Study on Mechanical Properties and Hydration Characteristics of Bauxite-GGBFS Alkali-Activated Materials, Based on Composite Alkali Activator and Response Surface Method
Abstract
1. Introduction
1.1. Research Background
1.2. Literature Review
1.2.1. Exploration of Hydration Activation Enhancement Methods
1.2.2. Multivariate Analysis
2. Materials and Methods
2.1. Materials
2.2. Response Surface Experimental Method
2.3. Sample Preparation
2.4. Test Method
2.4.1. Workability Analysis Method
2.4.2. Mechanical Performance Analysis Method
2.4.3. Analysis of Hydration Products
2.4.4. Microstructural Analysis of Hydration Products
3. Results and Analysis
3.1. Response Surface Modeling and Optimization
3.1.1. Modeling Regression
3.1.2. Model Applicability and Significance Analysis
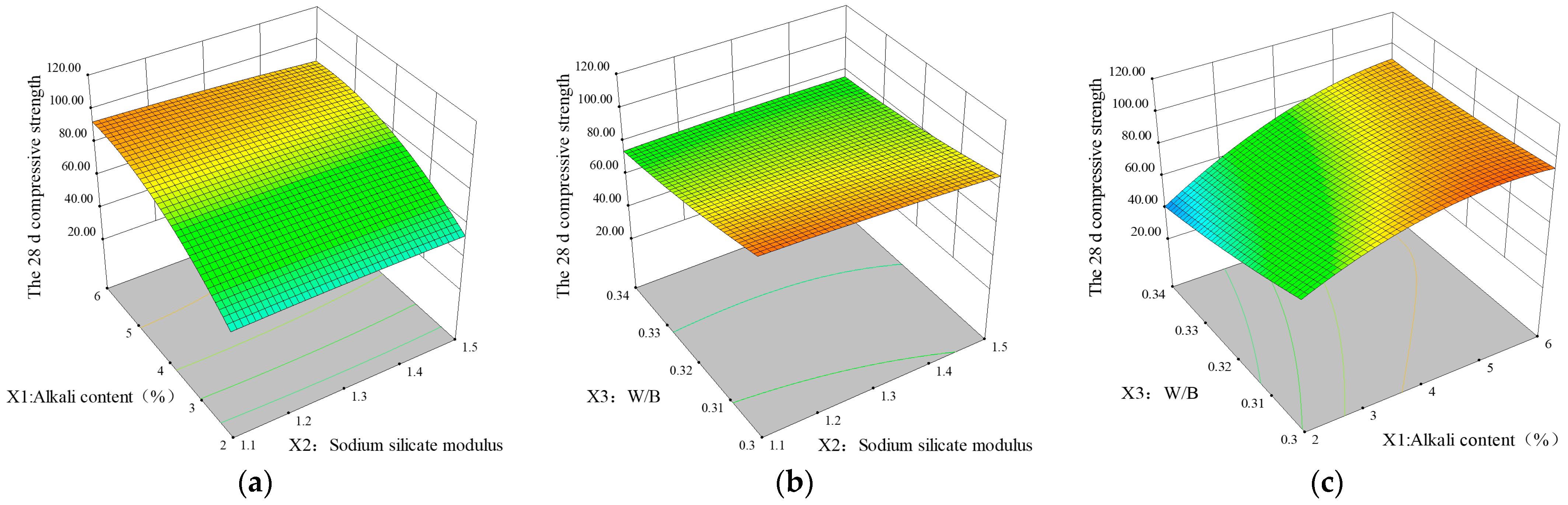
3.1.3. Corresponding Surface Analysis
3.1.4. Model Validation and Optimization Results
3.2. Macroscopic Performance Analysis
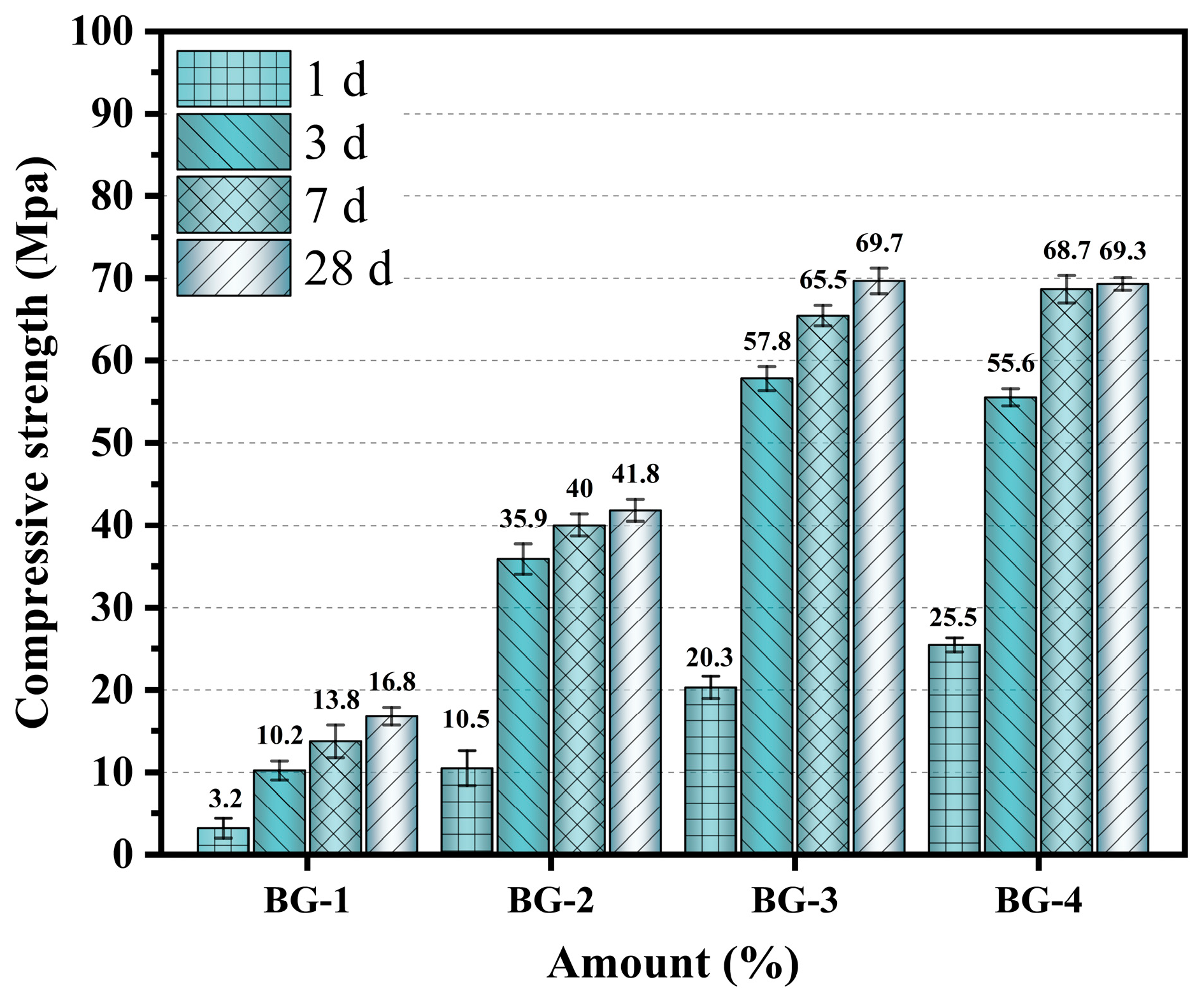
3.2.1. Mechanical Properties
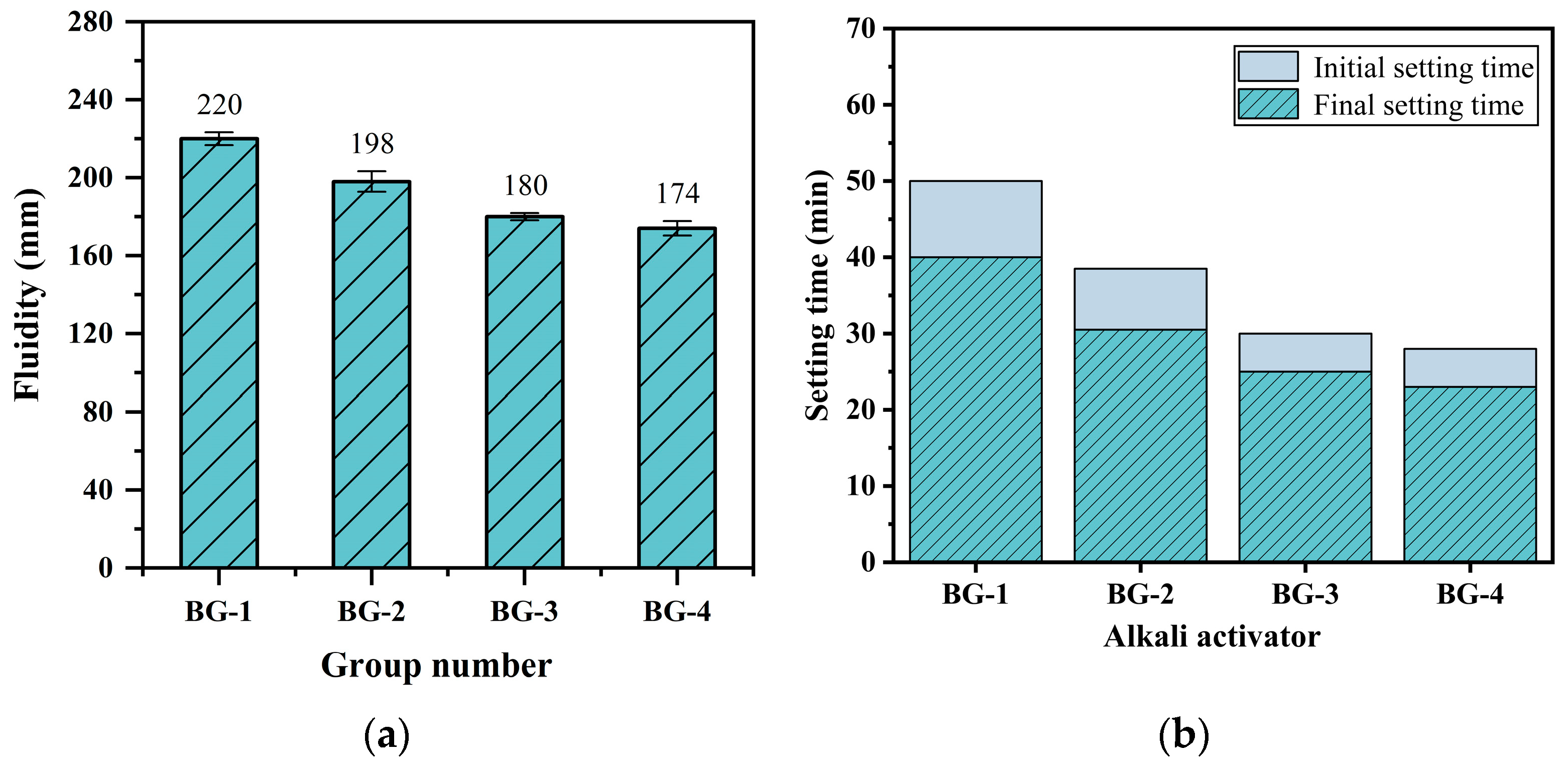
3.2.2. Flowability and Setting Time
3.3. Hydration Product Analysis
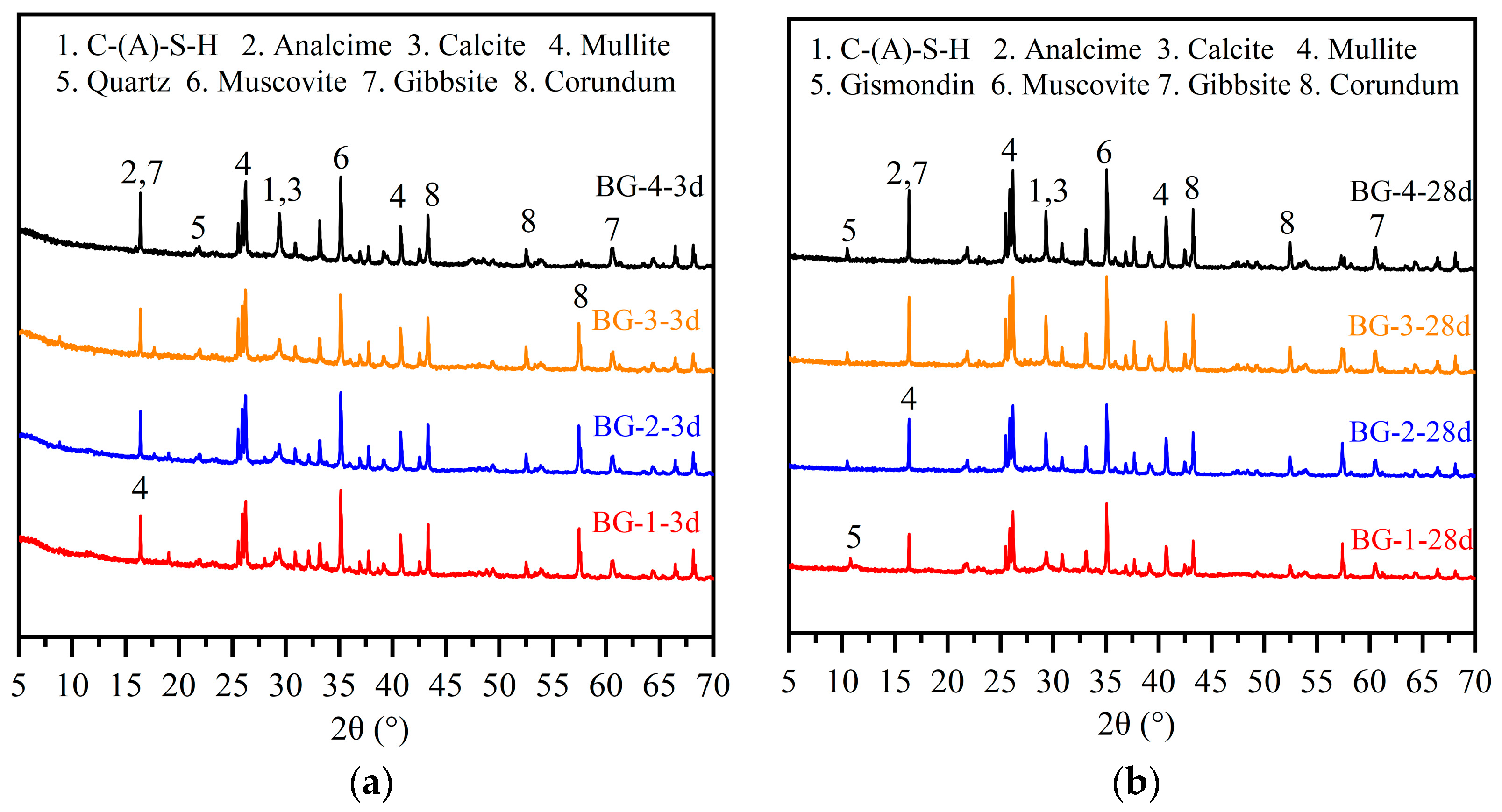
3.3.1. XRD Analysis
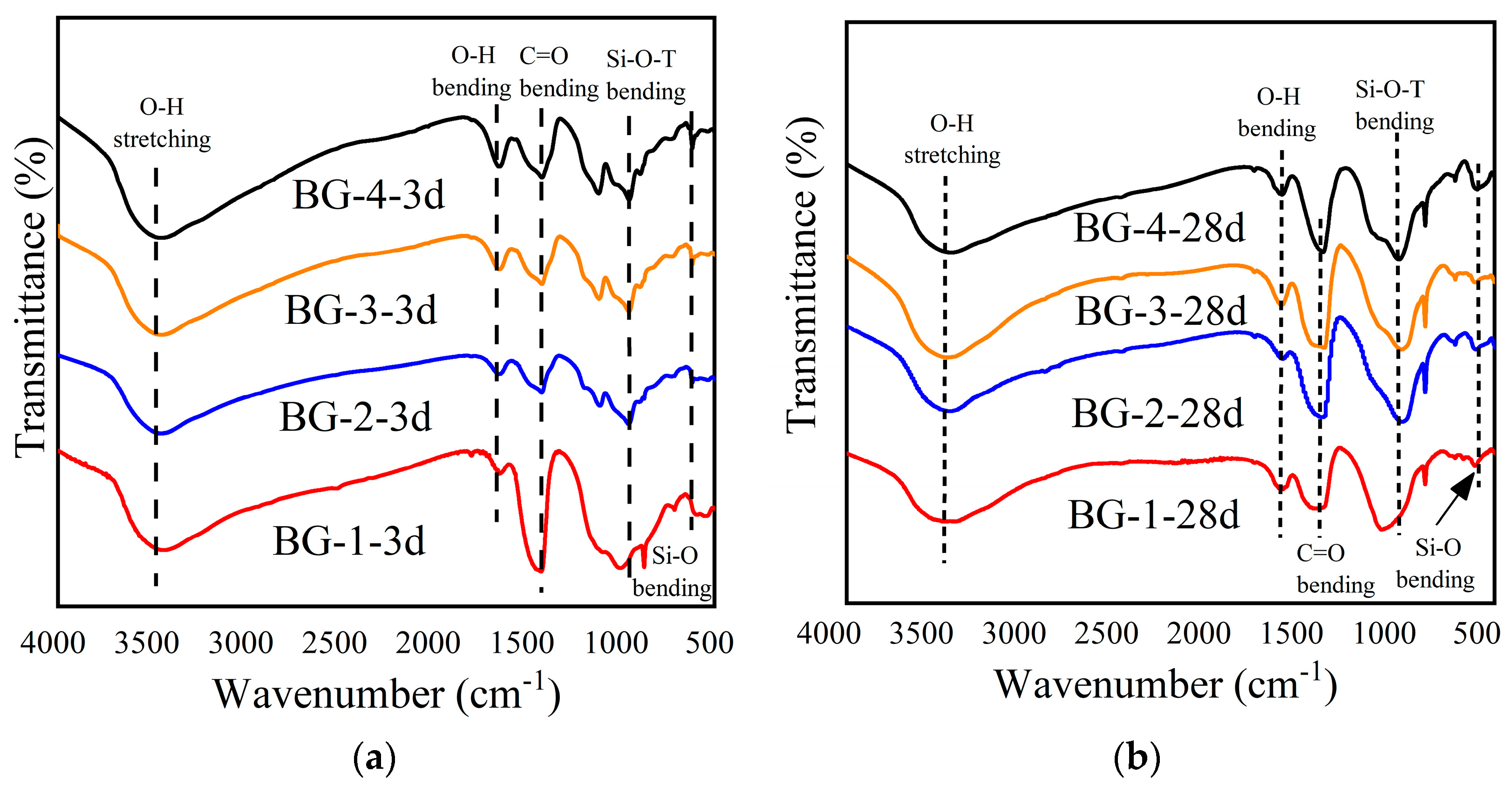
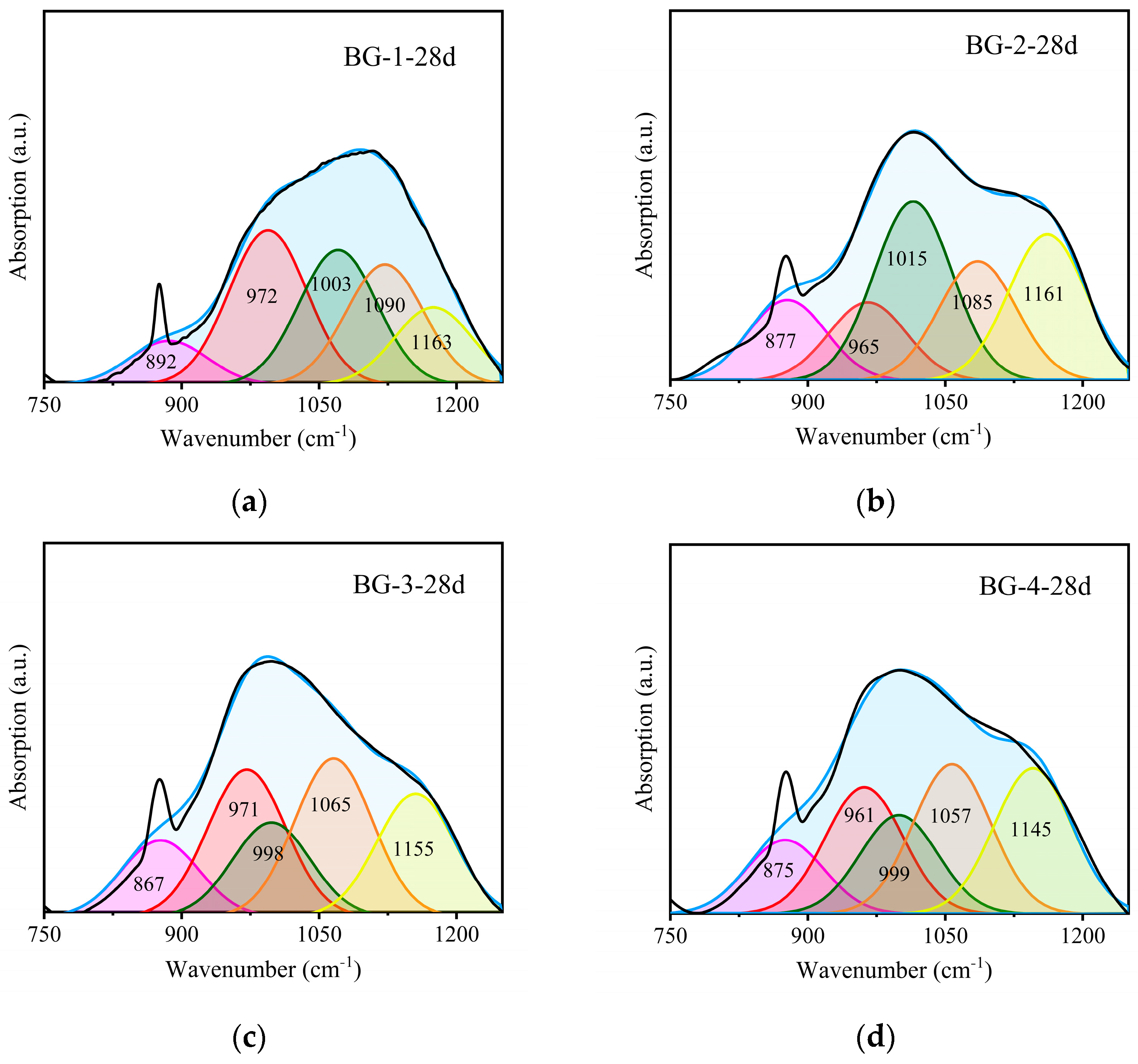
3.3.2. FTIR Analysis
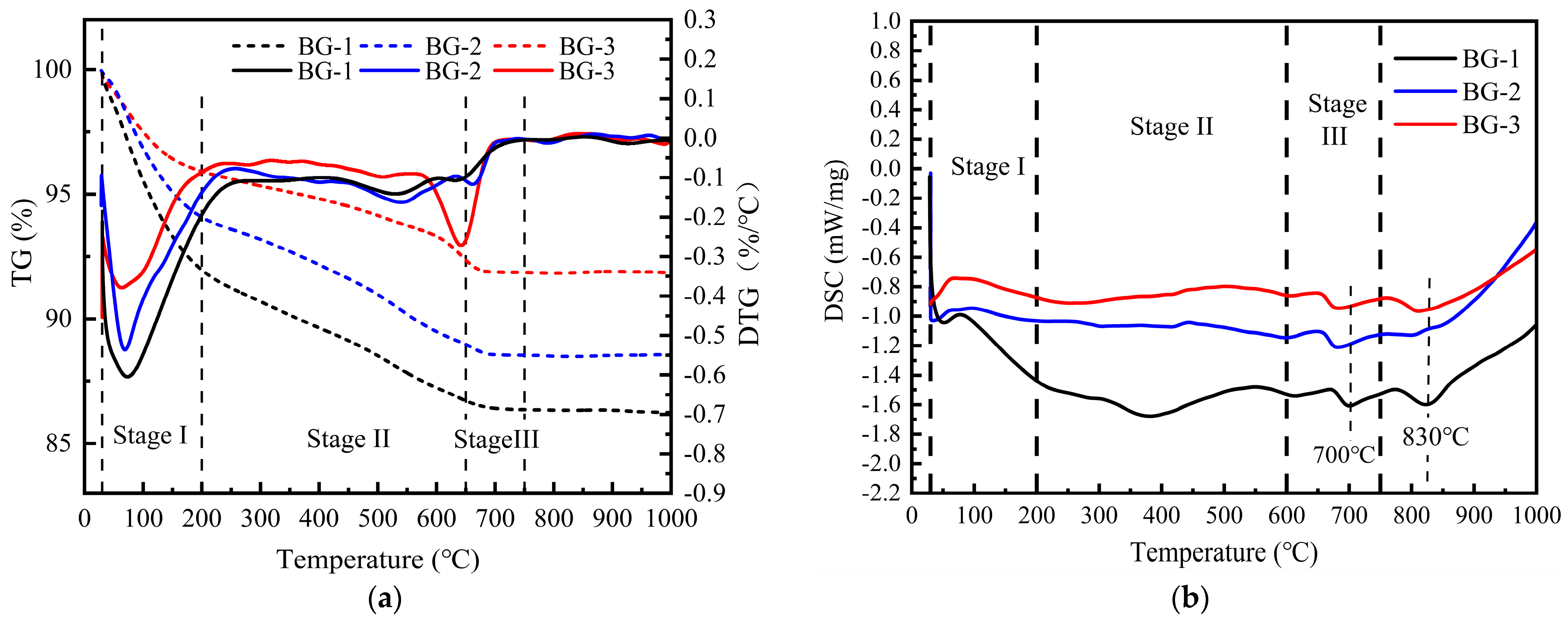
3.3.3. TG–DSC Analysis
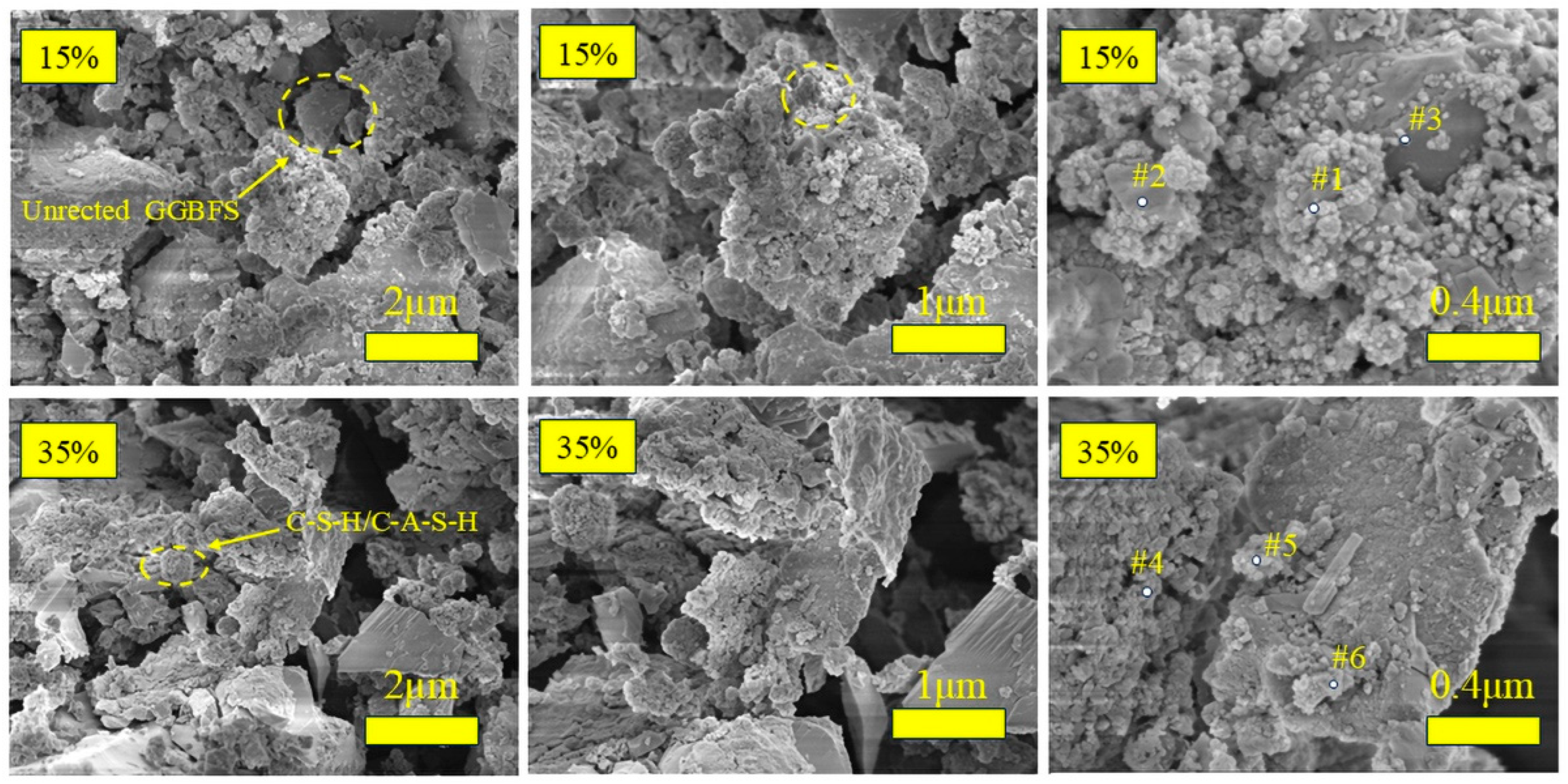
3.3.4. SEM + EDS Analysis
4. Discussion
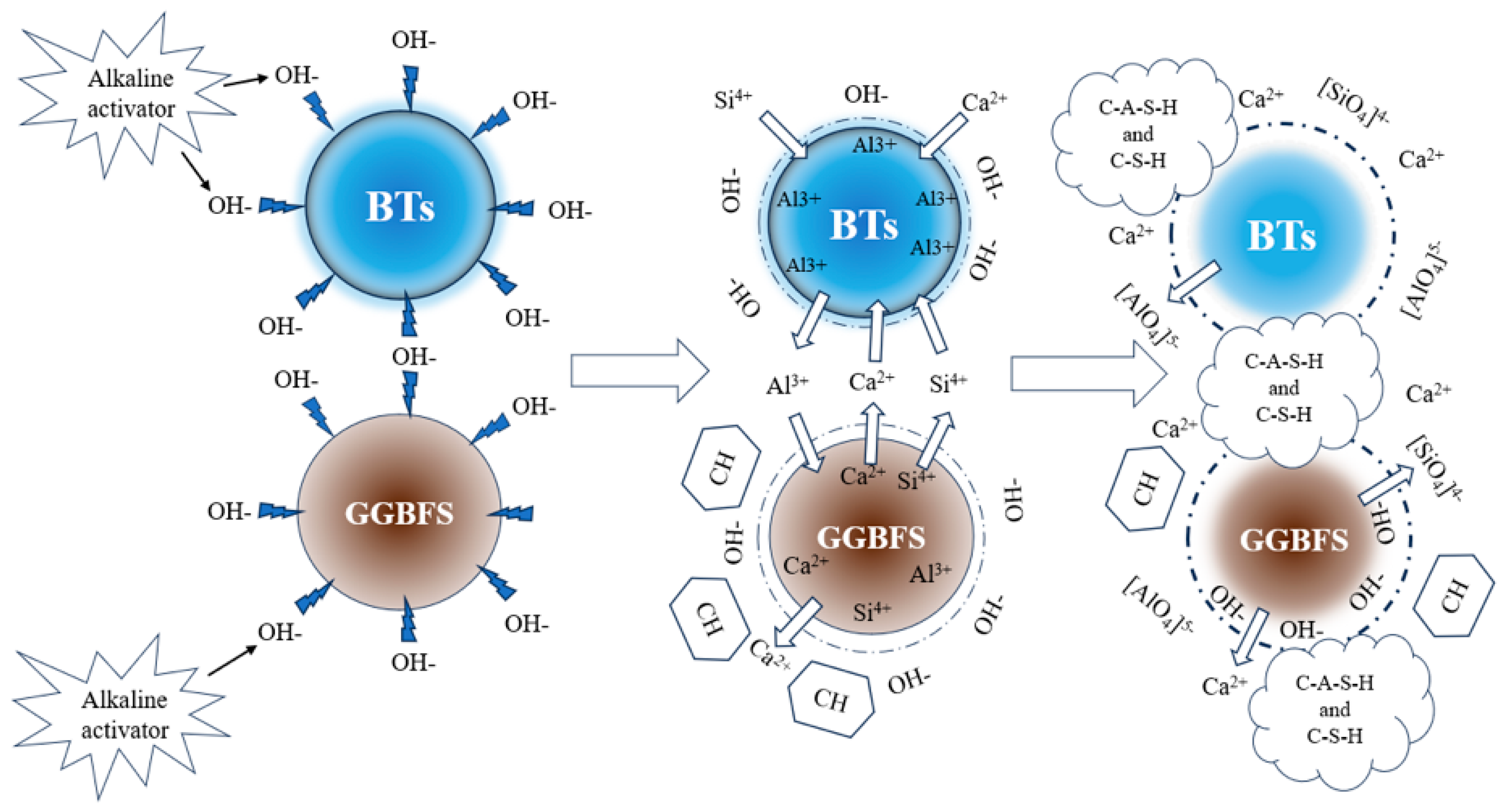
4.1. Discussion of Hydration Mechanism
- (1)
