Effect of Ce-Y Composite Addition on the Inclusion Evolution in T91 Heat-Resistant Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
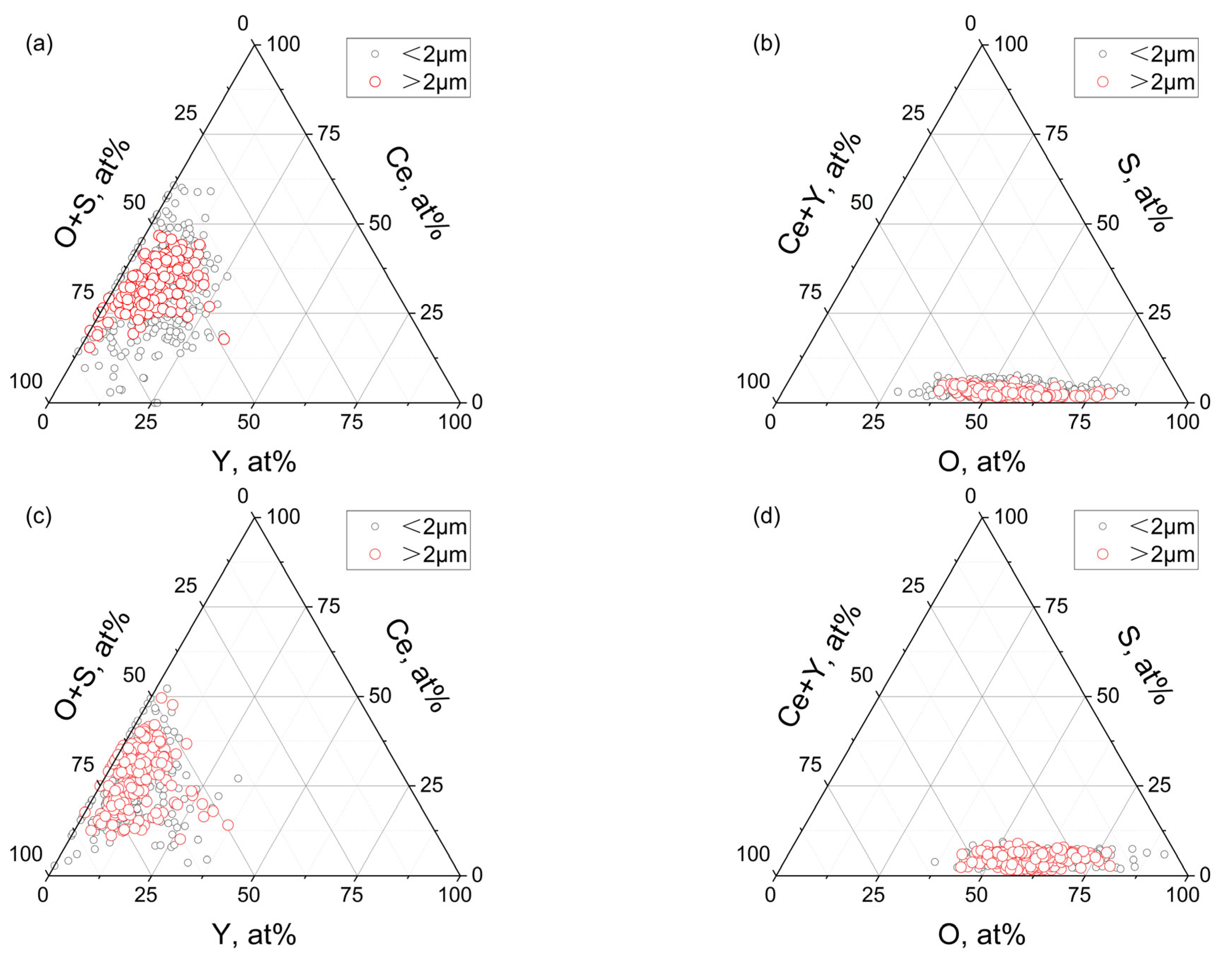
3.1. Morphology and Distribution of Inclusions
3.2. Effect of Rare Earth on the Type, Size, and Number Density of Inclusions
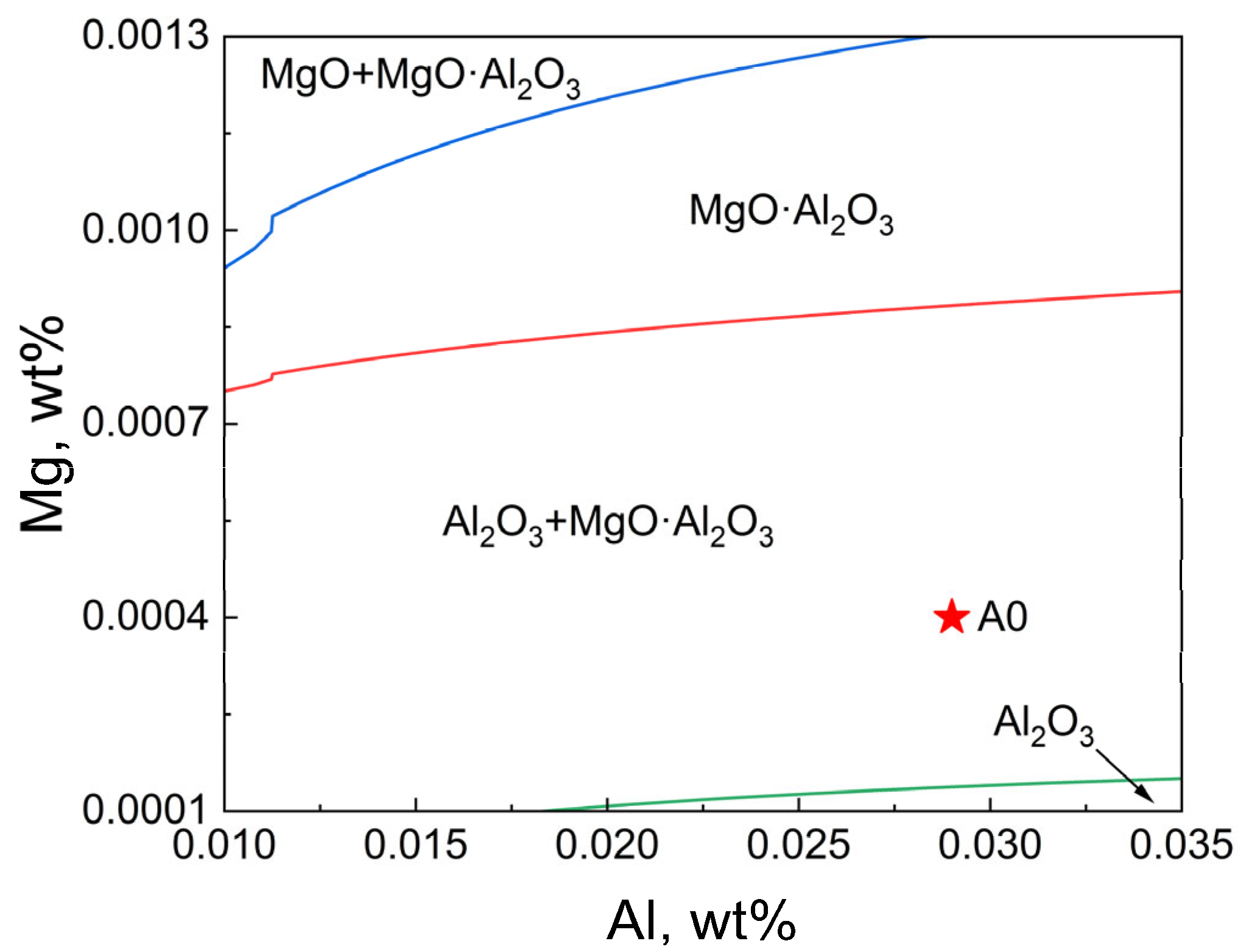
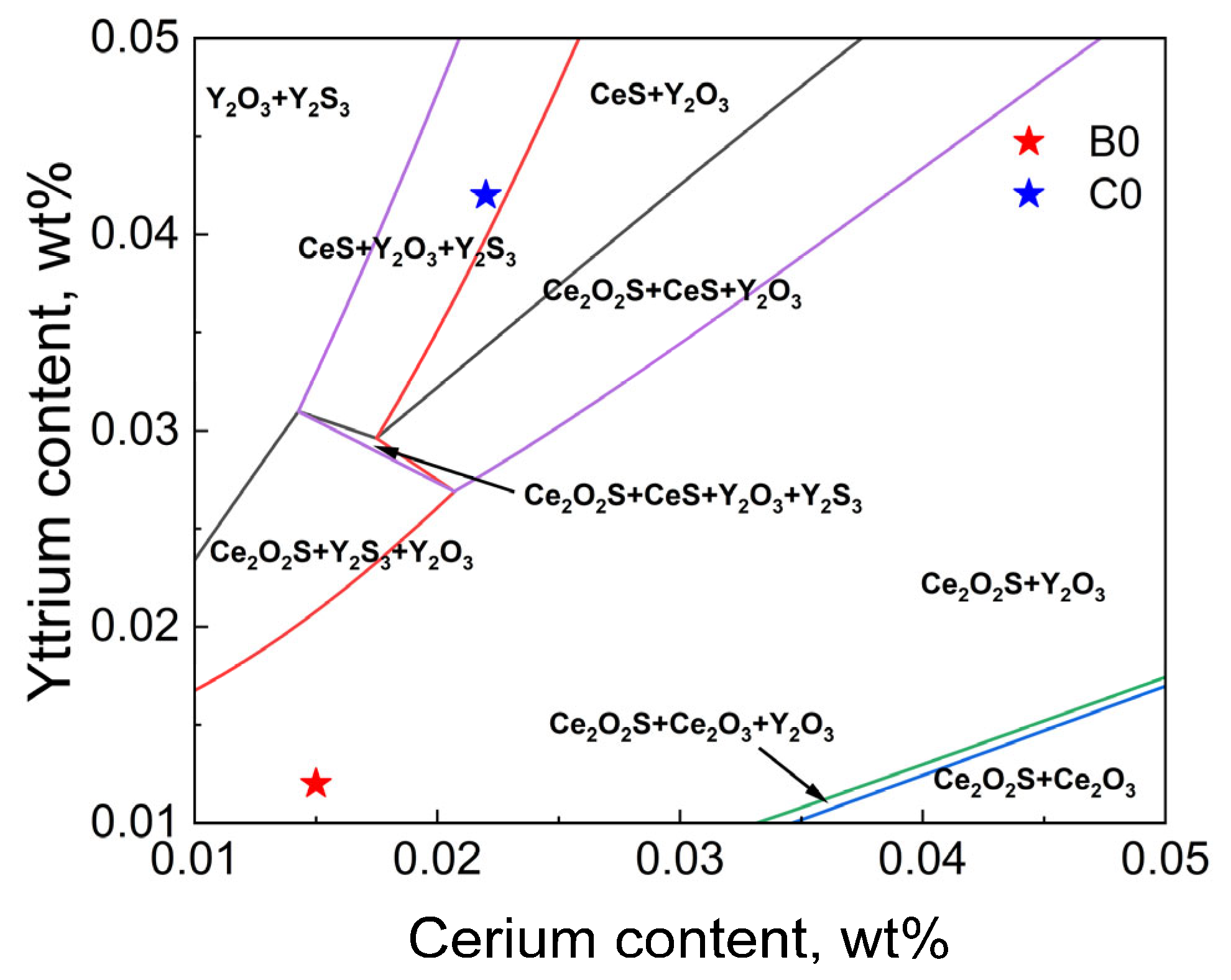
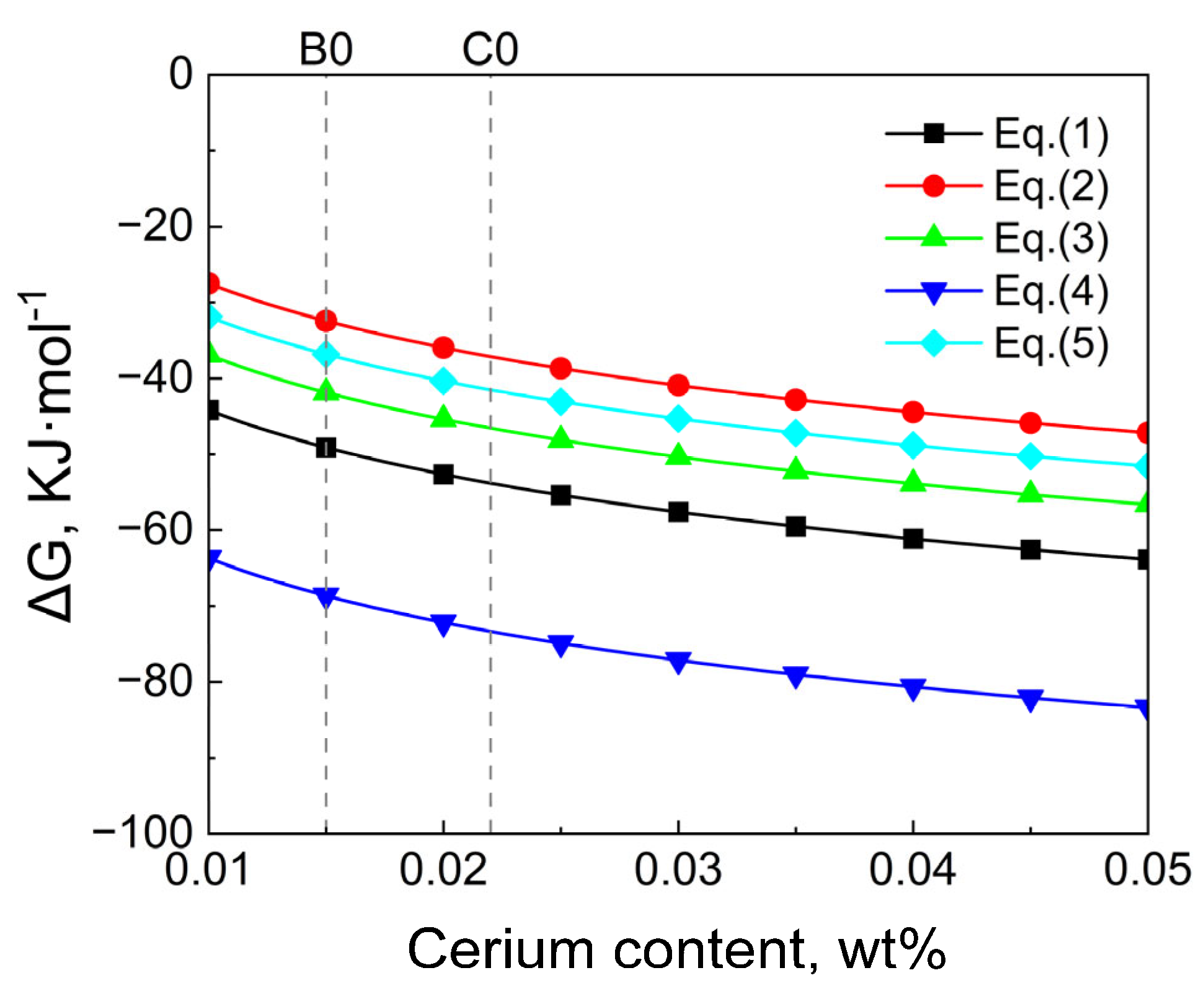
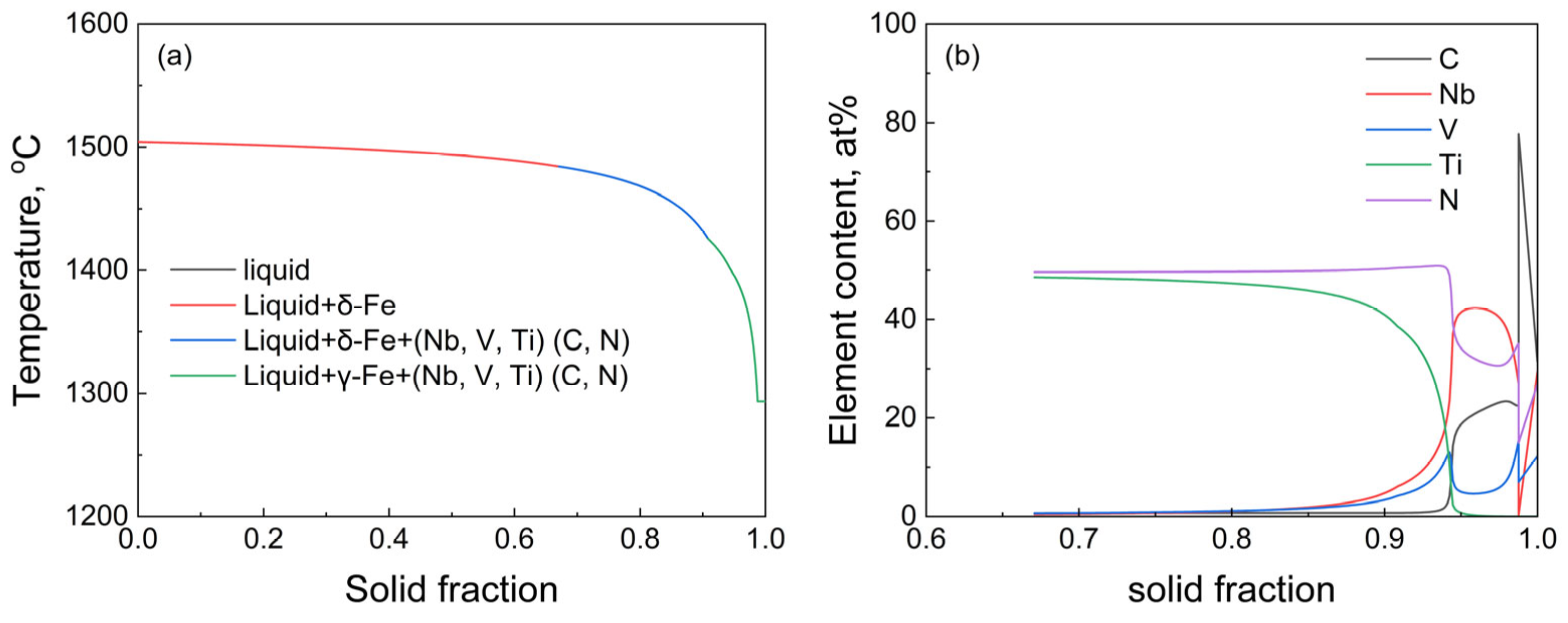
3.3. Thermodynamic Analysis of Inclusion Formation in T91 Steel
3.4. Analysis of the Influence Mechanism of Inclusions on Precipitated Phase
4. Conclusions
- The addition of rare earth Ce and Y makes the inclusions in T91 heat-resistant steel modified from angular Mg-Al-O oxides, (Nb, V, Ti)(C, N) carbonitrides and Mg-Al-O+(Nb, V, Ti)(C, N) composite inclusions to spherical Ce-Y-O, Ce-Y-O-S inclusions, and Ce-Y+Nb(C, N) composite inclusions. Compared with the sample without rare earth elements, after adding 0.015 wt.% Ce + 0.012 wt.% Y and 0.022 wt.% Ce + 0.042 wt.% Y, the number density of inclusions in T91 heat-resistant steel increased from 25/mm2 to 82/mm2 and 118/mm2, while the average size decreased significantly from 2.8 μm to 1.7 μm and 1.9 μm. It can be seen that due to the combined effect of the diffusion mechanism and the agglomeration mechanism, the further increase in rare earth content in a certain range will make the size of rare earth inclusions have an increasing trend.
