The Role of Organic Materials in Shaping the Content of Trace Elements in Iron-Contaminated Soil
Highlights
- Soil iron contamination leads to an increase in Fe, Mn, Cu, and Co and a decrease in Cd, Pb, Cr, and Zn in the soil.
- Organic material is effective in reducing the content of some trace elements (TEs), especially Cd and Zn, in Fe-contaminated soils.
- Other TEs content in the soil was increased after humic acids application, especially Co, Ni, and Pb.
- Humic acids have a greater effect than Fe contamination on the content of most TEs in the soil.
Abstract
1. Introduction
2. Materials and Methods
2.1. Pot Vegetation Experiment
2.2. Analytical Methods
2.3. Statistical Methods
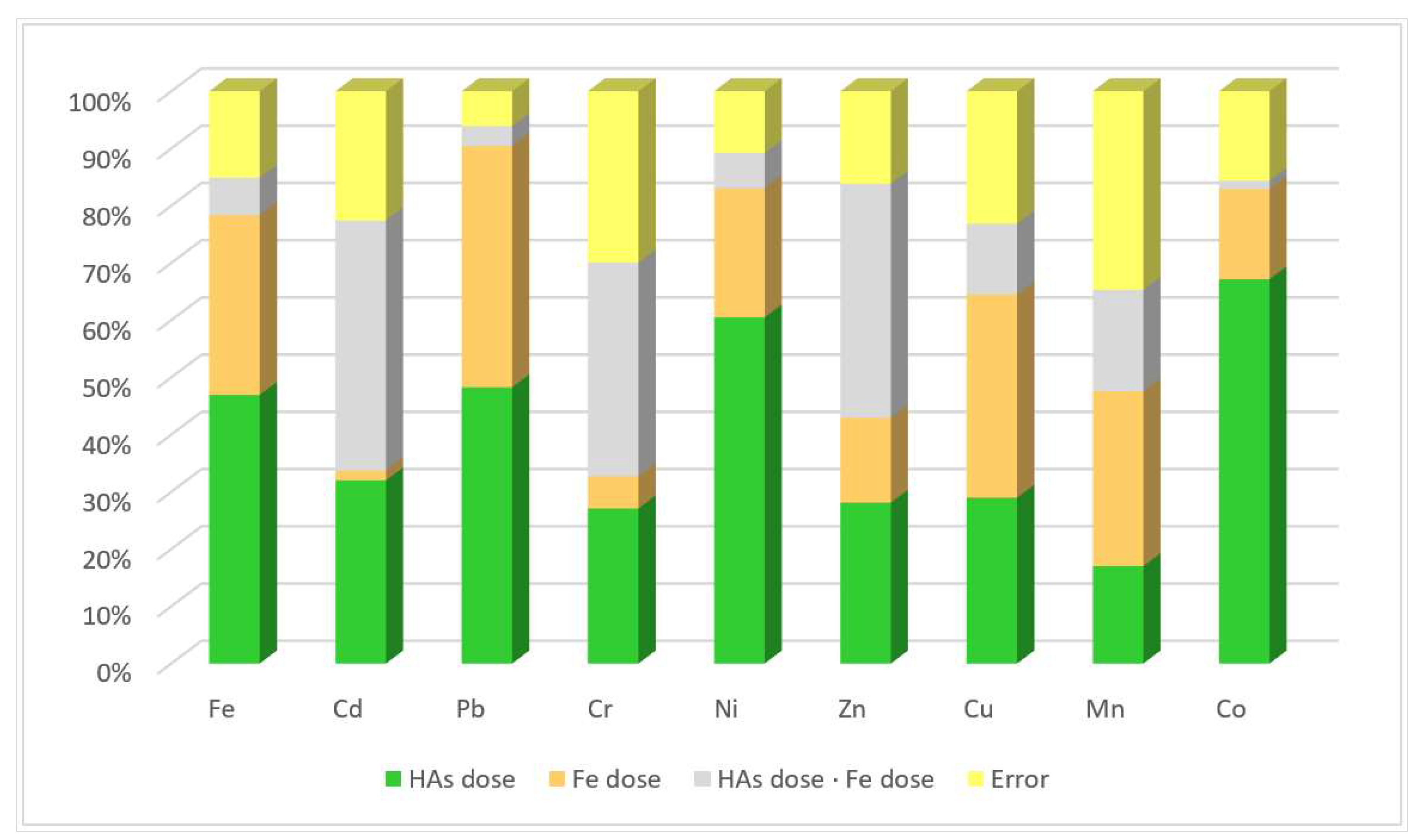
- η2—relative impact of factors,
- SS factor—sum of squares for a given factor,
- Total SS—sum of squares for all factors.