- Plasticizing effect: The addition of GGBFS optimizes the particle size distribution of the mixture (GGBFS, D50 = 22.9 μm; BTs, D50 = 45.77 μm). The GGBFS contains a certain amount of small particles, which can fill the pore space between the particles of the BTs to improve the particle gradation of the system, and the particles that fill in the gaps fill the gap between the original replacement water and free water, to promote the hydration reaction process and change the work performance.
- (2)
- Enhancement: As a highly active silica-aluminate precursor, the active Ca component of GGBFS rapidly participates in the reaction and promotes the formation of the gel phase under the action of the activator. Alkalinity destroys the silica–oxygen protective layer on the GGBFS surface. Because [SiO4]− and [AlO4]5− have not yet been dissolved in large quantities, when a large amount of Ca2+ accumulates in the solution up to a specific concentration, Ca(OH)2 crystals precipitate, which are enriched around the GGBFS and bauxite particles to provide a nucleation point for hydration product formation. By increasing the availability of reactive silicon and calcium in AAMs, GGBFS promotes the formation of C-S-H gels, which can coexist with N-A-S-H gels or form hybridized C(N)-A-S-H gels, ultimately increasing the mechanical strength. The progressive addition of GGBFS results in the continuous generation of C-S-H and C-A-S-H gels, leading to pore filling between the precursor particles and a reduction in the porosity of the hardened paste.
4.2. Discussion of Insufficient Research Contributions
- (1)
