Friction and Wear Performances and Mechanisms of Graphite/Copper Composites Under Electrical Contact in Marine Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Microstructure and Hardness
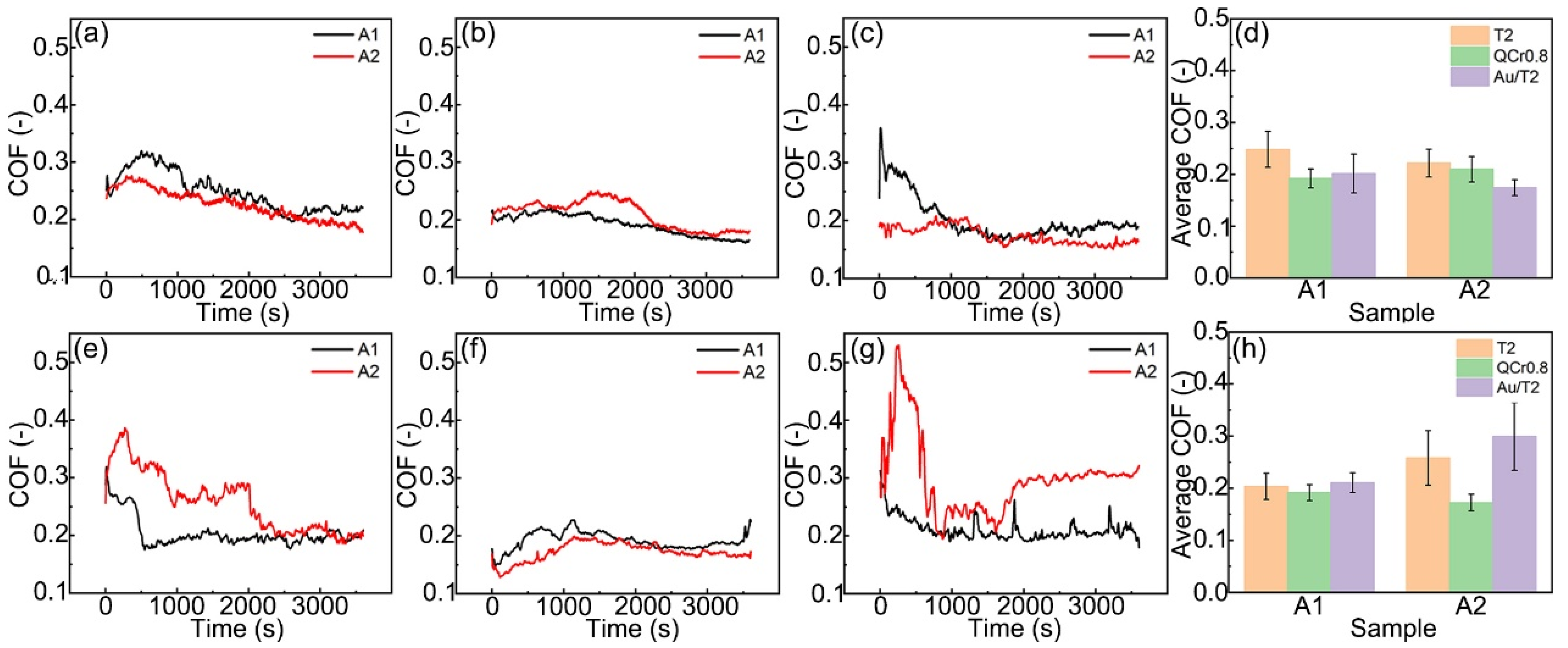
3.2. Friction Coefficient
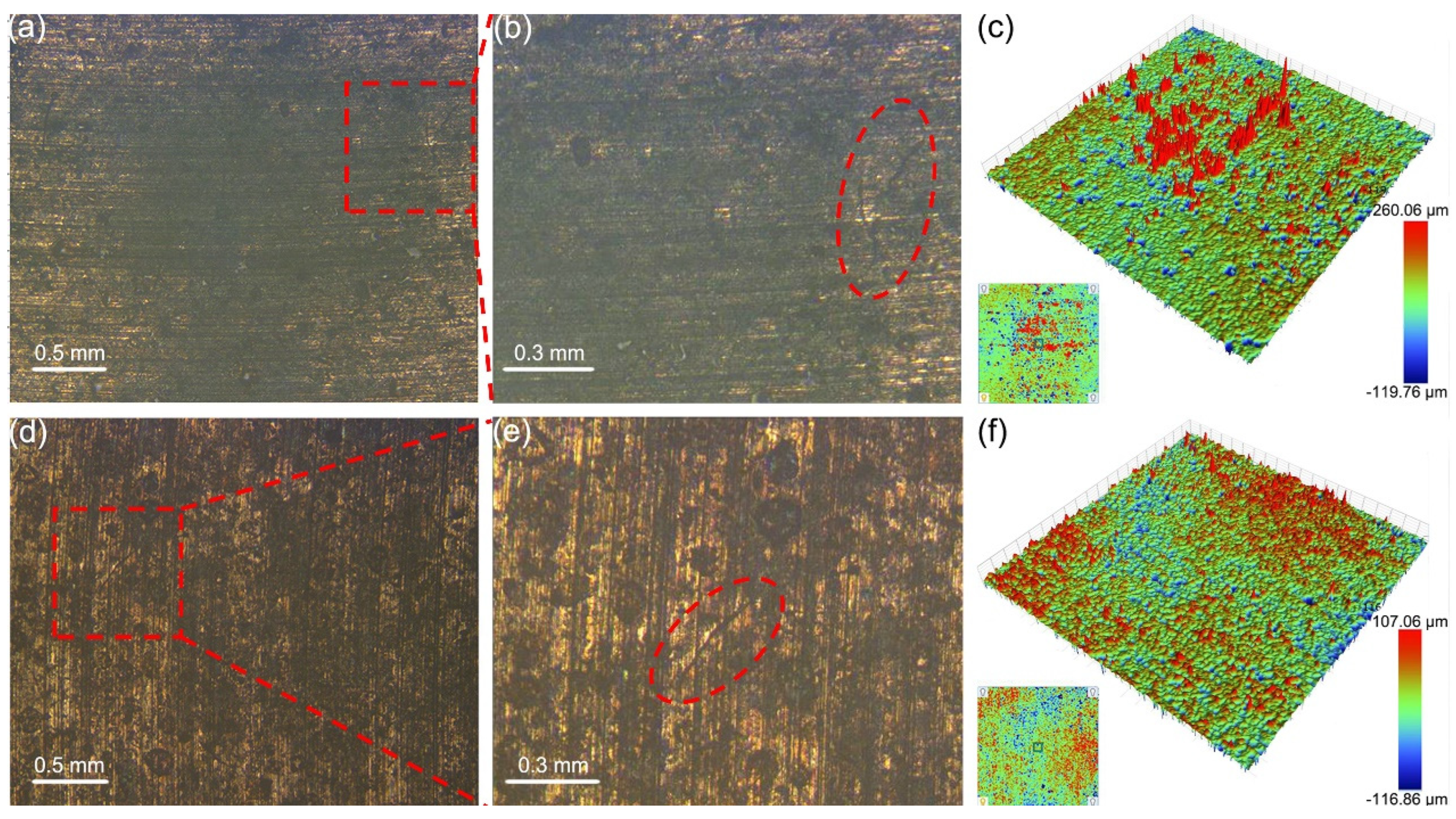
