Chem. Phys. PCCP 2007, 9, 2619–2629. [Google Scholar]
- Gu, T. New Understandings of Biocorrosion Mechanisms and their Classifications. J. Microb. Biochem. Technol. 2012, 4, iii–vi. [Google Scholar]
- Sherar, B.W.A.; Power, I.M.; Keech, P.G.; Mitlin, S.; Southam, G.; Shoesmith, D.W. Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion. Corros. Sci. 2011, 53, 955–960. [Google Scholar] [CrossRef]
- Deng, X.; Okamoto, A. Energy Acquisition via Electron Uptake by the Sulfate-Reducing Bacterium Desulfovibrio ferrophilus IS5. J. Jpn. Soc. Extrem. 2018, 16, 67–75. [Google Scholar]
- Deng, X.; Dohmae, N.; Nealson, K.H.; Hashimoto, K.; Okamoto, A. Multi-heme cytochromes provide a pathway for survival in energy-limited environments. Sci. Adv. 2018, 4, eaao5682. [Google Scholar] [CrossRef]
- Costa, N.L.; Clarke, T.A.; Philipp, L.-A.; Gescher, J.; Louro, R.O.; Paquete, C.M. Electron transfer process in microbial electrochemical technologies: The role of cell-surface exposed conductive proteins. Bioresour. Technol. 2018, 255, 308–317. [Google Scholar] [CrossRef]
- Jia, R.; Yang, D.; Abd Rahman, H.B.; Gu, T. Laboratory testing of enhanced biocide mitigation of an oilfield biofilm and its microbiologically influenced corrosion of carbon steel in the presence of oilfield chemicals. Int. Biodeterior. Biodegrad. 2017, 125, 116–124. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Jia, R.; Dou, W.; Kumseranee, S.; Punpruk, S.; Li, X.; Gu, T. Distinguishing two different microbiologically influenced corrosion (MIC) mechanisms using an electron mediator and hydrogen evolution detection. Corros. Sci. 2020, 177, 108993. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, D.; Li, Y.; Yang, K.; Gu, T. Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 2015, 101, 14–21. [Google Scholar] [CrossRef]
- Li, H.; Xu, D.; Li, Y.; Feng, H.; Liu, z.; Li, X.; Gu, T.; Yang, K. Extracellular Electron Transfer Is a Bottleneck in the Microbiologically Influenced Corrosion of C1018 Carbon Steel by the Biofilm of Sulfate-Reducing Bacterium Desulfovibrio vulgaris. PLoS ONE 2015, 10, e0136183. [Google Scholar]
- Saunders, S.H.; Tse, E.C.M.; Yates, M.D.; Otero, F.J.; Trammell, S.A.; Stemp, E.D.A.; Barton, J.K.; Tender, L.M.; Newman, D.K. Extracellular DNA Promotes Efficient Extracellular Electron Transfer by Pyocyanin in Pseudomonas aeruginosa Biofilms. Cell 2020, 182, 1–14. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, D.; Huang, L.; Lou, Y.; Muhadesi, J.; Qian, H.; Zhou, E.; Wang, B.; Li, X.; Jiang, Z.; et al. Responses of soil microbiome to steel corrosion. Npj Biofilms Microbiomes 2021, 7, 6. [Google Scholar] [CrossRef]
- Tang, H.; Yang, C.; Ueki, T.; Pittman, C.C.; Xu, D.; Woodard, T.L.; Holmes, D.E.; Gu, T.; Wang, F.; Lovley, D.R. Stainless steel corrosion via direct iron-to-microbe electron transfer by Geobacter species. ISME J. 2021, 15, 3084–3093. [Google Scholar] [CrossRef]
- Xu, D.; Gu, T. Carbon source starvation triggered more aggressive corrosion against carbon steel by the Desulfovibrio vulgaris biofilm. Int. Biodeterior. Biodegrad. 2014, 91, 74–81. [Google Scholar] [CrossRef]