- Exploring simplified preparation methods for bauxite residue-based cementitious materials: Research should focus on low-energy, efficient activation methods, as well as the potential for combining bauxite residue with other solid wastes (e.g., spontaneous coal gangue and carbide slag) and adjusting their workability and performance. These improvements would facilitate large-scale production and processing, making these materials more suitable for use in construction operations and building industries.
- (2)
- Investigating the mechanisms of microstructural evolution to optimize material performance: Understanding the microstructural evolution and strengthening mechanisms of cementitious materials is crucial for enhancing the performance of AAMs. This requires more accurate microstructural data analysis techniques (e.g., determining the new mineral phase content via X-ray diffraction (XRD), measuring the leaching ratio of precursor elements, and using nuclear magnetic resonance (NMR) to clarify polymerization degree changes) and the development of new research methods (e.g., machine learning for image recognition of inert material distribution, establishing specific hydration heat models, and compact packing models). Defining the pathways for microstructural enhancement is vital for improving performance.
- (3)
- Further evaluating environmental impacts: Future research should focus on the carbon emissions associated with the entire lifecycle of AAM production, from the generation, transportation, and preparation of bauxite residue (and other raw materials) to the final AAM product. Carbon reduction rates and industrial waste recycling rates should be quantified. In addition, establishing energy and carbon emission data for the entire process, from precursor production to AAM preparation, is essential. The integration of industrial and biomass wastes, along with a circular economy model, will accelerate the transition of the industry toward greener, low-carbon practices.
5. Conclusions
- (1)
- Based on the experimental data, a quadratic polynomial regression model was established to predict the 7 d and 28 d mechanical strengths of the BX-GGBFS alkali-activated cement material; variance analysis revealed that the model had a high coefficient of determination (f3c = 0.9803, f28c = 0.9789), and the model fitted well. The alkali content (X1) and water–solid ratio (X3) significantly affect the compressive strength, and the optimum mixture ratio is as follows: alkali content, 4%; sodium silicate modulus, 1.3; and water–solid ratio, 0.32. The error between the predicted and measured values was within the acceptable range.
- (2)
- The enhancement of the mechanical properties of AABGs by GGBFS was more pronounced. As the percentage of GGBFS increased, the strength increased continuously. However, when the GGBFS content exceeded 35%, the strength rate increased, and the optimization effect appeared to decrease. Active Ca2+ ions from GGBFS rapidly participated in the reaction, promoting gel formation under alkaline conditions. The hydration product gels filled the pores between the precursor particles, and the improved particle size distribution refined the pore structure, thereby enhancing the material strength.