3. Results
3.1. Iron
3.2. Other Trace Elements
3.3. Relations Beetwen Heavy Metals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brevik, E.C.; Cerdà, A.; Mataix-Solera, J.; Pereg, L.; Quinton, J.N.; Six, J.; Van Oost, K. The interdisciplinary nature of SOIL. Soil 2015, 1, 117–129. [Google Scholar] [CrossRef]
- Baveye, P.C.; Baveye, J.; Gowdy, J. Soil “ecosystem” services and natural capital: Critical appraisal of research on uncertain ground. Front. Environ. Sci. 2016, 4, 41. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Guo, M. Soil health assessment and management: Recent development in science and practices. Soil Syst. 2021, 5, 61. [Google Scholar] [CrossRef]
- Jones, J.D. Iron availability and management considerations: A 4R approach. Crops Soils 2020, 53, 32–37. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; He, J.Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Onyango, D.A.; Entila, F.; Dida, M.M.; Ismail, A.M.; Drame, K.N. Mechanistic understanding of Fe toxicity tolerance in contrasting rice varieties from Africa: 1. Morpho-physiological and biochemical responses. Funct. Plant Biol. 2019, 46, 93–105. [Google Scholar] [CrossRef]
- Mahender, A.; Swamy, B.P.M.; Anandan, A.; Ali, J. Tolerance of iron-deficient and -toxic soil conditions in rice. Plants 2019, 8, 31. [Google Scholar] [CrossRef]
- Ning, X.; Lin, M.; Huang, G.; Mao, J.; Gao, Z.; Wang, X. Research progress on iron absorption, transport, and molecular regulation strategy in plants. Front. Plant Sci. 2023, 14, 1190768. [Google Scholar] [CrossRef]
- Ghasemi-Fasaei, R.; Ronaghi, A. Interaction of iron with copper, zinc, and manganese in wheat as affected by iron and manganese in a calcareous soil. J. Plant Nutr. 2008, 31, 839–848. [Google Scholar] [CrossRef]
- Herlihy, J.H.; Long, T.A.; McDowell, J.M. Iron homeostasis and plant immune responses: Recent insights and translational implications. J. Biol. Chem. 2020, 295, 13444–13457. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Hafeez, M.B.; Shaukat, K.; Wahid, A.; Hasanuzzaman, M. Fe toxicity in plants: Impacts and remediation. Physiol. Plant. 2021, 173, 201–222. [Google Scholar] [CrossRef]
- Aung, M.S.; Masuda, H. How does rice defend against excess iron?: Physiological and molecular mechanisms. Front. Plant Sci. 2020, 11, 1102. [Google Scholar] [CrossRef]
- Matthus, E.; Wu, L.B.; Ueda, Y.; Höller, S.; Becker, M.; Frei, M. Loci, genes, and mechanisms associated with tolerance to ferrous iron toxicity in rice (Oryza sativa L.). Theor. Appl. Genet. 2015, 128, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Gülser, F.; Yavuz, H.Ý.; Gökkaya, T.H.; Sedef, M. Effects of Fe sources and doses on plant growth criteria in soybean seedlings. Eurasian J. Soil Sci. 2019, 8, 298–303. [Google Scholar] [CrossRef][Green Version]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Reis, S.; Pavia, I.; Carvalho, A.; Moutinho-Pereira, J.; Correia, C.; Lima-Brito, J. Seed priming with Fe and zinc in bread wheat: Effects in germination, mitosis and grain yield. Protoplasma 2018, 255, 1179–1194. [Google Scholar] [CrossRef]
- Audebert, A.; Sahrawat, K.L. Mechanisms for iron toxicity tolerance in lowland rice. J. Plant Nutr. 2000, 23, 1877–1885. [Google Scholar] [CrossRef]
- Chérif, M.; Audebert, A.; Fofana, M.; Zouzou, M. Evaluation of iron toxicity on lowland irrigated rice in West Africa. Tropicultra 2009, 27, 88–92. Available online: http://www.tropicultura.org/text/v27n2/88.pdf (accessed on 15 February 2025).