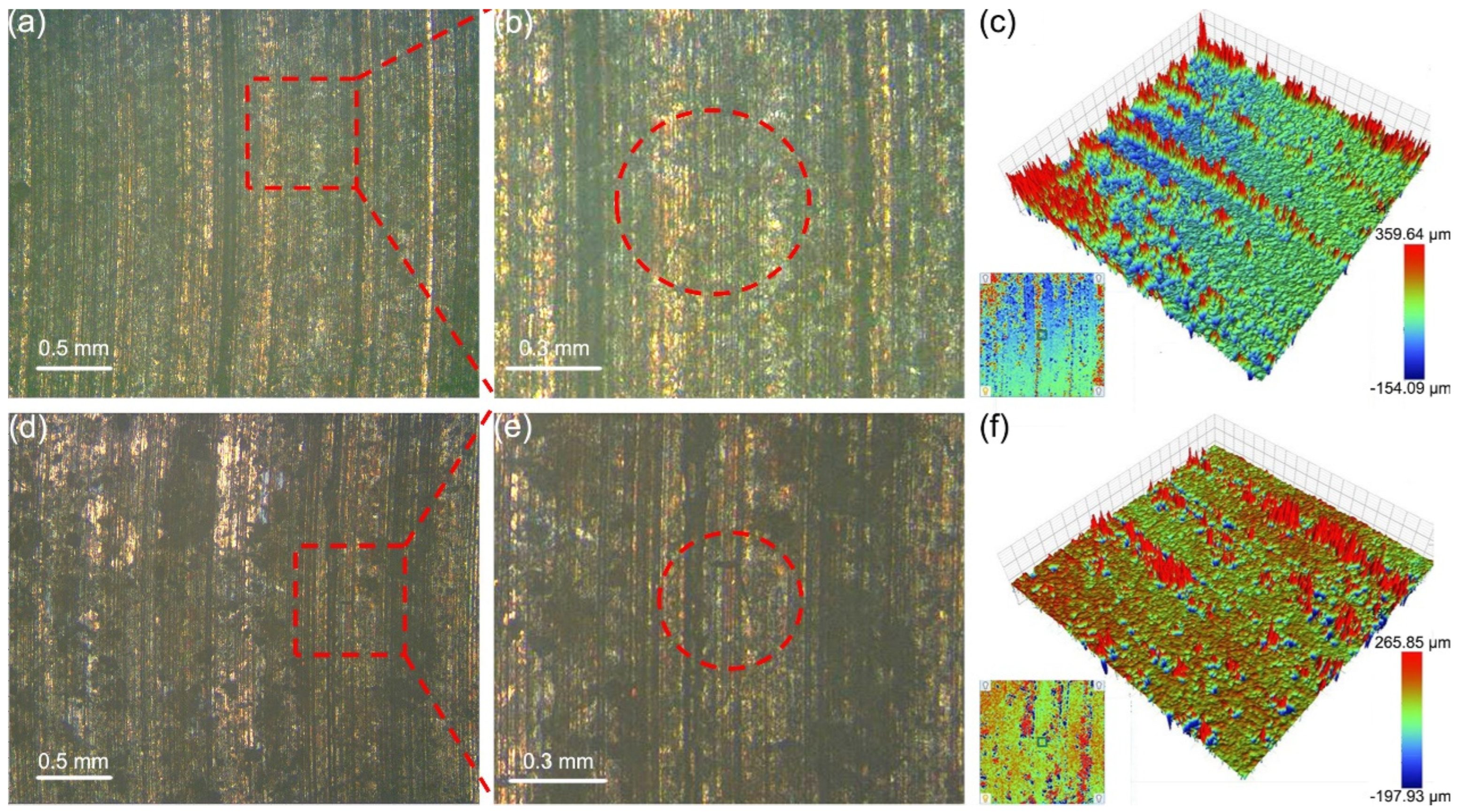
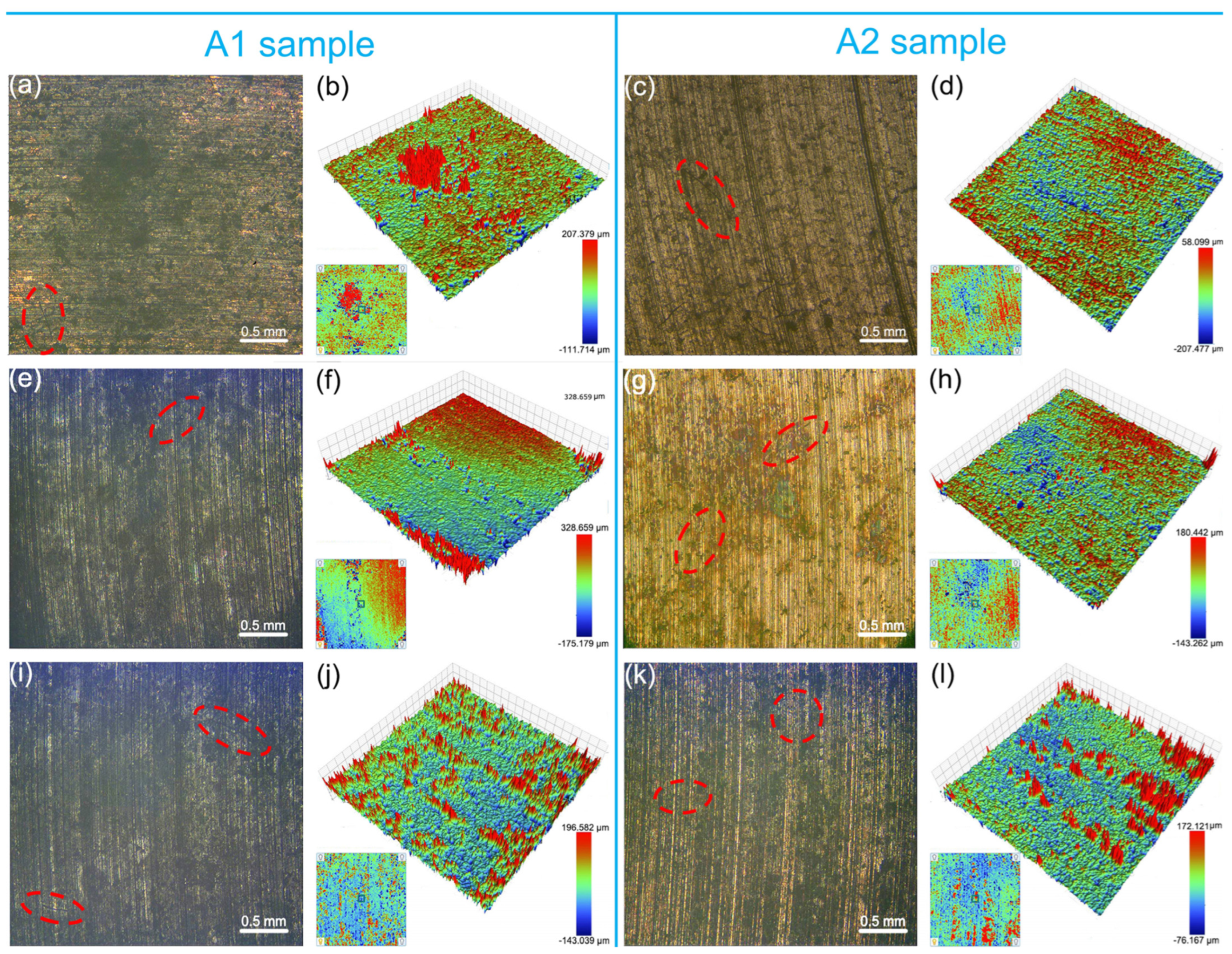
3.3. Macroscopic Wear Morphology and Wear Rate
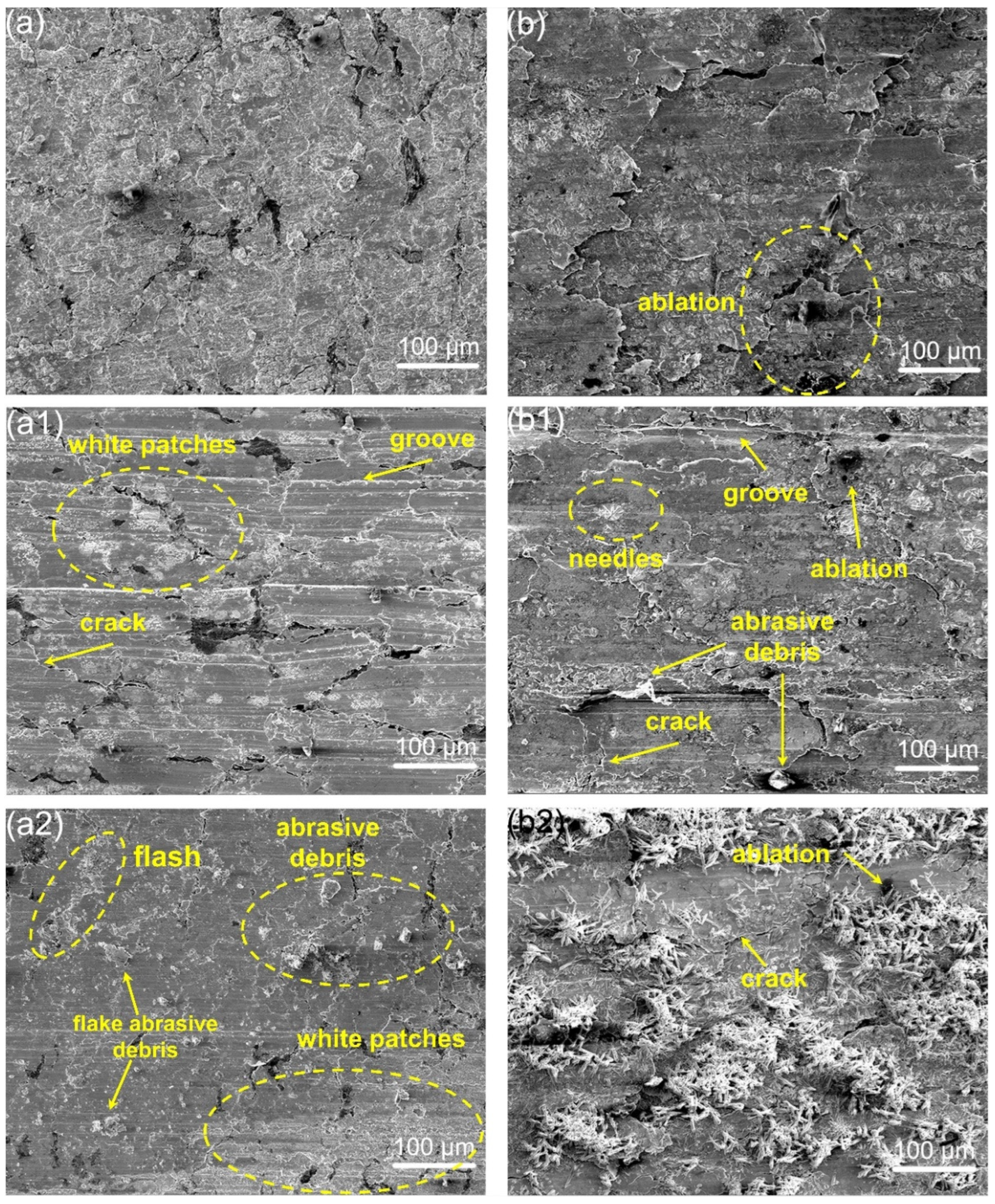