- (3)
- With the incorporation of GGBFS, the fluidity and setting time of the AABG system decreased gradually. Although GGBFS improved the particle size distribution and enhanced the flowability, the presence of Ca2+ ions accelerated the depolymerization of precursor particles and the formation of hydration products (C-A-S-H and C-S-H gels), leading to rapid setting of the slurry.
- (4)
- XRD, SEM–EDS, FTIR, and TG-DSC analyses demonstrated that the hydration products of AABG were predominantly C-S-H and C-(N)-A-S-H gels in the form of SiQ3 and SiQ4 tetrahedra, accompanied by a minor presence of analcime and gismondine. As the GGBFS doping level increased, the calcium-to-silicon (Ca/Si) ratio of the hydration product gradually decreased from 0.97 to 1.21, 0.7, and 1.0. Concurrently, the silicon-to-aluminum (Si/Al) ratio increased from 0.51 to 1.71, 0.64, and 1.27. This indicates an increase in the content of silicate structures and a more intricate gel network, contributing to enhanced mechanical properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Dong, C.; Huang, P.; Sun, Q.; Li, M.; Chai, J. Experimental Study on the Characteristics of Activated Coal Gangue and Coal Gangue-Based Geopolymer. Energies 2020, 13, 2504. [Google Scholar] [CrossRef]
- Wei, J.; Cen, K. Empirical Assessing Cement CO2 Emissions Based on China’s Economic and Social Development during 2001–2030. Sci. Total Environ. 2019, 653, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in Understanding Alkali-Activated Materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic Polymeric New Materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Ding, Y.; Dai, J.-G.; Shi, C.-J. Mechanical Properties of Alkali-Activated Concrete: A State-of-the-Art Review. Constr. Build. Mater. 2016, 127, 68–79. [Google Scholar] [CrossRef]
- Tchadjié, L.N.; Ekolu, S.O.; Quainoo, H.; Tematio, P. Incorporation of Activated Bauxite to Enhance Engineering Properties and Microstructure of Volcanic Ash Geopolymer Mortar Composites. J. Build. Eng. 2021, 41, 102384. [Google Scholar] [CrossRef]
- Zainudeen, N.M.; Mohammed, L.; Nyamful, A.; Adotey, D.; Osae, S.K. A Comparative Review of the Mineralogical and Chemical Composition of African Major Bauxite Deposits. Heliyon 2023, 9, e19070. [Google Scholar] [CrossRef]
- Alves, Z.; Senff, L.; Sakkas, K.; Yakoumis, I.; Labrincha, J.A.; Novais, R.M. Synthesis of Geopolymer Composites Using Bauxite Residue-Based Spheres as Aggregate: Novel and Eco-Friendly Strategy to Produce Lightweight Building Materials. Cem. Concr. Compos. 2024, 148, 105478. [Google Scholar] [CrossRef]
- Konduru, H.; Karthiyaini, S. Enhancing Solidification in One-Part Geopolymer Systems through Alkali-Thermal Activation of Bauxite Residue and Silica Fume Integration. Case Stud. Constr. Mater. 2024, 21, e03444. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, W.; Shi, D. Effect of Elevated Temperature on the Properties of Geopolymer Synthesized from Calcined Ore-Dressing Tailing of Bauxite and Ground-Granulated Blast Furnace Slag. Constr. Build. Mater. 2014, 69, 41–48. [Google Scholar] [CrossRef]
- Ke, W.; Zhang, X.; Zhu, F.; Wu, H.; Zhang, Y.; Shi, Y.; Hartley, W.; Xue, S. Appropriate Human Intervention Stimulates the Development of Microbial Communities and Soil Formation at a Long-Term Weathered Bauxite Residue Disposal Area. J. Hazard. Mater. 2021, 405, 124689. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, T.; Lv, G.; Chao, X.; Yang, X. Extraction and Utilization of Valuable Elements from Bauxite and Bauxite Residue: A Review. Bull. Environ. Contam. Toxicol. 2022, 109, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, J.; Ma, X.; Hu, X.; Wu, Z.; Shi, C. Use of Bauxite Tailing for the Production of Fine Lightweight Aggregates. J. Clean. Prod. 2022, 372, 133603. [Google Scholar] [CrossRef]
- Zhou, L.; Gou, M.; Guan, X. Hydration Kinetics of Cement-Calcined Activated Bauxite Tailings Composite Binder. Constr. Build. Mater. 2021, 301, 124296. [Google Scholar] [CrossRef]
- Alex, T.C.; Kumar, R.; Roy, S.K.; Mehrotra, S.P. Mechanical Activation of Al-Oxyhydroxide Minerals—A Review. Miner. Process. Extr. Metall. Rev. 2016, 37, 1–26. [Google Scholar] [CrossRef]
- Djobo, Y.J.N.; Elimbi, A.; Dika Manga, J.; Djon Li Ndjock, I.B. Partial Replacement of Volcanic Ash by Bauxite and Calcined Oyster Shell in the Synthesis of Volcanic Ash-Based Geopolymers. Constr. Build. Mater. 2016, 113, 673–681. [Google Scholar] [CrossRef]
- Boum, R.B.E.; Kaze, C.R.; Nemaleu, J.G.D.; Djaoyang, V.B.; Rachel, N.Y.; Ninla, P.L.; Owono, F.M.; Kamseu, E. Thermal Behaviour of Metakaolin–Bauxite Blends Geopolymer: Microstructure and Mechanical Properties. SN Appl. Sci. 2020, 2, 1358. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Wang, Q.; Zhang, W.; Li, Z. Sustainable Alkali-Activated Materials: Leveraging Spontaneous Combustion Coal Gangue for Enhanced Cementitious Performance. Mater. Today Commun. 2024, 41, 111044. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, W.; Shi, D. Properties of an Aged Geopolymer Synthesized from Calcined Ore-Dressing Tailing of Bauxite and Slag. Cem. Concr. Res. 2017, 100, 23–31. [Google Scholar] [CrossRef]
- Bian, Z.; Jin, G.; Ji, T. Effect of Combined Activator of Ca(OH)2 and Na2CO3 on Workability and Compressive Strength of Alkali-Activated Ferronickel Slag System. Cem. Concr. Compos. 2021, 123, 104179. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Zhang, X.; Tan, L.; Mei, Y. Mechanical Performance Optimization and Microstructural Mechanism Study of Alkali-Activated Steel Slag–Slag Cementitious Materials. Buildings 2024, 14, 1204. [Google Scholar] [CrossRef]
- Ji, X.; Wang, Z.; Zhang, H.; Wang, X.; Huo, J.; Zhang, T. Optimization Design and Characterization of Slag Cementitious Composites Containing Carbide Slag and Desulfurized Gypsum Based on Response Surface Methodology. J. Build. Eng. 2023, 77, 107441. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, H.; Li, H.; Jiang, Z.; Zhu, H.; Ju, J.W.; Yan, Z. Multiscale Modelling for the Ultra-High Performance Concrete: From Hydration Kinetics to Macroscopic Elastic Moduli. Constr. Build. Mater. 2020, 247, 118541. [Google Scholar] [CrossRef]
- Shao, W.; Zha, W.; Zhou, X.; Xu, T. Experimental Study Based on Box–Behnken Design and Response Surface Methodology for Optimization Proportioning of Activated Lithium Slag Composite Cement-Based Cementitious Materials. Materials 2024, 17, 2651. [Google Scholar] [CrossRef]
- Mucsi, G.; Kumar, S.; Csőke, B.; Kumar, R.; Molnár, Z.; Rácz, Á.; Mádai, F.; Debreczeni, Á. Control of Geopolymer Properties by Grinding of Land Filled Fly Ash. Int. J. Miner. Process. 2015, 143, 50–58. [Google Scholar] [CrossRef]
- Hounsi, A.D.; Lecomte-Nana, G.L.; Djétéli, G.; Blanchart, P. Kaolin-Based Geopolymers: Effect of Mechanical Activation and Curing Process. Constr. Build. Mater. 2013, 42, 105–113. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, X.; He, X.; Guo, Y.; Deng, X.; Su, Y.; Yang, J.; Wang, Y. Utilization of Lithium Slag by Wet-Grinding Process to Improve the Early Strength of Sulphoaluminate Cement Paste. J. Clean. Prod. 2018, 205, 536–551. [Google Scholar] [CrossRef]
- Duarte, M.S.; Almada, B.S.; José dos Santos, W.; Lima Bessa, S.A.; Cesar da Silva Bezerra, A.; Paulino Aguilar, M.T. Influence of Mechanical Treatment and Magnetic Separation on the Performance of Iron Ore Tailings as Supplementary Cementitious Material. J. Build. Eng. 2022, 59, 105099. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.; Wang, Q. Performance Study of Alkali-Activated Phosphate Slag-Granulated Blast Furnace Slag Composites: Effect of the Granulated Blast Furnace Slag Content. Arch. Civ. Mech. Eng. 2023, 23, 181. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, W.; Zhang, Y.; Xue, C.; Xu, Z.; Gao, Q.; Xiao, C.; Zhao, Q. Low Carbon Binder Preparation from Slag-Red Mud Activated by MSWI Fly Ash-Carbide Slag: Hydration Characteristics and Heavy Metals’ Solidification Behavior. J. Clean. Prod. 2022, 374, 134007. [Google Scholar] [CrossRef]
- Alventosa, K.M.L.; White, C.E. The Effects of Calcium Hydroxide and Activator Chemistry on Alkali-Activated Metakaolin Pastes. Cem. Concr. Res. 2021, 145, 106453. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Huo, W.; Song, S.; Zhang, J.; Yao, K.; Hu, L. Efficient Use of Steel Slag in Alkali-Activated Fly Ash-Steel Slag-Ground Granulated Blast Furnace Slag Ternary Blends. Constr. Build. Mater. 2020, 259, 119814. [Google Scholar] [CrossRef]
- Ghorbani, S.; Stefanini, L.; Sun, Y.; Walkley, B.; Provis, J.L.; De Schutter, G.; Matthys, S. Characterisation of Alkali-Activated Stainless Steel Slag and Blast-Furnace Slag Cements. Cem. Concr. Compos. 2023, 143, 105230. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, T.; Yang, Z.; Wang, C.; Wu, H. Influence of Different Activators on Microstructure and Strength of Alkali-Activated Nickel Slag Cementitious Materials. Constr. Build. Mater. 2020, 235, 117449. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A Review: The Comparison between Alkali-Activated Slag (Si + Ca) and Metakaolin (Si + Al) Cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- GB/T 2419–2005 (2005); Test Method for Fluidity of Cement Mortar. National Standard of the People’s Republic of China: Beijing, China, 2005.