- Thermodynamic calculations show that after the T91 heat-resistant steel with Ce and Y added undergoes homogenization at 1200 °C for 24 h and forging, the internal transformation of inclusions causes Y to accumulate in the inner layer. Meanwhile, the diffusion of Ce into inclusions in the matrix occurs Y2S3→CeS and Y2O3 + Y2S3→Ce2O2S. Eventually, the inclusion layer containing Ce is formed on the surface of inclusions. Therefore, it can be inferred that the increase in Ce content in steel makes the above reaction more likely to occur.
- During the solidification process of T91 heat-resistant steel, primary (Nb, V, Ti)(C, N) phases precipitate. However, the primary precipitation phase has not been detected in the steel after adding rare earths, and with the increase in the content of rare earths, the amount of Nb-containing composite phases on the surface of inclusions decreases. Combined with the calculation of the lattice mismatch, only Ce2O3 has a moderate heterogeneous nucleation effect with (Nb, V, Ti)(C, N). Therefore, appropriately increasing the Y/Ce ratio can reduce the formation of Ce2O3 and further avoid the formation of the primary precipitation phase (Nb, V, Ti)(C, N), which helps to improve the dispersion strengthening effect of the nanoscale secondary precipitation phase MX. Therefore, it is necessary to study the effect of different Y/Ce ratios on the size, number, and distribution of nano-scale secondary precipitated phase MX, so revealing the mechanism of rare earth elements in enhancing creep strength and steam oxidation resistance, thereby improving the service performance of