- Dou, W.; Jia, R.; Jin, P.; Liu, J.; Chen, S.; Gu, T. Investigation of the mechanism and characteristics of copper corrosion by sulfate reducing bacteria. Corros. Sci. 2018, 144, 237–248. [Google Scholar] [CrossRef]
- Guan, F.; Duan, J.; Zhai, X.; Wang, N.; Zhang, J.; Lu, D.; Hou, B. Interaction between sulfate-reducing bacteria and aluminum alloys—Corrosion mechanisms of 5052 and Al-Zn-In-Cd aluminum alloys. J. Mater. Sci. Technol. 2020, 36, 55–64. [Google Scholar] [CrossRef]
- Guan, F.; Zhai, X.; Duan, J.; Zhang, J.; Li, K.; Hou, B. Influence of sulfate-reducing bacteria on the corrosion behavior of 5052 aluminum alloy. Surf. Coat. Technol. 2017, 316, 171–179. [Google Scholar] [CrossRef]
- Duan, J.; Wu, S.; Zhang, X.; Huang, G.; Du, M.; Hou, B. Corrosion of carbon steel influenced by anaerobic biofilm in natural seawater. Electrochim. Acta 2008, 54, 22–28. [Google Scholar] [CrossRef]
- Yu, L.; Duan, J.; Du, X.; Huang, Y.; Hou, B. Accelerated anaerobic corrosion of electroactive sulfate-reducing bacteria by electrochemical impedance spectroscopy and chronoamperometry. Electrochem. Commun. 2013, 26, 101–104. [Google Scholar] [CrossRef]
- Yu, L.; Duan, J.; Zhao, W.; Huang, Y.; Hou, B. Characteristics of hydrogen evolution and oxidation catalyzed by Desulfovibrio caledoniensis biofilm on pyrolytic graphite electrode. Electrochim. Acta 2011, 56, 9041–9047. [Google Scholar] [CrossRef]
- Postgate, J. The Sulphate-Reducing Bacteria, 2nd ed.; Cambridge University Press: London, UK, 1984. [Google Scholar]
- Guan, F.; Liu, Z.; Dong, X.; Zhai, X.; Zhang, B.; Duan, J.; Wang, N.; Gao, Y.; Yang, L.; Hou, B. Synergistic effect of carbon starvation and exogenous redox mediators on corrosion of X70 pipeline steel induced by Desulfovibrio singaporenus. Sci. Total Environ. 2021, 788, 147573. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Zhai, X.; Duan, J.; Zhang, M.; Hou, B. Influence of Sulfate-Reducing Bacteria on the Corrosion Behavior of High Strength Steel EQ70 under Cathodic Polarization. PLoS ONE 2016, 11, e0162315. [Google Scholar] [CrossRef]
- Guan, F.; Yuan, X.; Duan, J.; Zhai, X.; Hou, B. Phenazine enables the anaerobic respiration of Pseudomonas aeruginosa via electron transfer with a polarised graphite electrode. Int. Biodeterior. Biodegrad. 2019, 137, 8–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Moloney, J. Electrochemical Corrosion Rate Measurement Under Iron Sulfide Deposit. Corrosion 2016, 72, 704–715. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, W.; Guo, N.; Zeng, Z.; Liu, T.; Wang, X. Molybdenum-mediated chemotaxis of Pseudoalteromonas lipolytica enhances biofilm-induced mineralization on low alloy steel surface. Corros. Sci. 2019, 159, 108123. [Google Scholar] [CrossRef]
- ASTMG1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2003.
- Liu, H.; Gu, T.; Lv, Y.; Asif, M.; Xiong, F.; Zhang, G.; Liu, H. Corrosion inhibition and anti-bacterial efficacy of benzalkonium chloride in artificial CO2-saturated oilfield produced water. Corros. Sci. 2017, 117, 24–34. [Google Scholar] [CrossRef]