- GB/T 17671–1999 (1999); Method of Testing Cements Determination of Strength. National Standard of the People’s Republic of China: Beijing, China, 1999.
- Li, Z.; Lu, D.; Gao, X. Optimization of Mixture Proportions by Statistical Experimental Design Using Response Surface Method—A Review. J. Build. Eng. 2021, 36, 102101. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Liu, K.; Wu, Y.; Ai, J.; Zhang, J. Preparation of Li4SiO4-Based Adsorbents with Coal Slag for High Temperature Cyclic CO2 Capture. Fuel 2022, 310, 121687. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, B.; Tan, H.; Liu, X.; Chen, P.; Luo, Z. Effect of TIPA on Mechanical Properties and Hydration Properties of Cement-Lithium Slag System. J. Environ. Manag. 2020, 276, 111274. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, Y. Influence of Waste Glass Powder Usage on the Properties of Alkali-Activated Slag Mortars Based on Response Surface Methodology. Constr. Build. Mater. 2018, 181, 527–534. [Google Scholar] [CrossRef]
- Srinivasa, A.S.; Swaminathan, K.; Yaragal, S.C. Microstructural and Optimization Studies on Novel One-Part Geopolymer Pastes by Box-Behnken Response Surface Design Method. Case Stud. Constr. Mater. 2023, 18, e01946. [Google Scholar] [CrossRef]
- Shi, D.; Ye, J.; Zhang, W. Effects of Activator Content on Properties, Mineralogy, Hydration and Microstructure of Alkali-Activated Materials Synthesized from Calcium Silicate Slag and Ground Granulated Blast Furnace Slag. J. Build. Eng. 2020, 32, 101791. [Google Scholar] [CrossRef]
- Cao, X.; Yang, W.; Liu, S.; Fang, L.; Liu, R.; Ma, R. Durability of Calcium-Rich Municipal Solid Waste Incineration Fly Ash-Based Geopolymer to Sulfate and Sulfuric Acid. Constr. Build. Mater. 2023, 405, 133389. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, W.; Wang, J.; Xu, Z.; Wang, S.; Zhao, Q.; Zhou, J. Geopolymer Bricks Prepared by MSWI Fly Ash and Other Solid Wastes: Moulding Pressure and Curing Method Optimisation. Chemosphere 2022, 307, 135987. [Google Scholar] [CrossRef] [PubMed]
- GB 175-2007 (2007); Common Portland Cement. National Standard of the People’s Republic of China: Beijing, China, 2007.
- Kamath, M.; Prashant, S.; Kumar, M. Micro-Characterisation of Alkali Activated Paste with Fly Ash-GGBS-Metakaolin Binder System with Ambient Setting Characteristics. Constr. Build. Mater. 2021, 277, 122323. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhang, Y.; Gu, X. Research on Hydration Characteristics of OSR-GGBFS-FA Alkali-Activated Materials. Constr. Build. Mater. 2024, 411, 134321. [Google Scholar] [CrossRef]
- Guan, X.; Jiang, L.; Fan, D.; Garcia Hernandez, A.; Li, B.; Do, H. Molecular Simulations of the Structure-Property Relationships of N-A-S-H Gels. Constr. Build. Mater. 2022, 329, 127166. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Rees, G.J.; Hanna, J.V.; van Deventer, J.S.J.; Provis, J.L. Phase Evolution of C-(N)-A-S-H/N-A-S-H Gel Blends Investigated via Alkali-Activation of Synthetic Calcium Aluminosilicate Precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Li, H.; Bai, C.; Wang, Z.; Yang, T.; Gu, T. Microwave-Thermal-Assisted Curing Method on Geopolymer Preparation from Panzhihua High-Titanium Slag by Alkali Activation. Constr. Build. Mater. 2023, 400, 132614. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Wang, A.; Dong, B. Research on the Mechanical Properties and Microstructure of Fly Ash-Based Geopolymers Modified by Molybdenum Tailings. Constr. Build. Mater. 2023, 385, 131530. [Google Scholar] [CrossRef]