- Müller, C.; Kuki, K.N.; Pinheiro, D.T.; de Souza, L.R.; Siqueira Silva, A.I.; Loureiro, M.E.; Oliva, M.A.; Almeida, A.M. Differential physiological responses in rice upon exposure to excess distinct iron forms. Plant Soil 2015, 391, 123–138. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Iron toxicity in wetland rice and the role of other nutrients. J. Plant Nutr. 2005, 27, 1471–1504. [Google Scholar] [CrossRef]
- Deka, J.; Sarma, H. Heavy metal contamination in soil in an industrial zone and its relation with some soil properties. Arch. Appl. Sci. Res. 2012, 4, 831–836. Available online: https://www.scholarsresearchlibrary.com/articles/heavy-metal-contamination-in-soil-in-an-industrial-zone-and-its-relation-with-some-soil-properties.pdf (accessed on 15 February 2025).
- Xing, W.; Li, D.; Liu, G. Antioxidative responses of Elodea nuttallii (planch.) H. St. John to short-term Fe exposure. Plant Physiol. Biochem. 2010, 48, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Ahmed, S.F.; Santiago-Arenas, R.; Himanshu, S.K.; Mansour, E.; Cha-um, S.; Datta, A. Tolerance mechanism and management concepts of iron toxicity in rice: A critical review. Adv. Agron. 2023, 177, 215–257. [Google Scholar] [CrossRef]
- Audebert, A.; Fofana, M. Rice yield gap due to iron toxicity in West Africa. J. Agron. Crop Sci. 2009, 196, 66–76. [Google Scholar] [CrossRef]
- Leone, V.; Iovino, P.; Canzano, S.; Salvestrini, S.; Capasso, S. Water purification from humic acids by clinoptilolite-rich tuff. Environ. Eng. Manag. J. 2013, 12, 3–6. Available online: http://www.eemj.icpm.tuiasi.ro/pdfs/vol12/no11suppl/2_Leone_13.pdf (accessed on 12 February 2025).
- Yang, T.; Hodson, M.E. Investigating the use of synthetic humic-like acid as a soil washing treatment for metal contaminated soil. Sci. Total Environ. 2019, 647, 290–300. [Google Scholar] [CrossRef]
- Kandra, B.; Tall, A.; Vitková, J.; Procházka, M.; Šurda, P. Effect of humic amendment on selected hydrophysical properties of sandy and clayey soils. Water 2024, 16, 1338. [Google Scholar] [CrossRef]
- Michalska, J.; Turek-Szytow, J.; Dudło, A.; Surmacz-Górska, J. Characterization of humic substances recovered from the sewage sludge and validity of their removal from this waste. EFB Bioeconomy J. 2022, 2, 100026. [Google Scholar] [CrossRef]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J.; Borowik, A.; Kaczyński, P. Possibilities of restoring homeostasis of soil exposed to terbuthylazine by its supplementation with HumiAgra preparation. Appl. Soil Ecol. 2022, 178, 104582. [Google Scholar] [CrossRef]
- Bai, H.; Jiang, Z.; He, M.; Ye, B.; Wei, S. Relating Cd2+ binding by humic acids to molecular weight: A modeling and spectroscopic study. J. Environ. Sci. 2018, 70, 154–165. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221. [Google Scholar] [CrossRef]
- Škarpa, P.; Pospíšilová, L.; Bjelková, M.; Fiala, K.; Hlušek, J. Effect of organic matter and pH on the mobility of some heavy metals in soils of permanent grasslands in the foothills of the Hruby Jesenik Mts. Ecol. Chem. Eng. A 2011, 18, 347–1354. Available online: http://tchie.uni.opole.pl/ece_a/A_18_9/ECE_A_18(9-10).pdf (accessed on 15 January 2025).