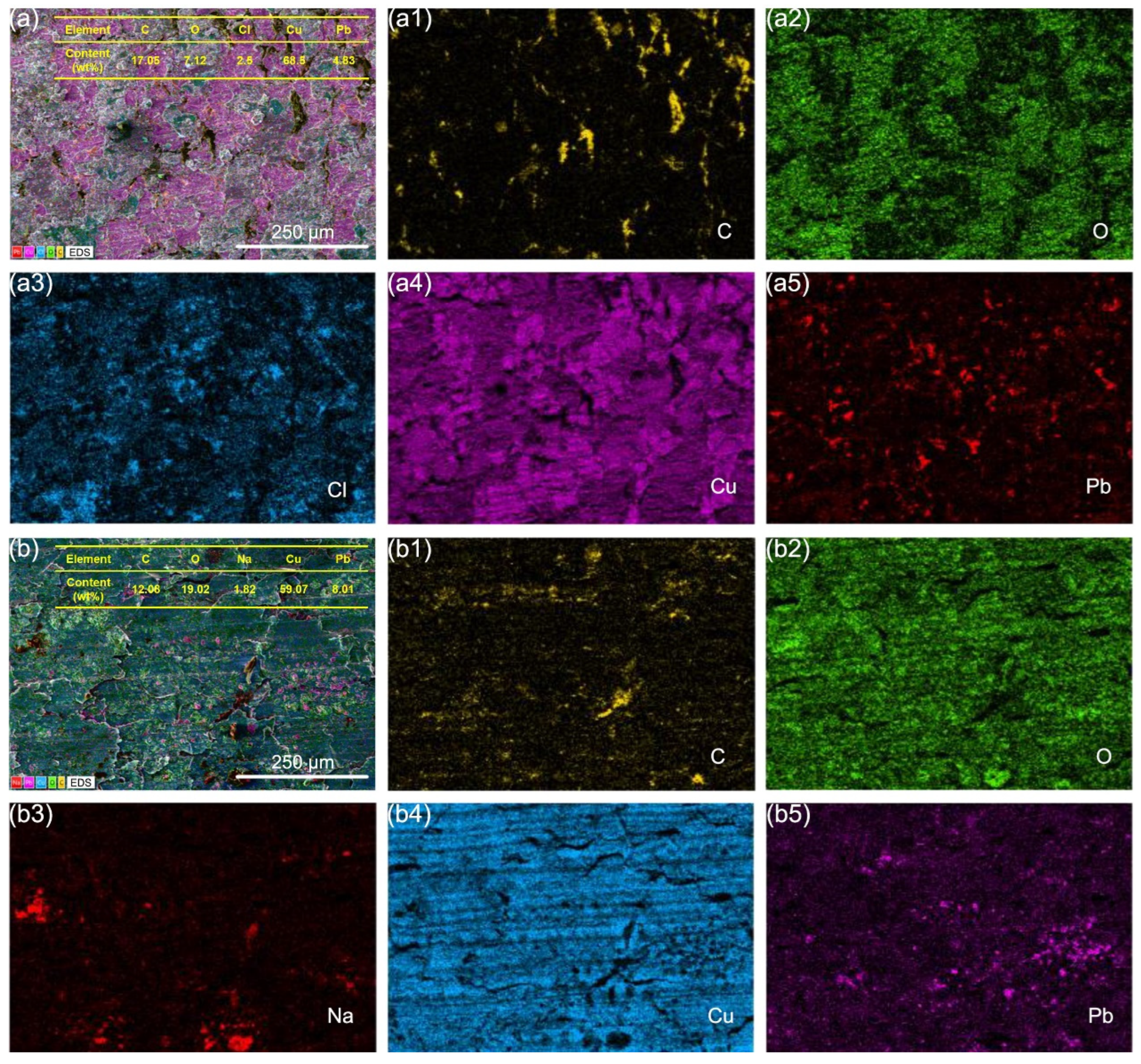
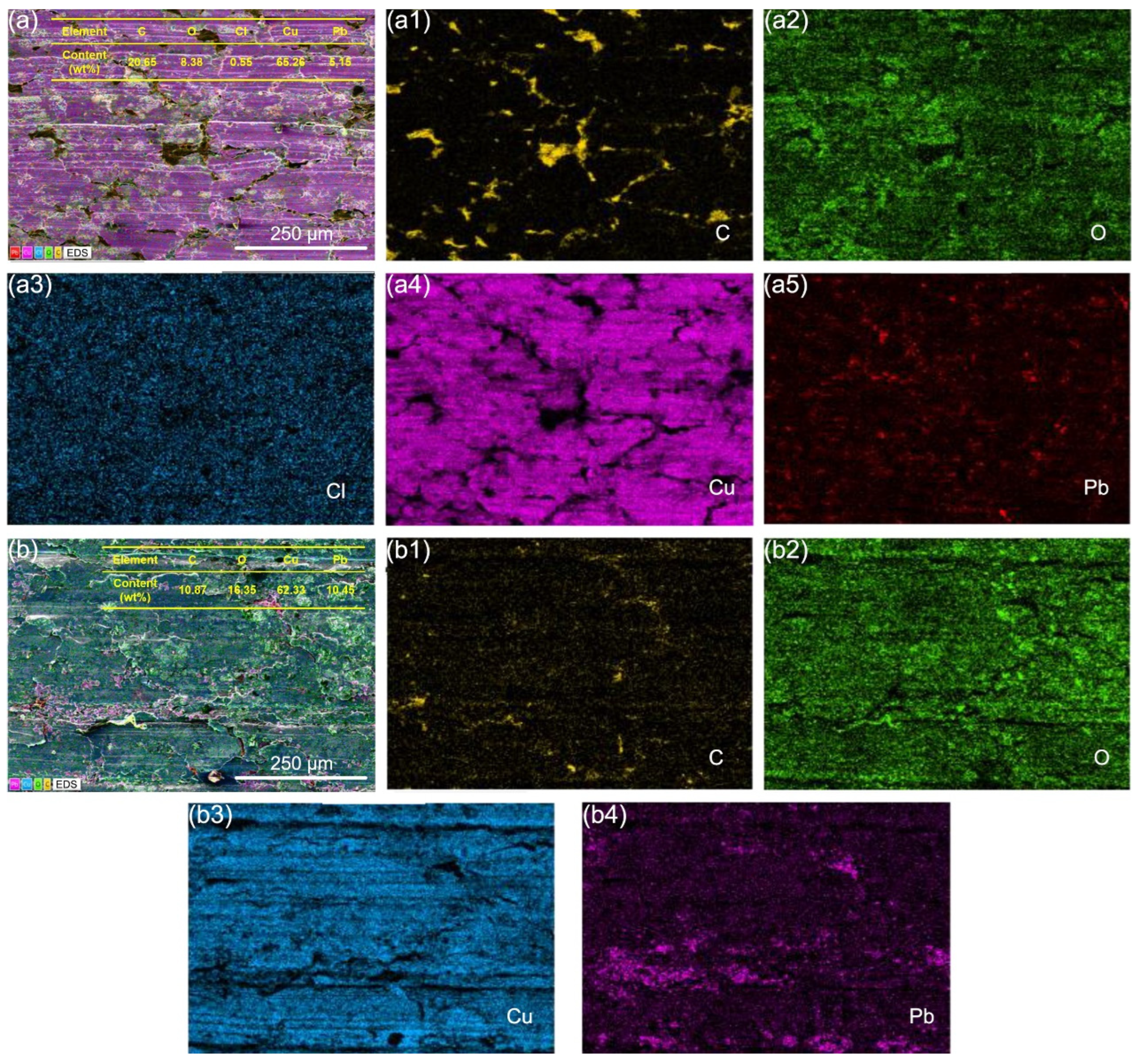
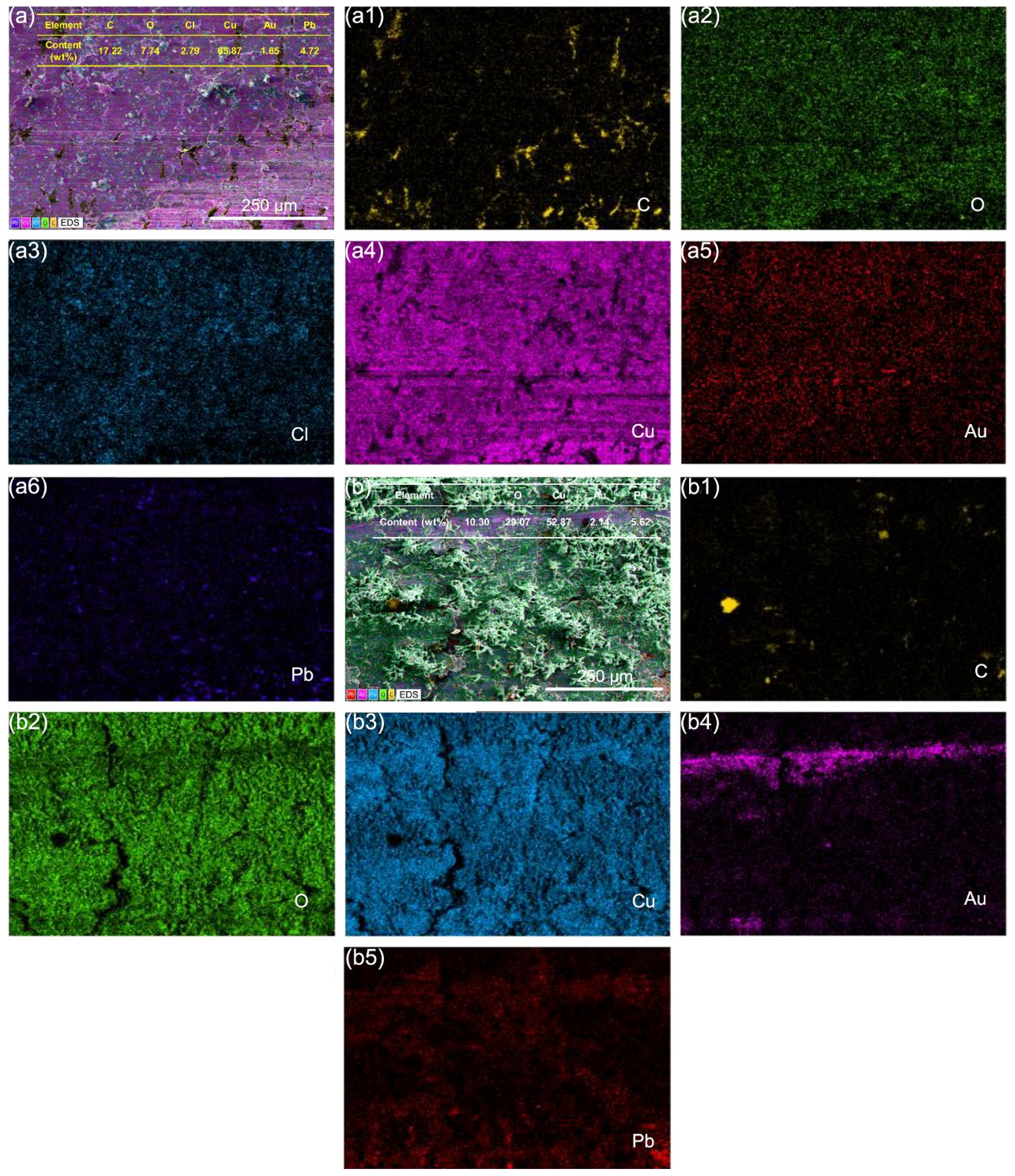
3.4. Microscopic Wear Morphology and Wear Mechanism
4. Application Recommendation
5. Conclusions
- (1)
- Two graphite/copper composites, differing in graphite content, displayed similar friction coefficients (ranging from 0.2 to 0.3) during the final stage of the friction process in both C1 and C2 conditions.