- Xia, M.; Muhammad, F.; Zeng, L.; Li, S.; Huang, X.; Jiao, B.; Shiau, Y.; Li, D. Solidification/Stabilization of Lead-Zinc Smelting Slag in Composite Based Geopolymer. J. Clean. Prod. 2019, 209, 1206–1215. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía de Gutierrez, R. Evolution of Binder Structure in Sodium Silicate-Activated Slag-Metakaolin Blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.; Li, L.; Wang, J.; Shao, N.; Wang, D. Microstructure and Phase Evolution of Alkali-Activated Steel Slag during Early Age. Constr. Build. Mater. 2019, 204, 158–165. [Google Scholar] [CrossRef]
- Cui, W.; Liu, J.; He, G.; Duan, W.; Li, X.; Dong, X. Evaluation of Active Silica-Alumina Content and Reactivity in Bayer Process Red Mud. Constr. Build. Mater. 2024, 418, 135502. [Google Scholar] [CrossRef]
- He, P.; Drissi, S.; Hu, X.; Liu, J.; Shi, C. Investigation on the Influential Mechanism of FA and GGBS on the Properties of CO2-Cured Cement Paste. Cem. Concr. Compos. 2023, 142, 105186. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; Van Deventer, J.S.J. Thermal Evolution of Metakaolin Geopolymers: Part 1—Physical Evolution. J. Non-Cryst. Solids 2006, 352, 5541–5555. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; Van Deventer, J.S.J. Modification of Phase Evolution in Alkali-Activated Blast Furnace Slag by the Incorporation of Fly Ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]

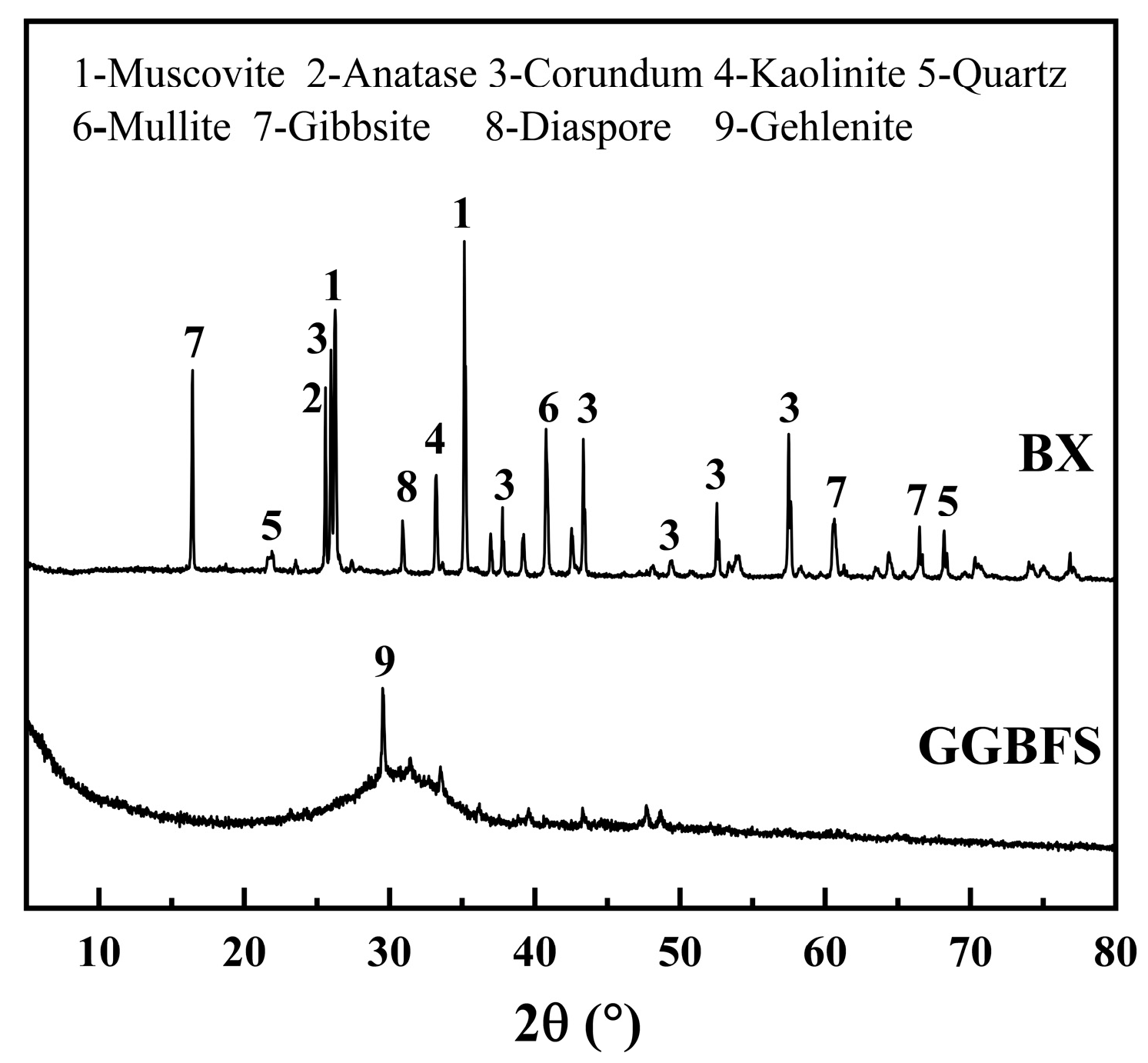
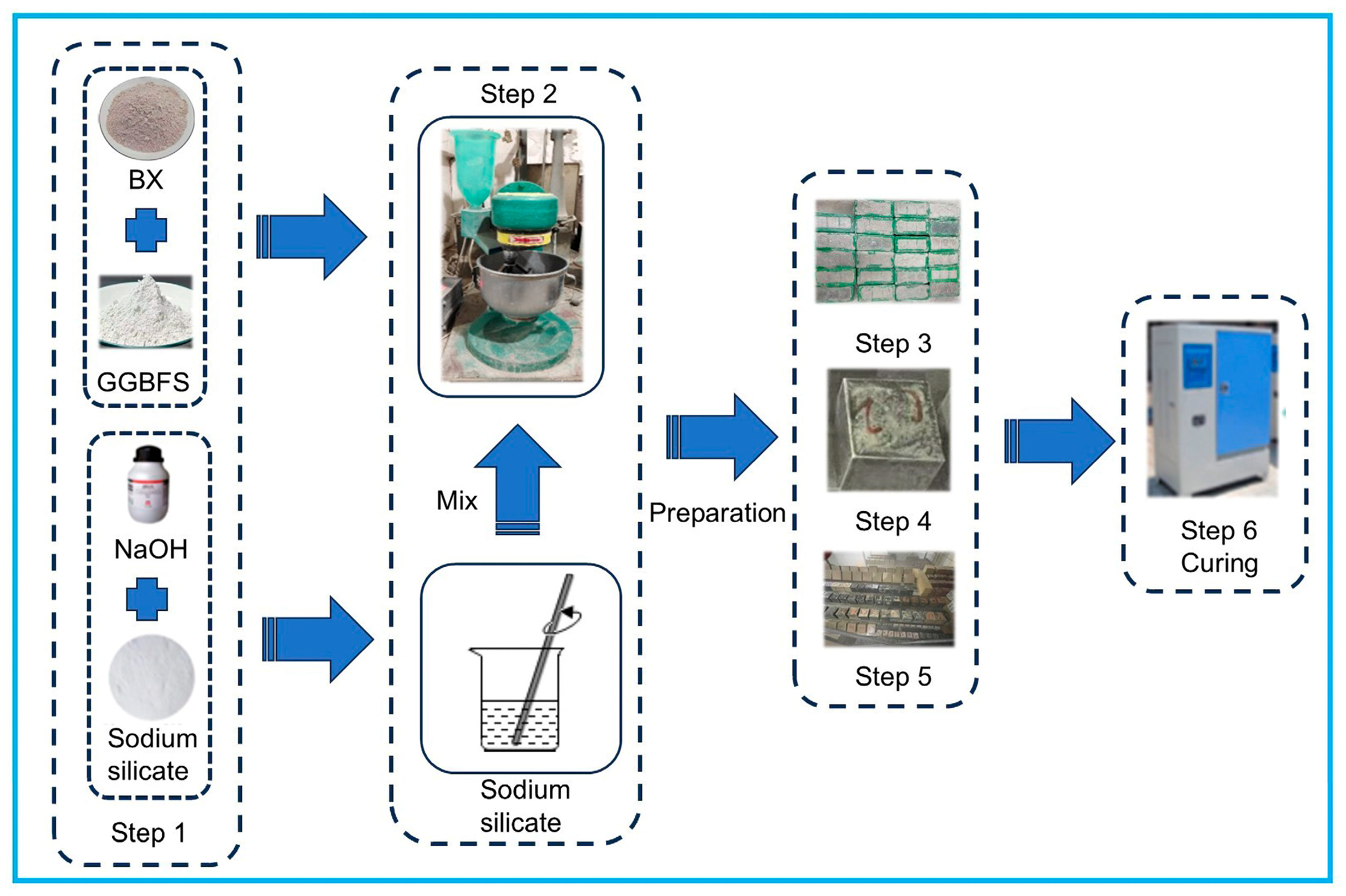
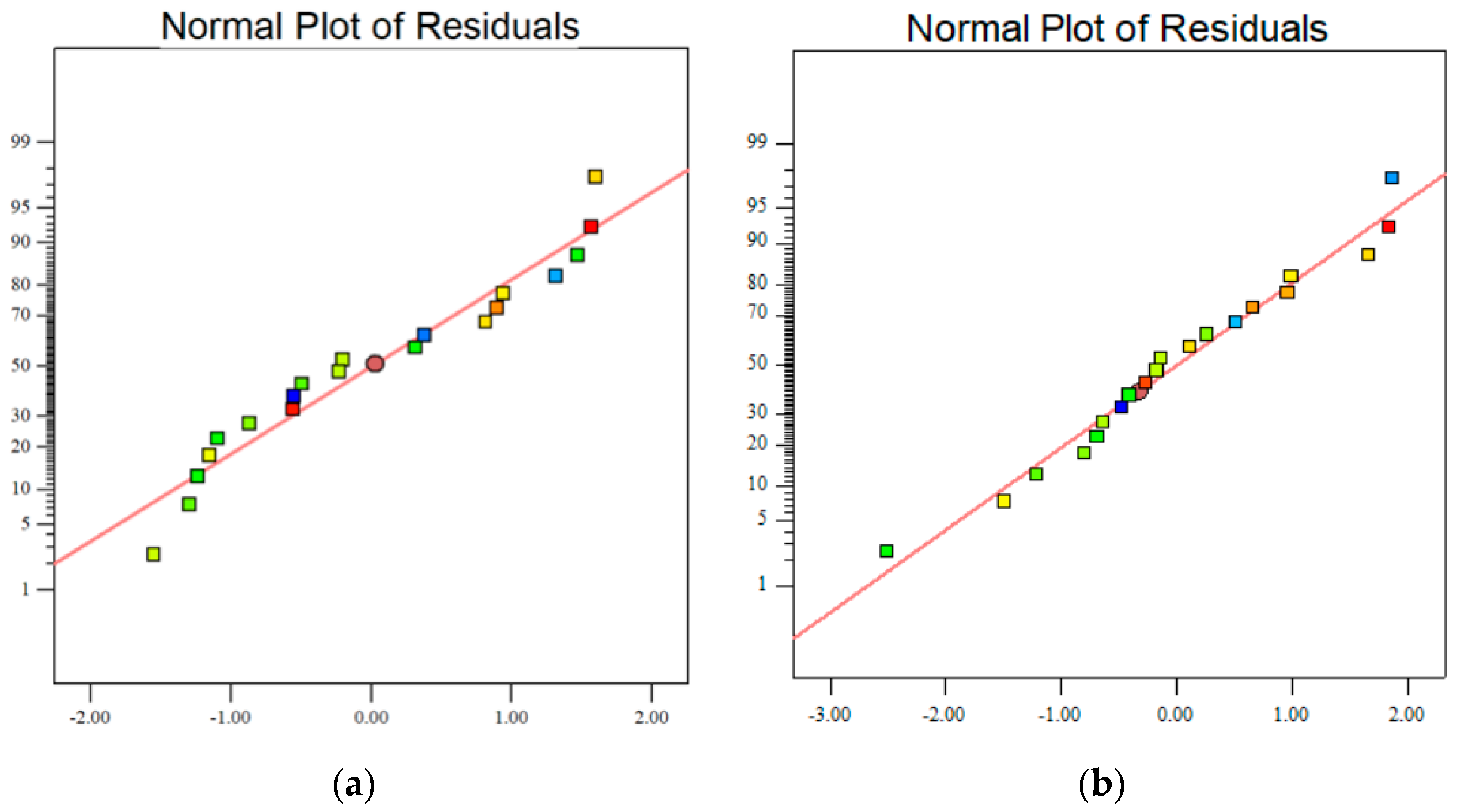
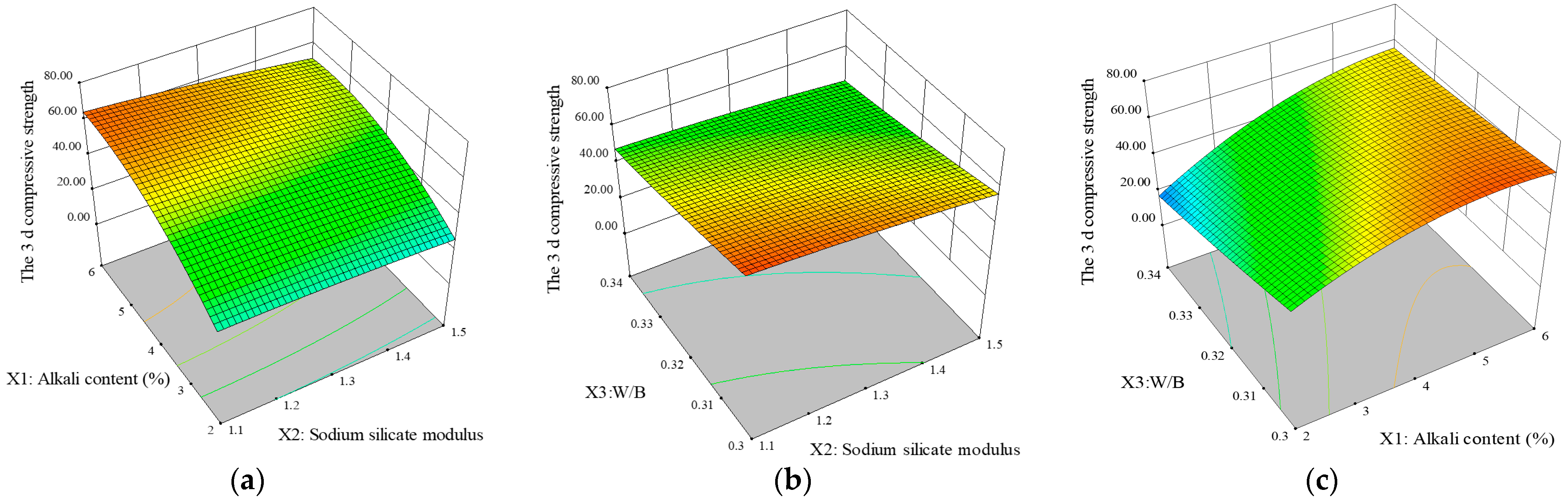

| Materials | CaO | SiO2 | Al2O3 | MgO | SO3 | Fe2O3 | K2O | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| BX | 0.562 | 21.049 | 69.234 | 0.092 | 0.009 | 1.997 | 0.189 | - | 6.868 |
| GGBFS | 50.219 | 25.615 | 12.070 | 5.175 | 2.408 | 0.314 | 0.301 | 0.408 | 3.49 |
| Independent Variable Factor | Code Level | ||||
|---|---|---|---|---|---|
| −1.5 (−α) | −1 (Low Level) | 0 (Center Level) | 1 (High Level) | 1.5 (α) | |
| X1 (%) | 1 | 2 | 4 | 6 | 7 |
| X2 (M) | 1 | 1.1 | 1.3 | 1.5 | 1.6 |
| X3 | 0.29 | 0.30 | 0.32 | 0.34 | 0.35 |
| Samples | m(GGBFS)/m(BX) | Alkaline Activators | W/C | |
|---|---|---|---|---|
| Modulus | Na2O (%) | |||
| 1 | 65:35 | 1 | 4 | 0.32 |
| 2 | 65:35 | 1.1 | 2 | 0.3 |
| 3 | 65:35 | 1.1 | 6 | 0.3 |
| 4 | 65:35 | 1.1 | 2 | 0.34 |
| 5 | 65:35 | 1.1 | 6 | 0.34 |
| 6 | 65:35 | 1.3 | 4 | 0.29 |
| 7 | 65:35 | 1.3 | 1 | 0.32 |
| 8 | 65:35 | 1.3 | 4 | 0.32 |
| 9 | 65:35 | 1.3 | 4 | 0.32 |
| 10 | 65:35 | 1.3 | 4 | 0.32 |
| 11 | 65:35 | 1.3 | 4 | 0.32 |
| 12 | 65:35 | 1.3 | 4 | 0.32 |
| 13 | 65:35 | 1.3 | 4 | 0.32 |
| 14 | 65:35 | 1.3 | 7 | 0.32 |
| 15 | 65:35 | 1.3 | 4 | 0.35 |
| 16 | 65:35 | 1.5 | 2 | 0.3 |
| 17 | 65:35 | 1.5 | 6 | 0.3 |
| 18 | 65:35 | 1.5 | 2 | 0.34 |
| 19 | 65:35 | 1.5 | 6 | 0.34 |
| 20 | 65:35 | 1.6 | 4 | 0.32 |
| Samples | Compressive Strength (MPa) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| f3c | 54.85 | 45.34 | 69.13 | 19.52 | 62.59 | 70.94 | 9.34 | 49.83 | 52.84 | 52.75 |