T91 steel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, L.; Jiang, Z.; Xiao, Z.; Huang, Q. Cracking and exfoliation behavior of oxide scale on T91 steel under different tensile stresses in oxygen-controlled lead-bismuth eutectic at 550 °C. Corros. Sci. 2021, 183, 109324. [Google Scholar] [CrossRef]
- Xu, Y.W.; Song, S.H.; Wang, J.W. Effect of rare earth cerium on the creep properties of modified 9Cr–1Mo heat-resistant steel. Mater. Lett. 2015, 161, 616–619. [Google Scholar] [CrossRef]
- Naoi, H.; Ohgami, M.; Liu, X.; Fujita, T. Effects of aluminum content on the mechanical properties of a 9Cr-0.5Mo-1.8W steel. Metall. Mater. Trans. A 1997, 28, 1195–1203. [Google Scholar] [CrossRef]
- Guo, X.; Tan, M.; Li, T.; Ju, L.; Dang, J.; Guo, H.; Zhao, Y. Formation mechanisms and three-dimensional characterization of composite inclusion of MnS-Al2O3 in high speed wheel steel. Mater. Charact. 2023, 197, 112669. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, L.; Zhang, L.; Wang, Y.; Shu, Y.; Zhou, Y. Investigation of RE-OS-As inclusions in high carbon steels. Metall. Mater. Trans. B 2017, 48, 2849–2858. [Google Scholar] [CrossRef]
- Imashuku, S.; Wagatsuma, K. Cathodoluminescence analysis of nonmetallic inclusions in steel deoxidized and desulfurized by rare-earth metals (La, Ce, Nd). Metall. Mater. Trans. B 2020, 51, 79–84. [Google Scholar] [CrossRef]
- Geng, R.; Geng, R.; Li, J.; Li, J.; Shi, C.; Shi, C.; Zhi, J.; Zhi, J.; Lü, B.; Lu, B. Effect of Ce-La on inclusion evolution in Al-killed high strength steel. Rev. Metall.-Cah. Inf. Tech. 2020, 117, 616. [Google Scholar] [CrossRef]