- Wyszkowski, M.; Kordala, N.; Brodowska, M.S. Trace element content in soils with nitrogen fertilisation and humic acids addition. Agriculture 2023, 13, 968. [Google Scholar] [CrossRef]
- Hriciková, S.; Kožárová, I.; Hudáková, N.; Reitznerová, A.; Nagy, J.; Marcinčák, S. Humic substances as a versatile intermediary. Life 2023, 13, 858. [Google Scholar] [CrossRef] [PubMed]
- Zhilin, D.M.; Schmitt-Kopplin, P.; Perminova, I.V. Reduction of Cr(VI) by peat and coal humic substances. Environ. Chem. Lett. 2004, 2, 141–145. [Google Scholar] [CrossRef]
- Gao, K.; Pearce, J.; Jones, J.; Taylor, C. Interaction between peat, humic acid and aqueous metal ions. Environ. Geochem. Health 1999, 21, 13–26. [Google Scholar] [CrossRef]
- Jung, H.; Kwon, S.; Kim, J.-H.; Jeon, J.-R. Which traits of humic substances are investigated to improve their agronomical value? Molecules 2021, 26, 760. [Google Scholar] [CrossRef]
- Chen, Q.; Qu, Z.; Ma, G.; Wang, W.; Dai, J.; Zhang, M.; Wei, Z.; Liu, Z. Humic acid modulates growth, photosynthesis, hormone and osmolytes system of maize under drought conditions. Agric. Water Manag. 2022, 263, 107447. [Google Scholar] [CrossRef]
- El-Bassiouny, H.S.M.; Bakry, A.B.; Abd El-Monem Attia, A.; Abd Allah, M.M. Physiological role of humic acid and nocotinamide on improving plant growth, yield, and mineral nutrient of wheat (Triticum durum) grown under newly reclaimed sandy soil. Agric. Sci. 2014, 5, 687–700. [Google Scholar] [CrossRef]
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface: From environmental aspects to molecular factors. Plant Signal Behav. 2010, 5, 635–643. [Google Scholar] [CrossRef]
- Sarlaki, E.; Kianmehr, M.H.; Marzban, N.; Shafizadeh, A.; Tajuddin, S.A.F.S.A.; Hu, S.; Tabatabaei, M.; Aghbashlo, M. Advances and challenges in humic acid production technologies from natural carbonaceous material wastes. Chem. Eng. J. 2024, 498, 155521. [Google Scholar] [CrossRef]
- Yang, F.; Antonietti, M. Artificial humic acids: Sustainable materials against climate change. Adv. Sci. 2020, 7, 1902992. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N.; Brodowska, M. Role of humic acids and nitrogen fertilisers in regulating macroelement content in maize biomass. J. Elem. 2023, 28, 1289–1309. [Google Scholar] [CrossRef]
- Wieczorek, K.; Turek, A.; Szczesio, M.; Wolf, W.M. Comprehensive evaluation of metal pollution in urban soils of a post-industrial city—A case of Łódź, Poland. Molecules 2020, 25, 4350. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources; International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; p. 236. Available online: https://www.isric.org/sites/default/files/WRB_fourth_edition_2022-12-18.pdf (accessed on 20 December 2024).
- Brodowska, M.S.; Wyszkowski, M.; Kordala, N. Use of organic materials to limit the potential negative effect of nitrogen on maize in different soils. Materials 2022, 15, 5755. [Google Scholar] [CrossRef]