| f28c | 81.56 | 70.33 | 98.42 | 41.85 | 92.72 | 103.56 | 30.82 | 78.64 | 82.19 | 82.08 |
| Samples | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| f3c | 57.75 | 50.91 | 56.18 | 57.25 | 40.83 | 37.67 | 53.36 | 16.51 | 49.63 | 48.25 |
| f28c | 82.08 | 79.91 | 86.13 | 87.42 | 70.01 | 67.28 | 85.81 | 44.33 | 91.43 | 79.77 |
| Compiled Source | f3c | f28c | ||
|---|---|---|---|---|
| FValue | pValue | FValue | pValue | |
| Model | 55.19 | <0.0001 | 51.66 | <0.0001 |
| X1 | 19.59 | 0.0013 | 1.66 | 0.2262 |
| X2 | 283.38 | <0.0001 | 297.55 | <0.0001 |
| X3 | 84.51 | <0.0001 | 58.60 | <0.0001 |
| X1X2 | 4.1 | 0.0704 | 1.57 | 0.2389 |
| X1X3 | 0.7 | 0.4215 | 2.51 | 0.1444 |
| X2X3 | 16.97 | 0.0021 | 23.28 | 0.0007 |
| X12 | 0.96 | 0.35 | 0.36 | 0.5645 |
| X22 | 85.49 | <0.0001 | 76.80 | <0.0001 |
| X32 | 0.97 | 0.349 | 2.97 | 0.1153 |
| Lack of fit | 1.14 | 0.4435 | 1.19 | 0.4257 |
| R2 | 0.9803 | 0.9789 | ||
| R2adj | 0.9625 | 0.9660 | ||
| R2pre | 0.9773 | 0.9777 | ||
| C.V | 6.60% | 4.88% | ||
| Independent Variable | Low Level | High Level |
|---|---|---|
| X1 (%) | 4 | 6 |
| X2 (M) | 1.1 | 1.3 |
| X3 | 0.3 | 0.32 |
| SiQ0 | SiQ1 | SiQ2 | SiQ3 | SiQ4 | (SiQ3 + SiQ4)/(SiQ1 + SiQ2) | |
|---|---|---|---|---|---|---|
| BG-1-28 d | 8.124 | 27.101 | 26.488 | 26.731 | 11.557 | 0.714 |
| BG-2-28 d | 13.405 | 13.026 | 29.804 | 19.850 | 23.915 | 1.022 |
| BG-3-28 d | 12.757 | 24.652 | 15.752 | 26.493 | 20.346 | 1.159 |
| BG-4-28 d | 7.851 | 4.814 | 29.118 | 36.134 | 22.084 | 1.305 |
| Temperature | BG-1 | BG-2 | BG-3 |
|---|---|---|---|
| 30–200 °C | 10.102 | 6.443 | 4.566 |
| 200–600 °C | 2.508 | 3.366 | 4.208 |
| 600–750 °C | 0.968 | 0.579 | 0.245 |
| Point | C | O | Na | Mg | Al | Si | Ca |
|---|---|---|---|---|---|---|---|
| Point #1 | 15.79 | 42.55 | 3.18 | 18.32 | 18.54 | 9.48 | 9.23 |
| Point #2 | 14.69 | 41.04 | 2.4 | 1.5 | 7.46 | 12.81 | 15.53 |
| Point #3 | 11.55 | 40.17 | 2.26 | 1.51 | 21.82 | 14.34 | 8.36 |
| Point #4 | 15.64 | 46.5 | 2.46 | 2.54 | 10.14 | 12.9 | 9.81 |
| Point #5 | 13.7 | 42.06 | 2.27 | 2.4 | 19.01 | 12.16 | 10.4 |
| Point #6 | 15.8 | 47.83 | 1.72 | 1.34 | 11.13 | 11.05 | 11.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Chen, H.; Zhang, Y. Study on Mechanical Properties and Hydration Characteristics of Bauxite-GGBFS Alkali-Activated Materials, Based on Composite Alkali Activator and Response Surface Method. Materials 2025, 18, 1466. https://doi.org/10.3390/ma18071466
Wang L, Chen H, Zhang Y. Study on Mechanical Properties and Hydration Characteristics of Bauxite-GGBFS Alkali-Activated Materials, Based on Composite Alkali Activator and Response Surface Method. Materials. 2025; 18(7):1466. https://doi.org/10.3390/ma18071466
Chicago/Turabian StyleWang, Lilong, Hongkai Chen, and Yannian Zhang. 2025. "Study on Mechanical Properties and Hydration Characteristics of Bauxite-GGBFS Alkali-Activated Materials, Based on Composite Alkali Activator and Response Surface Method" Materials 18, no. 7: 1466. https://doi.org/10.3390/ma18071466
APA StyleWang, L., Chen, H., & Zhang, Y. (2025). Study on Mechanical Properties and Hydration Characteristics of Bauxite-GGBFS Alkali-Activated Materials, Based on Composite Alkali Activator and Response Surface Method. Materials, 18(7), 1466. https://doi.org/10.3390/ma18071466