- Li, D. Low-oxygen rare earth steels. Nat. Mater. 2022, 21, 1137–1143. [Google Scholar] [CrossRef]
- Wang, H.; Bao, Y.P.; Zhi, J.G.; Duan, C.Y.; Gao, S.; Wang, M. Effect of rare earth Ce on the morphology and distribution of Al2O3 inclusions in high strength IF steel containing phosphorus during continuous casting and rolling process. ISIJ Int. 2021, 61, 657–666. [Google Scholar] [CrossRef]
- Geng, R.; Li, J.; Shi, C. Evolution of inclusions with Ce addition and Ca treatment in Al-killed steel during RH refining process. ISIJ Int. 2021, 61, 1506–1513. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, C.; Wang, S.; Ren, P.; Li, J. Reoxidation of liquid steel and evolution of inclusions during protective atmosphere electroslag remelting of Ce-containing heat-resistant stainless steel. J. Iron Steel Res. Int. 2024, 31, 1923–1935. [Google Scholar] [CrossRef]
- Jiang, X.; Li, G.; Tang, H.; Liu, J.; Cai, S.; Zhang, J. Modification of inclusions by rare earth elements in a high-strength oil casing steel for improved sulfur resistance. Materials 2023, 16, 675. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, G.; Liu, Y.; Wang, F.; Wang, Q. Cerium Addition Effect on Modification of Inclusions, Primary Carbides and Microstructure Refinement of H13 Die Steel. ISIJ Int. 2021, 61, 1850–1859. [Google Scholar] [CrossRef]
- Yan, J.; Gao, Y.; Liang, L.; Ye, Z.; Li, Y.; Chen, W.; Zhang, J. Effect of yttrium on the cyclic oxidation behaviour of HP40 heat-resistant steel at 1373 K. Corros. Sci. 2011, 53, 329–337. [Google Scholar] [CrossRef]