- (2)
- With the increase in counterpart material hardness, the interaction at the contact interface intensified, resulting in more plastic deformation on the surfaces of the graphite/copper samples, which in turn elevated the surface roughness. Meanwhile, graphite/copper composites of less graphite exhibited lower surface roughness due to higher hardness.
- (3)
- Under C1 and C2 conditions, both graphite/copper samples exhibited a negative wear rate, suggesting that the corrosive products generated during friction tests lead to an increase in the mass of the worn samples instead of decrease.
- (4)
- Under C1 conditions, the composite primarily underwent abrasive wear, while adhesive wear intensifies when grounded with harder Au/T2. Under C2 conditions, delamination wear and arc ablation became predominant, and minor amount of abrasive wear was also present. Similarly, adhesive wear arose when the composite was grounded with Au/T2. Furthermore, corrosive wear, particularly oxidative wear, was observed on the worn surfaces of all specimens.
- (5)
- In a seawater context, the electricity factor not only induced electrical damage to the graphite/copper surface but also aggravated corrosion, leading to an increased oxygen concentration on the wear surface while suppressing the formation of chlorine-containing corrosive substances.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.A.; Thompson, K.F.; Johnston, P.; Santillo, D. An overview of seabed mining including the current state of development, environmental impacts, and knowledge gaps. Front. Mar. Sci. 2018, 4, 418. [Google Scholar] [CrossRef]
- Pendleton, L.; Evans, K.; Visbeck, M. We need a global movement to transform ocean science for a better world. Proc. Natl. Acad. Sci. USA 2020, 117, 9652–9655. [Google Scholar] [CrossRef] [PubMed]
- Constant, C.; Kotarbinski, M.; Stefek, J.; Green, R.; DeGeorge, E.; Baring-Gould, I. Accelerating ocean-based renewable energy educational opportunities to achieve a clean energy future. Prog. Energy 2021, 3, 042002. [Google Scholar] [CrossRef]
- Zheng, Z.; Xie, J.; Liang, P.; Liu, J. Study on measurement technology and influencing factors of atmospheric salt fog concentration at the seaside—A case study of sanya, China. Asia-Pac. J. Atmos. Sci. 2023, 59, 311–326. [Google Scholar] [CrossRef]
- Cole, I.S.; Paterson, D.A. Possible effects of climate change on atmospheric corrosion in Australia. Corros. Eng. Sci. Technol. 2013, 45, 19–26. [Google Scholar] [CrossRef]
- Wood, R.J.K. Marine wear and tribocorrosion. Wear 2017, 376–377, 893–910. [Google Scholar] [CrossRef]
- Chen, Z.; He, X.; Xiao, C.; Kim, S. Effect of humidity on friction and wear—A critical review. Lubricants 2018, 6, 74. [Google Scholar] [CrossRef]
- Drach, A.; Tsukrov, I.; DeCew, J.; Aufrecht, J.; Grohbauer, A.; Hofmann, U. Field studies of corrosion behaviour of copper alloys in natural seawater. Corros. Sci. 2013, 76, 453–464. [Google Scholar] [CrossRef]
- Lv, W.Y.; Zheng, K.Q.; Wang, Y.B.; Liu, Z.W. Influence of electric current on the corrosion behavior of Cu for the electric contact in the isolation switch. Mater. Corros. 2019, 70, 1900–1906. [Google Scholar] [CrossRef]
- Rohatgi, P.K.; Ray, S.; Liu, Y. Tribological properties of metal matrix graphite particle composites. Int. Mater. Rev. 1992, 37, 129–149. [Google Scholar] [CrossRef]
- Bai, L.; Ge, Y.; Zhu, L.; Chen, Y.; Yi, M. Preparation and properties of copper-plated expanded graphite/copper composites. Tribol. Int. 2021, 161, 107094. [Google Scholar]
- Song, Y.; Yang, Z.; Jiao, J.; Li, W.; Zhao, M.; Shangguan, B.; Zhang, Y. Research on damage optimization of copper-carbon gradient composite materials for current-carrying friction. Tribol. Int. 2024, 195, 109635. [Google Scholar] [CrossRef]
- Opalek, A.; Emmer, S.; Cicka, R.; Beronska, N.; Oslanec, P., Jr.; Kovacik, J. Structure and thermal expansion of Cu-90 vol. % graphite composites. Materials 2021, 14, 7089. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ankit; Majhi, M.R.; Chaturvedi, R.K. Wear and friction behaviour of Cu-Gr-B4C composites and correlating to the topographical characteristics. Surf. Topogr. Metrol. Prop. 2024, 12, 015012. [Google Scholar]
- Li, H.; Liu, Y.; Zheng, B.; Wang, S.; Yi, Y.; Zhang, Y.; Li, W. On the tribological behaviors of Cu matrix composites with different Cu-coated graphite content. J. Mater. Res. Technol. 2023, 25, 83–94. [Google Scholar] [CrossRef]