- US-EPA Method 3051A. Microwave Assisted Acid Digestion of Sediment, Sludges, Soils, and Oils; United States Environmental Protection Agency: Washington, DC, USA, 2007; pp. 1–30. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf (accessed on 15 December 2024).
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods for Analysis and Evaluation of Soil and Plant Properties; Institute of Environmental Protection: Warsaw, Poland, 1991; pp. 1–334. [Google Scholar]
- Wyszkowski, M.; Kordala, N. Mineral and organic materials as factors reducing the effect of petrol on heavy metal content in soil. Materials 2024, 17, 3528. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System), version 13.3; TIBCO Software Inc.: Palo Alto, CA, USA, 2021. [Google Scholar]
- Becker, M.; Asch, F. Iron toxicity in rice—Conditions and management concepts. J. Plant Nutr. Soil Sci. 2005, 168, 558–573. [Google Scholar] [CrossRef]
- Kicińska, A.; Wikar, J. Ecological risk associated with agricultural production in soils contaminated by the activities of the metal ore mining and processing industry—Example from southern Poland. Soil Tillage Res. 2021, 205, 104817. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Wang, L.; Li, H.; Zhang, S.; Yang, J.; Liu, N.; Han, X. Effects of long-term application of Cl-containing fertilizers on chloride content and acidification in brown soil. Sustainability 2023, 15, 8801. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Dąbkowska-Naskręt, H.; Jaworska, H.; Rydlewska, M. Effect of salinity on the mobility of trace metals in soils near a soda chemical factory. J. Elem. 2020, 25, 501–512. [Google Scholar] [CrossRef]
- Sipos, P.; Choib, C.; Németha, T.; Szalaic, Z.; Pókaa, T. Relationship between iron and trace metal fractionation in soils. Chem. Speciat. Bioavailab. 2014, 26, 21–30. [Google Scholar] [CrossRef]
- Fazekašová, D.; Fazekaš, J. Soil quality and heavy metal pollution assessment of iron ore mines in Nizna Slana (Slovakia). Sustainability 2020, 12, 2549. [Google Scholar] [CrossRef]
- Tarnawczyk, M.; Uzarowicz, Ł.; Perkowska-Pióro, K.; Pędziwiatr, A.; Kwasowski, W. Effect of land reclamation on soil properties, mineralogy and trace-element distribution and availability: The example of technosols developed on the tailing disposal site of an abandoned Zn and Pb mine. Minerals 2021, 11, 559. [Google Scholar] [CrossRef]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Soil–plant transfer of trace elements—An environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Xu, T.; Nan, F.; Jiang, X.; Tang, Y.; Zeng, Y.; Zhang, W.; Shi, B. Effect of soil pH on the transport, fractionation, and oxidation of chromium(III). Ecotoxicol. Environ. Saf. 2020, 195, 110459. [Google Scholar] [CrossRef]
- Neina, D. The Role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2022, 73, e13203. [Google Scholar] [CrossRef]