- Chen, L.; Ma, X.; Wang, L.; Ye, X. Effect of rare earth element yttrium addition on microstructures and properties of a 21Cr–11Ni austenitic heat-resistant stainless steel. Mater. Des. 2011, 32, 2206–2212. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, L.; Liu, Y.; Cui, L.; Yang, W. Transformation of cerium-containing inclusions in ultra-low-carbon aluminum-killed steels during solidification and cooling. J. Mater. Res. Technol. 2020, 9, 8197–8206. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Li, J.; Wu, J. Transformation of Oxide Inclusions in Stainless Steel Containing Yttrium during Isothermal Heating at 1473 K. Metals 2019, 9, 961. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Zhang, J.; Zhou, L. Precipitation behaviors of titaniferous inclusions in cerium-containingg IF steel. J. Iron Steel Res. 2015, 27, 49–52. [Google Scholar] [CrossRef]
- Wang, R.; Bao, Y.; Yan, Z.; Li, D.; Kang, Y. Comparison between the surface defects caused by Al2O3 and TiN inclusions in interstitial-free steel auto sheets. Int. J. Miner. Metall. Mater. 2019, 26, 178–185. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Zhang, F.; Fu, X.; Li, H.; Yang, C. Experimental and DFT study on cerium inclusions in clean steels. J. Rare Earths 2021, 39, 477–486. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.; Su, Y. Effect of adding cerium on microstructure and morphology of Ce-based inclusions formed in low-carbon steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Y.; Zhang, S.; Zhang, L. Effect of rare earth element yttrium modified inclusion on corrosion properties of stainless steel. Mater. Today Commun. 2024, 39, 109185. [Google Scholar] [CrossRef]