- Liu, X.L.; Cai, Z.B.; Xiao, Q.; Shen, M.X.; Yang, W.B.; Chen, D.Y. Fretting wear behavior of brass/copper-graphite composites as a contactor material under electrical contact. Int. J. Mech. Sci. 2020, 184, 105703. [Google Scholar]
- Yang, Z.; Ge, Y.; Xu, Z.; Bao, S.; Zhang, Y.; Zhang, J. Effect of carbon content on friction and wear properties of copper matrix composites at high speed current-carrying. Materials 2019, 12, 2881. [Google Scholar] [CrossRef]
- Yang, Z.; Ge, Y.; Zhang, X.; Bao, S.; Zhang, Y.; Wang, Y. Effect of particle size on current-carrying friction and wear properties of copper-graphite composites by spark plasma sintering. Materials 2019, 12, 2825. [Google Scholar] [CrossRef]
- Grandin, M.; Wiklund, U. Wear phenomena and tribofilm formation of copper/copper-graphite sliding electrical contact materials. Wear 2018, 398, 227–235. [Google Scholar]
- Xu, E.; Huang, J.; Li, Y.; Zhu, Z.; Cheng, M.; Li, D.; Zhong, H.; Liu, J.; Jiang, Y. Graphite cluster/copper-based powder metallurgy composite for pantograph slider with well-behaved mechanical and wear performance. Powder Technol. 2019, 344, 551–560. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, J.K.; Xiao, S.X.; Xu, G.M.; Chen, J.; Zhang, C. Effect of electrical current on the tribological property of Cu-graphite brush. Tribol. Lett. 2024, 72, 29. [Google Scholar]
- Gan, Z.Y.; Liu, Y.; Lü, X.Q.; Tan, Y.N.; Zou, J.P. Lubrication mechanism of copper-based solid self-lubricating materials by lead. Mater. Sci. Eng. Powder Metall. 2018, 23, 347–353. [Google Scholar]
- Li, Y.S.; Zhang, K.; Zhang, G.H.; Xu, C.F.; Hu, Z.; Wang, Y.F. Heat resistance and friction properties of copper-based friction materials with different graphite content. Powder Metall. Ind. 2023, 33, 68–73. [Google Scholar]
- Kumar, D.; Bharti, A.; Azam, S.M.; Kumar, N.; Tripathi, H. Investigations of mechanical properties of copper matrix hybrid composite. In Advances in Mechanical Engineering, International Conference on Recent Innovations and Developments in Mechanical Engineering (ICRIDME), Shillong, India, 8–10 November 2018; Springer: Singapore; Natl. Inst. Technol. Meghalaya: Shillong, India, 2020; pp. 671–676. [Google Scholar]
- Hu, J.; Li, H.; Li, J.; Huang, J.; Kong, J.; Zhu, H.; Xiong, D. Structure, mechanical and tribological properties of TaCx composite films with different graphite powers. J. Alloys Compd. 2020, 832, 153769. [Google Scholar]
- Escherova, J.; Krbata, M.; Kohutiar, M.; Barenyi, I.; Chochlikova, H.; Eckert, M.; Jus, M.; Majersky, J.; Janik, R.; Dubcova, P. The influence of Q & T heat treatment on the change of tribological properties of powder tool steels ASP2017, ASP2055 and their comparison with steel X153CrMoV12. Materials 2024, 17, 974. [Google Scholar] [CrossRef]
- Kohutiar, M.; Krbata, M.; Escherova, J.; Eckert, M.; Mikus, P.; Jus, M.; Polasek, M.; Janik, R.; Dubec, A. The influence of the geometry of movement during the friction process on the change in the tribological properties of 30CrNiMo8 steel in contact with a G40 steel ball. Materials 2023, 17, 127. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, J.; Culliton, D.; Melentiev, R.; Fang, F. Tribological study on the surface modification of metal-on-polymer bioimplants. Front. Mech. Eng. 2022, 17, 26. [Google Scholar]



| Material ID | C (wt.%) | Pb (wt.%) | Cu (wt.%) |
|---|---|---|---|
| A1 | 45 | 3 | 52 |
| A2 | 30 | 3 | 67 |
| Operation Condition | Voltage (V) |
|---|---|
| C1 | 0 |
| C2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Wang, C.; Xu, W.; Cheng, W.; Wu, H.; Li, H. Friction and Wear Performances and Mechanisms of Graphite/Copper Composites Under Electrical Contact in Marine Environments. Materials 2025, 18, 1516. https://doi.org/10.3390/ma18071516
Wang N, Wang C, Xu W, Cheng W, Wu H, Li H. Friction and Wear Performances and Mechanisms of Graphite/Copper Composites Under Electrical Contact in Marine Environments. Materials. 2025; 18(7):1516. https://doi.org/10.3390/ma18071516
Chicago/Turabian StyleWang, Nenghui, Chuanfeng Wang, Wenhu Xu, Weiping Cheng, Haihong Wu, and Hongsheng Li. 2025. "Friction and Wear Performances and Mechanisms of Graphite/Copper Composites Under Electrical Contact in Marine Environments" Materials 18, no. 7: 1516. https://doi.org/10.3390/ma18071516
APA StyleWang, N., Wang, C., Xu, W., Cheng, W., Wu, H., & Li, H. (2025). Friction and Wear Performances and Mechanisms of Graphite/Copper Composites Under Electrical Contact in Marine Environments. Materials, 18(7), 1516. https://doi.org/10.3390/ma18071516