- Vargas, C.; Pérez-Esteban, J.; Escolástico, C.; Masaguer, A.; Moliner, A. Phytoremediation of Cu and Zn by vetiver grass in mine soils amended with humic acids. Environ. Sci. Pollut. Res. 2016, 23, 13521–13530. [Google Scholar] [CrossRef] [PubMed]
- Hattab, N.; Soubrand, M.; Guegan, R.; Motelica-Heino, M.; Bourrat, X.; Faure, O.; Bouchardon, J.L. Effect of organic amendments on the mobility of trace elements in phytoremediated techno-soils: Role of the humic substances. Environ. Sci. Pollut. Res. 2014, 21, 10470–10480. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z. The role of soil organic matter in trace element bioavailability and toxicity. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Fragouli, P.G.; Roulia, M.; Vassiliadis, A.A. Macromolecular Size and architecture of humic substances used in the Dyes’ adsorptive removal from water and soil. Agronomy 2023, 13, 2926. [Google Scholar] [CrossRef]
- Lado, L.R.; Hengl, T.; Reuter, H.I. Heavy metals in European soils: A geostatistical analysis of the FOREGS Geochemical database. Geoderma 2008, 148, 189–199. [Google Scholar] [CrossRef]
- Burlakovs, J.; Kļaviņš, M.; Osinska, L.; Purmalis, O. The impact of humic substances as remediation agents to the speciation forms of metals in soil. APCBEE Procedia 2013, 5, 192–196. [Google Scholar] [CrossRef]
- Dauletbay, A.; Serikbayev, B.A.; Kamysbayev, D.K.; Kudreeva, L.K. Interaction of metal ions with humic acids of brown coals of Kazakhstan. J. Exp. Nanosci. 2020, 15, 406–416. [Google Scholar] [CrossRef]
- Adusei-Gyamfi, J.; Ouddane, B.; Rietveld, L.; Cornard, J.P.; Criquet, J. Natural organic matter-cations complexation and its impact on water treatment: A critical review. Water Res. 2019, 160, 130–147. [Google Scholar] [CrossRef]
- Raj, A.; Mandal, J.; Kumari, P.B. Organo-metallic complex and its implications. J. Pharmacogn. Phytochem. 2020, 9, 491–500. [Google Scholar] [CrossRef]
- Kwiatkowska, J. The effect of organic amendments on the phytoavailability of heavy metals in polluted soil. Ecohydrol. Hydrobiol. 2006, 6, 181–186. [Google Scholar] [CrossRef]
- Ozkutlu, F.; Torun, B.; Cakmak, I. Effect of zinc humate on growth of soybean and wheat in zinc-deficient calcareous soil. Commun. Soil Sci. Plant Anal. 2006, 37, 2769–2778. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef]
- Pérez Esteban, J.; Escolastico, C.; Masaguer, A.; Vargas, C.; Moliner, A. Soluble organic carbon and pH of organic amendments affect metal mobility and chemical speciation in mine soils. Chemosphere 2014, 103, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Saha, L.; Bauddh, K. Phytomanagement of iron mine soil by Ricinus communis L. and garden soil. Chemosphere 2023, 313, 137534. [Google Scholar] [CrossRef]
- Kwiatkowska-Malina, J. Functions of organic matter in polluted soils: The effect of organic amendments on phytoavailability of heavy metals. Appl. Soil Ecol. 2018, 123, 542–545. [Google Scholar] [CrossRef]