- Ahmad, S.; Herrmann, M.; Mahmoud, M.M.; Leiste, H.; Lippmann, W.; Seifert, H.J. Crystallisation studies of RE2O3-Al2O3-SiO2 glasses under long heat-treatment conditions. J. Alloys Compd. 2016, 688, 762–774. [Google Scholar] [CrossRef]
- Yang, Y.; Zhan, D.; Lei, H.; Li, Y.; Liu, X.; Jiang, Z.; Zhang, H. Research on Inclusion Evolution during Re-Heating Process in Ti-Zr Deoxidized Low Carbon Steel. Metall. Mater. Trans. B 2021, 52, 1839–1853. [Google Scholar] [CrossRef]
- Yang, Y.; Zhan, D.; Lei, H.; Qiu, G.; Jiang, Z.; Zhang, H. Formation of Non-Metallic Inclusion and Acicular Ferrite in Ti–Zr Deoxidized Steel. ISIJ Int. 2019, 59, 1545–1551. [Google Scholar] [CrossRef]
- Yin, H.; Shibata, H.; Emi, T.; Suzuki, M. Characteristics of Agglomeration of Various Inclusion Particles on Molten Steel Surface. ISIJ Int. 1997, 37, 946–955. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, C.; Liu, C.; Liu, C. Agglomeration Characteristics of Various Inclusions in Al-killed Molten Steel Containing Rare Earth Element. Metall. Mater. Trans. B—Process Metall. Mater. Process. Sci. 2020, 51, 2585–2595. [Google Scholar] [CrossRef]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]

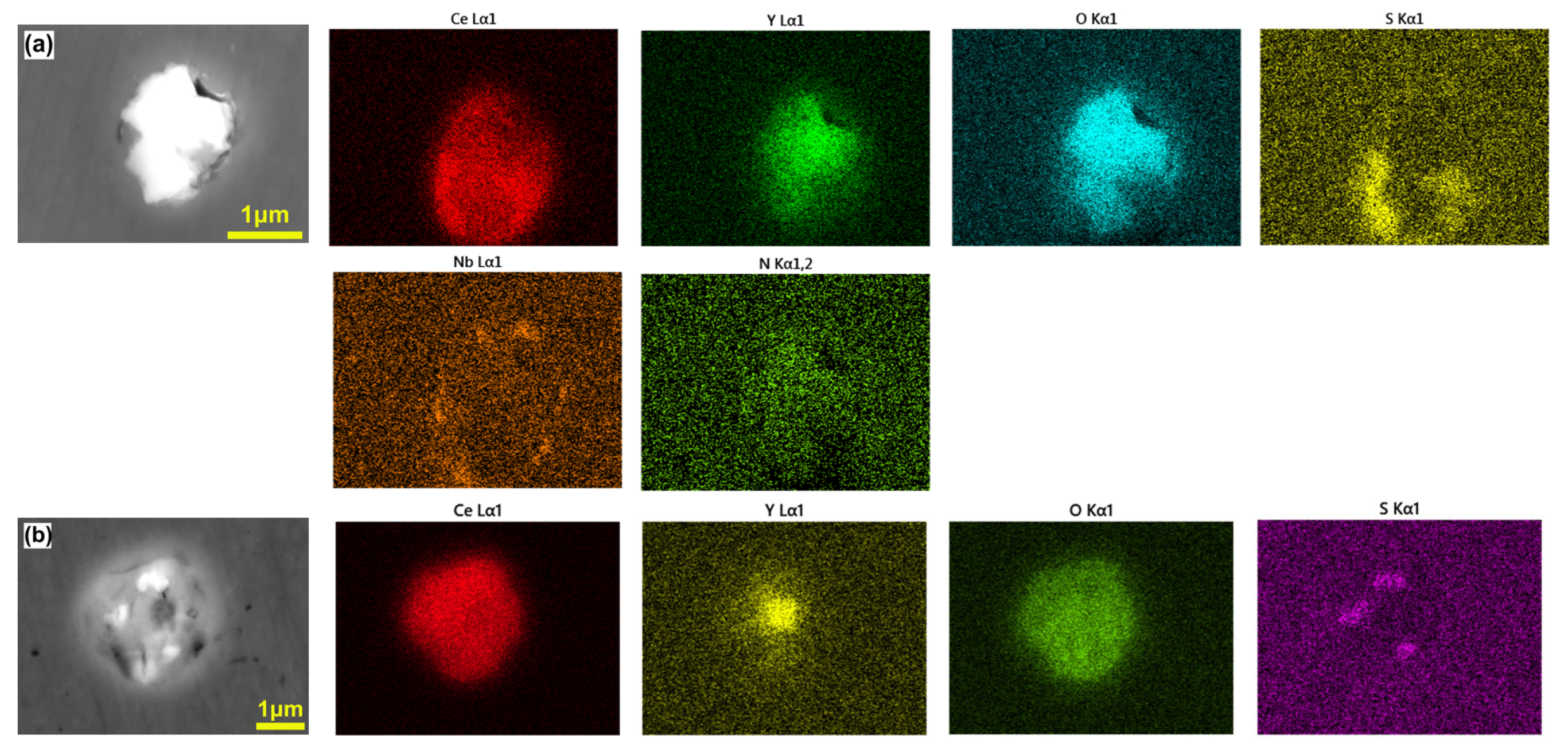
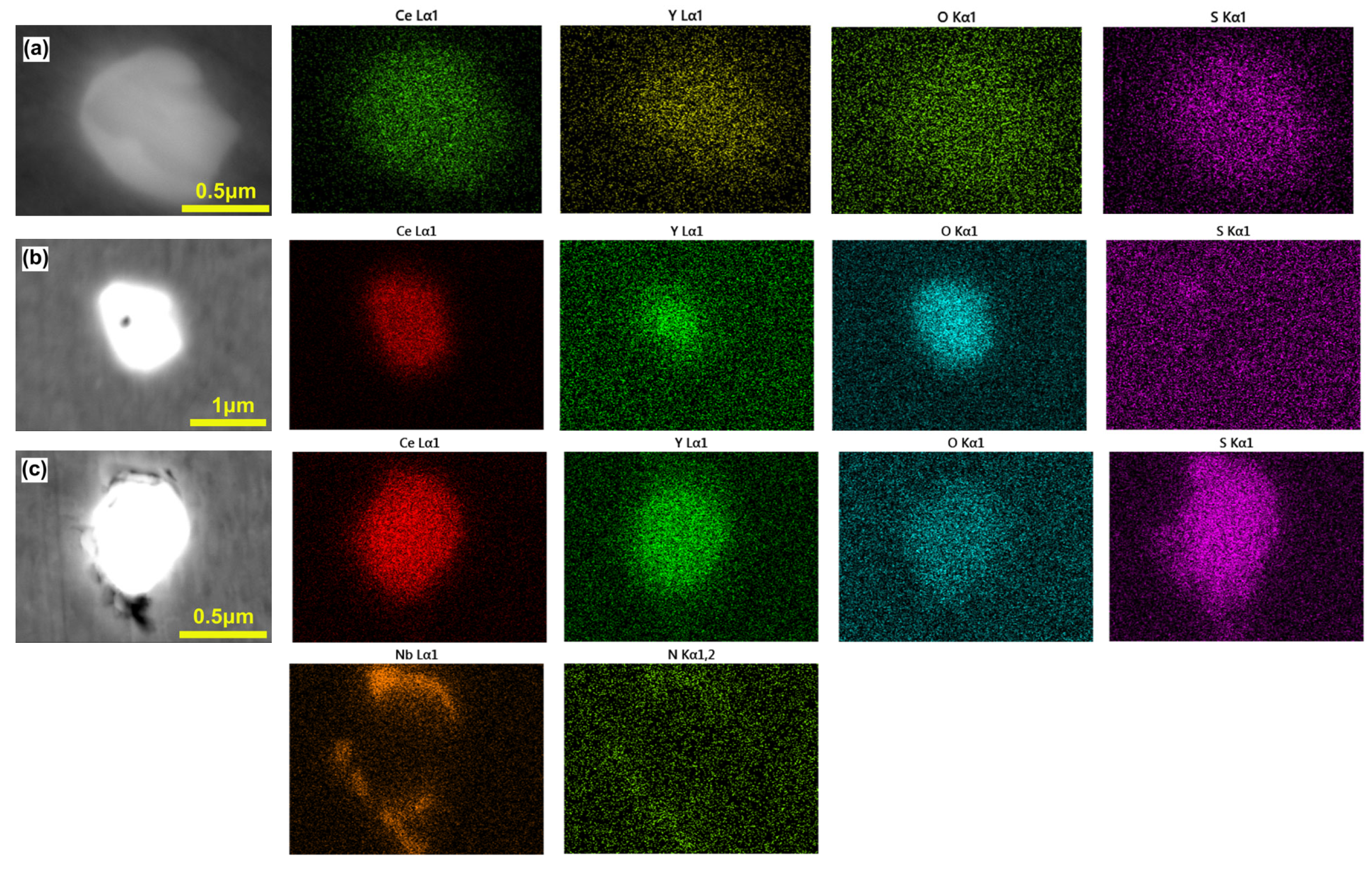
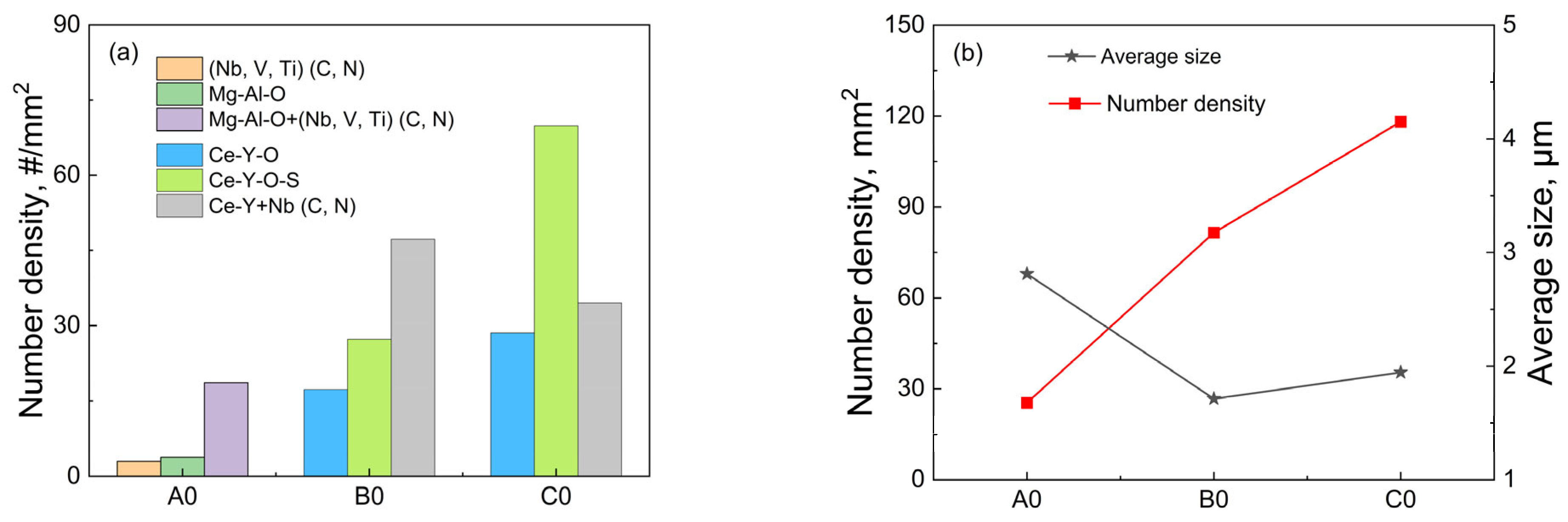