- Hamilton, W.A. Microbially Influenced Corrosion as a Model System for the Study of Metal Microbe Interactions: A Unifying Electron Transfer Hypothesis. Biofouling 2003, 19, 65–76. [Google Scholar] [CrossRef]
- Wang, D.; Yang, C.; Saleh, M.A.; Alotaibi, M.D.; Mohamed, M.E.; Xu, D.; Gu, T. Conductive magnetite nanoparticles considerably accelerated carbon steel corrosion by electroactive Desulfovibrio vulgaris biofilm. Corros. Sci. 2022, 205, 110440. [Google Scholar] [CrossRef]
- Jia, R.; Tan, J.L.; Jin, P.; Blackwood, D.J.; Xu, D.; Gu, T. Effects of biogenic H2S on the microbiologically influenced corrosion of C1018 carbon steel by sulfate reducing Desulfovibrio vulgaris biofilm. Corros. Sci. 2018, 130, 1–11. [Google Scholar] [CrossRef]
- San, N.O.; Nazır, H.; Dönmez, G. Microbially influenced corrosion and inhibition of nickel–zinc and nickel–copper coatings by Pseudomonas aeruginosa. Corros. Sci. 2014, 79, 177–183. [Google Scholar] [CrossRef]
- Deng, X.; Dohmae, N.; Kaksonen, A.H.; Okamoto, A. Biogenic Iron Sulfide Nanoparticles to Enable Extracellular Electron Uptake in Sulfate-Reducing Bacteria. Angew. Chem. Int. Ed. 2020, 59, 5995–5999. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, J. An Introduction of Electrochemical Impedance Spectroscopy Science; Science: Beijing, China, 2002; pp. 86–106. [Google Scholar]
- Little, B.J.; Lee, J.S.; Ray, R.I. The influence of marine biofilms on corrosion: A concise review. Electrochim. Acta 2008, 54, 2–7. [Google Scholar] [CrossRef]
- Peme, T.; Olasunkanmi, L.O.; Bahadur, I.; Adekunle, A.S.; Kabanda, M.M.; Ebenso, E.E. Adsorption and Corrosion Inhibition Studies of Some Selected Dyes as Corrosion Inhibitors for Mild Steel in Acidic Medium: Gravimetric, Electrochemical, Quantum Chemical Studies and Synergistic Effect with Iodide Ions. Molecules 2015, 20, 16004–16029. [Google Scholar] [CrossRef]
- Jain, A.; Gazzola, G.; Panzera, A.; Zanoni, M.; Marsili, E. Visible spectroelectrochemical characterization of Geobacter sulfurreducens biofilms on optically transparent indium tin oxide electrode. Electrochim. Acta 2011, 56, 10776–10785. [Google Scholar]
- Jain, A.; Zhang, X.; Pastorella, G.; Connolly, J.O.; Barry, N.; Woolley, R.; Krishnamurthy, S.; Marsili, E. Electron transfer mechanism in Shewanella loihica PV-4 biofilms formed at graphite electrode. Bioelectrochemistry 2012, 87, 28–32. [Google Scholar] [PubMed]
- Marsili, E.; Rollefson, J.B.; Baron, D.B.; Hozalski, R.M.; Bond, D.R. Microbial Biofilm Voltammetry: Direct Electrochemical Characterization of Catalytic Electrode-Attached Biofilms. Appl. Environ. Microbiol. 2008, 74, 7329–7337. [Google Scholar]
- Venzlaff, H.; Enning, D.; Srinivasan, J.; Mayrhofer, K.J.J.; Hassel, A.W.; Widdel, F.; Stratmann, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef]
- Murugan, M.; Miran, W.; Masuda, T.; Lee, D.S.; Okamoto, A. Biosynthesized Iron Sulfide Nanocluster Enhanced Anodic Current Generation by Sulfate Reducing Bacteria in Microbial Fuel Cells. ChemElectroChem 2018, 5, 4015–4020. [Google Scholar] [CrossRef]