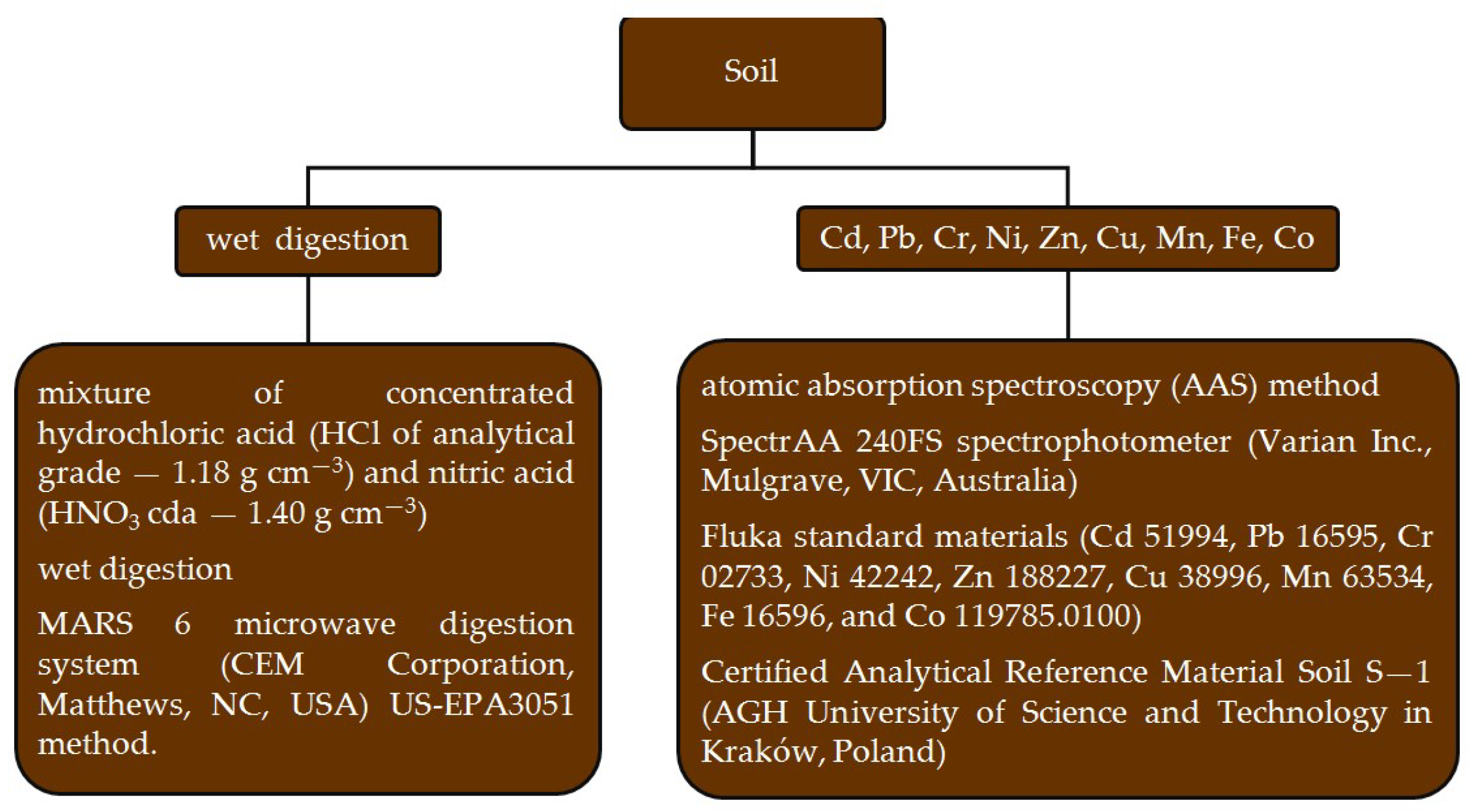
| Parameter | Content |
|---|---|
| pH value in 1 M KCl dm−3 | 6.51 |
| Cation exchange capacity—CEC (mmol + kg−1 DM) | 82.83 |
| Total organic carbon—TOC (g kg−1 DM) | 3.183 |
| Total nitrogen (g kg−1 DM) | 0.313 |
| Available form of (mg kg−1 DM): | |
| P | 128.8 |
| K | 112.0 |
| Mg | 49.55 |
| Total Fe (g kg−1 DM) | 10.46 |
| Total other TEs (mg kg−1 DM): | |
| Cd | 0.241 |
| Pb | 15.86 |
| Cr | 45.36 |
| Ni | 14,20 |
| Zn | 21.10 |
| Cu | 6.556 |
| Mn | 163.9 |
| Co | 2.081 |
| Fe Dose mg kg−1 of Soil | Humic Acids (HAs) Addition in g kg−1 of Soil | ||||
|---|---|---|---|---|---|
| 0 | 0.3 | 0.6 | 0.9 | Average | |
| Iron | |||||
| 0 | 10,549 a | 11,326 a–c | 11,918 cd | 12,241 c–e | 11,509 A |
| 250 | 10,751 ab | 12,037 c–e | 12,287 c–e | 12,627 de | 11,926 B |
| 500 | 11,754 b–d | 12,142 c–e | 12,768 de | 12,721 de | 12,346 C |
| 750 | 12,064 c–e | 12,641 de | 13,089 e | 12,732 de | 12,632 C |
| Average | 11,280 A | 12,037 B | 12,516 B | 12,580 C | 12,103 |
| r | 0.965 | 0.965 | 0.997 | 0.876 | 0.996 |
| Cadmium | |||||
| 0 | 0.229 d | 0.183 a–d | 0.127 a–b | 0.121 a | 0.165 A |
| 250 | 0.225 d | 0.187 a–d | 0.117 a | 0.157 a–d | 0.172 A |
| 500 | 0.203 cd | 0.183 a–d | 0.135 a–c | 0.199 b–d | 0.180 A |
| 750 | 0.117 a | 0.211 d | 0.155 a–d | 0.189 a–d | 0.168 A |
| Average | 0.194 B | 0.191 B | 0.134 A | 0.167 B | 0.171 |
| r | −0.884 | 0.767 | 0.817 | 0.902 | 0.348 |
| Lead | |||||
| 0 | 9.05 a–b | 10.60 b–e | 12.09 c–f | 12.95 e–g | 11.17 C |
| 250 | 10.49 b–e | 12.57 d–g | 14.91 gh | 16.69 h | 13.67 D |
| 500 | 6.69 a | 8.86 a–b | 10.08 b–d | 13.63 f–g | 9.82 B |
| 750 | 6.39 a | 6.38 a | 9.47 bc | 11.35 b–f | 8.40 A |
| Average | 8.16 A | 9.60 B | 11.64 C | 13.66 D | 10.76 |
| r | −0.776 | −0.804 | −0.668 | −0.453 | −0.701 |
| Fe Dose mg kg−1 of Soil | HAs Addition in g kg−1 of Soil | ||||
|---|---|---|---|---|---|
| 0 | 0.3 | 0.6 | 0.9 | Average | |
| Chromium | |||||
| 0 | 54.34 a–d | 50.70 a–c | 54.71 a–d | 56.96 b–d | 54.18 A |
| 250 | 54.30 a–d | 55.36 b–d | 56.96 b–d | 55.47 b–d | 55.52 A |
| 500 | 53.00 a–d | 55.54 b–d | 61.25 c–d | 52.68 a–c | 55.62 A |
| 750 | 47.31 a–b | 54.81 b–d | 63.81 d | 43.66 a | 52.40 A |
| Average | 52.24 A | 54.10 A | 59.18 B | 52.19 A | 54.43 |
| r | −0.865 | 0.705 | 0.993 | −0.925 | −0.450 |
| Nickel | |||||
| 0 | 7.66 a | 13.84 c–d | 13.97 c–d | 15.07 c–e | 12.64 A |
| 250 | 9.24 a–b | 15.44 c–f | 14.00 c–d | 17.04 d–f | 13.93 A |
| 500 | 14.22 c–e | 15.62 d–f | 16.88 d–f | 19.01 f | 16.43 B |
| 750 | 11.84 bc | 15.76 d–f | 16.65 d–f | 17.88 ef | 15.53 B |
| Average | 10.74 A | 15.17 B | 15.38 B | 17.25 C | 14.63 |
| r | 0.783 | 0.859 | 0.877 | 0.808 | 0.857 |
| Zinc | |||||
| 0 | 20.59 a–e | 17.50 ab | 22.13 c–e | 23.83 d–f | 21.01 B |
| 250 | 21.65 b–e | 16.93 a | 26.67 f | 21.48 b–e | 21.68 B |