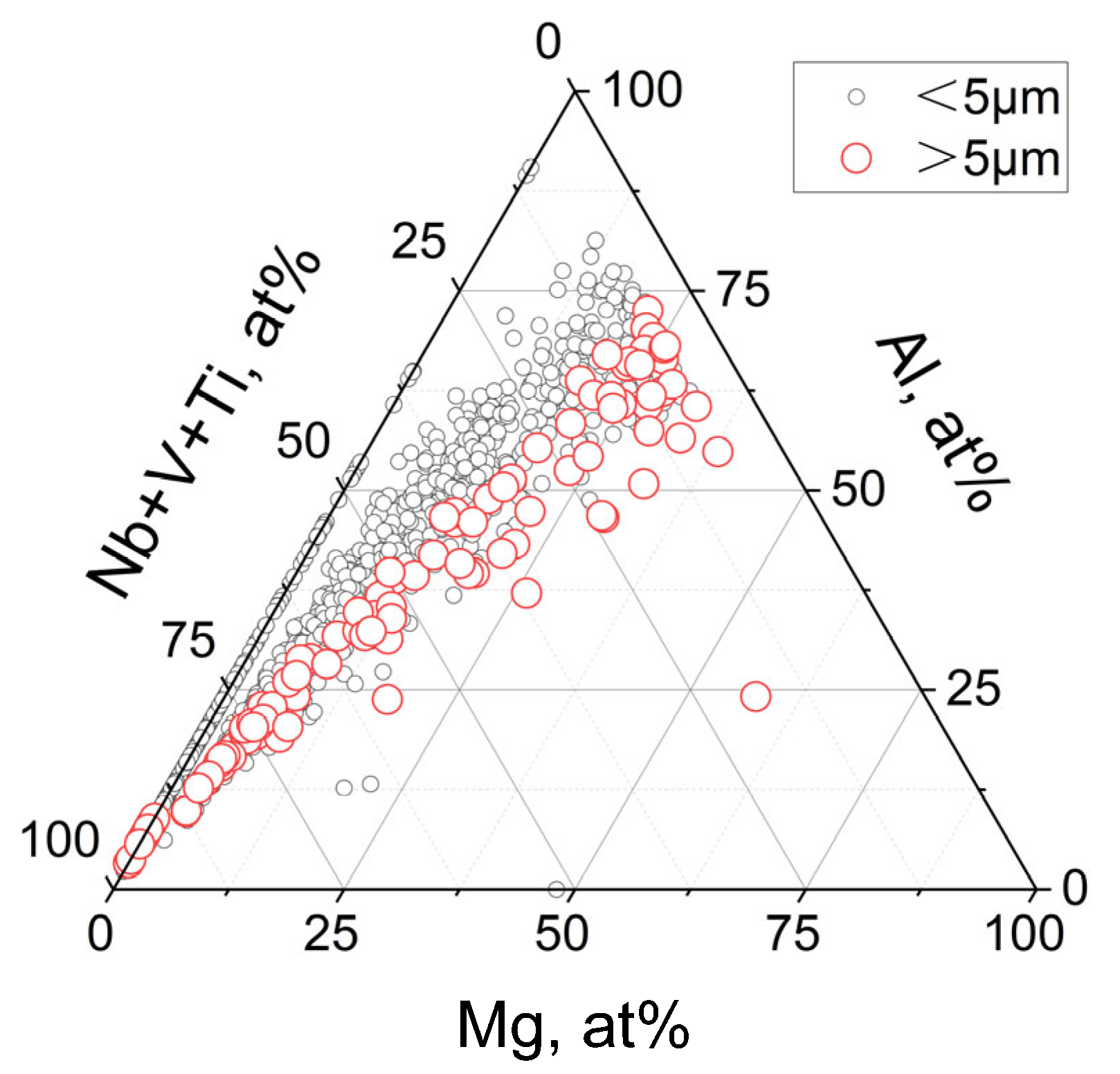
| Steel | C | Si | V | Cr | Mo | Nb | Mn | Al | Ti | N | O | S | Ce | Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A0 | 0.10 | 0.49 | 0.22 | 9.36 | 1.02 | 0.073 | 0.50 | 0.029 | 0.0006 | 0.047 | 0.0022 | 0.001 | 0 | 0 |
| B0 | 0.11 | 0.49 | 0.20 | 9.05 | 0.97 | 0.070 | 0.46 | 0.0044 | 0.0015 | 0.039 | 0.0012 | 0.0012 | 0.015 | 0.012 |
| C0 | 0.10 | 0.54 | 0.21 | 9.24 | 1.05 | 0.065 | 0.46 | 0.0045 | 0.0010 | 0.066 | 0.0006 | 0.0014 | 0.022 | 0.042 |
| Formula of Reactions | (1873 K) |
|---|---|
| (i,j) | C | N | O | Si | Mn | S | Al | Ce | Y |
|---|---|---|---|---|---|---|---|---|---|
| O | −0.45 | 0.057 | −0.2 | −0.131 | −0.021 | −0.133 | −3.9 | −0.57 | −16.3 |
| Ce | 0.397 | −6.612 | −5.03 | 0.13 | −10.34 | −2.67 | −0.008 | ||
| S | 0.11 | 0.01 | −0.27 | 0.063 | −0.026 | −0.028 | 0.035 | −2.36 | −0.55 |
| Al | 0.091 | −0.058 | −6.6 | 0.0056 | 0.035 | 0.03 | 0.045 | −0.5114 | |
| Y | −0.22 | −3.55 | −90.7 | −7.34 | −0.006 |
| Phase | Crystal Type | Lattice Parameters (10−10 m) |
|---|---|---|
| BCC | cubic | a = b = c = 2.867 |
| Al2O3 | hexagonal | a = b = 4.759, c = 12.99 |
| NbN | cubic | a = b = c = 4.442 |
| TiN | cubic | a = b = c = 4.235 |
| Ce2O3 | hexagonal | a = b = 3.891, c = 6.059 |
| Ce2O2S | hexagonal | a = b = 4.002, c = 6.888 |
| Y2O3 | cubic | a = b = c = 10.607 |
| CeS | cubic | a = b = c = 5.776 |
| Inclusion Type | Inclusion//NbN | [uvw]s | [uvw]n | d[uvw]s | d[uvw]n | θ | |
|---|---|---|---|---|---|---|---|
| Al2O3 | (0001)//(100) | [100] | 4.759 | 4.442 | 0 | 6.2 | |