- Yuan, S.; Liang, B.; Zhao, Y.; Pehkonen, S.O. Surface chemistry and corrosion behaviour of 304 stainless steel in simulated seawater containing inorganic sulphide and sulphate-reducing bacteria. Corros. Sci. 2013, 74, 353–366. [Google Scholar]
- Dietrich, L.E.P.; Price-Whelan, A.; Petersen, A.; Whiteley, M.; Newman, D.K. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 2006, 61, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Jain, A. Extracellular electron transfer mechanism in Shewanella loihica PV-4 biofilms formed at indium tin oxide and graphite electrodes. Int. J. Electrochem. Sci. 2013, 8, 1778. [Google Scholar]
- Cordas, C.M.; Guerra, L.T.; Xavier, C.; Moura, J.J.G. Electroactive biofilms of sulphate reducing bacteria. Electrochim. Acta 2008, 54, 29–34. [Google Scholar] [CrossRef]
- Beese-Vasbender, P.F.; Grote, J.-P.; Garrelfs, J.; Stratmann, M.; Mayrhofer, K.J.J. Selective microbial electrosynthesis of methane by a pure culture of a marine lithoautotrophic archaeon. Bioelectrochemistry 2015, 102, 50–55. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, X.; Yin, J.; Xu, S.; Zhang, Y.; Xie, D.; Li, Z. Geobacter sulfurreducens adapts to low electrode potential for extracellular electron transfer. Electrochim. Acta 2016, 191, 743–749. [Google Scholar] [CrossRef]

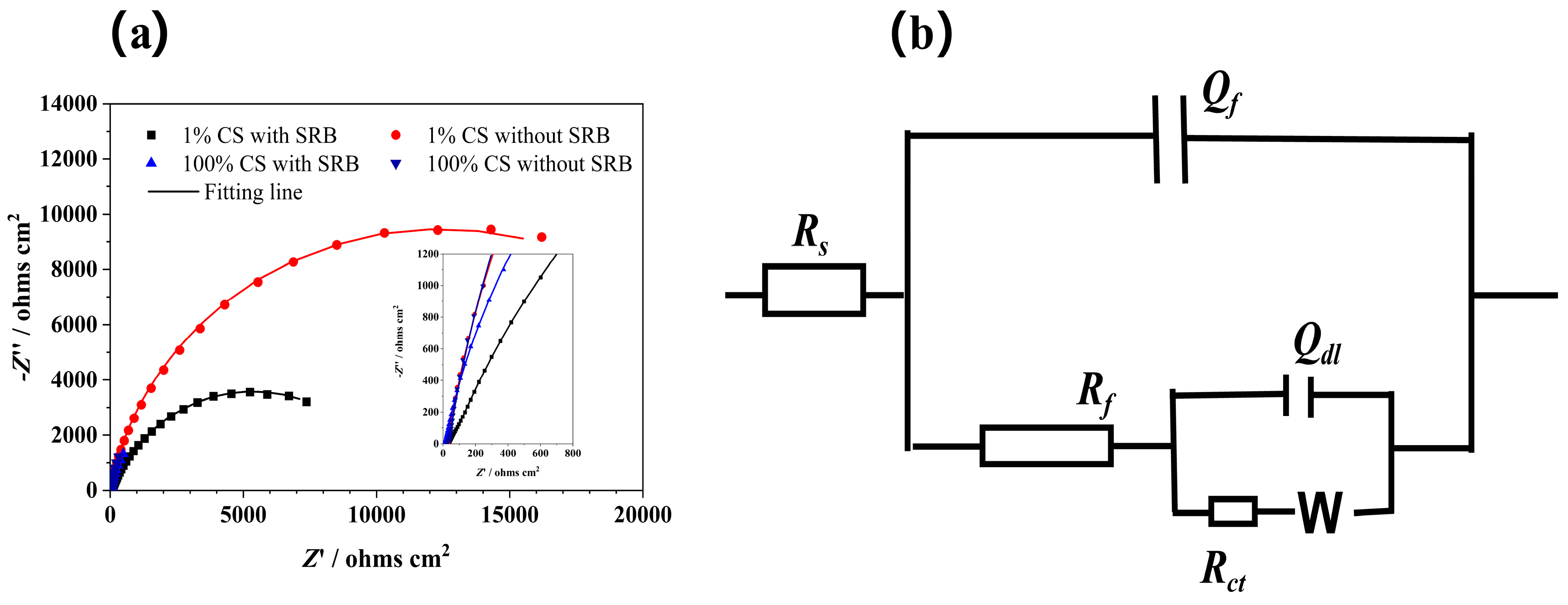


| Conditions | Rs (Ohms cm2) | Qf | Rf (Ohms cm2) | Qdl | Rct (Ohms cm2) | ||
|---|---|---|---|---|---|---|---|
| Yf (×10−4 S cm−2 Sn) | nf | Ydl (×10−4 S cm−2 Sn) | ndl | ||||
| 1% CS with SRB | 21.66 ± 1.18 | 1.12 ± 0.15 | 0.75 ± 0.35 | 15.10 ± 2.36 | 5.79 ± 2.80 | 0.80 ± 0.034 | 12,850 ± 212.13 |
| 1% CS without SRB | 20.46 ± 5.54 | 3.67 ± 0.78 | 0.67 ± 0.29 | 13.72 ± 2.79 | 2.10 ± 0.97 | 0.91 ± 0.0078 | 27,450 ± 70.71 |
| 100% CS with SRB | 11.34 ± 0.21 | 64.1 ± 1.17 | 0.86 ± 0.004 | 12.20 ± 0.85 | 14.9 ± 0.80 | 0.97 ± 0.03 | 10,410 ± 130 |
| 100% CS without SRB | 22.22 ± 2.66 | 23.4 ± 5.04 | 0.97± 0.03 | 17.80 ± 0.22 | 60.7 ± 10.5 | 0.89 ± 0.05 | 17,610 ± 390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, K.; Guan, F.; Zhai, X.; Jiao, G.; Sang, Y.; Jing, M.; Duan, J. Distinguishing the Contribution of Extracellular Electron Transfer in the Desulfovibrio caledoniensis-Induced Total Corrosion of Q235 Carbon Steel. Materials 2025, 18, 1613. https://doi.org/10.3390/ma18071613
Fan K, Guan F, Zhai X, Jiao G, Sang Y, Jing M, Duan J. Distinguishing the Contribution of Extracellular Electron Transfer in the Desulfovibrio caledoniensis-Induced Total Corrosion of Q235 Carbon Steel. Materials. 2025; 18(7):1613. https://doi.org/10.3390/ma18071613
Chicago/Turabian StyleFan, Keliang, Fang Guan, Xiaofan Zhai, Guanhua Jiao, Yugang Sang, Min Jing, and Jizhou Duan. 2025. "Distinguishing the Contribution of Extracellular Electron Transfer in the Desulfovibrio caledoniensis-Induced Total Corrosion of Q235 Carbon Steel" Materials 18, no. 7: 1613. https://doi.org/10.3390/ma18071613
APA StyleFan, K., Guan, F., Zhai, X., Jiao, G., Sang, Y., Jing, M., & Duan, J. (2025). Distinguishing the Contribution of Extracellular Electron Transfer in the Desulfovibrio caledoniensis-Induced Total Corrosion of Q235 Carbon Steel. Materials, 18(7), 1613. https://doi.org/10.3390/ma18071613