| 500 | 24.23 ef | 18.91 a–c | 20.19 a–e | 17.87 a–c | 20.30 B |
| 750 | 18.46 a–c | 18.65 a–c | 19.67 a–d | 17.84 a–c | 18.66 A |
| Average | 21.23 BC | 18.00 A | 22.17 C | 20.26 B | 20.41 |
| r | −0.205 | 0.747 | −0.562 | −0.950 | −0.839 |
| Fe Dose mg kg−1 of Soil | HAs Addition in g kg−1 of Soil | ||||
|---|---|---|---|---|---|
| 0 | 0.3 | 0.6 | 0.9 | Average | |
| Copper | |||||
| 0 | 5.331 a | 5.444 a–c | 5.859 a–c | 5.919 a–c | 5.638 A |
| 250 | 5.339 a | 5.912 a–c | 6.538 a–c | 6.070 a–c | 5.965 A |
| 500 | 6.198 bc | 6.032 a–c | 6.666 a–c | 6.153 a–c | 6.262 A |
| 750 | 6.108 a–c | 6.439 a–c | 6.688 a–c | 6.153 a–c | 6.347 A |
| Average | 5.744 A | 5.957 A | 6.438 A | 6.074 A | 6.053 |
| r | 0.869 | 0.979 | 0.862 | 0.918 | 0.973 |
| Manganese | |||||
| 0 | 155.5 a–c | 153.2 ab | 145.3 a | 170.2 a–c | 156.1 A |
| 250 | 163.4 a–c | 160.7 a–c | 180.1 bc | 174.7 bc | 169.7 B |
| 500 | 163.5 a–c | 168.6 a–c | 180.1 bc | 175.7 bc | 172.0 B |
| 750 | 165.2 a–c | 172.2 a–c | 181.8 c | 176.0 bc | 173.8 B |
| Average | 161.9 A | 163.7 A | 171.8 AB | 174.2 B | 167.9 |
| r | 0.867 | 0.989 | 0.799 | 0.883 | 0.888 |
| Cobalt | |||||
| 0 | 2.006 a | 2.018 a–c | 3.217 cd | 3.283 c–e | 2.631 A |
| 250 | 2.250 ab | 2.041 c–e | 3.225 c–e | 3.335 de | 2.713 B |
| 500 | 2.302 b–d | 2.409 c–e | 3.891 de | 3.582 de | 3.046 C |
| 750 | 2.661 c–e | 2.753 de | 4.181 e | 4.126 de | 3.430 C |
| Average | 2.305 A | 2.305 B | 3.629 B | 3.582 C | 2.955 |
| r | 0.963 | 0.954 | 0.947 | 0.929 | 0.968 |
| Variable | Fe | Cd | Pb | Cr | Ni | Zn | Cu | Mn |
|---|---|---|---|---|---|---|---|---|
| Cd | −0.421 ** | |||||||
| Pb | 0.191 | −0.249 | ||||||
| Cr | 0.160 | −0.119 | 0.149 | |||||
| Ni | 0.792 ** | −0.202 | 0.306 * | 0.074 | ||||
| Zn | −0.105 | −0.305 * | 0.215 | 0.321 * | −0.183 | |||
| Cu | 0.700 ** | −0.336 * | −0.056 | 0.215 | 0.530 ** | 0.026 | ||
| Mn | 0.640 ** | −0.211 | 0.119 | 0.163 | 0.393 ** | 0.132 | 0.541 ** | |
| Co | 0.646 ** | −0.325 * | 0.267 | 0.138 | 0.586 ** | 0.084 | 0.559 ** | 0.494 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszkowski, M.; Kordala, N. The Role of Organic Materials in Shaping the Content of Trace Elements in Iron-Contaminated Soil. Materials 2025, 18, 1522. https://doi.org/10.3390/ma18071522
Wyszkowski M, Kordala N. The Role of Organic Materials in Shaping the Content of Trace Elements in Iron-Contaminated Soil. Materials. 2025; 18(7):1522. https://doi.org/10.3390/ma18071522
Chicago/Turabian StyleWyszkowski, Mirosław, and Natalia Kordala. 2025. "The Role of Organic Materials in Shaping the Content of Trace Elements in Iron-Contaminated Soil" Materials 18, no. 7: 1522. https://doi.org/10.3390/ma18071522
APA StyleWyszkowski, M., & Kordala, N. (2025). The Role of Organic Materials in Shaping the Content of Trace Elements in Iron-Contaminated Soil. Materials, 18(7), 1522. https://doi.org/10.3390/ma18071522