| [102] | 4.759 | 4.966 | 3.43 | ||||
| [001] | 8.243 | 8.884 | 0 | ||||
| Ce2O3 | (0001)//(110) | [001] | 3.891 | 4.442 | 0 | 9.3 | |
| 3.891 | 3.847 | 5.26 | |||||
| 7.782 | 6.663 | 10.53 | |||||
| Ce2O2S | (0001)//(110) | [001] | 4.002 | 4.442 | 0 | 13.3 | |
| 4.002 | 3.847 | 5.26 | |||||
| 6.932 | 9.423 | 0 | |||||
| Y2O3 | (111)//(110) | 7.500 | 3.141 | 0 | 13.5 | ||
| 12.991 | 3.847 | 15 | |||||
| [001] | 7.500 | 2.221 | 30 | ||||
| CeS | (111)//(110) | 7.074 | 3.847 | 15 | 18.9 | ||
| [001] | 4.084 | 2.221 | 30 | ||||
| 4.084 | 5.441 | 5.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, G.; Shi, C.; Tang, Z.; Jia, L.; Zhao, Y.; Wang, S.; He, X. Effect of Ce-Y Composite Addition on the Inclusion Evolution in T91 Heat-Resistant Steel. Materials 2025, 18, 1459. https://doi.org/10.3390/ma18071459
Liu J, Li G, Shi C, Tang Z, Jia L, Zhao Y, Wang S, He X. Effect of Ce-Y Composite Addition on the Inclusion Evolution in T91 Heat-Resistant Steel. Materials. 2025; 18(7):1459. https://doi.org/10.3390/ma18071459
Chicago/Turabian StyleLiu, Jun, Gen Li, Chengbin Shi, Zhengxin Tang, Lei Jia, Yu Zhao, Shijun Wang, and Xikou He. 2025. "Effect of Ce-Y Composite Addition on the Inclusion Evolution in T91 Heat-Resistant Steel" Materials 18, no. 7: 1459. https://doi.org/10.3390/ma18071459
APA StyleLiu, J., Li, G., Shi, C., Tang, Z., Jia, L., Zhao, Y., Wang, S., & He, X. (2025). Effect of Ce-Y Composite Addition on the Inclusion Evolution in T91 Heat-Resistant Steel. Materials, 18(7), 1459. https://doi.org/10.3390/ma18071459







